Significance
Our results show that a standardized laboratory psychosocial stressor causes a greater inflammatory response in young healthy participants with an urban upbringing in the absence of pets, relative to young healthy participants with a rural upbringing in the presence of farm animals. In view of the known links between persistent inflammatory states and psychiatric disturbances, and considering that many stress-associated physical and mental disorders are more prevalent in environments offering a narrow range of microbial exposures, we feel that our findings are of general interest and significance. Moreover, we feel our study is timely, as urbanization and the associated socioeconomic consequences are increasing.
Keywords: TSST, urban, rural, inflammation, old friends
Abstract
Urbanization is on the rise, and environments offering a narrow range of microbial exposures are linked to an increased prevalence of both physical and mental disorders. Human and animal studies suggest that an overreactive immune system not only accompanies stress-associated disorders but might even be causally involved in their pathogenesis. Here, we show in young [mean age, years (SD): rural, 25.1 (0.78); urban, 24.5 (0.88)] healthy human volunteers that urban upbringing in the absence of pets (n = 20), relative to rural upbringing in the presence of farm animals (n = 20), was associated with a more pronounced increase in the number of peripheral blood mononuclear cells (PBMCs) and plasma interleukin 6 (IL-6) concentrations following acute psychosocial stress induced by the Trier social stress test (TSST). Moreover, ex vivo-cultured PBMCs from urban participants raised in the absence of animals secreted more IL-6 in response to the T cell-specific mitogen Con A. In turn, antiinflammatory IL-10 secretion was suppressed following TSST in urban participants raised in the absence of animals, suggesting immunoregulatory deficits, relative to rural participants raised in the presence of animals. Questionnaires, plasma cortisol, and salivary α-amylase, however, indicated the experimental protocol was more stressful and anxiogenic for rural participants raised in the presence of animals. Together, our findings support the hypothesis that urban vs. rural upbringing in the absence or presence of animals, respectively, increases vulnerability to stress-associated physical and mental disorders by compromising adequate resolution of systemic immune activation following social stress and, in turn, aggravating stress-associated systemic immune activation.
More than 50% of the world’s population currently lives in urban areas, projected to rise to 70% by 2050, with 50% of the urban population living in cities with more than 500,000 residents (1). At the same time, psychiatric disorders are more prevalent in urban vs. rural areas (2, 3). Given that psychosocial stress is a risk factor for many mental disorders (4), an altered neuronal social processing or an elevated acute cortisol stress response provide two possible distinct mechanisms underlying the higher urban prevalence of psychiatric disorders (5, 6). However, a lack of long-term and early-life exposure to stables and farm milk is also known to promote chronic inflammatory disorders, including asthma and allergies (7). Moreover, many stress-associated mental disorders are accompanied by an overreactive immune system and chronic low-grade inflammation (8, 9). Prospective human and mechanistic animal studies strengthen the idea that an exaggerated immune (re)activity plays a role in the development of mental disorders (10, 11). For example, individual differences in interleukin 6 (IL-6) secretion from ex vivo-stimulated immune cells predict susceptibility vs. resilience to a subsequently applied repeated social stressor in mice, while treatment with an anti–IL-6 antibody increases stress resilience (12). Furthermore, it is known that psychosocial stress promotes systemic immune activation and chronic low-grade inflammation (13), and that IL-6 responses to psychosocial stressors, such as the Trier social stress test (TSST) are exaggerated in those with a diagnosis of major depressive disorder and increased early life stress (8). Therefore, another possible mechanism predisposing those with an urban upbringing, relative to those with a rural upbringing, to develop mental disorders in which inflammation has been identified as a risk factor, is an exaggerated inflammatory response following psychosocial stress exposure. Increased inflammation in urban environments may be due to impaired immunoregulation, which is thought to be dependent, at least in part, on reduced exposure, especially during early life (14), to microorganisms with which mammals coevolved, as has been proposed by the “biodiversity” hypothesis (15), “missing-microbes” hypothesis (16), or “old-friends” hypothesis (17, 18), which all have been evoked to explain the epidemic of inflammatory disease in urban environments. Throughout human evolution, the interactions between these ancestral microbiota and the innate immune system promoted immunoregulation, as they were either part of host physiology (human microbiota), were harmless but inevitably contaminating air, food, and water (environmental microbiota), or were causing severe tissue damage when attacked by the host immune system (helminthic parasites) (16, 18). However, microbial biodiversity and, thus, overall contact with environmental and commensal microorganisms that were present during mammalian evolution and that play a role in setting up regulatory immune pathways, is progressively diminishing in high-income countries, particularly in urban areas. The latter is due to sanitation, drinking water treatment, excessive use of antibiotics, changes in diet, feeding of formula milk as a replacement for breast milk, increased caesarean section birth rates, as well as increased time spent within the built environment (16, 18–20). Of particular interest in this context is a recent study showing increased innate immune system activation in Hutterite compared with Amish farm children, and an ameliorating effect of dust extracts from Amish, but not Hutterite, homes on airway hyperreactivity and eosinophilia in a mouse model of allergic asthma (21). Living on single-family dairy farms with regular contact with farm animals in Amish farm children further goes along with four and six times lower asthma and allergic sensitization prevalence, respectively, compared with living on highly industrialized farms with little contact with farm animals in Hutterite farm children (21). Thus, another critical factor contributing to the diminishing contact with old friends in both urban and rural areas seems to be regular contact with animals. In accordance with this hypothesis, early exposure to both pets and farm animals is able to reduce the risk of childhood asthma and other inflammatory disorders (22, 23). Immigrant studies further suggest that differential contact with old friends, particularly during early life, accounts for differences in the prevalence of psychiatric disorders in rural vs. urban environments (18, 24).
To test whether urban, compared with rural, upbringing in the absence or presence of animals, respectively, is associated with an increased immune response to social stress, we recruited young, physically and emotionally healthy male participants (Table S1), raised during the first 15 y of life either in a city with more than 100,000 residents and in the absence of pets (urban) or on a farm keeping farm animals (rural). Pets were excluded for urban participants as they potently reduce the risk for inflammatory disorders (22), likely by facilitating contact with old friends. Participants were individually exposed to the TSST (25), and, before and after the TSST, heart rate and blood pressure were assessed, blood was drawn for collection of plasma and viable peripheral blood mononuclear cells (PBMCs), and saliva samples were collected for determination of α-amylase (Fig. S1). In addition, mental and physical health status, early life and perceived life stress, and subjective strain induced by TSST exposure were assessed using validated questionnaires (Fig. S1 and Table S2).
Materials and Methods
Recruiting.
This study was approved by the Ethics Committee of Ulm University and is registered at the German Clinical Trials Register (DRKS) (ID DRKS00011236). For recruitment, a flyer was designed asking for healthy male participants between 20 and 40 y of age who grew up (until the age of 15) either in a city with more than 100,000 residents and in the absence of pets (urban: n = 20) or on a farm keeping farm animals (rural: n = 20). Interested participants were then called, and those who turned out to be physically (asked whether they suffer from chronic physical disorders) and emotionally healthy [Structured Clinical Interview for DSM-IV Disorders (SCID-I) (telephone screening); Fig. S1 and Table S2], nonsmoking, caucasian, nondrug taking (nonsteroidal antiinflammatory drug, cannabis, etc.), nonexcessive exercising (i.e., <4 h per week), nontraumatized (during early life, adolescence, and adulthood), nonacutely (within the last 6 mo) bereaved or divorced, had a body mass index (BMI) between 20 and 30, gave their informed consent, and were invited to participate in the present study (Table S1). For further details, see SI Materials and Methods.
Experimental Procedure (Fig. S1).
On the test day itself, basal physical and emotional health statuses of the participants were assessed, employing validated questionnaires [list of complaints for quantitative analysis of current bodily and general complaints (BL); State–Trait Anxiety Inventory (STAI-S) Questionnaire]. Before (−5 min) and after (5, 15, 60, 90, and 120 min) the TSST, heart rate and diastolic blood pressure (DBP) and systolic blood pressure (SBP) were assessed [for calculation of median arterial pressure according to the formula: DBP + (SBP – DBP)/3], blood was drawn in EDTA- and lithium heparin-coated monovettes for collection of plasma and PBMCs, respectively, and saliva samples were collected for determination of α-amylase concentration. After the fifth blood draw (90-min time point), STAI-S was used again to assess subjective strain induced by the TSST procedure. After the sixth blood draw (120 min), the catheter was removed, and mental health status [Hospital Anxiety and Depression Scale–German Version (HADS-D); SCID-I (affective part)], early life [Childhood Experience of Care and Abuse Questionnaire (CECA-Q); Childhood Trauma Questionnaire (CTQ)], and perceived life stress [Perceived Stress Scale-4 (PSS-4)] were assessed using validated questionnaires. For further details, see SI Materials and Methods.
TSST.
Acute psychosocial stress was induced using the TSST, which was performed as described earlier (25), with minor modifications. For details, see SI Materials and Methods.
Blood Pressure and Heart Rate.
Blood pressure and heart rate of the participants were determined at time points −5, 5, 15, 60, 90, and 120 min, using a digital brachial blood pressure monitor (Boso Medicus Control; Bosch + Sohn). For further details, see SI Materials and Methods.
Blood Draw.
Blood (7.5 mL at each time point) was collected from an indwelling venous catheter in the nondominant arm (inserted at −60 min) at time points −5 min (5 min before the start of the TSST), 5 min (5 min after termination of the TSST), 15 min, 60 min, 90 min, and 120 min into chilled EDTA-coated monovettes. The latter were centrifuged (1,000 × g/15 min, 4 °C) immediately after each blood draw, and plasma was aliquoted and stored at −80 °C until further processing. Additionally, 9 mL of blood were collected at each time point into lithium-heparin–coated monovettes and stored at room temperature until blood from all time points was drawn for subsequent isolation and ex vivo stimulation of PBMCs.
PBMC Isolation and Stimulation.
Nine milliliters of blood were transferred from lithium-heparin–coated monovettes into Leucosep tubes (Greiner Bio-One), which were prepared beforehand with Ficoll Paque (GE Healthcare Life Sciences) according to the manufacturer’s instructions. The number of viable PBMCs (identified using trypan blue staining) was determined using an automated cell counter (TC20 Automated Cell Counter; Bio-Rad Laboratories). A total of 2.5 × 105 cells was then cultured in 96-well plates, either under basal conditions or in the presence of Con A (final concentration, 2.5 µg/mL) or lipopolysaccharide (LPS) (final concentration, 1 µg/mL) at 37 °C and 5% CO2 for 24 h. Supernatants were collected afterward and stored at −80 °C until further analysis. For further details, see SI Materials and Methods.
ELISA.
Plasma samples and supernatants from PBMC stimulations were analyzed using commercially available ELISA kits according to the manufacturers’ instructions. For further details, see SI Materials and Methods.
Determination of Salivary α-Amylase Concentrations.
Salivary α-amylase as a surrogate marker of sympathetic nervous system activity was measured as described earlier (26). For further details, see SI Materials and Methods.
Statistics.
For statistical comparisons, the software package IBM SPSS statistics (version 22.0) and Stata (version 14.2 SE) (StataCorp 2016; StataCorp LP) were used. For details, see SI Materials and Methods.
Results
Sample Characteristics.
Experimental groups did not differ in any of the socioeconomic parameters assessed, but significantly more rural vs. urban participants had regular contact with pets and/or farm animals during adulthood (Table S1). Furthermore, we did not detect any differences in early life (CECA-Q, CTQ) or perceived life stress (PSS-4) between groups (Fig. S1 and Table S2). SCID-I and BL scores (Fig. S1 and Table S2), assessed during telephone screening (SCID-I), at −60 min before TSST (BL), or at 120 min after TSST (SCID-I), indicated that mental and physical health status were also not affected by upbringing.
Effects of Upbringing and/or TSST on Emotionality.
Anxiety levels in the STAI-S, both at −60 (P = 0.014) and 90 (P = 0.005) min, and in the HADS-D (P = 0.027) were higher in rural vs. urban participants raised in the presence or absence of animals, respectively, as were scores in threat (P = 0.005), challenge (P = 0.032), primary appraisal (P = 0.004), and stress index (P = 0.025) in the Primary Appraisal Secondary Appraisal Scale (PASA) (Fig. S1 and Table S2).
Effects of Upbringing and/or TSST on Systemic Immune Activation.
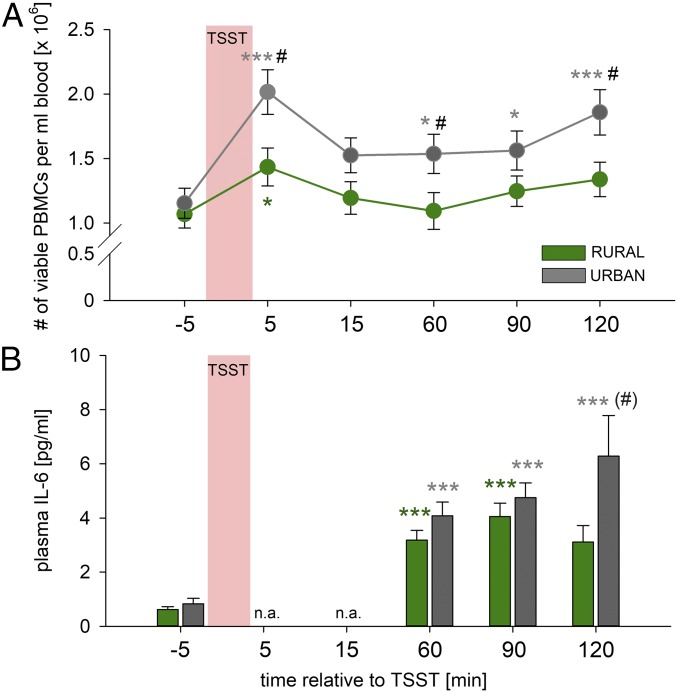
Basal numbers of viable PBMCs were not different between groups. However, compared with basal values (at −5 min), while PBMC counts in rural participants raised in the presence of animals were increased only transiently at 5 min (P = 0.015), PBMC counts in urban participants raised in the absence of animals were increased at 5 (P < 0.001), 60 (P = 0.023), 90 (P = 0.018), and 120 min [P < 0.001; factor time: F(5,185) = 12.621, P < 0.001], resulting in higher PBMC counts in participants with an urban, vs. rural, upbringing at 5 (P = 0.015), 60 (P = 0.040), and 120 min [P = 0.023; factor upbringing: F(1,37) = 5.272, P = 0.027; factor time by upbringing: F(5,185) = 2.112, P = 0.066; Fig. 1A]. Basal plasma IL-6 concentrations were not different between groups. Plasma IL-6 concentrations were increased in both experimental groups compared with respective basal values [factor time: F(3,105) = 23.836, P < 0.001; Fig. 1B] at 60 (urban, P < 0.001; rural, P < 0.001) and 90 min (urban, P < 0.001; rural, P < 0.001). However, participants with an urban upbringing in the absence of animals, relative to those with a rural upbringing in the presence of animals, showed a prolonged increase in plasma IL-6 concentrations compared with respective basal values at 120 min (P < 0.001), consistent with an overall interaction between upbringing and time [factor time by upbringing: F(3,105) = 3.118, P = 0.029; Fig. 1B].
Fig. 1.
Effects of urban vs. rural upbringing on Trier social stress test (TSST)-induced changes in peripheral blood mononuclear cell (PBMC) counts and plasma interleukin-6 (IL-6) concentrations. Urban compared with rural upbringing in the absence or presence of animals, respectively, was associated with an exaggerated increase in (A) the number (#) of viable PBMCs per milliliter of blood and (B) plasma IL-6 concentrations in response to the TSST. Plasma IL-10 concentrations were undetectable at all time points assessed. Data are presented as mean ± SEM (A) or + SEM (B). *P < 0.05, ***P ≤ 0.001 vs. respective basal (−5 min) group; #P ≤ 0.05 vs. respective rural upbringing in the presence of animals group. (#)P = 0.063 vs. respective rural upbringing in the presence of animals group. n.a., not assessed.
Effects of Upbringing and/or TSST on ex Vivo PBMC Cytokine Release.
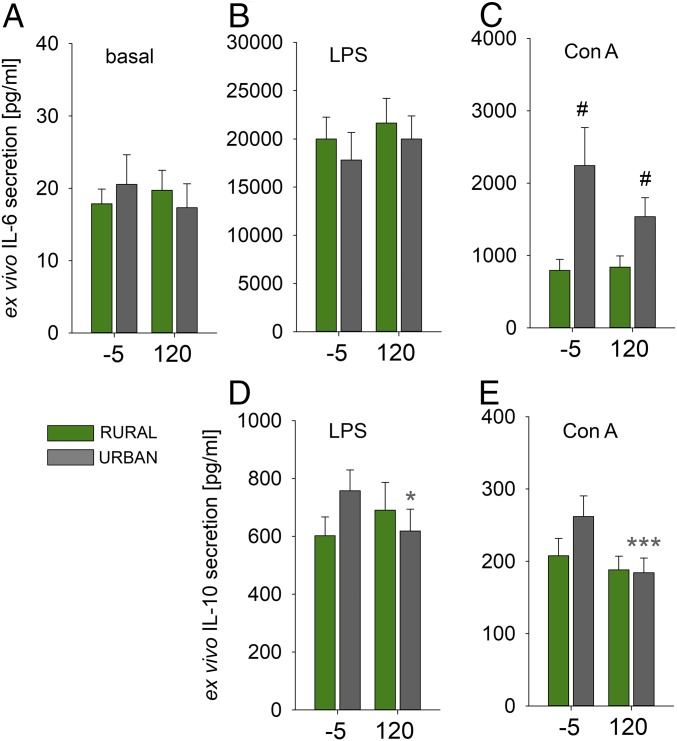
Basal ex vivo IL-6 secretion from isolated PBMCs (Fig. 2A) was comparable between groups, and unaffected by TSST exposure. Ex vivo IL-6 secretion from isolated PBMCs during Con A [factor upbringing: F(1,37) = 13.728, P = 0.001; Fig. 2C], but not LPS (Fig. 2B), stimulation was significantly increased in participants with an urban vs. rural upbringing, in the absence or presence of animals, respectively, at −5 (P = 0.012) and 120 min (P = 0.029). Ex vivo IL-10 secretion from isolated PBMCs was lower following TSST exposure, but only in participants with an urban upbringing in the absence of animals, both in the presence of LPS [factor time by treatment: F(1,37) = 7.922, P = 0.008; P = 0.035; Fig. 2D] and Con A [factor time: F(1,38) = 12.399, P = 0.001; factor time by treatment: F(1,38) = 4.518, P = 0.040; P < 0.001; Fig. 2E].
Fig. 2.
Effects of urban vs. rural upbringing in the absence or presence of animals on Trier social stress test (TSST)-induced changes in ex vivo cytokine secretion from isolated peripheral blood mononuclear cells (PBMCs). Compared with rural participants raised in the presence of animals, urban participants raised in the absence of animals showed unaffected (A) basal and (B) lipopolysaccharide (LPS), but increased (C) Con A-induced ex vivo secretion of interleukin-6 (IL-6), both at the −5-min and the 120-min time point of the TSST. IL-10 secretion was undetectable under basal conditions, but lower in both (D) LPS and (E) Con A-stimulated PBMCs from urban participants raised in the absence of animals, but not rural participants raised in the presence of animals, assessed at the 120-min time point of the TSST compared with IL-10 values assessed at the −5-min time point of the TSST. Data are presented as mean + SEM. *P < 0.05, ***P ≤ 0.001 vs. respective basal (−5-min) group; #P ≤ 0.05 vs. respective rural upbringing in the presence of animals group.
Effects of Upbringing and/or TSST Exposure on Hypothalamic–Pituitary–Adrenal Axis, Sympathetic Nervous System, and Cardiovascular System.
Rural vs. urban upbringing, in the presence or absence of animals, respectively, was associated with higher absolute plasma cortisol concentrations both at basal (−5 min; P = 0.039) and at the 5-min (P = 0.030) time point [factor upbringing: F(1,33) = 5.246, P = 0.029; Fig. S2A]. Compared with basal values, plasma cortisol concentrations were increased in both groups (both P < 0.001) at 5 min [factor time: F(5,165) = 26.978, P < 0.001; Fig. S2A], with a comparable delta increase (5 min – basal) (Fig. S2A inlay). Basal (−5 min) salivary α-amylase concentrations were not different between groups. Salivary α-amylase [factor time: F(5,180) = 25.723, P < 0.001; Fig. S2B] was increased in both groups at 5 min (urban, P < 0.001; rural, P < 0.001) and/or 15 min (rural, P = 0.001) compared with respective basal values. Basal mean arterial blood pressure was not different between groups. Mean arterial blood pressure [factor time: F(5,185) = 59.241, P < 0.001; Fig. S2C] was increased in both groups at 5 min (urban, P < 0.001; rural, P < 0.001) and 15 min (urban, P = 0.001; rural, P < 0.001) compared with respective basal values and a main effect of time was found for heart rate [F(5,185) = 14.810, P < 0.001; Fig. S2D].
Discussion
Here, we showed an increased systemic immune activation in response to a standardized laboratory social stressor in healthy participants with an urban upbringing in the absence of pets, relative to healthy participants with a rural upbringing in the presence of farm animals, even though questionnaires, plasma cortisol, and salivary α-amylase indicated that the experimental protocol was more stressful and anxiogenic for the latter. These data are in line with the biodiversity, missing-microbes, and old-friends hypotheses, which propose that the rapid rise in inflammatory physical and mental diseases in modern societies is due in part to a lack of exposure to immunoregulatory microorganisms (16–18). Another possible explanation for the increased immune reactivity following TSST in participants with an urban upbringing in the absence of animals, relative to participants with a rural upbringing in the presence of animals, might be that natural landscapes provide a stronger positive health effect compared with urban landscapes, resulting in accelerated short-term recovery from stress or mental fatigue, faster physical recovery from illness, and long-term overall improvement on people’s health and well-being (27). A brief nature experience, a 90-min walk in a natural, but not urban, setting, further decreases both self-reported rumination and neural activity in the subgenual prefrontal cortex (28), a brain region previously shown to respond with decreased activity to Montreal Imaging Stress Task exposure in rural vs. urban participants (5).
Acute psychosocial stress was induced using the TSST (25), well known for its ability to elevate PBMC counts (29) and plasma concentrations of IL-6 (8). Consistent with these earlier studies, TSST exposure in the current study increased the number of viable PBMCs in both experimental groups, 5 min following stressor termination, compared with respective basal values (at −5 min). However, while PBMC counts in rural participants raised in the presence of animals were increased only at 5 min, PBMC counts in urban participants raised in the absence of animals were increased at 5, 60, 90, and 120 min following TSST, indicating a more pronounced immune activation in urban vs. rural participants raised in the absence or presence of animals, respectively, in response to a social stressor. This is further supported by the fact that PBMC counts of urban participants raised in the absence of animals were elevated compared with rural participants raised in the presence of animals at the 5-, 60-, and 120-min time points following TSST exposure. Although we did not perform differential blood counts in the current study, increased lymphocyte and unaffected monocyte counts following TSST have been reported in previous studies (30, 31), suggesting that the exaggerated PBMC mobilization in urban vs. rural participants raised in the absence or presence of animals, respectively, in the present study is mainly mediated by the lymphocyte compartment. Again, consistent with well-known TSST effects (32–34), plasma IL-6 concentrations were increased in both groups 60 and 90 min following TSST compared with respective basal values. Importantly and consistent with the exaggerated stress-induced PBMC mobilization, this increase was again more pronounced in urban vs. rural participants raised in the absence or presence of animals, respectively. While plasma IL-6 concentrations in rural participants raised in the presence of animals peaked at 90 min and were not different from baseline at 120 min, levels in urban participants raised in the absence of animals were elevated until at least 120 min, indicating a prolonged inflammatory response following TSST exposure. Changes to plasma IL-6 after 120 min or before 60 min were not expected (32) and, thus, not studied here, but our results suggest that increased IL-6 might persist beyond the 120-min time point in urban participants raised in the absence of animals. As plasma IL-6 at baseline, 60 min, and 90 min did not differ between the groups, basal and acute stress-induced immune activation seem to be unaffected by upbringing, whereas immunoregulatory capacity responsible for adequate resolution of stress-induced immune activation seems to be compromised in urban participants raised in the absence of animals.
Basal ex vivo IL-6 secretion between PBMCs from both groups was comparable and TSST independent, suggesting that upbringing effects on plasma IL-6 concentrations were due to changes in circulating PBMC numbers rather than to individual cell activity. In contrast, ex vivo IL-6 secretion during Con A, but not LPS, stimulation was significantly increased in urban vs. rural participants raised in the absence or presence of animals, respectively, at baseline conditions and 120 min following TSST. The latter suggests increased cellular reactivity of the adaptive (Con A), but not the innate (LPS), immune system toward immunologic stimuli in urban participants raised in the absence of animals, which is in line with the fact that, based on previous studies (30, 31), mainly lymphocytes are mobilized by TSST. Although TSST exposure in the present study did not sensitize proinflammatory ex vivo cytokine secretion as described earlier (29), increased ex vivo cytokine secretion toward immunologic stimuli has been reported for individuals with a diagnosis of depression (35) or posttraumatic stress disorder (9). Of note, as these studies employed immunologic stimuli specific for either T cells and, thus, adaptive immunity (i.e., phytohemagglutinin) (35), or for monocytes and, thus, innate immunity (i.e., LPS) (9, 36), it remains to be investigated whether different psychiatric disorders, like anxiety disorders, mood disorders, as well as trauma- and stressor-related disorders, go along with, or are promoted by, activation of either the innate or adaptive immune system. Data from studies on healthy school teachers indicate that not only lymphocytes but also monocytes of individuals that experience high levels of effort–reward imbalance are more likely to show a pronounced inflammatory response to a mitogen signal (36).
Interestingly, in support of the above-reported plasma IL-6 findings suggesting immunoregulatory deficits in urban participants raised in the absence of animals, ex vivo PBMC IL-10 secretion was inhibited by TSST only in this group, both in the presence of LPS (Fig. 2D) and Con A (Fig. 2E). As the stress-protective and immunoregulatory effects of repeated immunization with Mycobacterium vaccae, a soil-derived, saprophytic bacterium with immunoregulatory and antiinflammatory activity, are mediated by the induction of Treg and IL-10 secretion (37), and as IL-10–deficient mice are prone to develop inflammatory disorders (38), these findings are in accordance with the increased risk for both inflammatory somatic and mental disorders in urban vs. rural participants (2, 3). Importantly, increased inflammatory TSST responses also have been reported in other young healthy individuals at risk for mental disorders, with response magnitudes predicting disease incidence (33, 39, 40).
In contrast to the immunologic results reported above and in contrast to findings reported by Steinheuser et al. (6), the TSST reactivity of the hypothalamic–pituitary–adrenal (HPA) axis, sympathetic nervous system, and cardiovascular system were comparable, or significantly more pronounced, in rural vs. urban participants raised in the presence or absence of animals, respectively. In detail, compared with respective baseline values, plasma cortisol concentrations were increased in both groups 5 min following TSST, with the delta increase being comparable as well. In line with these findings, salivary α-amylase, a surrogate marker for sympathetic nervous system activity (26), and medial arterial pressure were increased in both groups at 5 and/or 15 min, compared with respective basal values, and a main effect of time was found for heart rate. Interestingly, rural vs. urban upbringing in the presence or absence of animals, respectively, was associated with higher plasma cortisol concentrations, both at baseline and 5 min after the TSST; likewise, rural vs. urban upbringing in the presence or absence of animals, respectively, was associated with a delayed normalization of salivary α-amylase, suggesting that the experimental setup and procedure were more stressful and aversive for the former group. This hypothesis is supported by increased anxiety levels in the STAI-S, both at −60 and 90 min, and in the HADS-D, as well as with increased scores in threat, challenge, primary appraisal, and stress index in the PASA reported by rural vs. urban participants raised in the presence or absence of animals, respectively. Given that glucocorticoids during acute stress have been shown to facilitate immune activation (41–43), it is unlikely that the transiently elevated basal cortisol concentrations in rural vs. urban participants raised in the presence or absence of animals, respectively, are involved in mediating the decreased TSST-induced immune activation in the former group. This is further in line with data showing that stress-induced mobilization of bone marrow myeloid cells is mediated by β3-adrenergic signaling (44) and data suggesting that TSST-induced nuclear factor-κB (NF-κB) activation in PBMCs is likely mediated by adrenoreceptor-mediated signaling (45). However, TSST-induced NF-κB activation in PBMCs was also shown to negatively correlate with plasma cortisol response (46). However, as the significant main effect for factor upbringing on plasma cortisol levels did not hold when controlling for BMI, education, income, and current daily contact with pets and/or farm animals, the effects of upbringing on plasma cortisol levels were dependent on one or more of these covariates.
Thus, although an increased HPA axis (re)activity has been associated with several psychiatric disorders (47), our data do not support, or even are contrasting to, the hypothesis that the increased prevalence of mental disorders reported in urban vs. rural areas (2, 3) is due to an exaggerated HPA axis (re)activity. Furthermore, we did not detect any differences in early life (CECA-Q; CTQ) or perceived life stress (PSS-4) between groups, making it also unlikely that “a more demanding and stressful social urban environment” (5) contributes to or even mediates the increased disease prevalence in the urban vs. rural population in other studies (2, 3). Of note, SCID-I and BL scores, assessed during telephone screening (SCID-I), at −60 min before TSST (BL), or at 120 min after TSST (SCID-I), indicated that mental and physical health status were not affected by upbringing.
Interestingly, highly industrialized farming with low contact with farm animals, relative to traditional farming with regular contact with farm animals, is paralleled by increased innate immune system activation and higher prevalences of asthma and allergic sensitization, conditions that are characterized by dysregulation of both innate and acquired immune function (21). Thus, it is likely that the protective effect of rural vs. urban upbringing in the presence or absence of animals, respectively, on TSST-induced immune activation seen in the present study is rather due to differences in regular animal contact during early life than to the degree of urbanization per se between the groups. The latter interpretation would be in line with data indicating that incidence rates of certain cancer types as well as cardiovascular disorders in the United States, both representing illnesses associated with inflammation, are higher or at least decreasing more slowly in rural compared with urban environments (48, 49). The trend toward more and more industrialized farming and mechanization of farm work in the last decades (50, 51) and, consequently, the lack of regular and intense animal contact, might explain why many earlier studies showed lower incidence rates of these disorders in rural vs. urban areas before 2007 (49).
Our study has several strengths but also some limitations that warrant consideration. Notable strengths are the use of an objective and highly standardized stress test, the combination of both in vivo and ex vivo techniques to assess immune (re)activity, and repeated in vivo measures of physiologic and immunologic parameters in the same individuals over a period of 120 min, taking into account the temporal dynamics of the stress response. Another strength is that all significant main and interaction effects reported in the current manuscript, except the main effect for factor upbringing reported for plasma cortisol levels, were still detectable after adding BMI, high income, high education, and current daily contact with pets and/or farm animals as covariates (using a linear mixed model approach). This strongly argues for the robustness of our findings and for the critical role of an urban vs. rural upbringing in the absence of presence of animals, respectively, on these parameters. One limitation is the cross-sectional design of our study and the relatively small sample size, which, nevertheless, is representative of the sample sizes in the few previous studies available assessing plasma cytokine levels following TSST exposure (8, 36). Given that women are more likely to develop mood disorders compared with men (52), another limitation of the study is that only male participants were used. As Stein et al. (21) particularly emphasize the role of regular animal contact in promoting immunoregulation in farmers, yet another limitation of our study is that we did not include participants raised in urban areas in the presence of animals and in rural areas in the absence of animals. Additional limitations are that we did not take into account possible differences in participants’ mode of delivery at birth, antibiotic usage during first years of life, feeding of formula milk as a replacement for breast milk, or diet. All these factors are well known to affect the microbiome and microbiome–gut–brain axis, and consequently immune (re)activity, stress responsiveness, and behavior (14, 53, 54). Given the pronounced differences in TSST-induced PBMC mobilization between urban and rural participants raised in the absence or presence of animals, respectively, another limitation of the current study is that we do not have cell compositional data that would allow us to draw conclusions on the particular cell type(s) mediating this effect.
Despite these limitations, we believe that our experimental approach contributes significantly to our understanding of possible biological mechanisms underlying increased risk for inflammatory diseases, as well as increased vulnerability to mental health disorders where inappropriate inflammation is thought to be a risk factor, for those raised in areas offering a narrow range of microbial exposures. Our findings reveal an increased systemic immune activation and a compromised resolution of inflammation in urban vs. rural participants, raised in the absence and presence of animals, respectively, when exposed to an acute social stressor, likely mediated by differences in early animal contact, although validated questionnaires and plasma cortisol data clearly argue for rural participants raised in the presence of animals perceiving the experimental procedure as more stressful and anxiogenic.
Supplementary Material
Acknowledgments
We gratefully thank all participants and people contributing to this study. We also thank P. Hornischer and U. Binder (both from the Laboratory for Molecular Psychosomatics, Clinic for Psychosomatic Medicine and Psychotherapy, Ulm University) and Dr. M. T. Rojewski (Institute for Transfusion Medicine, Ulm University) for their excellent technical assistance. We further are grateful to L. Hackl, B. Schembera, P. Marquardt, A. Bauer, B. Häringer, T. Richter, L. Hermann, M. Tisch, M. Zeh, F. Weinreich, H. Holzrichter, and J. Kunze (all Ulm University) for participating as jury members in the TSST and to M. C. Flux (Department of Integrative Physiology and Center for Neuroscience, University of Colorado Boulder) for assistance with statistical analysis. Furthermore, we thank Prof. Dr. M. Wirsching (University of Freiburg) for his critical and helpful project discussions.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1719866115/-/DCSupplemental.
References
- 1.United Nations, Department of Economic and Social Affairs, Population Division 2014. World Urbanization Prospects: The 2014 Revision, Highlights (United Nations, New York), ST/ESA/SER.A/352.
- 2.Peen J, Schoevers RA, Beekman AT, Dekker J. The current status of urban-rural differences in psychiatric disorders. Acta Psychiatr Scand. 2010;121:84–93. doi: 10.1111/j.1600-0447.2009.01438.x. [DOI] [PubMed] [Google Scholar]
- 3.Vassos E, Agerbo E, Mors O, Pedersen CB. Urban-rural differences in incidence rates of psychiatric disorders in Denmark. Br J Psychiatry. 2016;208:435–440. doi: 10.1192/bjp.bp.114.161091. [DOI] [PubMed] [Google Scholar]
- 4.Langgartner D, Füchsl AM, Uschold-Schmidt N, Slattery DA, Reber SO. Chronic subordinate colony housing paradigm: A mouse model to characterize the consequences of insufficient glucocorticoid signaling. Front Psychiatry. 2015;6:18. doi: 10.3389/fpsyt.2015.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lederbogen F, et al. City living and urban upbringing affect neural social stress processing in humans. Nature. 2011;474:498–501. doi: 10.1038/nature10190. [DOI] [PubMed] [Google Scholar]
- 6.Steinheuser V, Ackermann K, Schönfeld P, Schwabe L. Stress and the city: Impact of urban upbringing on the (re)activity of the hypothalamus-pituitary-adrenal axis. Psychosom Med. 2014;76:678–685. doi: 10.1097/PSY.0000000000000113. [DOI] [PubMed] [Google Scholar]
- 7.Riedler J, et al. ALEX Study Team Exposure to farming in early life and development of asthma and allergy: A cross-sectional survey. Lancet. 2001;358:1129–1133. doi: 10.1016/S0140-6736(01)06252-3. [DOI] [PubMed] [Google Scholar]
- 8.Pace TW, et al. Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. Am J Psychiatry. 2006;163:1630–1633. doi: 10.1176/ajp.2006.163.9.1630. [DOI] [PubMed] [Google Scholar]
- 9.Gola H, et al. Posttraumatic stress disorder is associated with an enhanced spontaneous production of pro-inflammatory cytokines by peripheral blood mononuclear cells. BMC Psychiatry. 2013;13:40. doi: 10.1186/1471-244X-13-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khandaker GM, Pearson RM, Zammit S, Lewis G, Jones PB. Association of serum interleukin 6 and C-reactive protein in childhood with depression and psychosis in young adult life: A population-based longitudinal study. JAMA Psychiatry. 2014;71:1121–1128. doi: 10.1001/jamapsychiatry.2014.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kivimäki M, et al. Long-term inflammation increases risk of common mental disorder: A cohort study. Mol Psychiatry. 2014;19:149–150. doi: 10.1038/mp.2013.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hodes GE, et al. Individual differences in the peripheral immune system promote resilience versus susceptibility to social stress. Proc Natl Acad Sci USA. 2014;111:16136–16141. doi: 10.1073/pnas.1415191111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rohleder N. Stimulation of systemic low-grade inflammation by psychosocial stress. Psychosom Med. 2014;76:181–189. doi: 10.1097/PSY.0000000000000049. [DOI] [PubMed] [Google Scholar]
- 14.Lynch SV, Boushey HA. The microbiome and development of allergic disease. Curr Opin Allergy Clin Immunol. 2016;16:165–171. doi: 10.1097/ACI.0000000000000255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hanski I, et al. Environmental biodiversity, human microbiota, and allergy are interrelated. Proc Natl Acad Sci USA. 2012;109:8334–8339. doi: 10.1073/pnas.1205624109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blaser MJ. The theory of disappearing microbiota and the epidemics of chronic diseases. Nat Rev Immunol. 2017;17:461–463. doi: 10.1038/nri.2017.77. [DOI] [PubMed] [Google Scholar]
- 17.Lowry CA, et al. The microbiota, immunoregulation, and mental health: Implications for public health. Curr Environ Health Rep. 2016;3:270–286. doi: 10.1007/s40572-016-0100-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rook GA, Lowry CA, Raison CL. Microbial “Old Friends,” immunoregulation and stress resilience. Evol Med Public Health. 2013;2013:46–64. doi: 10.1093/emph/eot004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martínez I, et al. The gut microbiota of rural papua new guineans: Composition, diversity patterns, and ecological processes. Cell Rep. 2015;11:527–538. doi: 10.1016/j.celrep.2015.03.049. [DOI] [PubMed] [Google Scholar]
- 20.Stamper CE, et al. The microbiome of the built environment and human behavior: Implications for emotional health and well-being in postmodern western societies. Int Rev Neurobiol. 2016;131:289–323. doi: 10.1016/bs.irn.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 21.Stein MM, et al. Innate immunity and asthma risk in amish and hutterite farm children. N Engl J Med. 2016;375:411–421. doi: 10.1056/NEJMoa1508749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fall T, et al. Early exposure to dogs and farm animals and the risk of childhood asthma. JAMA Pediatr. 2015;169:e153219. doi: 10.1001/jamapediatrics.2015.3219. [DOI] [PubMed] [Google Scholar]
- 23.Mubanga M, et al. Dog ownership and the risk of cardiovascular disease and death—a nationwide cohort study. Sci Rep. 2017;7:15821. doi: 10.1038/s41598-017-16118-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rook GA, Raison CL, Lowry CA. Childhood microbial experience, immunoregulation, inflammation and adult susceptibility to psychosocial stressors and depression in rich and poor countries. Evol Med Public Health. 2013;2013:14–17. doi: 10.1093/emph/eos005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kirschbaum C, Pirke KM, Hellhammer DH. The “Trier social stress test”—a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- 26.Thoma MV, Kirschbaum C, Wolf JM, Rohleder N. Acute stress responses in salivary alpha-amylase predict increases of plasma norepinephrine. Biol Psychol. 2012;91:342–348. doi: 10.1016/j.biopsycho.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 27.Velarde MD, Fry G, Tveit M. Health effects of viewing landscapes—landscape types in environmental psychology. Urban For Urban Green. 2007;6:199–212. [Google Scholar]
- 28.Bratman GN, Hamilton JP, Hahn KS, Daily GC, Gross JJ. Nature experience reduces rumination and subgenual prefrontal cortex activation. Proc Natl Acad Sci USA. 2015;112:8567–8572. doi: 10.1073/pnas.1510459112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buske-Kirschbaum A, Kern S, Ebrecht M, Hellhammer DH. Altered distribution of leukocyte subsets and cytokine production in response to acute psychosocial stress in patients with psoriasis vulgaris. Brain Behav Immun. 2007;21:92–99. doi: 10.1016/j.bbi.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 30.Rohleder N, Wolf JM, Piel M, Kirschbaum C. Impact of oral contraceptive use on glucocorticoid sensitivity of pro-inflammatory cytokine production after psychosocial stress. Psychoneuroendocrinology. 2003;28:261–273. doi: 10.1016/s0306-4530(02)00019-7. [DOI] [PubMed] [Google Scholar]
- 31.Geiger AM, Pitts KP, Feldkamp J, Kirschbaum C, Wolf JM. Cortisol-dependent stress effects on cell distribution in healthy individuals and individuals suffering from chronic adrenal insufficiency. Brain Behav Immun. 2015;50:241–248. doi: 10.1016/j.bbi.2015.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McInnis CM, et al. Response and habituation of pro- and anti-inflammatory gene expression to repeated acute stress. Brain Behav Immun. 2015;46:237–248. doi: 10.1016/j.bbi.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McInnis CM, et al. Measures of adiposity predict interleukin-6 responses to repeated psychosocial stress. Brain Behav Immun. 2014;42:33–40. doi: 10.1016/j.bbi.2014.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thoma MV, et al. Stronger hypothalamus-pituitary-adrenal axis habituation predicts lesser sensitization of inflammatory response to repeated acute stress exposures in healthy young adults. Brain Behav Immun. 2017;61:228–235. doi: 10.1016/j.bbi.2016.11.030. [DOI] [PubMed] [Google Scholar]
- 35.Maes M, et al. Relationships between interleukin-6 activity, acute phase proteins, and function of the hypothalamic-pituitary-adrenal axis in severe depression. Psychiatry Res. 1993;49:11–27. doi: 10.1016/0165-1781(93)90027-e. [DOI] [PubMed] [Google Scholar]
- 36.Bellingrath S, Rohleder N, Kudielka BM. Effort-reward-imbalance in healthy teachers is associated with higher LPS-stimulated production and lower glucocorticoid sensitivity of interleukin-6 in vitro. Biol Psychol. 2013;92:403–409. doi: 10.1016/j.biopsycho.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 37.Reber SO, et al. Immunization with a heat-killed preparation of the environmental bacterium Mycobacterium vaccae promotes stress resilience in mice. Proc Natl Acad Sci USA. 2016;113:E3130–E3139. doi: 10.1073/pnas.1600324113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kühn R, Löhler J, Rennick D, Rajewsky K, Müller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 39.Carpenter LL, et al. Association between plasma IL-6 response to acute stress and early-life adversity in healthy adults. Neuropsychopharmacology. 2010;35:2617–2623. doi: 10.1038/npp.2010.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Derry HM, et al. Lower subjective social status exaggerates interleukin-6 responses to a laboratory stressor. Psychoneuroendocrinology. 2013;38:2676–2685. doi: 10.1016/j.psyneuen.2013.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dhabhar FS, McEwen BS. Enhancing versus suppressive effects of stress hormones on skin immune function. Proc Natl Acad Sci USA. 1999;96:1059–1064. doi: 10.1073/pnas.96.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rosenberger PH, et al. Surgical stress-induced immune cell redistribution profiles predict short-term and long-term postsurgical recovery. A prospective study. J Bone Joint Surg Am. 2009;91:2783–2794. doi: 10.2106/JBJS.H.00989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dhabhar FS, et al. Short-term stress enhances cellular immunity and increases early resistance to squamous cell carcinoma. Brain Behav Immun. 2010;24:127–137. doi: 10.1016/j.bbi.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Heidt T, et al. Chronic variable stress activates hematopoietic stem cells. Nat Med. 2014;20:754–758. doi: 10.1038/nm.3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bierhaus A, et al. A mechanism converting psychosocial stress into mononuclear cell activation. Proc Natl Acad Sci USA. 2003;100:1920–1925. doi: 10.1073/pnas.0438019100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wolf JM, Rohleder N, Bierhaus A, Nawroth PP, Kirschbaum C. Determinants of the NF-kappaB response to acute psychosocial stress in humans. Brain Behav Immun. 2009;23:742–749. doi: 10.1016/j.bbi.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 47.Holsboer F. The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology. 2000;23:477–501. doi: 10.1016/S0893-133X(00)00159-7. [DOI] [PubMed] [Google Scholar]
- 48.Zahnd WE, et al. Rural-urban differences in cancer incidence and trends in the United States. Cancer Epidemiol Biomarkers Prev. 2017:cebp.0430.2017. doi: 10.1158/1055-9965.EPI-17-0430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kulshreshtha A, Goyal A, Dabhadkar K, Veledar E, Vaccarino V. Urban-rural differences in coronary heart disease mortality in the United States: 1999–2009. Public Health Rep. 2014;129:19–29. doi: 10.1177/003335491412900105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cossman JS, James WL, Cosby AG, Cossman RE. Underlying causes of the emerging nonmetropolitan mortality penalty. Am J Public Health. 2010;100:1417–1419. doi: 10.2105/AJPH.2009.174185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Patterson PD, Moore CG, Probst JC, Shinogle JA. Obesity and physical inactivity in rural America. J Rural Health. 2004;20:151–159. doi: 10.1111/j.1748-0361.2004.tb00022.x. [DOI] [PubMed] [Google Scholar]
- 52.Kessler RC, et al. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Arch Gen Psychiatry. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- 53.Stilling RM, Dinan TG, Cryan JF. Microbial genes, brain & behaviour—epigenetic regulation of the gut-brain axis. Genes Brain Behav. 2014;13:69–86. doi: 10.1111/gbb.12109. [DOI] [PubMed] [Google Scholar]
- 54.Cryan JF, Dinan TG. Mind-altering microorganisms: The impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. 2012;13:701–712. doi: 10.1038/nrn3346. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.