Significance
Pressure overload triggers responses in cardiomyocytes and noncardiomyocytes, leading to pressure overload hypertrophy (POH). Here, we show that cardiac resident macrophages regulate compensatory myocardial adaptation to POH, while nonresident infiltrating macrophages are detrimental. At early-phase POH, pressure overload induces cardiac resident macrophage proliferation, which is regulated by Kruppel-like factor 4. At late-phase POH, pressure overload also induces Ly6Chi monocyte infiltration, and its blockade improves myocardial angiogenesis and preserves cardiac function. Mechanistically, the differential impact of these two macrophage subsets on myocardial angiogenesis may underlie the cardiac phenotype. These findings provide insights regarding the role of cardiac resident and nonresident macrophages, conceptually update the view of myocardial angiogenesis, and identify monocyte infiltration as a therapeutic target for nonischemic cardiomyopathy.
Keywords: cardiac macrophage, angiogenesis, pressure overload hypertrophy
Abstract
Nonischemic cardiomyopathy (NICM) resulting from long-standing hypertension, valvular disease, and genetic mutations is a major cause of heart failure worldwide. Recent observations suggest that myeloid cells can impact cardiac function, but the role of tissue-intrinsic vs. tissue-extrinsic myeloid cells in NICM remains poorly understood. Here, we show that cardiac resident macrophage proliferation occurs within the first week following pressure overload hypertrophy (POH; a model of heart failure) and is requisite for the heart’s adaptive response. Mechanistically, we identify Kruppel-like factor 4 (KLF4) as a key transcription factor that regulates cardiac resident macrophage proliferation and angiogenic activities. Finally, we show that blood-borne macrophages recruited in late-phase POH are detrimental, and that blockade of their infiltration improves myocardial angiogenesis and preserves cardiac function. These observations demonstrate previously unappreciated temporal and spatial roles for resident and nonresident macrophages in the development of heart failure.
Cardiomyopathy is a disease of the heart muscle that decreases the heart’s ability to pump blood and meet the body’s energetic demands. Cardiomyopathy can be broadly classified as ischemic cardiomyopathy (ICM) or nonischemic cardiomyopathy (NICM). In ICM, interruption of coronary artery blood flow, as seen in myocardial infarction (MI), is the primary cause of cardiac dysfunction. In NICM, cardiac muscle function can be compromised by either intrinsic gene mutation (hypertrophic cardiomyopathy) or extrinsic stress, such as pressure overload secondary to chronic hypertension or valve diseases [pressure overload hypertrophy (POH)]. With respect to NICM, accumulating clinical evidence suggests that small vessel dysfunction is a strong independent predictor of clinical deterioration and death in patients (1–4). Further, experimental studies suggest that inadequate myocardial angiogenesis is a critical determinant of the transition from compensatory hypertrophy to heart failure (5–7).
Macrophages are found in virtually every tissue and are critical for homeostasis and stress-induced responses. Macrophages broadly consist of two classes: resident macrophages and blood-borne infiltrating macrophages. Resident macrophages originate from yolk sac-derived erythromyeloid progenitors (EMPs), reside in tissue, perform homeostatic functions, and self-maintain locally (8–10). In general, infiltrating macrophages arise from circulating classic Ly6Chi (inflammatory) monocytes and are recruited after inciting pathology through the CCL2–CCR2 chemotaxis pathway (8, 11). Nonclassic Ly6Clo (patrolling) monocytes also patrol the luminal side of the endothelium and extravasate in response to both septic and aseptic tissue injury, playing a protective or antiinflammatory role during tissue injury (12). The adult heart contains two major subsets of Ly6Clo/CCR2− resident macrophages that express different levels of MHC-II, which are EMP-derived resident macrophages self-maintained through local proliferation at steady state. In addition, there are two minor subsets of Ly6Chi macrophages (<2%) that differ in CCR2 levels (13). These Ly6Chi subsets are derived from hematopoietic stem cells, are maintained through monocyte infiltration, and help to resolve tissue injury (14). Recent studies suggest that in ICM (i.e., MI), tissue injury induces both local proliferation of resident macrophages and infiltration of monocytes in the site of injury to regulate wound healing and inflammation in the heart (9, 15). Further, macrophages also accumulate in the remote nonischemic zone of the myocardium after MI to mediate post-MI cardiac remodeling (16). By contrast, much less is known in the setting of NICM. For example, whether macrophages are involved in disease progression and, if so, the identity, origin, and function of such cardiac macrophages remain unknown.
Here, using a classic mouse POH model for NICM, we track resident and infiltrating macrophages in the myocardium during the early and late phases of POH and provide multiple lines of evidence showing previously unappreciated and distinct time-dependent roles of different subsets of cardiac macrophages in POH. Using the myeloid-specific Kruppel-like factor 4 (KLF4) mutant mouse, we also shed light on the molecular mechanism that regulates local proliferation of cardiac resident macrophages and a potential role that these cells play in regulating perfusion during POH.
Results
Pressure Overload Induces Local Proliferation of Cardiac Resident Macrophages.
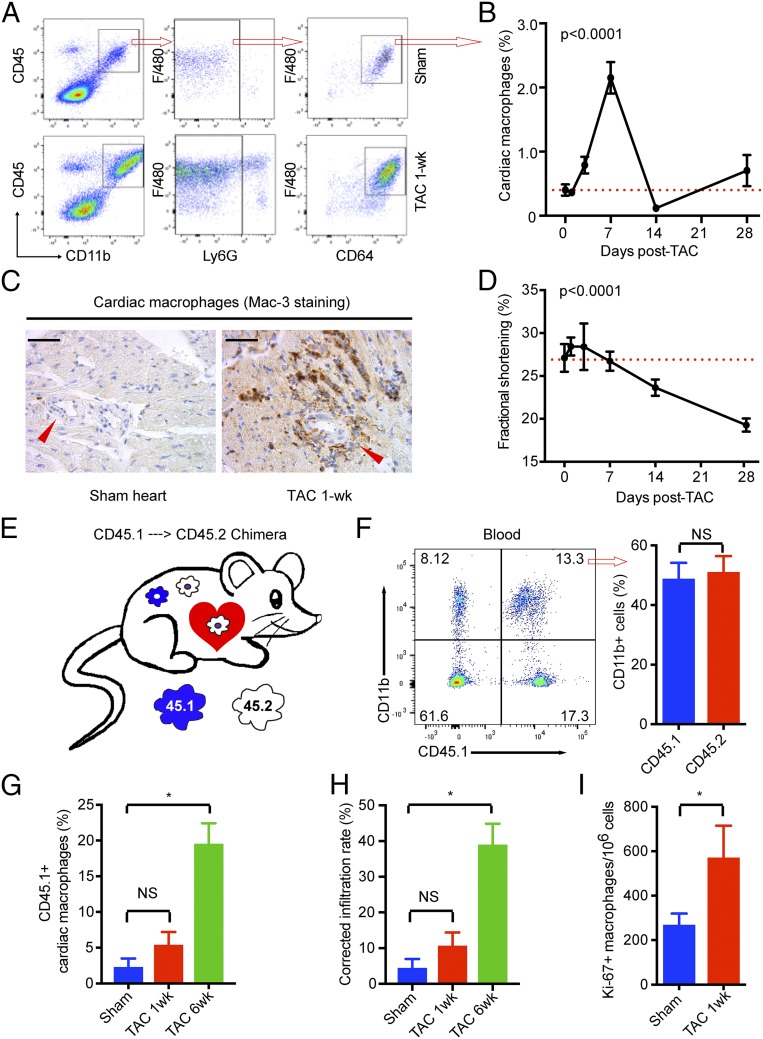
To understand the role of macrophages in POH, we first assessed macrophage numbers in the heart following transverse aortic constriction (TAC), a well-established model of pressure overload-induced hypertrophy and heart failure (i.e., POH). Hearts from sham or TAC animals were analyzed by flow cytometry. As shown in Fig. 1A, we found that TAC induced significant accumulation of myeloid cells (CD45+/CD11b+) in the myocardium. The majority of these cells are macrophages (Ly6G−/F4/80+/CD64+ subset; >95%), and the remainder are Ly6G+ neutrophils (<5%) (Fig. 1A). Time course studies showed that cardiac macrophages increased significantly by 3 d post-TAC, peaked at 7 d, returned to baseline after 2 wk, and then increased modestly at 4 wk after TAC (Fig. 1B). The presence of macrophages in the myocardium was further confirmed by anti–Mac-3 immunostaining, particularly in the perivascular region (Fig. 1C). This dynamic change in cardiac macrophage numbers is noteworthy as it correlates with two major phases of the cardiac response to TAC, namely, the compensatory cardiac hypertrophy with preserved contractile function phase that characterizes the first 7–10 d following TAC, followed by decompensation and development of heart failure between 2 and 4 wk after TAC (Fig. 1D).
Fig. 1.
Pressure overload induces local proliferation of cardiac resident macrophages at early phase and infiltration of monocytes at late phase. (A) Gating strategy for flow cytometry. (B) Dynamics of cardiac macrophages following TAC and its correlation with cardiac function. The dashed line indicates baseline. Cardiac macrophages were gated as live (live/dead stain-negative) CD45+CD11b+Ly6G−F4/80+CD64+ cells and are shown as a percentage vs. total heart cells. Cardiac function was expressed as left ventricular fractional shortening. (C) Immunostaining for Mac-3 to reveal macrophages in the myocardium, particularly in the perivascular region. Brown (DAB-positive) cells are macrophages. Arrowheads indicate vessels. (Scale bars, 100 μm.) (D) LV dysfunction following TAC. (E) Strategy to generate CD45.1-CD45.2 chimeric mice. Recipient CD45.2 mice were irritated with their head-chest shielded by lead block and transplanted via tail vein injection with bone marrow cells from CD45.1 donor mice. The resulting chimera mice carry both CD45.2 and CD45.1 cells in the blood but only have CD45.2 cardiac resident macrophages. (F) Blood (50 μL) was drawn from the tail vein at 8 wk posttransplantation and analyzed by FACS for CD45.1+ myeloid cells (CD45+CD11b+). (G) FACS analysis for CD45.1+ cardiac macrophages before and after TAC. (H) Corrected monocyte infiltration rate considering that only 50% of monocytes were CD45.1+ cells. (I) FACS analysis for Ki-67 expression in cardiac macrophages. (B and D) P values were calculated from one-way ANOVA. (F–I) *P < 0.05 by t test after Bonferroni correction (n = 5–8 for all data points). NS, not significant.
Next, we undertook multiple complementary approaches to determine if the increase in cardiac macrophages following TAC was from resident macrophages, blood monocytes recruited to the myocardium, or both. First, we utilized a recently described fluorescence-activated cell sorting (FACS) strategy in which the surface expression of CCR2 is low in resident macrophages but high in infiltrating macrophages (9). Importantly, we found that macrophages accumulating after 1-wk TAC are CCR2−, indicating that they are resident macrophages (SI Appendix, Fig. S1 A and B). Second, we showed that blockade of monocyte infiltration by RS-504393 (RS), a small-molecule CCR2 antagonist (9, 17), had no effect on cardiac macrophage populations (SI Appendix, Fig. S1 A and B). Consistently, most cardiac macrophages are Ly6Clo before and after 1-wk TAC (SI Appendix, Fig. S1 C and D). Finally, to validate if pressure overload only induces resident macrophage expansion, we utilized a CD45.1-CD45.2 chimeric mouse model, in which recipient CD45.2 mice were irradiated utilizing a thorax shield to minimize cardiac macrophage depletion. Following transplantation, the donor CD45.1+ bone marrow cells replaced circulating CD45.2+ blood cells without affecting cardiac resident macrophages (Fig. 1E). We chose the chimeric animals with ∼50% CD45.1+ myeloid cells (CD11b+) for the TAC model to ensure effective in vivo cell tracking (Fig. 1F). In sham animals, we observed that only 2.3% of cardiac macrophages are CD45.1+, confirming that cardiac macrophages are maintained locally at steady state (Fig. 1G). After 1-wk TAC, the CD45.1+ cardiac macrophages remained low at 5.4%, suggesting that no significant monocyte infiltration occurs at this early stage of POH (Fig. 1G). However, after 6-wk TAC, the CD45.1+ cardiac macrophages reached 20% (Fig. 1G), indicating monocyte infiltration in late-stage POH that is associated with the transition from cardiac compensation to decompensation. Because the CD45.1-CD45.2 chimeras subjected to TAC carried both CD45.1+ and CD45.2+ white blood cells in circulation (1:1 by experimental design), the above percentages of CD45.1+ macrophages only reflect 50% of the actual infiltrating cells. As such, the corrected rate of monocyte infiltration into the myocardium is about 5–10% at baseline and early-phase POH, and it increases to around 40% in late-phase POH (Fig. 1H).
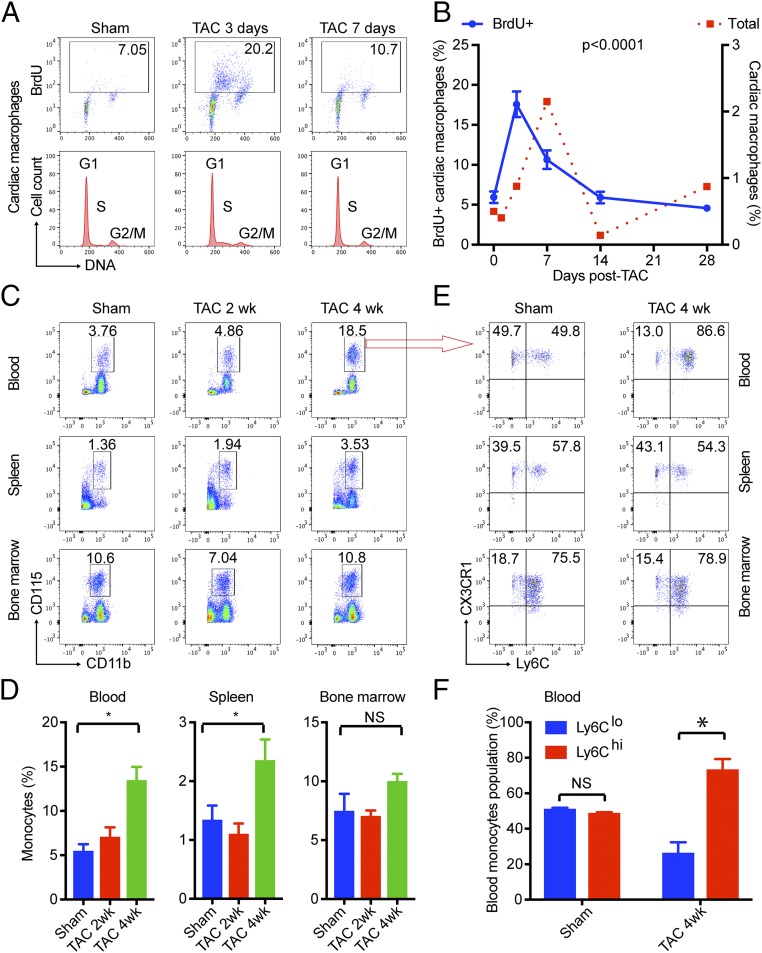
Because TAC increased cardiac macrophage numbers independent of monocyte infiltration at the early stage, we hypothesized that such cells have to be derived from local proliferation. Indeed, we found TAC significantly induced the expression of Ki-67 protein, a nuclear proliferation marker, in cardiac macrophages (Fig. 1I). The local proliferation of cardiac resident macrophages was further confirmed by in vivo BrdU pulse labeling. Mice undergoing sham or TAC surgery were injected i.p. with BrdU for 2 h. Because bone marrow-derived cells are not released into the circulation within this period (SI Appendix, Fig. S2), BrdU incorporation in the heart is limited to locally proliferating cells (9). We observed significant cell proliferation in cardiac macrophages 3 d post-TAC as demonstrated by BrdU incorporation and DNA S-phase populations by flow cytometry (Fig. 2 A and B). Of note, the pattern of macrophage proliferation (Fig. 2B, blue line) closely aligned with that of cardiac macrophage count (Fig. 2B, red dotted line) along the TAC time line, with proliferation peaking just before the cellular maximum at day 7 and subsequently decreasing to baseline by day 14. Collectively, these studies demonstrate that pressure overload induces local proliferation of cardiac resident macrophages in early-phase POH.
Fig. 2.
Local proliferation of cardiac resident macrophages and infiltration of blood-borne Ly6C+ monocytes. (A and B) TAC induced local proliferation of cardiac resident macrophages as assessed by BrdU pulse-labeling assay. DNA was stained with Hoechst 33342 for cycle analysis, and the S-phase was clearly visible in TAC 3-d samples. The time course curve of total cardiac macrophages (dotted red line) was overlaid to show the phase difference. (C and D) Monocytes (CD115+/CD11b+) in blood, spleen, and bone marrow before and after TAC. (E and F) Classic Ly6C+ monocytes and nonclassic Ly6C− monocytes before and after TAC. In B, P values were calculated from one-way ANOVA for BrdU data. In D and F, *P < 0.05 by t test (n = 5 for all data points). NS, not significant.
The late-phase monocyte infiltration (Fig. 1H) may result from an expanded circulating monocyte pool and/or greater infiltrative capacity of monocytes. To assess circulating monocyte numbers (18), we gated blood for monocytes (CD115+CD11b+) and found that 4-wk TAC (late-phase POH) significantly increased monocyte numbers in blood and spleen but not in bone marrow, indicating TAC-induced extramural monocytosis (Fig. 2 C and D). Peripheral blood monocytes are classified into three different populations based on expression of cell surface molecules and functions. In humans, these consist of CD14hiCD16− (classic monocytes), CD14loCD16lo (intermediate monocytes), and CD14loCD16hi (nonclassic monocytes). In mice, the equivalent populations are Ly6ChiCD62L+CD43−CCR2+ (classic monocytes), Ly6CintCD62L−CD43+CCR2− (intermediate monocytes), and Ly6CloCD62L−CD43+CCR2− (nonclassic monocytes) (12). Given the heterogeneity of these populations, we sought to determine if one population predominantly contributes to monocyte infiltration after TAC. Interestingly, TAC induced an increase in Ly6Chi monocytes in blood but not in spleen or bone marrow (Fig. 2 E and F). These data demonstrate that pressure overload induces circulating Ly6Chi classic monocyte infiltration during late-phase POH.
Cardiac Resident Macrophages Are Required for Myocardial Adaptation in POH.
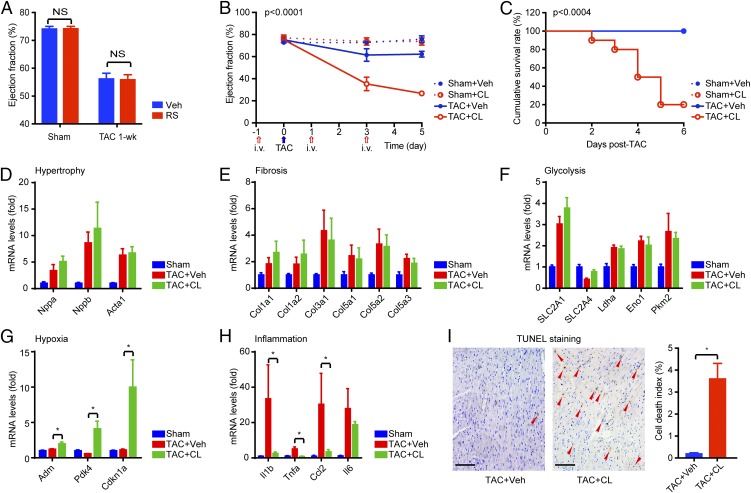
We next sought to determine the functional importance of resident macrophages in the early adaptive response to TAC. As there is no specific method to specifically deplete cardiac resident macrophages, we undertook a stepwise elimination approach to distinguish the unique roles of cardiac resident vs. infiltrating macrophages. We first treated mice with the CCR2 antagonist RS to block infiltration of blood-borne CCR2+Ly6Chi classic monocytes (15, 17, 19). RS administration had no impact on cardiac macrophage numbers or cardiac function before and after 1 wk post-TAC (Fig. 3A and SI Appendix, Fig. S1). Next, we subjected animals to clodronate liposomes (CLs), which deplete both circulating and resident macrophages (20). Mice with CL administration for a week (500 μg/kg i.v. every other day) did not exhibit any symptom of sickness or heart dysfunction as reported previously (21). However, when subjected to TAC, the macrophage-depleted animals (CL-injected) developed acute heart failure following TAC as revealed by serial echocardiography studies, while cardiac function was well maintained in PBS-liposome [vehicle (Veh)]–treated animals (Fig. 3B). CL-injected animals also exhibited striking postoperative mortality, with ∼80% dead within 6 d, while a 100% survival rate was observed in all other groups as expected (Fig. 3C). Postmortem autopsy revealed similar heart weight increases after TAC but heavier lungs in the CL-treated TAC group, indicating lung edema secondary to heart failure (SI Appendix, Fig. S3A). Spleen weight was similarly reduced by CL in both the sham and TAC groups, confirming efficient macrophage/monocyte depletion (SI Appendix, Fig. S3A). Gene expression analysis by quantitative real-time PCR (qPCR) revealed no difference in hypertrophy, fibrosis, or glycolysis genes (Fig. 3 D–F). However, the expression of hypoxia-inducible genes, including Adm, Pdk4, and p21 (Cdkn1a), was significantly higher in the macrophage-depleted TAC (TAC+CL) group compared with the normal TAC (TAC+Veh) group (Fig. 3G), indicating that TAC induced more severe hypoxia stress in the macrophage-depleted myocardium. TAC also induced expression of inflammatory markers, including IL-1β, TNF-α, and CCL2 (MCP1), which was abrogated following macrophage depletion (Fig. 3H), suggesting that inflammation was not the reason for heart failure in early-phase TAC. Histological studies confirmed no significant differences in cardiomyocyte hypertrophy (wheat germ agglutinin staining), fibrosis (collagen staining), or capillary density (CD31 staining) in the myocardium after CL treatment (SI Appendix, Fig. S3B). However, we detected profound cardiomyocyte death in macrophage-depleted hearts (Fig. 3I and SI Appendix, Fig. S4A). The mechanism of cell death of these cardiomyocytes appeared to be nonapoptotic, as demonstrated by negative caspase-3 activation/cleavage (SI Appendix, Fig. S4B). These data underscore the importance of resident macrophages in the early-phase cardiac adaptation to POH.
Fig. 3.
Cardiac resident macrophages are requisite for cardiac adaptation to POH. (A) Blocking monocyte infiltration by RS had no effect on POH. (B) Left ventricular function following clodronate-mediated macrophage depletion and TAC (n = 8–10 in each group). The P value was calculated from two-way ANOVA. The blue arrow indicates the time of TAC, and red arrows indicate the time of liposome injections. (C) Postoperation survival rates. P values were calculated from log-rank between TAC+CL and TAC+Veh groups. (D–H) Gene expression in the heart after 4-d TAC ± CL (n = 5). *P < 0.05 by t test with Bonferroni correction. (I) Cell death detected by TUNEL staining in the myocardium after 4-d TAC ± CL. Red arrowheads indicate nuclei of dead cells. *P < 0.05 by t test (n = 5). NS, not significant. (Scale bars, 200 μm.)
While these data underscore the importance of resident macrophages in early-phase cardiac adaptation to POH, the role of cardiac resident macrophages in late-phase adaptation remains unexplored. To address this, we allowed early compensation to occur after TAC and subsequently depleted macrophages via three alternate-day doses of CL beginning at day 14 post-TAC. As with pre-TAC macrophage depletion, an 80% mortality rate was observed after three injections of CL 3 wk post-TAC (SI Appendix, Fig. S5A). It is noteworthy that CL administration trigged a quick transition from cardiac compensation [ejection fraction (EF) ∼ 60%] to heart failure (EF < 30%) as revealed by echocardiography (SI Appendix, Fig. S5B). CL administration at this point of TAC exhibited little effect on further development of cardiac hypertrophy or fibrosis (SI Appendix, Fig. S5C), but resulted in profound cardiomyocyte death (SI Appendix, Fig. S5 D–F). Because blocking peripheral monocyte infiltration did not promote cardiac dysfunction in early-phase (Fig. 3A) or late-phase-TAC (see Fig. 5), these late-stage macrophage depletion studies implicate cardiac resident macrophages in maintaining cardioprotection throughout the POH disease course.
KLF4 Regulates Proliferation of Cardiac Resident Macrophages.
Most tissue resident macrophages, including cardiac resident macrophages, are maintained by self-renewal (9, 22). While our understanding of how resident macrophages proliferate is limited, a recent seminal study by Sieweke and coworkers (23) provides critical insights. Specifically, it was found that primary macrophages from MafB/c-Maf double-deficient (Maf-DKO) mice demonstrated indefinite KLF4-dependent proliferative capacity when cultured with macrophage colony-stimulating factor (M-CSF) (23). Therefore, we utilized myeloid-specific KLF4-deficient mice (Lyz2-Cre:KLF4flox/flox; designated K4-cKO) (24) to investigate if KLF4 regulates cardiac macrophage proliferation in the context of POH.
Given that Lyz2-Cre is active in macrophages and neutrophils but not in monocytes (21), we first sought to explore any potential role for neutrophils in this model. The infiltration of Ly6G+ neutrophils was marginal after TAC (Fig. 1A), and depletion of neutrophils using the anti-Ly6G antibody (clone 1A8) before TAC demonstrated very little effect on POH (SI Appendix, Fig. S6). Therefore, these data supported our decision to focus on the role of KLF4-deficient macrophages.
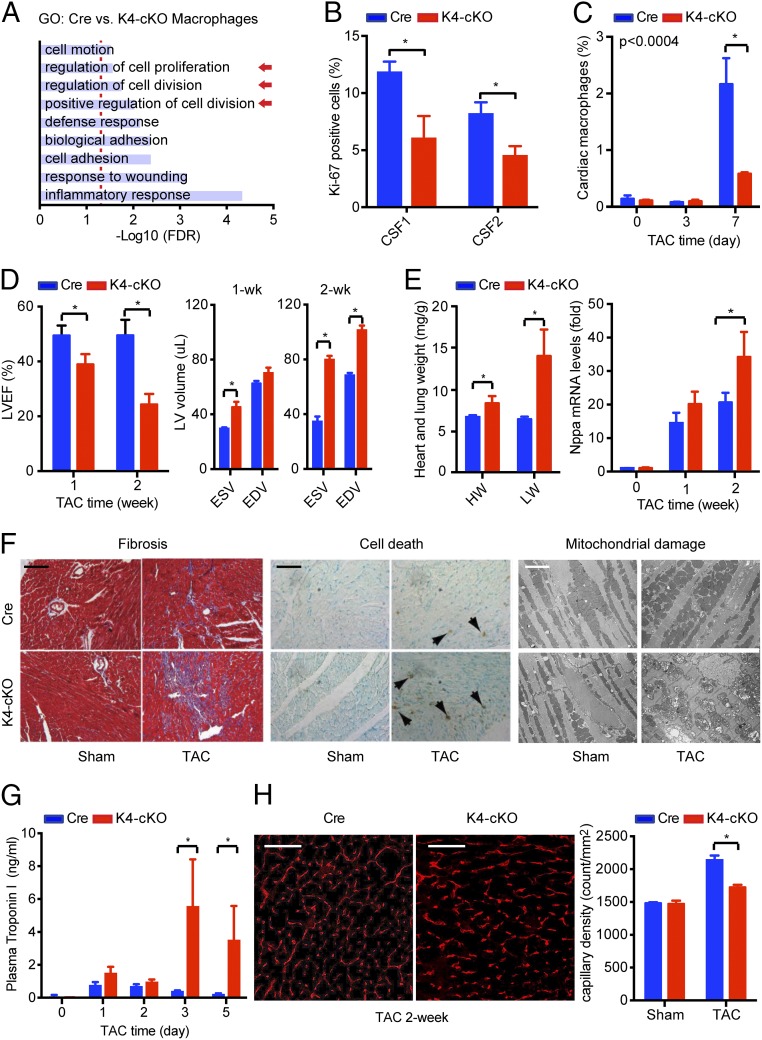
We first performed transcriptomic analysis of primary macrophages from Lyz2-Cre (designated Cre) and K4-cKO mice via RNA-sequencing (RNA-seq) and found 238 genes that were differentially expressed (false discovery rate < 0.05). Gene ontology (GO) analysis demonstrated enrichment in multiple pathways of cell proliferation and division (Fig. 4A). Importantly, when treated in vitro with CSF1 and CSF2, KLF4-deficient primary macrophages showed less accumulation of Ki-67 protein (Fig. 4B and SI Appendix, Fig. S7A), demonstrating impaired proliferative capacity. Next, we subjected the K4-cKO and Cre animals to TAC. FACS analysis demonstrated that TAC-induced cardiac macrophage accumulation was significantly reduced in the K4-cKO group compared with that of Cre (Fig. 4C). Consistent with fewer cardiac macrophages, myeloid KLF4 deficiency impaired cardiac resident macrophage proliferation in vivo following TAC as demonstrated by reduced BrdU incorporation (SI Appendix, Fig. S7B).
Fig. 4.
Myeloid KLF4 regulates cardiac macrophage proliferation in POH. (A) RNA-seq studies with primary peritoneal macrophages from Cre and K4-cKO mice. A total of 283 genes were differentially regulated between the Cre and K4-cKO groups, and gene ontology (GO) term enrichment analysis revealed nine pathways that were functionally enriched [false discovery rate (FDR) < 0.05]. Arrows indicate pathways of interest. (B) KLF4-deficient peritoneal macrophages exhibited an impaired proliferative response to CSF1/2 stimulation (48 h). *P < 0.05. (C) KLF4-deficiency impaired TAC-induced cardiac macrophage proliferation (n = 10). *P < 0.05. (D) Myeloid KLF4 deficiency impaired cardiac function after TAC, resulting in dilated cardiomyopathy at 2 wk post-TAC (n = 8–10). *P < 0.05. EDV, end-diastolic volume; ESV, end-systolic volume; LVEF, left ventricular ejection fraction. (E) Myeloid KLF4 deficiency accelerated TAC-induced cardiac hypertrophy. Heart weight (HW; milligrams) and lung weight (LW; milligrams) at 2 wk post-TAC were normalized to body weight (grams) (n = 6 in each group). *P < 0.05 by t test with Bonferroni correction. (F) TAC induced more fibrosis, cell death, and mitochondrial damage in K4-cKO hearts. Samples were from 1-wk TAC and sham mice. (Black scale bar, 100 μm; white scale bar, 2 μm.) (G) TAC induced higher plasma cardiac troponin I levels in the K4-cKO group (n = 5). *P < 0.05 with Bonferroni correction. (H) Impaired angiogenesis in K4-cKO hearts after TAC as revealed by myocardial capillary density using CD31 immunostaining (n = 5). *P < 0.05. (Scale bars, 100 μm.).
Post-TAC serial echocardiography revealed that K4-cKO mice rapidly develop a dilated cardiomyopathy characterized by reduced left ventricular EF (Fig. 4D), elevated heart and lung weight, and expression of the cardiac hypertrophy marker gene Nppa (Fig. 4E). Histological analyses exhibited enhanced fibrosis and cell death, as well as severe mitochondrial damage in K4-cKO hearts post-TAC (Fig. 4F). Plasma cardiac troponin I levels were significantly higher in K4-cKO mice post-TAC, indicating myocardial injury (Fig. 4G). K4-cKO hearts exhibited significantly impaired angiogenesis as demonstrated by CD31 immunostaining and the dysregulation of angiogenesis genes (Fig. 4H and SI Appendix, Fig. S8 A and B). Although not induced by TAC, macrophage KLF4 appeared to be required for optimal expression of a potent angiogenesis factor, VEGFA, in vitro and in the heart (SI Appendix, Fig. S8 C and D). These data, paired with the observation that many cardiac macrophages reside in the perivascular region (Fig. 1C), suggest that cardiac macrophages may have a potential role in angiogenesis or other vascular responses. In addition to impaired angiogenesis, we observed that K4-cKO hearts exhibited enhanced fibrosis after TAC (Fig. 4F). However, qPCR analysis using a panel of 84 fibrosis-related genes (Qiagen RT2 Profiler PCR array) revealed no significant difference between Cre and K4-cKO cardiac macrophages (SI Appendix, Fig. S8E). These observations, coupled with the appreciation of reduced KLF4-deficient cardiac macrophage numbers (Fig. 4C), suggest that enhanced fibrosis seen in the K4-cKO heart was likely secondary to myocardial injury (Fig. 4 F and G).
Because KLF4 also regulates macrophage M1/M2 polarization (24, 25) and gene expression in KLF4-deficient macrophages exhibited enriched inflammation pathways (Fig. 4A), we tested if the macrophage polarization state contributes to the phenotype observed after TAC. Consistent with our previous report (24), K4-cKO hearts at 1 wk post-TAC showed lower expression of multiple M2 genes (SI Appendix, Fig. S9A). However, the expression of M1 genes in K4-cKO hearts was also lower, indicating the absence of excessive inflammation (SI Appendix, Fig. S9B). We attribute this phenomenon to reduced cardiac macrophage numbers in K4-cKO hearts after TAC (Fig. 4C). We further studied two classic M2-deficient animals (26), namely, hematopoietic STAT6-KO mice (via transplantation of STAT6-KO bone marrow to WT recipients) and myeloid IL4Ra-KO mice (Lyz2-Cre:IL4Raflox/flox), and found that biased M1/M2 polarization had no effect on POH (SI Appendix, Fig. S9 C and D). As such, we conclude that the role of KLF4 in cardiac macrophages in POH is not simply defined by M1/M2 polarization but is secondary to its regulation of cardiac resident macrophage proliferation and their angiogenic potential (Fig. 4C and SI Appendix, Fig. S8D).
Infiltrating Macrophages Are Detrimental to the Heart in Late-Phase POH.
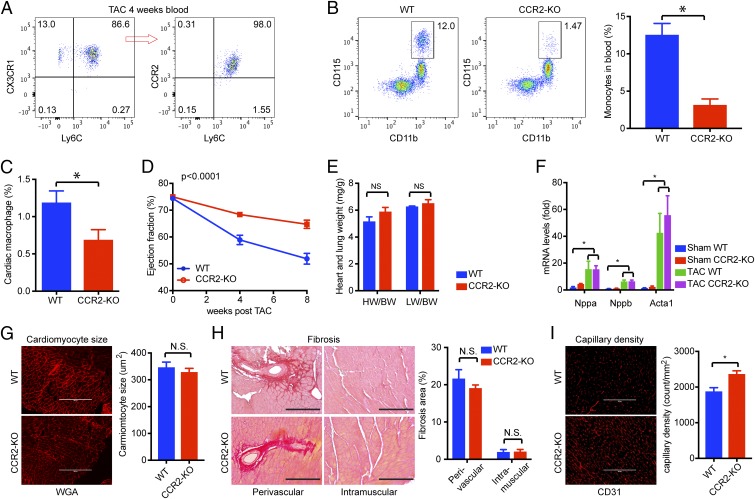
While our studies indicate that blood-borne macrophages are not required for the early-phase protective compensatory response to POH, they do infiltrate the myocardium during late-stage POH (Fig. 1G). Because heart failure is often associated with immune cell infiltration and inflammation (27–29), we asked if blood-borne macrophages contribute to late-phase decompensation of POH. As shown in Fig. 2F, TAC increased circulating Ly6Chi monocytes during late-phase POH. These monocytes were further characterized as Ly6ChiCX3CR1+CCR2+ classic monocytes (Fig. 5A). To block such monocytes, we utilized the CCR2-KO mouse, which is known to have reduced circulating classic monocytes due to impaired CCL2/CCR2-mediated trafficking from the bone marrow (30, 31). When subjected to 4-wk TAC, the circulating monocytes remained much lower (∼25%) in CCR2-KO mice than WT control mice (Fig. 5B). Consistently, overall macrophage numbers in late-phase POH were also lower (∼50%) within CCR2-KO myocardium (Fig. 5C).
Fig. 5.
Blockade-infiltrating macrophages preserved cardiac function in late-phase POH. (A) Classic Ly6C+CCR2+ monocytes increased in blood after 4 wk of TAC. A representative FACS plot from five samples is shown. (B) Reduced monocyte numbers in CCR2-KO mice after 4 wk of TAC (n = 5). *P < 0.05 by t test. (C) Reduced cardiac macrophage numbers in CCR2-KO hearts after 4 wk of TAC (n = 5). *P < 0.05 by t test. (D) Left ventricular function assessed by echocardiography. The P value is shown as P (genotype * time) calculated from two-way ANOVA. (E) Heart weight and lung weight. (F) Expression of hypertrophy marker genes. (G) Cardiomyocyte cross-section area. Cell membranes were outlined by wheat germ agglutinin (WGA) staining. (H) Fibrosis assessed by PicoSirus Red staining, with collagen stained in red. Perivascular and intramuscular areas are shown. Fibrosis was quantified as the percentage of the fibrotic area in total by ImageJ (NIH) software. (I) Myocardial capillary density assessed by CD31 staining. All tissue samples were assessed at 8 wk post-TAC (n = 5–8). *P < 0.05. NS, not significant. (Scale bars, 200 μm.)
Serial echocardiography for 8 wk post-TAC demonstrated that WT mice gradually develop cardiac dysfunction during late-phase POH, while CCR2-KO mice maintain relatively normal cardiac function (EF at 8 wk post-TAC: WT: 52 ± 2.0% vs. CCR2-KO: 65 ± 1.5%; P < 0.0001) (Fig. 5D). Although there was no difference in cardiac hypertrophy or fibrosis between WT and CCR2-KO groups (Fig. 5 E–H), myocardial capillary density was significantly higher in the CCR2-KO group (capillary counts per 1 mm2 of myocardium: WT: 1,872 ± 113 vs. CCR2-KO: 2,359 ± 98; ∼26% increase; P < 0.0038) (Fig. 5I), suggesting that CCR2-KO mice were able to maintain superior angiogenesis during the 8 wk of TAC. Finally, blocking CCR2 signaling in WT animals using RS preserved cardiac function in an established POH model (RS oral administration 2 wk after TAC) without affecting cardiac hypertrophy (SI Appendix, Fig. S10). Collectively, these data demonstrate that blocking monocyte/macrophage infiltration maintains angiogenesis and preserves cardiac function without affecting cardiac hypertrophy or fibrosis in late-phase POH. Taken together, these data demonstrate that late-phase infiltration of Ly6ChiCX3CR1+CCR2+ classic monocytes contributes to cardiac dysfunction in POH and, conversely, that blockade of infiltrating macrophages (genetically by CCR2-KO or pharmaceutically by CCR2 antagonist) preserves heart function, in part, through improved angiogenesis.
Discussion
The heart contains resident macrophages and can recruit infiltrating macrophages upon stress. As cardiac resident and infiltrating macrophages have separate origins and reside in distinct geographic locations, it is not surprising that they have different functions as well. In the present study using a mouse model of NICM, we show that resident macrophage proliferation and angiogenic activity in the initial period following pressure overload stress are critical for cardiac adaptation and function. Furthermore, cardiac resident macrophages are essential to maintain cardiac function even in the late phase of POH. By contrast, in the late phase of POH, infiltrating monocytes/macrophages promote decompensation and blockade of this ingress is ameliorative. These findings are aligned with recent observations that in models of acute and chronic post-MI remodeling, resident macrophages are salutary, while infiltrating macrophages are detrimental (15, 16). Further, in neonatal hearts, tissue macrophages have been shown to regulate cardiac angiogenesis and regeneration after apical resection or MI (15, 32). Collectively, the current work, along with these recent findings, underscores the differential roles of tissue-intrinsic and tissue-extrinsic macrophages in the cardiac response to injury (summarized in Fig. 6).
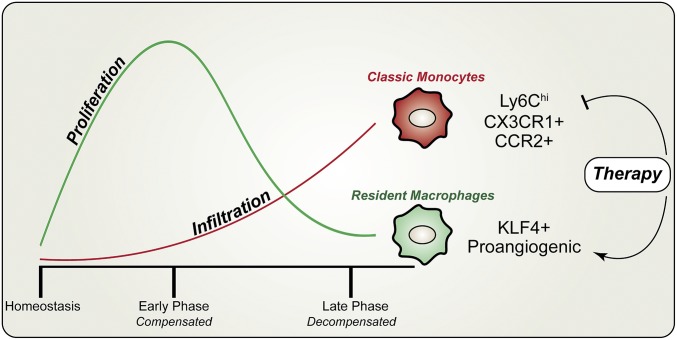
Fig. 6.
Dynamic changes and distinct roles of cardiac macrophage subsets in POH. Our studies have demonstrated a two-phase response of cardiac macrophages to pressure overload. (i) During the early phase of POH, there is robust local proliferation of resident macrophages that is crucial for cardiac compensation to pressure overload. (ii) During the late phase of POH, the infiltration of inflammatory monocytes increases, which promotes the transition to cardiac decompensation. Mechanistically, transcription factor KLF4 is required for resident macrophage proliferation and the infiltrating monocytes are Ly6ChiCX3CR1+CCR2+ classic monocytes. Finally, this model implies that these two subsets of cardiac macrophages can be targeted for therapeutic gain, either by blocking monocytes (i.e., RS administration) or by promoting resident macrophages (i.e., prolonged proliferation), or by simultaneously targeting both subsets for a healthy balance.
An important aspect of our work relates to the identification of KLF4 as a critical regulator of resident macrophage proliferation and angiogenic function. We found that myeloid KLF4 deficiency resulted in a marked reduction in local proliferation of cardiac resident macrophages and the total number of cardiac macrophages 7 d after TAC. Pathway analysis of RNA-seq studies in KLF4-deficient macrophages highlighted enrichment for pathways that control cellular proliferation. These findings are particularly intriguing considering prior work linking KLF4 to macrophage proliferation (23). The upstream mechanism following POH that leads to KLF4 induction remains incompletely understood. However, a recent study by Fujiu et al. (33) reported that kidney-derived CSF2 activated proliferation of cardiac resident macrophages in response to TAC. We observed that myeloid KLF4 deficiency significantly diminished macrophage proliferation in response to CSF2 (as well as CSF1; Fig. 4B). As such, it is possible that kidney-derived CSF2 induces KLF4-dependent resident macrophage proliferation. Future studies will be needed to delineate the detailed mechanisms by which KLF4 regulates proliferation of resident macrophages in vivo and whether KLF4 is universally required for resident macrophage proliferation in other tissues.
Another important observation relates to myeloid KLF4 control of angiogenesis. We observed that myeloid KLF4 KO animals demonstrated a marked susceptibility to heart failure, likely due to reduced angiogenic capacity leading to cardiomyocyte death (Fig. 4). Consistent with this, myeloid KLF4 KO mice demonstrated significant cardiac troponin levels in plasma and clear evidence of cardiac mitochondrial damage. The basis for myeloid KLF4 control of angiogenesis remains incompletely understood, but we note that expression of a number of proangiogenic factors, such VEGFA, is reduced and that expression of potent antiangiogenic factors, such as Thbs1, is increased in the K4-cKO hearts. The reduced expression of VEGFA is further confirmed in cardiac macrophages isolated from K4-cKO hearts. An intriguing and unanswered question relates to how the relatively small number of resident macrophages can impact angiogenesis throughout the heart. As macrophages often cluster around blood vessels, it is possible that they may release VEGF into the coronary circulation to impact the heart more broadly. Alternatively, the acute heart failure (<3 d) observed in macrophage-depleted animals (Fig. 3B) suggests that cardiac macrophages may also regulate arteriogenesis in POH as reported previously (34). Additional studies will be required to address these considerations. Finally, while we and others have also shown that KLF4 regulates macrophage polarization (24, 25), the present study excludes the significance of macrophage M1/M2 polarization in POH using two additional M2-deficient mouse models, namely, hematopoietic STAT6-KO and myeloid-specific IL4Ra-KO mice, which exhibited normal cardiac adaptation to POH despite lack of classic M2 macrophages (26, 35). We also note that Aurora et al. (32) have reported that cardiac resident macrophages mainly mediate angiogenesis rather than inflammation, with no clear bias toward M1 or M2 in a neonatal myocardial injury model.
In addition to resident macrophage proliferation in early-phase POH (<7 d), there is significant infiltration of classic Ly6Chi monocytes during late-phase POH (>4 wk). Infiltration of these tissue-extrinsic monocytes/macrophages takes place during the cardiac decompensation phase, suggesting a potentially negative role in POH. Consistent with this view, our studies in CCR2-KO mice showed that blockade of infiltrating macrophages (monocytes) preserved cardiac function, likely through improved myocardial angiogenesis (Fig. 5). How infiltrating macrophages affect angiogenesis remains to be fully understood. Infiltrating macrophages may trigger inflammation and other responses that negatively regulate angiogenesis (32). Alternatively, infiltrating macrophages may negatively impact resident macrophages, which are endowed with angiogenic capacity (15). It is noteworthy that cardiac resident macrophages also persist into and clearly are requisite for the late-phase POH (SI Appendix, Fig. S5). As such, cardiac resident and infiltrating macrophage subsets may regulate POH through temporal and spatial activation and interaction between each other. In addition to their roles in angiogenesis as discussed above, cardiac resident macrophages have been reported to regulate electrical conduction of the heart (21). Although CL-mediated macrophage depletion is insufficient to induce arrhythmia in unstressed baseline hearts (21), it is possible that loss of resident macrophages may result in arrhythmia in a more susceptible substrate, such as the hypertrophied heart (36). For instance, some of the post-TAC mortality in CL-treated TAC animals might result from sudden death (Fig. 3C and SI Appendix, Fig. S5A).
Heart failure affects more than 26 million people worldwide and (37, 38) is associated with a devastating ∼50% mortality within 5 y of diagnosis (39). Current therapies aimed at reducing neurohormonal stress and improving hemodynamics are only partially effective, underscoring the need for orthogonal strategies based on newly identified molecular and cellular mechanisms. The studies here highlight the differential impact of cardiac resident and nonresident macrophages in an experimental model of heart failure. Further, they suggest that inhibition of blood-borne macrophages through blockade of the CCR2 pathway can be exploited for therapeutic gain.
Materials and Methods
Detailed materials and methods can be found in SI Appendix. RNA-seq data are deposited in the Gene Expression Omnibus (accession no. GSE107016). All experimental procedures were approved by the Institutional Animal Care and Use Committee of Case Western Reserve University School of Medicine and were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals (40).
Supplementary Material
Acknowledgments
We thank Ajay Chawla for providing the IL4Ra-cKO mice, Simone Edelheit and Alexander Miron for assistance in RNA-seq studies, Mike Sramkoski for assistance in flow cytometry, and Sarah Liao for artistic assistance. This work was supported by American Heart Association (AHA) National Scientist Development Grant 12SDG12070077 (to X.L.), AHA Postdoctoral Fellowship 17POST33650110 (to Y.S.), National Natural Science Foundation of China Grant 81400347 (to L.Z.), NIH Grants T32GM007250 and F30HL139014 (to D.R.S.), NIH Grants R35 HL135789 and R01 DK111468-01 (to M.K.J.), and the Elisabeth Severance Prentiss Foundation Grant (to M.K.J.). This work was also generously supported by Tom F. Peterson.
Footnotes
Conflict of interest statement: X.L. and M.K.J. have a patent application (serial no. 62/644,792).
This article is a PNAS Direct Submission.
Data deposition: The data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, https://www.ncbi.nlm.nih.gov/geo (accession no. GSE107016).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1720065115/-/DCSupplemental.
References
- 1.Cecchi F, et al. Coronary microvascular dysfunction and prognosis in hypertrophic cardiomyopathy. N Engl J Med. 2003;349:1027–1035. doi: 10.1056/NEJMoa025050. [DOI] [PubMed] [Google Scholar]
- 2.Treasure CB, et al. Hypertension and left ventricular hypertrophy are associated with impaired endothelium-mediated relaxation in human coronary resistance vessels. Circulation. 1993;87:86–93. doi: 10.1161/01.cir.87.1.86. [DOI] [PubMed] [Google Scholar]
- 3.Maron BJ, Wolfson JK, Epstein SE, Roberts WC. Intramural (“small vessel”) coronary artery disease in hypertrophic cardiomyopathy. J Am Coll Cardiol. 1986;8:545–557. doi: 10.1016/s0735-1097(86)80181-4. [DOI] [PubMed] [Google Scholar]
- 4.Frey N, Luedde M, Katus HA. Mechanisms of disease: Hypertrophic cardiomyopathy. Nat Rev Cardiol. 2011;9:91–100. doi: 10.1038/nrcardio.2011.159. [DOI] [PubMed] [Google Scholar]
- 5.Sano M, et al. p53-induced inhibition of Hif-1 causes cardiac dysfunction during pressure overload. Nature. 2007;446:444–448. doi: 10.1038/nature05602. [DOI] [PubMed] [Google Scholar]
- 6.Prosdocimo DA, et al. Kruppel-like factor 15 is a critical regulator of cardiac lipid metabolism. J Biol Chem. 2014;289:5914–5924. doi: 10.1074/jbc.M113.531384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Izumiya Y, et al. Vascular endothelial growth factor blockade promotes the transition from compensatory cardiac hypertrophy to failure in response to pressure overload. Hypertension. 2006;47:887–893. doi: 10.1161/01.HYP.0000215207.54689.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hashimoto D, et al. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity. 2013;38:792–804. doi: 10.1016/j.immuni.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Epelman S, et al. Embryonic and adult-derived resident cardiac macrophages are maintained through distinct mechanisms at steady state and during inflammation. Immunity. 2014;40:91–104. doi: 10.1016/j.immuni.2013.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gomez Perdiguero E, et al. Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature. 2015;518:547–551. doi: 10.1038/nature13989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein-1 (MCP-1): An overview. J Interferon Cytokine Res. 2009;29:313–326. doi: 10.1089/jir.2008.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Misharin AV, et al. Nonclassical Ly6C(-) monocytes drive the development of inflammatory arthritis in mice. Cell Rep. 2014;9:591–604. doi: 10.1016/j.celrep.2014.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cohen HB, Mosser DM. Cardiac macrophages: How to mend a broken heart. Immunity. 2014;40:3–5. doi: 10.1016/j.immuni.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 14.Frantz S, Nahrendorf M. Cardiac macrophages and their role in ischaemic heart disease. Cardiovasc Res. 2014;102:240–248. doi: 10.1093/cvr/cvu025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lavine KJ, et al. Distinct macrophage lineages contribute to disparate patterns of cardiac recovery and remodeling in the neonatal and adult heart. Proc Natl Acad Sci USA. 2014;111:16029–16034, and erratum (2016) 113:E1414. doi: 10.1073/pnas.1406508111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sager HB, et al. Proliferation and recruitment contribute to myocardial macrophage expansion in chronic heart failure. Circ Res. 2016;119:853–864. doi: 10.1161/CIRCRESAHA.116.309001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mirzadegan T, et al. Identification of the binding site for a novel class of CCR2b chemokine receptor antagonists: Binding to a common chemokine receptor motif within the helical bundle. J Biol Chem. 2000;275:25562–25571. doi: 10.1074/jbc.M000692200. [DOI] [PubMed] [Google Scholar]
- 18.Terrazas C, et al. Ly6Chi inflammatory monocytes promote susceptibility to Leishmania donovani infection. Sci Rep. 2017;7:14693. doi: 10.1038/s41598-017-14935-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raghu H, et al. CCL2/CCR2, but not CCL5/CCR5, mediates monocyte recruitment, inflammation and cartilage destruction in osteoarthritis. Ann Rheum Dis. 2017;76:914–922. doi: 10.1136/annrheumdis-2016-210426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Rooijen N, Sanders A. Liposome mediated depletion of macrophages: Mechanism of action, preparation of liposomes and applications. J Immunol Methods. 1994;174:83–93. doi: 10.1016/0022-1759(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 21.Hulsmans M, et al. Macrophages facilitate electrical conduction in the heart. Cell. 2017;169:510–522.e20. doi: 10.1016/j.cell.2017.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jenkins SJ, et al. IL-4 directly signals tissue-resident macrophages to proliferate beyond homeostatic levels controlled by CSF-1. J Exp Med. 2013;210:2477–2491. doi: 10.1084/jem.20121999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aziz A, Soucie E, Sarrazin S, Sieweke MH. MafB/c-Maf deficiency enables self-renewal of differentiated functional macrophages. Science. 2009;326:867–871. doi: 10.1126/science.1176056. [DOI] [PubMed] [Google Scholar]
- 24.Liao X, et al. Krüppel-like factor 4 regulates macrophage polarization. J Clin Invest. 2011;121:2736–2749. doi: 10.1172/JCI45444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kapoor N, et al. Transcription factors STAT6 and KLF4 implement macrophage polarization via the dual catalytic powers of MCPIP. J Immunol. 2015;194:6011–6023. doi: 10.4049/jimmunol.1402797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tugal D, Liao X, Jain MK. Transcriptional control of macrophage polarization. Arterioscler Thromb Vasc Biol. 2013;33:1135–1144. doi: 10.1161/ATVBAHA.113.301453. [DOI] [PubMed] [Google Scholar]
- 27.Dick SA, Epelman S. Chronic heart failure and inflammation: What do we really know? Circ Res. 2016;119:159–176. doi: 10.1161/CIRCRESAHA.116.308030. [DOI] [PubMed] [Google Scholar]
- 28.Fu M. Inflammation in chronic heart failure: What is familiar, what is unfamiliar? Eur J Heart Fail. 2009;11:111–112. doi: 10.1093/eurjhf/hfn039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ismahil MA, et al. Remodeling of the mononuclear phagocyte network underlies chronic inflammation and disease progression in heart failure: Critical importance of the cardiosplenic axis. Circ Res. 2014;114:266–282. doi: 10.1161/CIRCRESAHA.113.301720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 31.Tsou CL, et al. Critical roles for CCR2 and MCP-3 in monocyte mobilization from bone marrow and recruitment to inflammatory sites. J Clin Invest. 2007;117:902–909. doi: 10.1172/JCI29919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aurora AB, et al. Macrophages are required for neonatal heart regeneration. J Clin Invest. 2014;124:1382–1392. doi: 10.1172/JCI72181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fuji UK, et al. A heart-brain-kidney network controls adaptation to cardiac stress through tissue macrophage activation. Nat Med. 2017;23:611–622. doi: 10.1038/nm.4326. [DOI] [PubMed] [Google Scholar]
- 34.Takeda Y, et al. Macrophage skewing by Phd2 haplodeficiency prevents ischaemia by inducing arteriogenesis. Nature. 2011;479:122–126. doi: 10.1038/nature10507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nguyen KD, et al. Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature. 2011;480:104–108. doi: 10.1038/nature10653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McLenachan JM, Henderson E, Morris KI, Dargie HJ. Ventricular arrhythmias in patients with hypertensive left ventricular hypertrophy. N Engl J Med. 1987;317:787–792. doi: 10.1056/NEJM198709243171302. [DOI] [PubMed] [Google Scholar]
- 37.Benjamin EJ, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics-2017 update: A report from the American Heart Association. Circulation. 2017;135:e146–e603, and erratum (2017) 136:e196. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ambrosy AP, et al. The global health and economic burden of hospitalizations for heart failure: Lessons learned from hospitalized heart failure registries. J Am Coll Cardiol. 2014;63:1123–1133. doi: 10.1016/j.jacc.2013.11.053. [DOI] [PubMed] [Google Scholar]
- 39.Mozaffarian D, et al. Writing Group Members; American Heart Association Statistics Committee Stroke Statistics Subcommittee Heart disease and stroke statistics-2016 update: A report from the American Heart Association. Circulation. 2016;133:e38–e360, and erratum (2016) 133:e599. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 40.National Research Council . Guide for the Care and Use of Laboratory Animals. 8th Ed National Academies Press; Washington, DC: 2011. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.