Significance
Climate change is causing widespread damage to the world’s tropical coral reefs, via increases in cyclones and mass bleaching. Healthy populations of reef fishes facilitate recovery from such events, and recruitment of juvenile fish is influenced by acoustic cues that guide larval orientation, habitat selection, and settlement to reefs. Our matched recordings of Australia’s Great Barrier Reef before and after recent severe degradation demonstrate major changes to natural reef sound. In field experiments using these recordings, we show the potential impact of such acoustic changes. Postdegradation reef sounds were less attractive to young fishes than their predegradation equivalents. Reductions in fish settlement, caused by acoustic changes, may threaten the recovery potential of degraded coral reefs.
Keywords: acoustics, climate change, coral reefs, Great Barrier Reef, settlement
Abstract
Coral reefs are increasingly degraded by climate-induced bleaching and storm damage. Reef recovery relies on recruitment of young fishes for the replenishment of functionally important taxa. Acoustic cues guide the orientation, habitat selection, and settlement of many fishes, but these processes may be impaired if degradation alters reef soundscapes. Here, we report spatiotemporally matched evidence of soundscapes altered by degradation from recordings taken before and after recent severe damage on Australia’s Great Barrier Reef. Postdegradation soundscapes were an average of 15 dB re 1 µPa quieter and had significantly reduced acoustic complexity, richness, and rates of invertebrate snaps compared with their predegradation equivalents. We then used these matched recordings in complementary light-trap and patch-reef experiments to assess responses of wild fish larvae under natural conditions. We show that postdegradation soundscapes were 8% less attractive to presettlement larvae and resulted in 40% less settlement of juvenile fishes than predegradation soundscapes; postdegradation soundscapes were no more attractive than open-ocean sound. However, our experimental design does not allow an estimate of how much attraction and settlement to isolated postdegradation soundscapes might change compared with isolated predegradation soundscapes. Reductions in attraction and settlement were qualitatively similar across and within all trophic guilds and taxonomic groups analyzed. These patterns may lead to declines in fish populations, exacerbating degradation. Acoustic changes might therefore trigger a feedback loop that could impair reef resilience. To understand fully the recovery potential of coral reefs, we must learn to listen.
Coral reefs are subject to intense and increasing damage from anthropogenic climate change (1–3). The likelihood of reefs recovering from degradation and returning to environments characterized by live coral, as opposed to undergoing phase shifts to persistent macroalgal-dominated states, is determined by reef resilience (4, 5). The abundance and composition of fish communities is an important component of reef resilience (6, 7). Populations of many reef fishes are sustained by recruitment, whereby young fish with a pelagic larval stage use a range of sensory cues to detect, orient toward, and settle to suitable benthic habitats at night (8–10). Degraded reefs receive lower rates of settlement (11, 12), compromising recovery potential (13). However, investigations of the mechanisms causing reduced recruitment in degraded habitats are thus far limited to laboratory choice-tests and focus only on visual and olfactory cues (e.g., refs. 13–15).
Acoustic cues are important for fish recruitment because they facilitate offshore detection of reefs by young fishes at the end of a planktonic larval phase (16–18). Further, reef sounds can act as indicators of habitat quality, with acoustic parameters varying across reefs that are home to different sound-producing communities (19, 20). In this study, we compared nocturnal soundscapes from coral reefs around Lizard Island, a continental midshelf island in Australia’s northern Great Barrier Reef (GBR), before and after the most severe period of degradation in their recorded history (21). Recordings of 10 lagoonal reefs were taken in November 2012 and repeated in the same locations and times and under similar conditions in November 2016. In the intervening period, three major disturbance events had caused considerable reef degradation: Cyclone Ita occurred in April 2014 [Category 5; 40% reductions in lagoonal reef coral cover and significantly altered fish-community dynamics (22, 23)] and was followed by Cyclone Nathan in March 2015 (Category 3) and the most severe global mass-bleaching event on record in early 2016 [over 60% of live coral bleached (21)].
We then used two complementary field experiments to assess the impact of changes in the soundscape associated with degradation on the attractiveness of reef-sound and settlement behaviors of young reef fishes. Light traps (investigating presettlement larval preferences) and patch reefs (investigating juvenile settlement behavior) were coupled with loudspeakers broadcasting playback of pre- and postdegradation reef sound and an ambient-sound (open-ocean) control. The abundance of larvae and juveniles associated with each sound treatment was used to assess the relative attractiveness of pre- and postdegradation coral reef soundscapes to settlement-stage fishes.
Results and Discussion
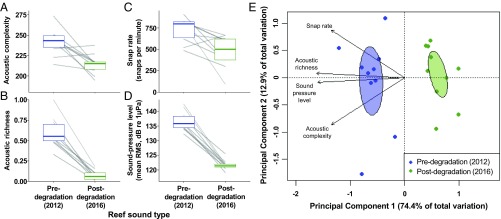
Pre- and postdegradation sound recordings differed across four complementary ecoacoustic indices: the 2012 predegradation recordings had significantly higher acoustic complexity indices (ACI), acoustic richness (AR), invertebrate snap rates (SR), and sound-pressure levels (SPL) than their 2016 postdegradation equivalents (Wilcoxon signed-rank tests, n = 10; ACI: V = 52, P = 0.010; AR: V = 55, P = 0.002; SR: V = 52, P = 0.010; SPL: V = 55, P = 0.002; Fig. 1 A–D and Table 1). Principal Component Analysis identified pre- and postdegradation recordings as separate groups (Fig. 1E), whose division was supported by significant differences in multivariate analysis (PERMANOVA: F1,18 = 11.59, P = 0.005, 999 permutations). These consistent differences between the same reefs before and after degradation are considerably greater than previously reported natural temporal variation in reef soundscapes (e.g., refs. 24 and 25) and are likely to significantly impact both their acoustic characteristics and the distance from reefs at which they are audible to larval fishes. Mechanistically, soundscape change might be the result of a decreased abundance of biophonic organisms [for example, the degradation-linked changes to the study site’s fish communities (22, 23)], changes in the soniferous behavior of organisms [for example, lower snapping rates in shrimp exposed to unfavorable environmental conditions (26)], or a combination of both possibilities.
Fig. 1.
Acoustic analysis of pre- and postdegradation nocturnal reef soundscapes. (A) Acoustic complexity index, (B) acoustic richness, (C) invertebrate snap rate, and (D) sound-pressure level calculated from 30-s site-matched recordings (FFT size = 512) of nocturnal reef noise taken in November 2012 (predegradation) and November 2016 (postdegradation) (n = 10). Shown are results for each reef (gray lines) and overall median and 25% and 75% quartiles (colored boxes). (E) Principal Component Analysis based on a correlation matrix of the four ecoacoustic indices from site-matched pre- and postdegradation soundscapes. Areas of ellipses represent SEs of associated points.
Table 1.
Acoustic indices used to compare pre- and postdegradation reef recordings
| Acoustic index | Mechanism | Calculation method | Citations |
| Acoustic complexity index | Measures the variation in the intensity of changing frequencies over time. | The package Seewave (50) on R v3.3.0 (www.r-project.org). | Development: ref. 33 |
| Previous use: refs. 24 and 51–54 | |||
| Acoustic richness | Combines a previous Shannon–Weiner-based acoustic entropy index (55) with a rank-based incorporation of the median of the amplitude envelope. | The package Seewave (50) on R v3.3.0. | Development: ref. 34 |
| Previous use: ref. 54; similar Shannon–Weiner-based acoustic entropy indices used by refs. 51 and 55. | |||
| Snap rate | Counts the number of independent snap sounds occurring in 30 s, doubling this to achieve a per-minute value. | A custom-designed MATLAB (MathWorks, Inc.) algorithm detecting snap events (see Materials and Methods for details). | Development: Custom-designed. |
| Previous use: Similar algorithms used by refs. 51, 53, and 56–59. | |||
| Sound-pressure level | Measures the root-mean-squared amplitude level (dB re 1 µPa) across the full-frequency bandwidth (0.01–24 kHz) using a Hann window function (FFT size = 512). | The PAMGuide analysis package (38) on MATLAB. | Development: A simple measurement of sound intensity. |
| Previous use: refs. 19, 24, 57, 59, and 60. |
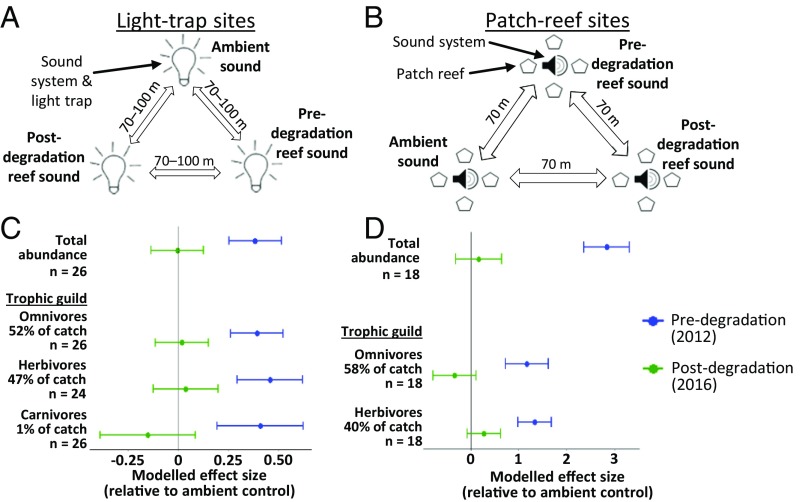
Complementary field experiments demonstrated that the attractiveness of reef sounds and settlement behaviors of young fishes were negatively affected by the changes in reef soundscapes arising from degradation. Triplicate sets of light traps (identical to “small traps” used in ref. 27) and patch reefs (similar to ref. 28) were deployed for 18 consecutive nights centered around the new moon of November 2016 (Fig. 2 A and B). Sound treatment significantly affected fish abundance in both the light-trap [Generalized Linear Mixed Model (GLMM): χ2 = 12.283, df = 2, P = 0.002; Fig. 2C, Fig. S1A, and Table S1] and the patch-reef [Linear Mixed Model (LMM): χ2 = 28.957, df = 2, P < 0.001; Fig. 2D, Fig. S1B, and Table S2] experiments. Playbacks of predegradation soundscapes were associated with higher total abundances of fishes than postdegradation soundscapes, which showed no significant difference to ambient controls [Tukey’s honest significant difference (HSD) tests, light traps: predegradation vs. postdegradation P = 0.007, predegradation vs. ambient P = 0.009, postdegradation vs. ambient P = 0.999; patch reefs: predegradation vs. postdegradation P < 0.001, predegradation vs. ambient P < 0.001, postdegradation vs. ambient P = 0.935]. Lower levels of attraction and settlement to postdegradation soundscapes might be facilitated by quieter reef sound propagating over a smaller area, resulting in detection by fewer fishes (see refs. 16, 17, and 29 for discussions of the spatial scale of reef-sound propagation). Alternatively, young fishes might exhibit qualitatively different behavioral responses to pre- and postdegradation reef soundscapes, irrespective of detectability. In either case, these results show that acoustics may be a key mechanistic driver of previously observed reductions in settlement of fishes to degraded reefs (13, 30).
Fig. 2.
Effects of sound treatment on abundance of recruiting reef fish. (A and B) Experimental setup at (A) light-trap and (B) patch-reef sites; traps and reefs were location-fixed, and sound treatments were rotated in a randomized counterbalanced block design. (C and D) Modeled effects of pre- and postdegradation reef-sound playback on abundance of fish collected from (C) light traps and (D) patch reefs, relative to an ambient-sound control. Shown are results for models analyzing total abundance and all trophic guilds with at least 50% frequency of occurrence. Each row represents a separate model; in each case, the y axis represents the baseline abundance associated with ambient controls. Points represent relative effect sizes associated with the fixed effect of sound treatment; error bars represent associated SEs. Total abundance of each trophic guild as a percentage of the experiment’s total catch and the number of experimental replicates analyzed (n) are given on the y axis. Results come from Generalized Linear Mixed Models and Linear Mixed Models, which all display statistically significant effects of sound treatment (P < 0.05; see Tables S1 and S2). For details of trophic guild classifications, see Dataset S1.
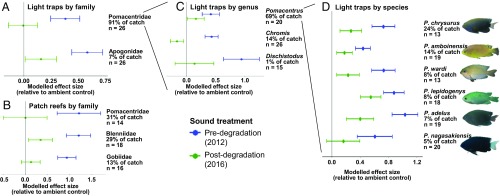
Differences among sound treatments in abundances of young fishes were consistent when tested at multiple trophic and taxonomic levels. All fishes were identified to family, those from the most abundant family (Pomacentridae: damselfishes) were identified to genus, and those from the most abundant genus (Pomacentrus) were identified to species. All fishes were further classified into broad trophic guilds as either herbivores, omnivores, or carnivores (Dataset S1). All identified trophic and taxonomic groups that occurred in at least 50% of replicates showed significant responses to sound treatment (light traps: GLMMs, χ2 = 6.712–38.997, df = 2, P ≤ 0.001–0.035; patch reefs: LMMs, χ2 = 7.115–18.078, df = 2, P ≤ 0.001–0.029; Figs. 2 C and D and 3, Figs. S1 C and D and S2, and Tables S1 and S2). Analyses of trophic guilds in both light-trap and patch-reef experiments always revealed significantly higher abundances associated with playback of predegradation soundscapes relative to postdegradation equivalents (Fig. 2 C and D, Fig. S1 C and D, and Tables S1 and S2). Further, there was no significant difference between abundances associated with postdegradation soundscapes and ambient controls for herbivores in both experiments (Fig. 2 C and D, Fig. S1 C and D, and Tables S1 and S2). Qualitatively similar patterns were observed across three families in the patch-reef experiment (Fig. 3B, Fig. S2B, and Table S2) and two families, three genera, and six species in the light-trap experiment (Fig. 3 A, C, and D, Fig. S2 A, C, and D, and Table S1); significant effects of sound treatment were taxonomically ubiquitous, with the predegradation treatment being the most attractive in all cases and significantly more attractive than the postdegradation treatment in all but one case. Additionally, there was no significant effect of sound treatment on the exponential Shannon–Weiner diversity index in either experiment (LMMs, light traps: χ2 = 1.245, df = 2, P = 0.537; patch reefs: χ2 = 4.045, df = 2, P = 0.132; Tables S1 and S2). It is important to note that several important fish families (e.g., Scaridae, Kyphosidae) are not typically attracted to either patch reefs or light traps, and the patterns described herein may not be taxonomically ubiquitous. However, the range of trophic and taxonomic groups covered by this study represents an important subsection of fishes common to coral reefs. For example, damselfishes represent up to 50% of biomass in reef-fish communities (31), and herbivorous fishes play particularly important functional roles in promoting reef resilience (6, 7). The reported differences in settlement therefore have potentially strong implications for wider reef health.
Fig. 3.
Effects of sound treatment at different taxonomic levels. Modeled effects of pre- and postdegradation reef-sound playback, relative to an ambient-sound control, on abundance of fishes in taxonomic groups with at least 50% frequency of occurrence. Shown are (A) families in light traps, (B) families on patch reefs, (C) genera in light traps, and (D) species in light traps, with total abundance as a percentage of the experiment’s total catch and number of replicates analyzed (n) given on the y axis. Each row represents a separate model; in each case, the y axis represents the baseline abundance associated with ambient controls. Points represent relative-effect sizes associated with the fixed effect of sound treatment; error bars are associated SEs. Results come from Generalized Linear Mixed Models and Linear Mixed Models that calculated statistically significant effects of the sound treatment (P < 0.05; see Tables S1 and S2). Images courtesy of Andy Lewis and Mark Shepherd (Life Island Field Guide, Sydney).
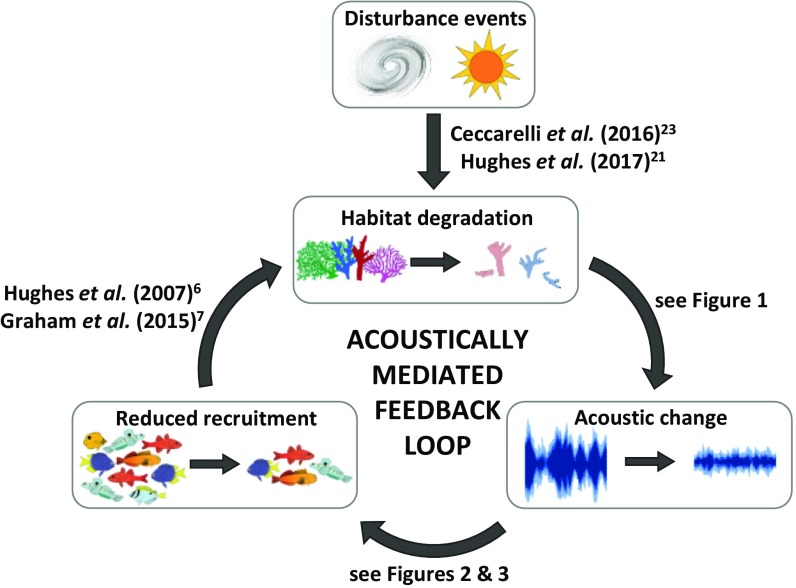
Our field-based study provides evidence for an acoustic link between reef degradation and two processes fundamental to fish recruitment: larval preferences and juvenile settlement behavior. These experimental results demonstrate that predegradation soundscapes are more attractive than postdegradation soundscapes. Future work could valuably explore how attractive the different soundscapes are in isolation because our experimental design does not allow an estimate of how much settlement to isolated postdegradation soundscape reefs might change compared with isolated predegradation reefs. Reduced recruitment potentiates a damaging feedback loop, whereby subsequent lower grazing rates associated with fewer herbivores might slow recovery and create the conditions for increasing macroalgal dominance (6, 7) (Fig. 4). This is of global concern, given current predictions of increasing frequency and severity of disturbances to reefs throughout the tropics (1–3). Acoustic changes caused by degradation may be an important determinant of the recovery potential of impacted coral reefs.
Fig. 4.
Schematic illustrating the potential for an acoustically mediated feedback loop that impairs reef recovery. Disturbance-induced habitat degradation causes acoustic change on reefs. This might reduce recruitment, further exacerbating degradation as a reduction in grazing facilitates macroalgal dominance. Evidence for each step provided on relevant arrows; pictures of fish adapted from ref. 13.
Materials and Methods
This study took place during November 2012 and November–December 2016 in the lagoon southwest of Lizard Island Research Station (14°40.8′S, 145°26.4′E; Fig. S3). Lizard Island is a continental midshelf island in the northern GBR with an extensive surrounding fringing reef and lagoonal system. Permission and ethical approval were granted by Lizard Island Research Station, Great Barrier Reef Marine Park Authority (G13/35909.1), James Cook University (A2408, A2361), and University of Exeter (2013/247).
Pre- and Postdegradation Acoustic Recordings.
Pre- and postdegradation recordings were site-matched to within 10 m by GPS coordinates and time-matched to within 45 min, 1–2 h after sunset. Neighboring sites were an average of 127 m apart (mean ± SE: 127.0 ± 36.5, range: 50–400; Fig. S3). Recordings were made during midtide in 2- to 6-m water depth (tidal range in the area is 0.5–2.7 m), in sea states between 0 and 2 on the Beaufort scale and never during rain. Sound-pressure recordings were taken in both 2012 and 2016 using an omnidirectional hydrophone (HiTech HTI-96-MIN with inbuilt preamplifier, manufacturer-calibrated sensitivity −164.3 dB re 1V/μPa; frequency range 0.002–30 kHz; calibrated by manufacturers; High Tech, Inc.) connected to a digital recorder (PCM-M10, 48 kHz sampling rate; Sony Corporation). The hydrophone was freely suspended 1 m above the seabed from a rope-anchored raft (>1.5-m maximum length) that contained the digital recorder and battery; this avoided unwanted noise from waves slapping on the hull of a larger vessel. Since many fishes primarily sense the particle-motion component of underwater sound (32), simultaneous particle-motion recordings were taken in 2016 using a calibrated triaxial accelerometer (M20L; sensitivity following a curve over the frequency range 0–2 kHz; calibrated by manufacturers; Geospectrum Technologies) connected to a digital 8-track recorder (F8 field recorder, sampling rate 48 kHz; Zoom Corporation). The accelerometer was connected to the same raft as the hydrophone but was fixed 1 m above the seabed to a weighted stand to prevent the triaxial sensors from moving in the water column. These recordings were used to assess the accuracy of playback in the particle-motion domain; suitable equipment for measurements of particle motion was not available in 2012. Spectrograms were manually inspected for incidences of external noise from equipment knocking; where this occurred, the affected sections were removed using Audacity 2.1.2 software (www.audacityteam.org).
The 10 spatiotemporally matched pairs of pre- and postdegradation recordings were compared over four acoustic indices used previously in marine ecoacoustic studies, each describing a different aspect of the soundscape (for full information, see Table 1). Analyses were performed on 30-s sections of recordings, full-range bandwidth, fast Fourier transform (FFT) size 512. The acoustic complexity index measures the variability of acoustic energy in the soundscape (33). Acoustic richness is an entropy-based index designed to be complementary to the acoustic complexity index (34). Snap rate is a count per minute of the number of independent snap sounds in reef noise. Snaps were defined as events that (i) exceed four SDs above median amplitude; (ii) drop below median amplitude again within 0.125 ms; and (iii) do not occur within a “dead zone” of 1 ms beyond previously counted signals (to avoid double-counting “echo” signals). These events are often associated with rapid claw closure by snapping shrimp [Alpheidae; the dominant biotic sound source in shallow tropical marine ecosystems (35, 36)]. Sound-pressure level is a measure of the average acoustic energy in the soundscape.
Comparisons between pre- and postdegradation recordings were made for each index using pairwise Wilcoxon tests. Multivariate analysis consisted of a pairwise permutational multivariate analysis of variance (PERMANOVA, 999 permutations) based on a Euclidean distance matrix and a Principal Component Analysis (PCA).
Experimental Assays.
Light traps assessed presettlement larval preferences and facilitated coverage of a wide and diverse taxonomic range as they attract large numbers of fish. Traps consisting of a single white fluorescent light housed in a Plexiglas chamber containing four horizontal tapered slits for fish entry (identical to small traps used by ref. 27) floated 0.5 m subsurface in 10–20 m water depth. Two triplicate sets of traps were deployed above sand flats 700–800 m from shore, 200 m from the nearest reef, with a distance of 70–100 m between within-set neighbors (Fig. 2 and Fig. S3).
Artificial patch reefs assessed the juvenile settlement behavior of wild fishes to ecologically realistic reef habitat in situ. Reefs consisting of 4 × 0.1 m3 of dead coral rubble arranged in a 2 × 2-m-square formation per reef (similar to ref. 28) were arranged on sand flats in 2–5 m water depth. One triplicate set of reefs was deployed on sand flats 100–200 m from shore, 100 m from the nearest reef, with a distance of 70 m between within-set neighbors.
To reduce issues of pseudoreplication, five different triplicate sets of playback tracks were used, with sound treatment rotated around the three location-fixed traps or reefs of a set each night within randomly counterbalanced blocks. Paired reef-sound playback tracks were created by continuously looping 30-s sections of one of five of the analyzed pairs of pre- or postdegradation reef-sound recordings. Ambient-sound controls were created by looping 10-s sections of open-ocean sound recordings, taken 2 km seaward beyond the outer Great Barrier Reef in November 2016, with equipment identical to reef-sound recordings and suspended 1 m below the surface. All tracks were created using Audacity and adjusted to achieve equivalence between the received broadband root-mean-squared average amplitude levels in recordings of playback and those in original recordings at 1 m distance [as per ref. 37; analyzed using SASLabPro v5.2.07 (Avisoft Bioacoustics); spectra shown in Fig. 5]. Playback systems consisted of a loudspeaker (University Sound UW-30; maximal output 156 dB re 1 μPa at 1 m, frequency response 0.1–10 kHz; Lubell Labs) powered by an amplifier (M033N, 18 W, frequency response 0.04–20 kHz; Kemo Electronic GmbH), an MP3 player (SanDisk Clip Jam), and a battery (12v 12Ah sealed lead-acid). Loudspeakers were suspended from floating buoys 1 m below the surface, 1 m away from light traps, and from submerged stands 1 m above the seabed in the middle of patch reefs. Recordings of playback were taken from 1 m away from the loudspeaker at the patch-reef experimental site using the same protocol as original recordings; power spectral densities were compared for reef sound and ambient recordings, and recordings of their playback, using the PAMGuide (38) and paPAM (32) analysis packages on MATLAB. Playback using loudspeakers is known to be an imperfect replication of original recordings, but analyses of spectral content and sound levels showed that differences in the characteristics of the original recordings were retained in playback in both sound-pressure and particle-motion domains (Fig. 5).
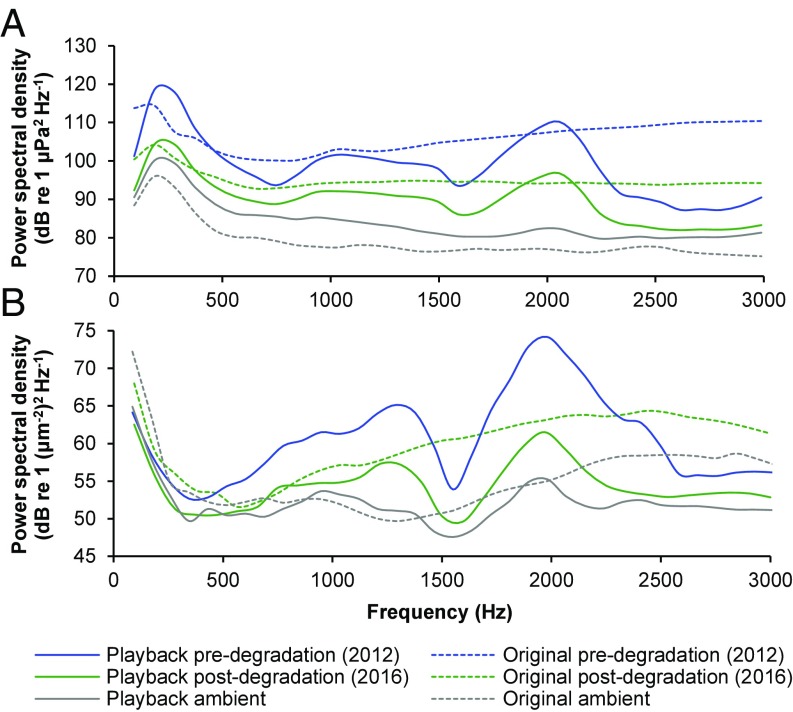
Fig. 5.
Power spectral density of original and played-back sound recordings. Mean spectral content in (A) sound-pressure and (B) particle-motion domains of all original field recordings of reef noise and ambient conditions (dashed lines) and playback of those recordings at experimental sites (solid lines). Thirty-second sections of all five triplicate sets of recordings were combined and analyzed across 0–3,000 Hz as the likely hearing range of many coral reef fish larvae (50, 51) (spectrum level units averaged, Hamming window function, FFT length = 512). Predegradation (2012) field recordings were taken only in the sound-pressure domain.
There is considerable uncertainty and likely to be high variability in the hearing thresholds of different larval fishes (see refs. 16, 17, 39, and 40). This means that accurate quantification of the distances at which loudspeakers were audible to fishes in this study is impossible. However, starting and stopping playback at any of the three experimental reefs did not affect the mean sound level at either of its adjacent reefs in either the sound-pressure or particle-motion domain, suggesting there was no overlap of playback tracks between neighboring reefs.
Juvenile settlement is a predominantly nocturnal behavior, so playback tracks were started 0.5–1 h before sunset and fish were collected from traps and reefs the next morning for identification and counting by an experimentally blind observer (T.A.C.G.). Light-trap catches were collected 0.5–1 h after sunrise, with data from the whole set discounted if any of the lights in the three traps were not on at collection; this occurred on 10/36 occasions. Patch-reef collections were performed 2–4 h after sunrise with the aid of SCUBA, clove oil, and hand nets; Apogonidae were not counted in this experiment, as their disparate shoaling behavior and low site-fidelity prevented accurate capture with this method. Reefs were cleaned daily with a brush to prevent buildup of algal growth. All fish were transported back to shore for identification and released unharmed onto lagoon reefs later the same day, at a minimum distance of 750 m from the experimental sites. Nonsettlement-stage (i.e., postsettlement juvenile and adult) and nonreef (e.g., Clupeidae and Scombridae) fishes were not counted in either experiment, but were removed from patch reefs daily. Fish collected by light traps were briefly (<15 s) placed in trays of shallow water (<1 cm), photographed in lateral view, and counted and identified from photographs later; patch-reef fish were identified on shore.
Differences between sound treatments in fish abundance and diversity in both the light-trap and the patch-reef experiments were evaluated using mixed models on raw count data in R. Date, playback track ID, and trap or reef ID (nested within experimental site ID for the light-trap experiment) were included as random terms, and significant effects of sound treatment were confirmed by comparisons with a null model and further analyzed with post hoc Tukey’s HSD testing. Light-trap abundance data were tested using negative binomial GLMMs to correct for positive skew, adjusting theta values to minimize AIC scores; all other models used LMMs. In all models, visual examination of residual plots did not reveal any obvious deviations from homoscedasticity or normality. Effect sizes ±SE and variance components for the random term ±SD are shown in model (Tables S1 and S2).
To assess whether differences in abundance among sound treatments were consistent across multiple trophic guilds, fishes were classified as either “herbivores,” “omnivores,” or “carnivores.” Trophic guilds were assigned at the same taxonomic level as fish IDs were completed (i.e., species within the genus Pomacentrus and genera within the family Pomacentridae were each assigned a separate trophic guild, and a single trophic guild was assigned to each of the other families). When considering genera and families, guilds were assigned to all species known to occur in the Lizard Island area [using lists compiled by the Lizard Island Field Guide (lifg.australianmuseum.net.au/Hierarchy.html)], and the most commonly occurring guild in the taxonomic group was chosen as the group’s trophic guild. All Blenniidae observed in the study were in the subfamily Salariinae, and all Serranidae observed were in the subfamily Epinephelinae; for these families, only the relevant subfamilies were considered. Classification was based on published literature (41–47) and FishBase (48), following ref. 49. Herbivores consisted of algal feeders, including scrapers, excavators, grazers, browsers, detritivores, and farmers. Omnivores included corallivores, planktivores, species targeting small benthic invertebrates, and species with a mixed diet. Carnivores included piscivores and species targeting large invertebrates (such as mollusks and crustaceans). For full details of trophic guild classifications, see Dataset S1. GLMM and LMM comparisons were repeated for all trophic guilds that occurred in at least 50% of replicates (n ≥ 13 for light traps; n ≥ 9 for patch reefs); this resulted in the analysis of all three trophic guilds in the light-trap experiment and omnivores and herbivores in the patch-reef experiment.
Similarly, to assess whether differences were consistent at multiple taxonomic levels, comparisons were repeated for all families that displayed at least 50% frequency of occurrence. This allowed analysis of two families in the light-trap experiment and three in the patch-reef experiment. Genera and species from the most abundant family (Pomacentridae in both experiments) were also analyzed individually if they displayed at least 50% frequency of occurrence; these conditions were met by three genera and six species in the light-trap experiment, but never occurred in the patch-reef experiment. It should be noted that as different subsets of the data were used for different trophic and taxonomic groups (i.e., each group did not occur in the same number of experimental replicates), exact values are not directly comparable across levels of trophic and taxonomic analysis. The number of fish in each trophic and taxonomic group that were analyzed represented at least 1% of the total catch of >20,000 fish. Diversity was analyzed at a family level using the exponential Shannon–Weiner diversity index.
Supplementary Material
Acknowledgments
We thank the staff at Lizard Island Research Station for logistical support; the McCormick Group, Emily Lester, Ellen D’Cruz, and Josh Temes for fieldwork assistance; Professor Innes Cuthill, Dr. Robert Ellis, Dr. Robert Thomas, Amy Morris-Drake, and Sam Houston for statistical advice; Dr. Danielle Dixson and True Glass Photography for assistance in figure preparation; and Professor Jane Memmot, Professor Rod Wilson, and Dr. Sophie Nedelec for comments on earlier versions of the manuscript. This work was supported by funding from the Natural Environment Research Council Research Grant NE/P001572/1 (to S.D.S. and A.N.R.), an NERC-Australian Institute of Marine Science CASE GW4+ Studentship NE/L002434/1 (to T.A.C.G.), and an NERC-Marine Scotland Science CASE GW4+ Studentship NE/L002434/1 (to H.R.H.); the Royal Society Research Grant RG160452 (to S.D.S. and A.N.R.); the University of Exeter (S.D.S.); the Australian Research Council Discovery Grant DP170103372 (to M.I.M.); the Australian Institute of Marine Science (M.G.M.); and Cefas (N.D.M.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: Raw data are available from the University of Exeter’s institutional repository at https://doi.org/10.24378/exe.265.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1719291115/-/DCSupplemental.
References
- 1.Spalding MD, Brown BE. Warm-water coral reefs and climate change. Science. 2015;350:769–771. doi: 10.1126/science.aad0349. [DOI] [PubMed] [Google Scholar]
- 2.Hughes TP, et al. Coral reefs in the Anthropocene. Nature. 2017;546:82–90. doi: 10.1038/nature22901. [DOI] [PubMed] [Google Scholar]
- 3.Hughes TP, et al. Spatial and temporal patterns of mass bleaching of corals in the Anthropocene. Science. 2018;359:80–83. doi: 10.1126/science.aan8048. [DOI] [PubMed] [Google Scholar]
- 4.Hughes TP, Graham NAJ, Jackson JBC, Mumby PJ, Steneck RS. Rising to the challenge of sustaining coral reef resilience. Trends Ecol Evol. 2010;25:633–642. doi: 10.1016/j.tree.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 5.Roff G, et al. Phase shift facilitation following cyclone disturbance on coral reefs. Oecologia. 2015;178:1193–1203. doi: 10.1007/s00442-015-3282-x. [DOI] [PubMed] [Google Scholar]
- 6.Hughes TP, et al. Phase shifts, herbivory, and the resilience of coral reefs to climate change. Curr Biol. 2007;17:360–365. doi: 10.1016/j.cub.2006.12.049. [DOI] [PubMed] [Google Scholar]
- 7.Graham NAJ, Jennings S, MacNeil MA, Mouillot D, Wilson SK. Predicting climate-driven regime shifts versus rebound potential in coral reefs. Nature. 2015;518:94–97. doi: 10.1038/nature14140. [DOI] [PubMed] [Google Scholar]
- 8.Doherty P, Fowler T. An empirical test of recruitment limitation in a coral reef fish. Science. 1994;263:935–939. doi: 10.1126/science.263.5149.935. [DOI] [PubMed] [Google Scholar]
- 9.Leis JM, Siebeck U, Dixson DL. How Nemo finds home: The neuroecology of dispersal and of population connectivity in larvae of marine fishes. Integr Comp Biol. 2011;51:826–843. doi: 10.1093/icb/icr004. [DOI] [PubMed] [Google Scholar]
- 10.Salles OC, et al. Coral reef fish populations can persist without immigration. Proc Biol Sci. 2015;282:20151311. doi: 10.1098/rspb.2015.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pratchett MS, et al. Effects of climate-induced coral bleaching on coral-reef fishes–Ecological and economic consequences. Oceanogr Mar Biol. 2008;46:251–296. [Google Scholar]
- 12.Nagelkerken I, Munday PL. Animal behaviour shapes the ecological effects of ocean acidification and warming: Moving from individual to community-level responses. Glob Change Biol. 2016;22:974–989. doi: 10.1111/gcb.13167. [DOI] [PubMed] [Google Scholar]
- 13.McCormick MI, Moore JAY, Munday PL. Influence of habitat degradation on fish replenishment. Coral Reefs. 2010;29:537–546. [Google Scholar]
- 14.Dixson DL, Abrego D, Hay ME. Reef ecology. Chemically mediated behavior of recruiting corals and fishes: A tipping point that may limit reef recovery. Science. 2014;345:892–897. doi: 10.1126/science.1255057. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.Scott A, Dixson DL. Reef fishes can recognize bleached habitat during settlement: Sea anemone bleaching alters anemonefish host selection. Proc Biol Sci. 2016;283:20152694. doi: 10.1098/rspb.2015.2694. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16.Mann DA, Casper BM, Boyle KS, Tricas TC. On the attraction of larval fishes to reef sounds. Mar Ecol Prog Ser. 2007;338:307–310. [Google Scholar]
- 17.Radford CA, Tindle CT, Montgomery JC, Jeffs AG. Modelling a reef as an extended sound source increases the predicted range at which reef noise may be heard by fish larvae. Mar Ecol Prog Ser. 2011;438:167–174. [Google Scholar]
- 18.Simpson SD, Piercy JJB, King J, Codling EA. Modelling larval dispersal and behaviour of coral reef fishes. Ecol Complex. 2013;16:68–76. [Google Scholar]
- 19.Bertucci F, Parmentier E, Lecellier G, Hawkins AD, Lecchini D. Acoustic indices provide information on the status of coral reefs: An example from Moorea Island in the South Pacific. Sci Rep. 2016;6:33326. doi: 10.1038/srep33326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Freeman L, Freeman S. Rapidly obtained ecosystem indicators from coral reef soundscapes. Mar Ecol Prog Ser. 2016;561:69–82. [Google Scholar]
- 21.Hughes TP, et al. Global warming and recurrent mass bleaching of corals. Nature. 2017;543:373–377. doi: 10.1038/nature21707. [DOI] [PubMed] [Google Scholar]
- 22.Brandl SJ, Emslie MJ, Ceccarelli DM. Habitat degradation increases functional originality in highly diverse coral reef fish assemblages. Ecosphere. 2016;7:01557. [Google Scholar]
- 23.Ceccarelli DM, Emslie MJ, Richards ZT. Post-disturbance stability of fish assemblages measured at coarse taxonomic resolution masks change at finer scales. PLoS One. 2016;11:e0156232. doi: 10.1371/journal.pone.0156232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Staaterman E, et al. Celestial patterns in marine soundscapes. Mar Ecol Prog Ser. 2014;508:17–32. [Google Scholar]
- 25.Kaplan MB, Mooney TA, Partan J, Solow AR. Coral reef species assemblages are associated with ambient soundscapes. Mar Ecol Prog Ser. 2015;533:93–107. [Google Scholar]
- 26.Rossi T, Connell SD, Nagelkerken I. Silent oceans: Ocean acidification impoverishes natural soundscapes by altering sound production of the world’s noisiest marine invertebrate. Proc Biol Sci. 2016;283:20153046. doi: 10.1098/rspb.2015.3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meekan MG, Wilson SG, Halford A, Retzel A. A comparison of catches of fishes and invertebrates by two light trap designs, in tropical NW Australia. Mar Biol. 2001;139:373–381. [Google Scholar]
- 28.Simpson SD, Meekan M, Montgomery J, McCauley R, Jeffs A. Homeward sound. Science. 2005;308:221. doi: 10.1126/science.1107406. [DOI] [PubMed] [Google Scholar]
- 29.Kaplan MB, Mooney TA. Coral reef soundscapes may not be detectable far from the reef. Sci Rep. 2016;6:31862. doi: 10.1038/srep31862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Booth DJ, Beretta GA. Changes in a fish assemblage after a coral bleaching event. Mar Ecol Prog Ser. 2002;245:205–212. [Google Scholar]
- 31.Ackerman JL, Bellwood DR. Reef fish assemblages: A re-evaluation using enclosed rotenone stations. Mar Ecol Prog Ser. 2000;206:227–237. [Google Scholar]
- 32.Nedelec SL, Campbell J, Radford AN, Simpson SD, Merchant ND. Particle motion: The missing link in underwater acoustic ecology. Methods Ecol Evol. 2016;7:836–842. [Google Scholar]
- 33.Pieretti N, Farina A, Morri D. A new methodology to infer the singing activity of an avian community: The acoustic complexity index (ACI) Ecol Indic. 2011;11:868–873. [Google Scholar]
- 34.Depraetere M, et al. Monitoring animal diversity using acoustic indices: Implementation in a temperate woodland. Ecol Indic. 2012;13:46–54. [Google Scholar]
- 35.Versluis M, Schmitz B, von der Heydt A, Lohse D. How snapping shrimp snap: Through cavitating bubbles. Science. 2000;289:2114–2117. doi: 10.1126/science.289.5487.2114. [DOI] [PubMed] [Google Scholar]
- 36.Cato DH. The biological contribution to the ambient noise in waters near Australia. Acoust Aust. 1992;20:76–80. [Google Scholar]
- 37.Radford CA, Stanley JA, Simpson SD, Jeffs AG. Juvenile coral reef fish use sound to locate habitats. Coral Reefs. 2011;30:295–305. [Google Scholar]
- 38.Merchant ND, et al. Measuring acoustic habitats. Methods Ecol Evol. 2015;6:257–265. doi: 10.1111/2041-210X.12330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wright KJ, Higgs DM, Cato DH, Leis JM. Auditory sensitivity in settlement-stage larvae of coral reef fishes. Coral Reefs. 2010;29:235–243. [Google Scholar]
- 40.Wright KJ, Higgs DM, Leis JM. Ontogenetic and interspecific variation in hearing ability in marine fish larvae. Mar Ecol Prog Ser. 2011;424:1–13. [Google Scholar]
- 41.Hiatt RW, Strasburg DW. Ecological relationships of the fish fauna on coral reefs of the Marshall Islands. Ecol Monogr. 1960;30:65–127. [Google Scholar]
- 42.Marnane MJ, Bellwood DR. Diet and nocturnal foraging in cardinalfishes (Apogonidae) at One Tree Reef, Great Barrier Reef, Australia. Mar Ecol Prog Ser. 2002;231:261–268. [Google Scholar]
- 43.Wilson SK, et al. Exploitation and habitat degradation as agents of change within coral reef fish communities. Glob Change Biol. 2008;14:2796–2809. [Google Scholar]
- 44.Green AL, Bellwood DR, Choat H. Monitoring Functional Groups of Herbivorous Reef Fishes as Indicators of Coral Reef Resilience: A Practical Guide for Coral Reef Managers in the Asia Pacific Region. Int Union Conserv Nature; Gland, Switzerland: 2009. [Google Scholar]
- 45.Pratchett MS, Hoey AS, Wilson SK, Messmer V, Graham NAJ. Changes in biodiversity and functioning of reef fish assemblages following coral bleaching and coral loss. Diversity (Basel) 2011;3:424–452. [Google Scholar]
- 46.Mouillot D, et al. Rare species support vulnerable functions in high-diversity ecosystems. PLoS Biol. 2013;11:e1001569. doi: 10.1371/journal.pbio.1001569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ashworth EC, Depczynski M, Holmes TH, Wilson SK. Quantitative diet analysis of four mesopredators from a coral reef. J Fish Biol. 2014;84:1031–1045. doi: 10.1111/jfb.12343. [DOI] [PubMed] [Google Scholar]
- 48.Froese R, Pauly D. 2017 FishBase. Available at www.fishbase.org. Accessed January 5, 2018.
- 49.Richardson LE, Graham NAJ, Pratchett MS, Hoey AS. Structural complexity mediates functional structure of reef fish assemblages among coral habitats. Environ Biol Fishes. 2017;100:193–207. [Google Scholar]
- 50.Sueur J, Aubin T, Simonis C. Seewave, a free modular tool for sound analysis and synthesis. Bioacoustics. 2008;18:213–226. [Google Scholar]
- 51.McWilliam JN, Hawkins AD. A comparison of inshore marine soundscapes. J Exp Mar Biol Ecol. 2013;446:166–176. [Google Scholar]
- 52.Sankupellay M, Towsey M, Truskinger A, Roe P. 2015 Visual fingerprints of the acoustic environment. Proceedings of the IEEE International Symposium on Big Data Visual Analytics (BDVA) (IEEE, New York). Available at https://eprints.qut.edu.au/88899/7/88899.pdf. Accessed January 21, 2017.
- 53.Butler J, Stanley JA, Butler MJ. Underwater soundscapes in near-shore tropical habitats and the effects of environmental degradation and habitat restoration. J Exp Mar Biol Ecol. 2016;479:89–96. [Google Scholar]
- 54.Harris SA, Shears NT, Radford CA, Reynolds J. Ecoacoustic indices as proxies for biodiversity on temperate reefs. Methods Ecol Evol. 2016;7:713–724. [Google Scholar]
- 55.Sueur J, Pavoine S, Hamerlynck O, Duvail S. Rapid acoustic survey for biodiversity appraisal. PLoS One. 2008;3:e4065. doi: 10.1371/journal.pone.0004065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Legg MW, Duncan AJ, Zaknich A, Greening MV. 2007 Analysis of impulsive biological noise due to snapping shrimp as a point process in time. OCEANS 2007 Europe (IEEE, New York). Available at citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.71.1224&rep=rep1&type=pdf. Accessed February 14, 2017.
- 57.Radford CA, Stanley JA, Tindle CT, Montgomery JC, Jeffs AG. Localised coastal habitats have distinct underwater sound signatures. Mar Ecol Prog Ser. 2010;401:21–29. [Google Scholar]
- 58.Nedelec SL, et al. Soundscapes and living communities in coral reefs: Temporal and spatial variation. Mar Ecol Prog Ser. 2015;524:125–135. [Google Scholar]
- 59.Rossi T, Connell SD, Nagelkerken I. The sounds of silence: Regime shifts impoverish marine soundscapes. Landsc Ecol. 2016;32:239–248. [Google Scholar]
- 60.Kennedy EV, Holderied MW, Mair JM, Guzman HM, Simpson SD. Spatial patterns in reef-generated noise relate to habitats and communities: Evidence from a Panamanian case study. J Exp Mar Biol Ecol. 2010;395:85–92. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.