Significance
Our findings demonstrate that metabolic supplementation of mucosal T cells, isolated from patients with active ulcerative colitis (UC), with N-acetylglucosamine (GlcNAc) leads to the enhancement of branched N-glycosylation on the T cell receptor, which was associated with the control of T cell activation and function. These results were validated in “glycoengineered” mouse models with severe colitis. Overall, our results open new avenues for a targeted-specific therapy in inflammatory bowel disease (IBD). The therapeutic use of GlcNAc (either alone or in combination with other antiinflammatory therapies) represents a simple immunomodulatory strategy in IBD, with absence of side effects, low costs, and the possibility of being used as a simple rescue therapy to avoid unnecessary toxic effects and step-up therapies in IBD.
Keywords: T lymphocytes, T cell receptor, adaptive immune response, branched N-glycosylation, intestinal inflammation
Abstract
Mucosal T lymphocytes from patients with ulcerative colitis (UC) were previously shown to display a deficiency in branched N-glycosylation associated with disease severity. However, whether this glycosylation pathway shapes the course of the T cell response constituting a targeted-specific mechanism in UC remains largely unknown. In this study, we demonstrated that metabolic supplementation of ex vivo mucosal T cells from patients with active UC with N-acetylglucosamine (GlcNAc) resulted in enhancement of branched N-glycosylation in the T cell receptor (TCR), leading to suppression of T cell growth, inhibition of the T helper 1 (Th1)/Th17 immune response, and controlled T cell activity. We further demonstrated that mouse models displaying a deficiency in the branched N-glycosylation pathway (MGAT5−/−, MGAT5+/−) exhibited increased susceptibility to severe forms of colitis and early-onset disease. Importantly, the treatment of these mice with GlcNAc reduced disease severity and suppressed disease progression due to a controlled T cell-mediated immune response at the intestinal mucosa. In conclusion, our human ex vivo and preclinical results demonstrate the targeted-specific immunomodulatory properties of this simple glycan, proposing a therapeutic approach for patients with UC.
Inflammatory bowel diseases (IBDs), encompassing Crohn’s disease and ulcerative colitis (UC), are chronic, relapsing, and life-long inflammatory disorders of the gastrointestinal tract affecting mainly young populations. The incidence of IBD is increasing worldwide, and the disease remains incurable, placing a heavy burden on populations by reducing patients’ quality of life and increasing disability (1). The current therapeutic strategies for IBD are limited by reduced effectiveness, high costs, and/or side effects. This scenario highlights the urgent need in the clinic of identifying novel molecular markers capable of being selectively targeted with new and optimized therapies. Future progress in IBD monitoring and therapy mostly depends on the identification of key mechanism(s) mediating intestinal inflammation that could be therapeutically targeted.
The immune system is tightly regulated by glycosylation, through the addition of carbohydrate structures (glycans) to key molecules (proteins) involved in innate and adaptive immune responses (2). The N-acetylglucosaminyltransferase V (GnT-V) is a glycosyltransferase encoded by the human MGAT5 gene that catalyzes the synthesis of β1,6-N-acetylglucosamine (GlcNAc) branched N-glycans, which are known to play pivotal roles in many glycoproteins in cancer (3–6) and also in T cell activity and function (7, 8). In homeostasis and self-tolerance, T cell activation [via T cell receptor (TCR) signaling] induces up-regulation of the MGAT5 gene, which, in turn, leads to GnT-V–mediated glycosylation of the TCR (9). Consequently, it can promote growth arrest of T cells early, by raising T cell activation thresholds via limiting TCR clustering at the immune synapse (and restricting TCR signaling), and, later, by increasing surface retention of growth inhibitory receptors such as cytotoxic T lymphocyte antigen-4 (CTLA-4) (9).
In fact, mice deficient in the MGAT5 gene display an increased susceptibility to autoimmune diseases (7, 10, 11). These mice lacking GnT-V function (no synthesis of β1,6-GlcNAc branched N-glycan structures) display an increased TCR clustering and increased T helper 1 (Th1) differentiation that result in a hyperimmune response in mouse models of multiple sclerosis (7, 10). Interestingly, in mouse models of experimental autoimmune encephalomyelitis (EAE) and type I diabetes, it was shown that supplementation with GlcNAc induces increased N-glycan branching, through increasing the hexosamine pathway, that was associated with inhibition of T cell growth and differentiation (12, 13), leading to delayed disease progression. Moreover, N-glycan branching was also found to regulate T cell development (14). Recently, it was demonstrated that branching N-glycans potentiate the differentiation of induced regulatory (iTreg) T cells over Th17 differentiation (15). Importantly, and in the setting of human immune-mediated disorders, we have recently found that patients with UC exhibit a deficiency in branched glycosylation (catalyzed by GnT-V) in mucosal T cells that was associated with disease severity. Patients with UC who have severe disease showed the most pronounced defect on branched N-glycans in intestinal T cells, together with a significant reduction of MGAT5 gene transcription in these cells (16).
In the present study, and building upon our previous findings in patients with UC (16), we have evaluated the impact of glycosylation, particularly the branched N-glycosylation pathway, in the regulation of the T cell-mediated immune response in patients with UC. We further explored whether this mechanism could be therapeutically targeted in vivo through a simple glycan-based strategy. Our results showed that metabolic supplementation of mucosal T cells, isolated from patients with active UC, with GlcNAc led to the enhancement of branched N-glycosylation on the TCR, controlling T cell activation and function. Preclinical data further demonstrated that GlcNAc treatment of MGAT5 null or heterozygous mice developing severe forms of induced colitis significantly controlled disease severity and progression due to suppression of the intestinal T cell-mediated immune response, with good clinical effects when GlcNAc was topically administered by enemas. Altogether, this study highlights the potential of glycans as novel immunomodulatory agents in IBD, warranting validation in human clinical trials.
Results
Ex Vivo GlcNAc Supplementation Increased Branched N-Glycosylation of T Cells from Patients with Active UC.
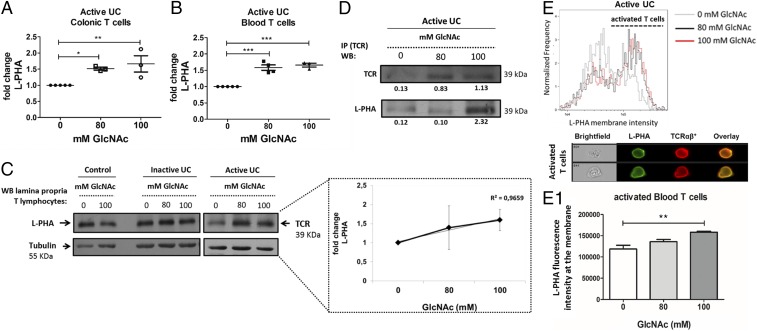
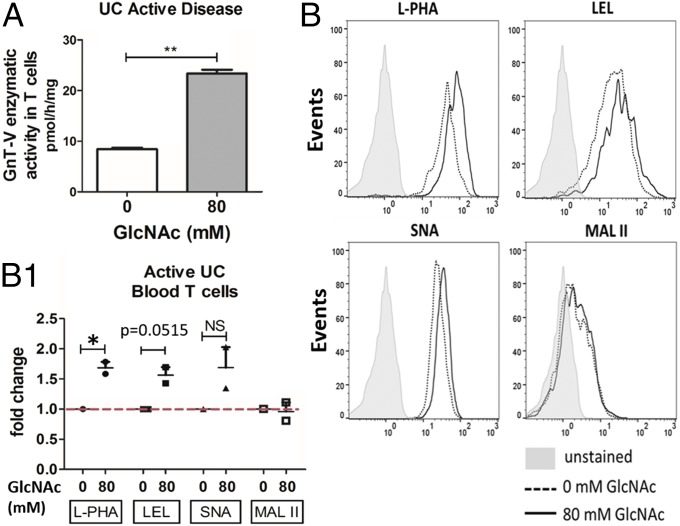
We have previously demonstrated that patients with UC display reduced branched N-glycosylation on mucosal T cells (16). To assess the ability of glycans as repairers of the above-mentioned mechanistic defect, we herein promoted, ex vivo, the hexosamine biosynthetic pathway (SI Appendix, Fig. S1A) in purified intestinal T cells by metabolic supplementation with GlcNAc. Previous studies showed that supplementation with GlcNAc increases the availability of the substrate (UDP-GlcNAc) to Golgi enzymes such as GnT-V, enhancing β1,6-GlcNAc branching N-glycans, particularly in T cells (13). To test this hypothesis, T cells (CD3+) were isolated ex vivo from both the intestinal lamina propria of fresh colonic biopsies and peripheral blood of patients with UC who have active disease and were supplemented with increasing doses of GlcNAc. Different GlcNAc concentrations (40 mM, 80 mM, and 100 mM) were tested, and 40 mM did not reveal major alterations compared with nontreated T cells (SI Appendix, Fig. S1B). The expression of β1,6-GlcNAc branched N-glycans on colonic T cells was evaluated by flow cytometry using Phaseolus vulgaris leukoagglutinating (L-PHA) lectin. We observed a dose-dependent increase of branched N-glycans on intestinal T cells upon GlcNAc supplementation across different patients (Fig. 1A). This increased modification with branched N-glycans was also observed in T cells isolated from peripheral blood mononuclear cells of patients with active UC displaying FSChigh and SSChigh light-scattering parameters, characteristic of activated T lymphocytes (Fig. 1B). The increased expression of β1,6-GlcNAc branched N-glycans was detected both on CD4+ and CD8+ T cells (SI Appendix, Fig. S1 C and D). No effects of GlcNAc treatment in the proportion of CD4+ and CD8+ T cell subsets in the cultures were observed (SI Appendix, Fig. S1 E and F), supporting that GlcNAc supplementation leads to a specific modification with branched glycans on T cells in a dose-dependent manner. Importantly, the enhancement of branched N-glycans was only observed in T cells from patients with active UC (Fig. 1C and SI Appendix, Fig. S1G). T cells from healthy controls and from patients with inactive disease did not show alterations in the levels of branched glycans upon treatment with increasing concentrations of GlcNAc (Fig. 1C and SI Appendix, Fig. S2), possibly due to the higher baseline branching comparing with patients with active UC. These results were further confirmed by other technical approaches. Increased expression of β1,6-GlcNAc branched N-glycans on a band the same size as the TCR β-chain (TCRβ) after GlcNAc supplementation was also detected by L-PHA blotting (Fig. 1C) and by TCR immunoprecipitation using lysates of lamina propria T lymphocytes (LPLs) purified from patients with UC (Fig. 1D). Interestingly, this increased branching of N-glycans after GlcNAc supplementation was found to occur in intestinal T cells from patients with UC with different Mayo subscores (with Mayo endoscopic subscores 1, 2, and 3) with a trend association with disease severity, as depicted in SI Appendix, Fig. S1G. The internalization of externally given GlcNAc was already demonstrated in cell lines (17). The specific effects of GlcNAc in enhancing branched glycosylation on T cells from patients with active UC was further demonstrated by the reversed effects on L-PHA mean fluorescence intensity when T cells were treated with the N-glycan branching inhibitors kifunensine (KF) and swainsonine (SW) (SI Appendix, Fig. S3) in T cells from biopsies and blood of patients with active UC. Moreover, the specific effects of GlcNAc in the enhancement of branched glycosylation in T cells was further validated by supplementation of T cells from patients with active UC with other glycan types such as d-mannose, which revealed no impact in branched N-glycan expression (SI Appendix, Fig. S3). To further validate these observations, we also performed imaging flow cytometry showing that TCRα/β+ cells display an increase of fluorescence intensity due to staining with L-PHA on the cell membrane. This increase was observed in T cells displaying blast-like morphology (Fig. 1 E and E1). Taken together, these results demonstrate that treating ex vivo T cells from patients with active UC with GlcNAc promotes the hexosamine biosynthetic pathway enhancing β1,6-GlcNAc branched N-glycans on the TCR, and thus restoring the deficiency on branched N-glycans previously shown in mucosal T cells from patients with UC (16). Next, we have determined the specificity of this enhancement of β1,6-GlcNAc N-glycan branching by analyzing the correspondent GnT-V enzymatic activity. Interestingly, and in line with our previous observations on MGAT5 gene transcription (16), T cells from patients with active UC displayed reduced GnT-V enzymatic activity compared with healthy controls (SI Appendix, Fig. S4). Our results showed that this reduced GnT-V enzymatic activity of T cells could be significantly recovered after metabolic supplementation with GlcNAc (Fig. 2A), which further supports the effects of GlcNAc in the enhancement of N-glycan branching mediated by GnT-V. In the N-glycosylation branching pathway, the β1,6-GlcNAc branched N-glycan catalyzed by GnT-V can be further extended with polylactosamine structures (ligands for galectins), which, in turn, can be terminally sialylated (SI Appendix, Fig. S1A). Our results showed that GlcNAc supplementation of ex vivo activated T cells led to increased expression of β1,6-GlcNAc branched N-glycans (as detected by L-PHA lectin) (Figs. 1E and 2 B and B1) with a trend of increased extension with polylactosamine structures, as indicated by staining with the Lycopersicon esculentum agglutinin (LEL) (Fig. 2 B and B1). Additionally, we determined whether there was a terminal addition of α2,6-linked sialic acid, recognized by binding of Sambucus nigra agglutinin (SNA), and/or α2,3-sialic acid, recognized by Maackia amurensis agglutinin (MAL-II). The results demonstrated a trend of increase in α2,6-linked sialic acid, and no consistent alterations in α2,3-sialic acid linkages were detected (Fig. 2 B and B1). Overall, our results support that ex vivo supplementation of T cells from patients with UC with GlcNAc has the potential to enhance the branched N-glycosylation on T cells, remodeling the T cell glycoprofile, which is ultimately expected to have an impact on the regulation of the immune response.
Fig. 1.
Ex vivo GlcNAc supplementation of T cells from patients with UC resulted in increased branching N-glycans. (A and B) CD3+ T cells from patients with active UC were cultured with different concentrations (millimolar) of GlcNAc, and the fold change of mean fluorescence intensity due to L-PHA staining was determined by flow cytometry. The scatter plots illustrate the mean ± SEM of five biological replicates. One-way ANOVA using the Newman–Keuls multiple comparison posttest: *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001. (C) Protein lysates of purified CD3+ T cells under GlcNAc treatment were subjected to L-PHA lectin blotting to evaluate the expression levels of β1,6-GlcNAc branched N-glycans on a protein band corresponding to the migration profile of the TCRβ. WB, Western blot. (Inset) Linear regression using mean values per treatment condition. (D) Immunoprecipitation (IP) of TCR followed by β1,6-GlcNAc branched N-glycan recognition with L-PHA. The density of bands is indicated below each band. (E) Imaging flow cytometry analysis (on an ImageStreamX) of L-PHA membrane distribution on TCR+ cells after GlcNAc supplementation in T cells isolated from blood of patients with active UC. Representative images of activated T cells display blast-like morphology showing colocalization (overlaid images) of TCRαβ and L-PHA staining on the cell membrane. (E1) Bars depict the mean ± SEM of L-PHA staining intensity on gated TCRαβ+ L-PHAhigh cells from three independent experiments. One-way ANOVA using Dunnett’s multiple comparison posttest: **P ≤ 0.01. In all experiments, results are normalized to the corresponding untreated condition (0 mM).
Fig. 2.
Remodeling of the glycosylation phenotype upon metabolic supplementation with GlcNAc. (A) Impact of GlcNAc supplementation on GnT-V activity was determined using a pool of lysates from treated vs. nontreated peripheral blood T cells, in three biological replicates of patients with active UC, from two independent technical experiments. Student’s t test: **P ≤ 0.01. (B) Flow cytometry evaluation of glycophenotype of T cells upon GlcNAc supplementation. (B1) Scatter plot: fold change of MFI due to staining with each lectin on T cells, in two biological replicates with different stages of disease severity (Mayo endoscopic scores 1 and 2), from two independent experiments. Results are normalized to the untreated condition, which was taken as 1. Student’s t test: *P ≤ 0.05. NS, not significant.
Shaping the T Cell-Mediated Immune Response in UC Through Increased Branching N-Glycans.
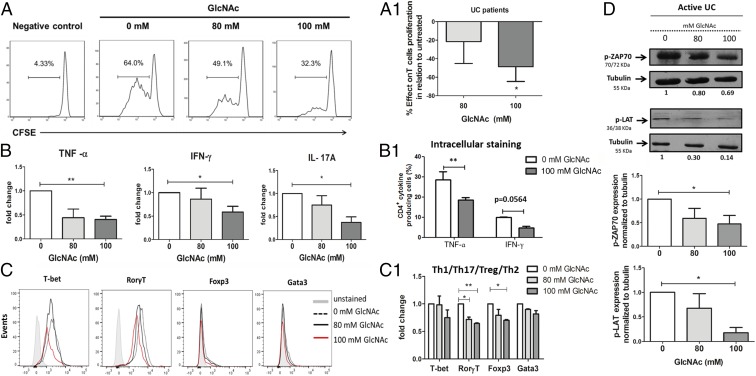
After demonstrating the ability of GlcNAc supplementation to repair the deficiency of branched N-glycans on ex vivo T cells, we next evaluated its impact on the modulation of T cell responsiveness. The metabolic supplementation with GlcNAc of ex vivo activated T cells from naive patients (without therapy) resulted in significant suppression of their proliferative response to anti-CD3/CD28 mAb stimulation (Fig. 3 A and A1). In addition, and importantly, the increased N-glycan branching on T cells resulted in a significant reduction in the production of the proinflammatory cytokines TNF-α, IFN-γ, and IL-17A, which are associated with UC pathogenesis (18), in a GlcNAc dose-dependent manner (Fig. 3B), supporting its effect on the control of Th1/Th17-type immune responses. The effects of GlcNAc on the suppression of proinflammatory cytokine production were found to be independent of cell death and/or decreased T cell proliferation. After normalization of cytokine concentrations to the respective T cell proliferation index (index of division), we still observed a reduction in the production of proinflammatory cytokines (SI Appendix, Fig. S5A). Moreover, the percentage of IFN-γ– and TNF-α–expressing cells among total CD4+ T cells, evaluated by intracellular flow cytometry, was decreased upon GlcNAc treatment (Fig. 3B1). The impact of GlcNAc supplementation on other cytokines, such as TGF-β and IL-10, is not significant (SI Appendix, Fig. S5 B and C). The specific effects of GlcNAc in suppressing proinflammatory cytokine production is further confirmed by inhibitors (KF and SW), which blocked the regulatory impact of branched N-glycans in T cell function (SI Appendix, Fig. S6). Next, we have evaluated the expression of the transcription factors (T-bet, RORγt, Foxp3, and Gata3) in T cells (CD3+) under ex vivo GlcNAc treatment. The results showed that GlcNAc supplementation was associated with a reduction in the expression of T-bet and RORγt, which corroborates the negative impact on the Th1/Th17-type response (Fig. 3 C and C1). The expression of Foxp3, a transcription factor associated with regulatory T cells as well as with human activated T cells (18), was also found to be reduced upon GlcNAc treatment. To gain insight into the molecular basis of this modulation of the T cell response through metabolic supplementation with GlcNAc, we analyzed its impact on the TCR signaling pathway. We observed that GlcNAc supplementation led to an inhibition of the phosphorylation of ZAP70 and LAT, thereby hindering T cell activity by controlling the TCR signaling pathway (Fig. 3D). Additionally, we evaluated whether GlcNAc supplementation had an impact on T cell apoptosis. Our results showed that T cells from patients with active UC treated with GlcNAc displayed an increased susceptibility to apoptosis compared with nontreated T lymphocytes as soon as 3 h after stimulation (SI Appendix, Fig. S7A). This effect was not seen in T cells from controls and patients with inactive UC, which demonstrated no differences, or even a trend to decrease apoptosis, respectively, at the same time points upon GlcNAc supplementation. This argues against a putative effect of hyperosmolarity in the observed increase of apoptosis/cell death in GlcNAc-supplemented T cells from patients with active UC and in controls (16) (SI Appendix, Fig. S7A). Treatment with the branched N-glycan inhibitors (KF and SW) did not reverse the effects of GlcNAc in apoptosis (SI Appendix, Fig. S7B). At 72 h, a significant decrease in branched glycosylation levels was observed with KF and SW (SI Appendix, Fig. S3); however, no differences in the apoptosis were detected (SI Appendix, Fig. S7B). The percentage of cell death was unchanged or even higher with SW and KF than with treatment with GlcNAc only (SI Appendix, Fig. S7B1). Taken together, these data collectively demonstrate that ex vivo GlcNAc supplementation of T cells from patients with active UC enhances the branched N-glycans and resulted in a significant suppression of T cell proliferation and TCR signaling, as well as controlled Th1/Th17-type immune responses.
Fig. 3.
Control of T cell-mediated immune response through enhancing branching N-glycosylation. (A) Purified CD3+ T cells from fresh biopsies of naive patients with active UC were labeled with CFSE and cultured with GlcNAc treatment. The gated cells in the histograms correspond to the percentage of live cells. (A1) Bar plot: the mean percentage of effect ± SEM due to GlcNAc supplementation on T cell proliferation in comparison to untreated cells. Results include four biological replicates. Student’s t test: *P ≤ 0.05. (B) Cytokine profile assessed by flow cytometry in the supernatants from ex vivo T cell cultures under GlcNAc supplementation. Bar plots: mean fold change ± SEM of cytokine concentrations (picograms per milliliter) in six biological replicates. Student’s t test: *P ≤ 0.05; **P ≤ 0.01. (B1) Evaluation of the percentage of IFN-γ– and TNF-α–producing CD4+ T cells treated vs. nontreated with GlcNAc. Bar plots: mean ± SEM percentage of CD4+cytokine-producing cells in three biological replicates from two independent experiments. Two-way ANOVA with Bonferroni postcorrection: **P ≤ 0.01. (C) Expression of the transcription factors (TFs) in CD4+CD45+ T cells isolated from patients with UC and analyzed by flow cytometry. Histogram overlays correspond to the expression of the indicated TFs observed upon GlcNAc supplementation (gray-shadowed histograms depict the respective unstained control). (C1) Bar plots: mean fold change in TF mean fluorescence intensity ± SEM in two biological replicates, from two independent experiments. Two-way ANOVA with Bonferroni postcorrection: *P ≤ 0.05; **P ≤ 0.01. (D) Western blot analysis of TCR signaling, p-ZAP70, and p-LAT assessed in T cell lysates from cultures supplemented with GlcNAc. Bar plots: mean ± SEM fold change of p-ZAP70 and p-LAT densities normalized to tubulin in five biological replicates, from three independent experiments. Student’s t test: *P ≤ 0.05. In all experiments, results are normalized to the corresponding untreated condition (0 mM), which was taken as 1.
Treatment with GlcNAc Reduces Disease Severity and Ameliorates Clinical Signs of Disease in Mice with Colitis.
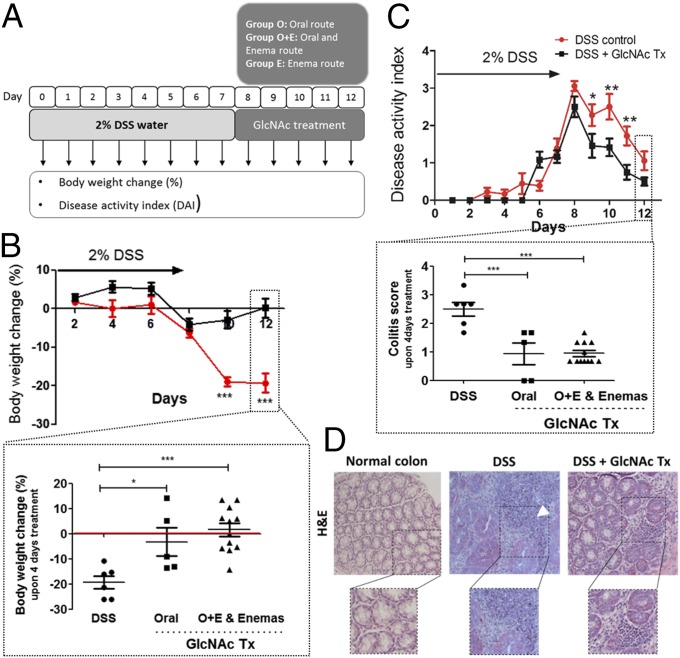
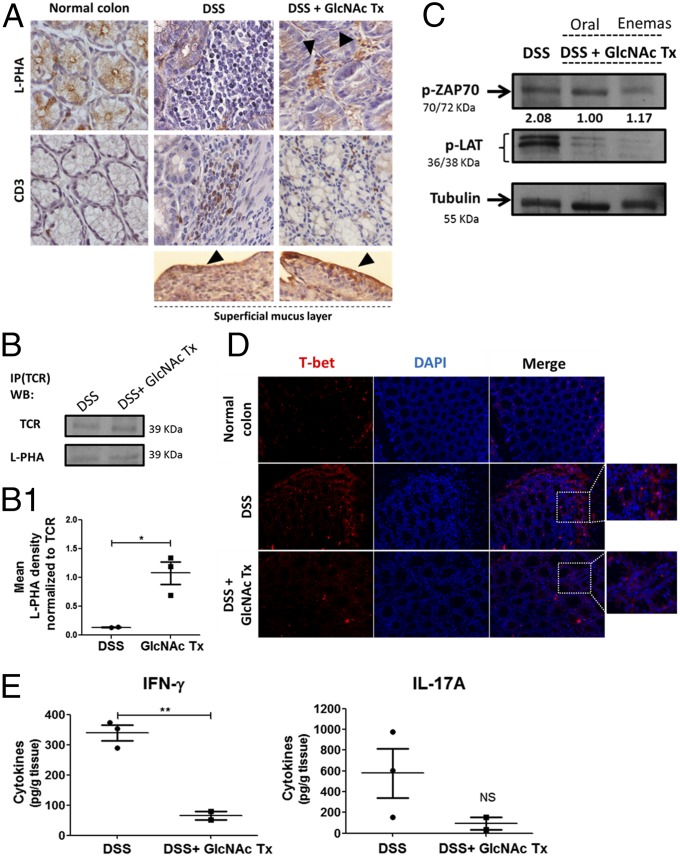
To determine whether dysregulation of branched N-glycans on TCR occurs in different experimental mouse models of colitis, we have evaluated two different chemically induced colitis mouse models, the dextran sodium sulfate (DSS)-induced and 2,4,6-trinitrobenzene sulfonic acid (TNBS)-induced models (19), in C57BL/6 wild-type mice. In both models, colitis was successfully induced, and we have observed a similar impact on the dysregulation of branched N-glycans in the TCR (SI Appendix, Fig. S8 A and B), which is in accordance with our previous observations on human patients with UC (16). The DSS-induced colitis model was selected as the ideal one to proceed with, since the rectal induction of disease in the TNBS model would interfere with the enema administration of GlcNAc. After disease onset using 2% DSS, GlcNAc was administered through two different routes: orally, by supplementing the drinking water with 0.25 mg/mL GlcNAc, and/or rectally, using 0.5 mg/mL GlcNAc enemas. With this approach, we assessed the therapeutic effects of GlcNAc on disease activity and on the control of intestinal inflammation. The experiment design is summarized in Fig. 4A. Our results showed that mice with colitis and treated with GlcNAc exhibited lower body weight loss (Fig. 4B) and significant improvements in disease activity index (DAI) (Fig. 4C) in comparison to mice with colitis not treated with GlcNAc (DSS control). Importantly, whenever animals received GlcNAc topically by enema administration (either alone or in combination with oral GlcNAc), the body weight changes were lower in comparison to animals receiving only oral GlcNAc. These results suggest that GlcNAc enemas may have promising topical effects on the control of disease severity (Fig. 4 B and C, Insets). Accordingly, macroscopic observation of the colon showed that mice with colitis displayed visible colonic edema (swelling of the bowel wall) that recovered upon GlcNAc treatment (SI Appendix, Fig. S8C). In addition, mice with colitis displayed extensive lymphocytic infiltrates (Fig. 4D, arrowhead) presenting with CD3+ cells (Fig. 5A) in the intestinal lamina propria, together with notable alterations of the glandular morphology. Furthermore, these animals also showed a reduced mucus layer (produced by glycoproteins at the glycocalyx), a natural barrier that confers protection from microbiota, preventing disease aggressiveness and progression (20). When animals with colitis were treated with GlcNAc, there was a decrease in the lymphocytic infiltrate and an improvement of the glandular architecture (Fig. 4D), which is compatible with disease remission (21). To further investigate the relationship between this overall improvement of disease severity through GlcNAc treatment and the levels of β1,6-GlcNAc branched N-glycans on LPLs, we performed L-PHA and CD3 histochemistry in the formalin-fixed, paraffin-embedded (FFPE) colonic specimens from the different groups of animals (Fig. 5A). The results showed that induction of colitis was accompanied by a notable intestinal lymphocytic infiltrate in the lamina propria, including CD3-expressing cells that expressed low levels of branched N-glycans. When mice were treated with GlcNAc, an increased expression of β1,6-branched glycans in the inflammatory infiltrate was observed (Fig. 5A, arrowheads). Importantly, the administration of GlcNAc also resulted in increased mucus lining, with augmented expression of branched glycans in glycoproteins in the superficial mucus layer, which is compatible with disease remission. Afterward, we investigated whether the TCR was particularly targeted by the observed enhancement of branched N-glycans in vivo. The results showed that GlcNAc treatment resulted in increased branching glycosylation on intestinal T cells, particularly in the TCR (Fig. 5 B and B1). To further address the mechanistic basis of the clinical efficacy of GlcNAc, we have evaluated the impact on T cell activity and signaling. Interestingly, LPLs from GlcNAc-treated mice displayed a decrease in the phosphorylation levels of ZAP70 and LAT that was more pronounced in mice treated with GlcNAc enemas (Fig. 5C). This result supports control of T cell activity through GlcNAc treatment, with effective results in topical/enema administration. Next, the effects of GlcNAc administration on the expression levels of T-bet, the transcription factor associated with proinflammatory Th1 cell polarization, were assessed in situ in the intestinal lamina propria. We have observed abundant cells expressing T-bet in lymphocytic infiltrates in mice with colitis that were markedly reduced in GlcNAc-treated mice (Fig. 5D, Insets). Furthermore, mice treated with GlcNAc revealed a significant reduction of IFN-γ production and a trend in the suppression of IL-17A secretion, further supporting that the enhancement of branched N-glycans by GlcNAc treatment controls Th1/Th17-type immune responses in vivo (Fig. 5E). Taken together, our in vivo results demonstrate a therapeutic effect of GlcNAc in a colitis-induced mouse model, revealing the immunomodulatory properties of this agent in the control of intestinal inflammation and, consequently, in the control of disease severity and progression.
Fig. 4.
Colitis-induced mouse model treated with GlcNAc displays significant control of disease severity and recovery from clinical signs. (A) Schematic representation of the in vivo study performed on C57BL/6 mice. (B and C) Body weight changes and DAI. (B) Effects of GlcNAc on body weight change (%) comparing DSS control and GlcNAc-treated animals. Graphs depict the mean ± SEM. Two-way ANOVA with Bonferroni postcorrection: ***P ≤ 0.001. (Inset) Discrimination of the effects of GlcNAc on body weight change (%) using different routes of administration. Scatter plots include the mean ± SEM. One-way ANOVA with Bonferroni postcorrection: *P ≤ 0.05; ***P ≤ 0.001. (C) DAI comparing mice with colitis that were untreated with those that were treated with GlcNAc. Plots depict the mean ± SEM. Two-way ANOVA with Bonferroni postcorrection: *P ≤ 0.05; **P ≤ 0.01. (Inset) Discrimination of the effect of GlcNAc on colitis scores using different routes of administration. Plots depict the mean ± SEM. One-way ANOVA with Bonferroni post correction: ***P ≤ 0.001. (D) Representative histological images (H&E) of colonic samples from mice [normal colon, DSS (DSS-induced colitis), and GlcNAc treatment (Tx) (DSS + GlcNAc Enema Tx)]. (Magnification: 40×.)
Fig. 5.
Colitis-induced mouse model treated with GlcNAc showed increased branched N-glycosylation associated with suppression of T cell function. (A) L-PHA histochemistry and CD3 immunohistochemistry. L-PHA lectin reactivity showed an increased expression of β1,6-branched structures in the intestinal inflammatory infiltrate (positive to CD3) as well as an increase in mucus lining in mice treated with GlcNAc enemas (arrowheads). (Magnification: 63×.) (B) Immunoprecipitation (IP) of TCR followed by β1,6-GlcNAc branched N-glycan recognition in mouse colon, DSS (DSS-induced colitis) vs. DSS + GlcNAc treatment (Tx). WB, Western blot. (B1) Scatter plot: ratio of densities of L-PHA reactivity normalized to that of TCR depicted as the mean ± SEM comparing DSS (n = 2) mice with DSS + GlcNAc Tx (n = 3) mice. Student’s t test: *P ≤ 0.05. (C) TCR signaling by Western blot analysis of the phosphorylation levels of ZAP70 and LAT in LPLs. Values of pZAP70 densities normalized to tubulin are indicated. (D) Immunofluorescence of T-bet in colonic sections of DSS vs. DSS + GlcNAc Tx. (Insets) T-bet–expressing cells at intestinal inflammatory infiltrate are highlighted. (Magnification: 20×.) (E) Concentration of IFN-γ and IL-17A in the supernatants of 24-h colonic explant cultures from DSS and DSS + GlcNAc Tx MGAT5wt (n = 5) mice by ELISA. Plots depict the mean ± SEM of two to three animals per group. Student’s t test: **P ≤ 0.01. NS, not significant.
Deficiency in Branched Glycosylation in MGAT5 Null or Heterozygous Mice Is Associated with Early-Onset Disease and Increased Severity of Colitis that Is Suppressed by GlcNAc Treatment.
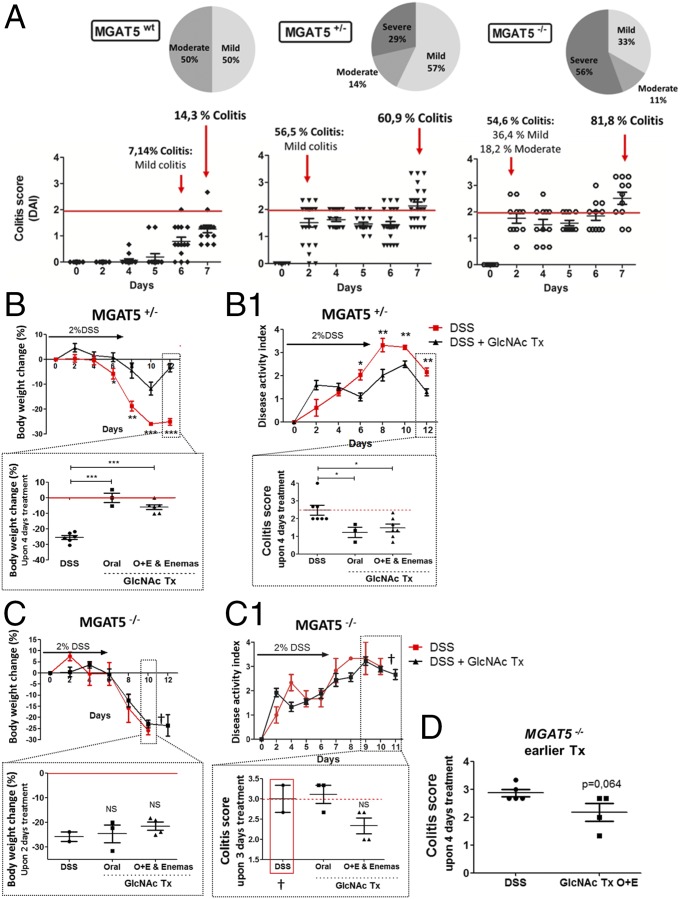
To gain insights into the targeted-specific therapeutic effects of GlcNAc, we used MGAT5 null or heterozygous mice that display a deficiency in branched N-glycosylation, mimicking the mechanistic defect described in humans (16). The impact on DSS-induced colitis onset and severity was assessed in MGAT5 heterozygous (+/−, partial deficiency on branched N-glycosylation) and MGAT5 knockout (−/−, absence of branched N-glycans) mice. Those genotypes represent intermediate N-glycans and the loss of one branch (β1,6-branching) of N-glycans, which may mimic mild/moderate versus severe deficiency on branched glycosylation. Our results showed that after DSS induction, MGAT5 null or heterozygous mice exhibited increased susceptibility to early-onset disease and to severe forms of colitis. At day 2 after DSS induction, more that 50% of MGAT5−/− mice developed both mild and moderate forms of colitis compared with WT mice, which only developed clinical signs of colitis at day 6 postinduction (Fig. 6A). Moreover, at the end of DSS induction period (day 7), and based on the DAI, we observed increased susceptibility to severe forms of colitis in MGAT5 null or heterozygous mice (Fig. 6A). As depicted in the pie chart in Fig. 6A, on day 7, more than 50% of MGAT5−/− mice exhibited severe forms of disease (scores ≥3) and 29% of MGAT5+/− mice developed severe colitis. In contrast, WT mice presented only mild/moderate forms of the disease. These results reinforce that MGAT5-mediated branched glycosylation has a strong impact on UC disease onset and progression.
Fig. 6.
MGAT5 null or heterozygous mice develop early-onset colitis and an increase in disease severity that is suppressed by GlcNAc treatment. (A) Evaluation of colitis onset and disease severity in MGAT5 null or heterozygous mice: C57BL/6 WT (n = 14), MGAT5+/− (n = 23), and MGAT5−/− (n = 11) mice. Active disease was defined when animals showed a DAI of ≥2, and three stages of severity were defined: mild (≥2 and <2.5), moderate (≥2.5 and <3), and severe (≥3). Average results of body weight change (B and C) and DAI (B1 and C1) of MGAT5+/− (n = 23) and MGAT5−/− (n = 9) mice, respectively, randomly distributed in controls and GlcNAc treatment groups are shown. DSS-induced colitis (DSS) vs. DSS treated with GlcNAc treatment (DSS + GlcNAc Tx). Animals showing severe signs of disease were euthanized (†). (B and B1, Insets) Discrimination of the efficiency of GlcNAc treatment (colitis scores) with different routes of administration upon 4 d of treatment. Graphs correspond to the mean ± SEM of 17 animals (three to seven animals per route of administration). Student’s t test (B and B1) and one-way ANOVA with Bonferroni postcorrection (B and B1, Insets): *P ≤ 0.05*; **P ≤ 0.01; ***P ≤ 0.001. Body weight changes of MGAT5−/− mice treated through different routes vs. nontreated upon 2 d of treatment (C) and DAI scores of MGAT5−/− mice treated (n = 7) vs. nontreated (n = 2) (C1) are shown. (C and C1, Insets) Discrimination of the efficiency of GlcNAc treatment (colitis scores) with different routes of administration upon 3 d of treatment. (D) Evaluation of the impact of early oral route (O) + enema route (E) GlcNAc treatment (starting on the second day of disease onset: 5–6 d after DSS induction) on the colitis scores (DAI of animals per group) of MGAT5−/− mice, comparing DSS (n = 5) with GlcNAc treated mice (n = 4).
Afterward, we tested the effect of GlcNAc treatment on the control of disease severity in mice with the different MGAT5 genotypes. As shown in Fig. 6 B and B1, MGAT5+/− mice with colitis and treated with GlcNAc presented lower body weight loss and lower DAI compared with nontreated controls. With regard to body weight changes and DAI scores, the same tendency was observed in MGAT5−/− mice treated with GlcNAc (Fig. 6 C and C1). Due to their higher susceptibility to colitis, nontreated MGAT5−/− animals were euthanized on day 10 since they reached the established humane end points. MGAT5−/− mice, besides developing a much more aggressive disease phenotype, were also less responsive to GlcNAc, which makes GlcNAc therapy in these mice more challenging. Nevertheless, the results in MGAT5+/− and MGAT5−/− mice (Fig. 6 B–D) showed that even in these mice, treatment with GlcNAc improved clinical scores compared with the scores of DSS mice. This beneficial effect was pronounced whenever GlcNAc was administered topically by enema (single or in combination with oral administration), as evidenced by lower body weight loss and lower colitis scores compared with DSS mice. Interestingly, when GlcNAc treatment was initiated earlier in disease course of MGAT5−/− mice, at day 5, when animals started to develop severe colitis, we observed a decrease of the colitis scores compared with nontreated diseased animals (DSS), which supports the benefits of treating these susceptible animals earlier with GlcNAc, as demonstrated in Fig. 6D.
GlcNAc Treatment of MGAT5 Null or Heterozygous Mice Attenuates Disease Progression by Controlling Th1/Th17-Type Immune Responses.
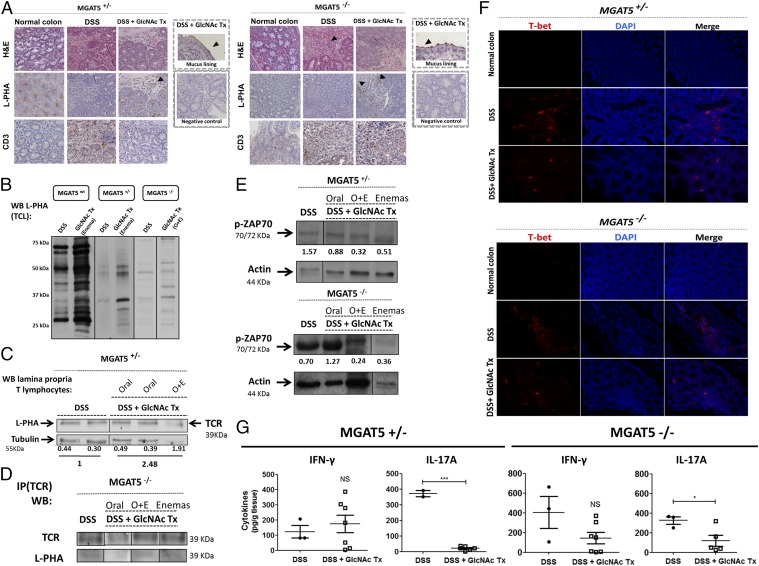
MGAT5 null and heterozygous mice showed disorganization of the glandular architecture and an increase of inflammatory infiltrates in the colonic mucosa after DSS-induced colitis that was improved overall upon GlcNAc treatment (Fig. 7A). Notably, the evaluation of β1,6-GlcNAc branched N-glycan levels on CD3+ LPLs confirmed that the induction of colitis was accompanied by decreased expression of branched N-glycans in LPLs from MGAT5+/− mice and by the absence of branched N-glycans in MGAT5−/− mice with induced colitis (DSS) (Fig. 7A). Interestingly, when mice of both MGAT5 genotypes were treated with GlcNAc, a recovery of β1,6-branched glycan expression was observed in the intestinal inflammatory infiltrate (Fig. 7A, arrowhead) and in the superficial mucus layer, which is compatible with mucosal healing. The positive detection of L-PHA staining in MGAT5 null mice upon GlcNAc supplementation was unexpected, as these mice lack the GnT-V enzyme. This positive detection was confirmed at the protein level by L-PHA blot (Fig. 7B and SI Appendix, Fig. S9A). In fact, the reactivity of L-PHA in MGAT5−/− mice is the lowest, compared with heterozygous and WT mice, but it is still positive. These observations may be in line with redundant effects of other GnTs at the Golgi (22–24) that, within an activated hexosamine pathway, may compensate for the absence of MGAT5 by producing the β1,6-GlcNAc branched glycans, although with a much lower yield of synthesis, as we have observed. In an attempt to explore the potential compensatory synthesis of β1,6-GlcNAc branched N-glycans in MGAT5 null mice, interestingly, we have observed that the MGAT5b gene [a homologous gene of MGAT5a that codifies the GnT-IX or GnT-Vb enzyme (25)] is apparently up-regulated in colonic T cells from MGAT5 null mice treated with GlcNAc compared with control mice (nontreated mice) (SI Appendix, Fig. S9B). MGAT5 null mice with DSS-induced colitis do not express MGAT5b. This preliminary evidence suggests GnT-IX/Vb as a potential candidate that might compensate for the synthesis of β1,6-GlcNAc branched glycans in MGAT5 null mice. This issue needs further investigation. We then assessed the enhancement of branched N-glycans specifically on T cells after GlcNAc treatment. MGAT5 null or heterozygous mice treated with GlcNAc showed an enhanced expression of branched N-glycans in the TCR compared with nontreated diseased animals (DSS) (Fig. 7 C and D). This effect was highlighted when animals were treated topically with GlcNAc enemas (Fig. 7 C and D). To explore the mechanistic effects of GlcNAc treatment in the T cell-mediated immune response, the impact on TCR signaling was evaluated in MGAT5 null and heterozygous mice. An overall decrease of ZAP70 phosphorylation, indicative of reduced TCR signaling, was detected in colonic T cells from GlcNAc-treated mice that was evident when GlcNAc was administered topically (Fig. 7E). This topical effect was particularly observed in MGAT5−/− mice, where oral treatment did not affect TCR signaling. The more marked effect achieved through GlcNAc enema administration suggests that this molecule may be more efficiently taken up by cells in this way, likely by increasing its local concentration, thus facilitating its entry into the hexosamine pathway and usage by glycosyltransferases other than GnT-V (SI Appendix, Fig. S9A) that may redundantly catalyze the branched N-glycans. These redundant effects need further investigation. Additionally, the evaluation of the Th1 proinflammatory response revealed that GlcNAc treatment in both genotypes was associated with reduced proportions of cells expressing T-bet in lymphocytic infiltrates compared with control mice with colitis (Fig. 7F). Notably, similar to our observations of GlcNAc treatment in T cells from patients with UC, colonic explants from MGAT5 null or heterozygous mice treated with GlcNAc indicate a trend for IFN-γ suppression, but with a more pronounced effect in reducing IL-17A. These results further support the impact of GlcNAc treatment and, consequently, the enhancement of branched N-glycans in controlling Th1/Th17-type immune responses also in the IBD in vivo model (Fig. 7G). Regarding the impact of GlcNAc on regulatory T cells, no apparent difference in the numbers of FoxP3-expressing cells was observed at the intestinal lamina propria comparing nontreated versus GlcNAc-treated mice of MGAT5wt and MGAT5−/− genotypes (SI Appendix, Fig. S9C). Nevertheless, further studies are needed to better characterize the regulatory effects of GlcNAc treatment in the different components of the immune response, such as in macrophages (SI Appendix, Fig. S9 D and E), as proposed by previous reports (26), and other T cell populations. Taken together, these data support the targeted-specific effects of GlcNAc that were able to repair the deficiency in branched glycosylation on T cells associated with MGAT5 deficiency, thus controlling progression of colitis.
Fig. 7.
GlcNAc treatment of MGAT5 null or heterozygous mice attenuates disease progression by controlling Th1/Th17-type immune responses. (A) Representative histological images (H&E) of colonic sections from MGAT5+/− and MGAT5−/− [normal colon, DSS-induced colitis (DSS), and GlcNAc treatment (DSS + GlcNAc Tx)] (Magnification: 20×.) DSS mice displayed visible signs of lymphocytic infiltrate in the intestinal lamina propria (arrowheads). L-PHA histochemistry and CD3 immunohistochemistry of mouse colon from the different groups. (Magnification: 20×.) (B) Evaluation of branching N-glycans on colonic total cell lysates from MGAT5wt, MGAT5+/−, and MGAT5−/− mice comparing DSS control with GlcNAc Tx enema by Western blot (WB). TCL, total cell lysate. (C) Protein lysates from LPLs isolated from the colon of MGAT5+/− mice with DSS (colitis) or treated (DSS + GlcNAc Tx) mice were subjected to L-PHA lectin blotting to evaluate the expression of β1,6-GlcNAc branched N-glycans on the TCR (39 kDa). L-PHA density normalized to tubulin is indicated for each case, and fold change differences of DSS vs. DSS + GlcNAc Tx are highlighted. (D) Immunoprecipitation (IP) of the TCR in total cell lysates from MGAT5−/− mouse colon followed by β1,6-GlcNAc branched N-glycan recognition. DSS vs. DSS + GlcNAc Tx with different routes of administration. (E) Analysis of the phosphorylation levels of ZAP70 in LPL lysates from colon of MGAT5+/− and MGAT5−/− mice. Values of pZAP70 normalized to actin in MGAT5+/− and MGAT5−/− mice are indicated. (F) Immunofluorescence of T-bet in MGAT5+/− and MGAT5−/− mice comparing normal colon, DSS, and DSS + GlcNAc Tx mice. (Magnification: 20×.) (G) Concentration of IFN-γ and IL-17A in the supernatants of 24-h colonic explant cultures from DSS and DSS + GlcNAc Tx MGAT5 heterozygous (n = 10) and null (n = 10) mice by ELISA. Plots depict the mean ± SEM of two to seven animals per group. Student’s t test: *P ≤ 0.05; ***P ≤ 0.001. NS, not significant.
Discussion
IBD is characterized by a substantial heterogeneity concerning disease onset, course, response to therapy, and progression to complications (e.g., hospitalization, need for surgery, cancer) (1). Moreover, and despite recent advances in IBD therapeutic resources, a high proportion of patients remain refractory to conventional treatment, and approximately half of the patients with UC do not achieve sustained remission (27). In addition, issues related to side effects and failure in therapy response highlight the need for more effective and targeted-specific drugs (28). We have recently demonstrated that patients with UC exhibit a deficiency in branched glycosylation on intestinal T cells due to a transcriptional reduction of the MGAT5 gene that accompanied disease severity (16).
Herein, we uncovered a prominent role for the branched glycosylation pathway in IBD pathogenesis, by shaping the course of the T cell response. This pathway is thus an attractive target for novel therapies. Indeed, we have shown here that checkpoints of T cell immune response in UC could be modulated by metabolic supplementation with the simple sugar GlcNAc. We showed that GlcNAc therapy concomitantly increased branched N-glycosylation on T cells and down-regulated T cell proinflammatory responses both ex vivo and in vivo. In line with these observations, it was previously reported that lack of β1,6-GlcNAc branched N-glycans, by targeted deletion of the locus encoding GnT-V, results in enhanced TCR signaling and increased susceptibility to multiple sclerosis (7, 10). Moreover, and in accordance with our results, the increase of N-glycan branching through GlcNAc salvage into the hexosamine pathway was associated with a decreased threshold in T cell activation and more stable CTLA-4 surface expression, which resulted in the inhibition of adoptively transferred EAE (7, 13).
The evidence presented here suggests that GlcNAc supplementation of T cells isolated from patients with active UC resulted in remodeling of the glycophenotype of T lymphocytes through a marked increase of β1,6-GlcNAc branched N-glycans and an increase of polylactosamine structures, the ligand for galectins (29), that can then be terminally sialylated, predominantly with α2,6-sialic acid residues. This glycan reprogramming on T cells was shown to translate into key immunomodulatory effects in UC. Importantly, the enhancement of branched N-glycans on T cells induced by GlcNAc supplementation led to the suppression of T cell proliferation; inhibition of T cell signaling; reduced production of the proinflammatory cytokines TNF-α, INF-γ, and IL1-7A; and controlled Th1- and Th17-type responses. Both Th responses have been associated with IBD pathogenesis (30). Furthermore, these results are in accordance with a very recent report using mouse T cell cultures, which showed the ability of GlcNAc to promote iTreg over Th17 differentiation (15).
The glycosylation of T cells can also have an impact on the susceptibility to cell death (31). Accordingly, treatment of T cells with GlcNAc induced an increased susceptibility to apoptosis, which is at the core of different regulatory processes controlling T cell activation and expansion, thus avoiding exacerbated inflammation (32). This effect of GlcNAc on apoptosis was dose-dependent and limited to T cells from patients with active UC (SI Appendix, Fig. S7B). In agreement, previous reports have shown that extension with polylactosamine structures, which are ligands for galectins (such as galectin 1), was associated with proapoptotic effects of CD4+ T cells (31, 33). Nevertheless, and given that treatment with KF and SW did not reverse the apoptotic effects induced by GlcNAc in the cells of patients with active UC, it cannot be excluded that factors other than branching glycans can also contribute to GlcNAc-mediated regulation of in vitro T cell apoptosis. Importantly, besides the TCR, the enhancement of branched N-glycosylation can also modify other receptors like the coreceptors CD4 and CD8, as well as the growth inhibitory receptor CTLA-4 (7, 10, 22). Moreover, CD45 and CD25 are also potential targets of branched glycosylation modification that can further contribute to the regulation of the T cell-mediated immune response through branching N-glycans (15, 34). Immunomodulation through GlcNAc-mediated enhancement of branched glycosylation, as described here, is a promising therapeutic approach to restore T cell homeostasis in IBD (SI Appendix, Fig. S10). Indeed, metabolic regulation of T cell function has been highlighted by recent research (35) and may be manipulated to reduce T cell-mediated inflammation (15, 36).
The preclinical data reported here provide the proof of concept supporting such a therapeutic approach in IBD. Deficiency of the MGAT5 gene was associated with higher susceptibility to severe forms of colitis and early-onset disease. These data highlight the prominent role of branched N-glycosylation in the pathogenesis of IBD, and are in accordance with previous studies on multiple sclerosis (11). GlcNAc supplementation improved clinical scores and was associated with a better disease course in mice developing the most severe disease phenotype (MGAT5−/−). These immune-suppressive effects catalyzed by GlcNAc were observed by both oral and enema administration routes, with promising effects when mice received GlcNAc topically via enemas. This topical effect of GlcNAc is in line with the ability of GlcNAc to be more efficiently taken up by the intestinal mucosa, thereby entering directly into the hexosamine pathway to increase branched glycosylation. Intriguingly, in MGAT5−/−, with the absence of MGAT5/GnT-V, expression of the β1,6-GlcNAc branched N-glycans detected by L-PHA lectin histochemistry and blotting was positive and slightly increased with GlcNAc treatment. This unexpected result may be in accordance with the fact that several glycosyltransferase-deficient mice exhibit no or only mild phenotypes due to redundancy or compensation of glycan functions. In fact, several family members of glycosyltransferases are known to be functionally redundant (37, 38). This potential redundant effect observed in MGAT5−/− mice treated with GlcNAc might be due to the fact that other Golgi glycosyltransferases within an activated hexosamine pathway triggered by UDP-GlcNAc may compensate for the lack of this specific glycan structure, thereby guaranteeing immune homeostasis. One of the candidate enzymes is the homologous GnT-Vb that may compensate for the synthesis of the branched glycans; however, further studies are needed to clarify this issue. Similar examples of the redundancy of glycosyltransferases were described, such as for FUT8-deficient mice (39). Nevertheless, this redundant effect observed upon GlcNAc supplementation needs to be further explored.
The clinical effects of GlcNAc were further evidenced by the decreased frequency of T-bet–expressing cells in colonic mucosa of treated mice, together with reduced release of the proinflammatory cytokine IL-17A of respective colonic explants.
GlcNAc is a naturally occurring amino sugar for which no adverse effects were reported in humans (40). It is available as a dietary supplement, and oral GlcNAc administration showed no toxicity in rats (41). Interestingly, 17 y ago, oral GlcNAc was described to promote intestinal lining through mucus production in children with severe treatment-resistant IBD (21). In that study, eight of the 12 children studied went into clinical remission, which could have resulted from the immunomodulatory effects of GlcNAc proposed here. Taken together, the combined evidence from both ex vivo and preclinical data provides proof of concept for the therapeutic use of GlcNAc (either alone or in combination with other antiinflammatory therapies) as a simple immunomodulatory strategy in IBD. Assessment of this strategy in clinical studies is currently ongoing. Some of the most relevant properties associated with GlcNAc treatment are the absence of side effects, low cost, and possibility of being used as a simple rescue therapy to avoid unnecessary toxic effects and step-up therapies in IBD.
Materials and Methods
Patient Selection and Colonic Biopsy Collection.
Fresh colonic biopsies were obtained from 75 patients (three patients in remission) diagnosed with UC and normal controls (n = 3) who underwent a scheduled colonoscopy (2014–2017) at the Gastroenterology Department of Centro Hospitalar do Porto–Hospital de Santo António (CHP-HSA), Porto, Portugal. All participants gave informed consent, and procedures were approved by the Ethics Committee of CHP/HSA [233/12(179-DEFI/177-CES)].
Isolation of CD3+ T Cells from Fresh Colonic Biopsies and Blood of Patients with Active UC: Ex Vivo Culture of T Cells.
After mechanical dissociation of colonic biopsies and blood density gradient centrifugation using Lymphoprep, CD3+ T cells (from biopsies and blood) were magnetically sorted using an EasySep Human T Cell Enrichment Kit (STEMCELL Technologies) following the manufacturer’s instructions. CD3+ T cells were cultured for 72 h with anti-CD3 mAb (clone OKT3) and soluble anti-CD28 mAb (clone CD28.2) (eBioscience). T cell cultures were supplemented with GlcNAc (Sigma and Wellesley Therapeutics, Inc.).
Imaging Flow Cytometry.
Imaging flow cytometry analysis was performed as previously described (42).
Flow Cytometry.
CD3+ T cells were stained with CD4 and CD8 (BD Biosciences) and fluorescein isothiocyanate (FITC)-conjugated L-PHA (Vector Laboratories), as well as with cell surface markers (CD4, CD45), intracellular antigens (T-bet, RORγt, Foxp3, and Gata3), and cytokine intracellular staining (TNF-α, IFN-γ). Various antibodies used for staining are described in SI Appendix, Table S1.
Proliferation Assay.
CD3+ T cells were purified from colonic biopsies and labeled with 5-(and-6)-carboxyfluorescein diacetate succinimidyl ester (CFSE) using a CellTrace CFSE Cell Proliferation Kit (Invitrogen), as described by Oliveira et al. (43).
Cytokine Production.
Supernatants from colonic T cell cultures were analyzed by flow cytometry using the BD Cytometric Bead Array Human Th1/Th2/Th17 Cytokine Kit (BD Biosciences) following the manufacturer’s instructions. Human TGF-β1 quantification was performed using ELISA kits (R&D Systems) according to the manufacturer’s instructions. The supernatants from mouse colonic explant cultures were concentrated using Amicon Ultra-2 mL Centrifugal Filters (Merck Millipore), according to manufacturer’s instructions. The levels of IFN-γ and IL-17A (anti-mouse, Ready-SET-Go! kits; eBioscience) and TNF-α and IL-6 (anti-mouse; Biolegend) were quantified by ELISA, according to the manufacturers’ instructions.
Western Blot and TCR Signaling.
TCR signaling and L-PHA lectin blot analysis (44), using T cell protein lysates (extracted with radioimmunoprecipitation buffer), were performed as described by Dias et al. (16). Incubation of phospho–Zap-70 [Tyr319/Syk (Tyr352)] rabbit mAb and anti–phospho-LAT (Tyr191) rabbit mAb (Cell Signaling Technologies) was performed. Goat anti-rabbit IgG-HRP mAb was used as a secondary antibody, and rabbit IgG antiactin (Santa Cruz Biotechnology) or mouse IgG antitubulin (Sigma) was used as a loading control.
Immunoprecipitation.
TCR immunoprecipitation, using total cell lysates obtained from mouse colons or from ex vivo human T cell cultures, was performed as previously described (16).
Glycophenotype.
T cells were incubated with biotinylated L-PHA, biotinylated LEL, biotinylated SNA, or biotinylated MAL-II (Vector Labs). Lectins were revealed with FITC-conjugated streptavidin.
Apoptosis Assays.
Apoptotic cells were identified by flow cytometry, using an FITC Annexin V Apoptosis Detection Kit I (BD Biosciences), following the manufacturer’s instructions.
Enzymatic Reaction and HPLC Analysis.
The GnT-V enzymatic activity analyses in T cells from patients with UC and controls were performed as previously described by Takamatsu et al. (45).
DSS- and TNBS-Induced Colitis and in Vivo GlcNAc Treatment.
Colitis was induced with DSS in C57BL/6, MGAT5 wild-type, heterozygous, and knockout mice (kindly provided by Michael Pierce, University of Georgia, Athens, GA) (19). The TNBS model was also performed using C57BL/6 mice (19). After disease onset, DSS mice were treated with GlcNAc (Sigma and Wellesley Therapeutics, Inc.) (12). LPLs were isolated from mouse colon as previously described (16).
Tissue Immunohistochemistry and Immunofluorescence.
FFPE colonic tissue slides were used for H&E staining and for immunohistochemistry with L-PHA and anti-CD3 mAb as well as Foxp3 and F4/80, as described, respectively, by Dias et al. (16) and Teixeira et al. (46). For T-bet immunofluorescence, mouse IgG1 T-bet–specific mAb (clone 4B10; Santa Cruz Biotechnology), goat anti-mouse Alexa 594 secondary antibody (Invitrogen), and DAPI staining were used.
Real-Time PCR.
Total RNA from isolated LPLs was extracted and the quantitative real-time PCR (qRT-PCR) was performed using TaqMan Gene Expression Assays (Applied Biosystems), as previously described (16). qRT-PCR was carried out in triplicates for the target gene MGAT5b (Taqman probe: Mm01252571_m1) and the endogenous control 18S (Hs99999901_s1) (Applied Biosystems) (16).
Statistics.
Statistical significance was assessed by one-way or two-way ANOVA using a Bonferroni’s, Dunnett’s, or Newman–Keuls multiple comparison posttest and, where appropriate, by an unpaired Student’s t test (two-tailed) using GraphPad Prism 5. P values of <0.05 were considered statistically significant.
More details can be found in SI Appendix, SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dr. Hiroaki Korekane and Fumi (RIKEN) for support in preparation of the fluorescent oligosaccharide acceptor substrate. We thank Dr. Michael Pierce for kindly providing the MGAT5 knockout mice. We also thank Paula Paíga (REQUIMTE/LAQV) for technical support with the HPLC system. The Institute of Molecular Pathology and Immunology of the University of Porto integrates the i3S research unit, which is partially supported by the Portuguese Foundation for Science and Technology (FCT). This article is a result of the project NORTE-01-0145-FEDER-000029, supported by the Norte Portugal Regional Programme (NORTE 2020) under the PORTUGAL 2020 Partnership Agreement through the European Regional Development Fund. This work was also funded by Fundo Europeu de Desenvolvimento Regional (FEDER) funds through the COMPETE 2020—Operacional Programme for Competitiveness and Internationalization (POCI), Portugal 2020, and by Portuguese funds through the FCT in the framework of the project (POCI-01/0145-FEDER-016601 and PTDC/DTP-PIC/0560/2014). S.S.P. acknowledges the European Crohn’s and Colitis Organization (ECCO) for ECCO Grant 2017, the Broad Medical Research Program at the Crohn’s and Colitis Foundation of America, and the Portuguese Group of Study in IBD (GEDII) for funding. A.M.D. [PD/BD/105982/2014], A.C. [SFRH/BPD/91623/2012], and M.S.P. [SFRH/BD/110148/2015] received funding from the FCT. M. Lima thanks the CHP for the research support.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. K.T. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1720409115/-/DCSupplemental.
References
- 1.Cosnes J, Gower-Rousseau C, Seksik P, Cortot A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology. 2011;140:1785–1794. doi: 10.1053/j.gastro.2011.01.055. [DOI] [PubMed] [Google Scholar]
- 2.Rudd PM, Elliott T, Cresswell P, Wilson IA, Dwek RA. Glycosylation and the immune system. Science. 2001;291:2370–2376. doi: 10.1126/science.291.5512.2370. [DOI] [PubMed] [Google Scholar]
- 3.Pinho SS, et al. The role of N-acetylglucosaminyltransferase III and V in the post-transcriptional modifications of E-cadherin. Hum Mol Genet. 2009;18:2599–2608. doi: 10.1093/hmg/ddp194. [DOI] [PubMed] [Google Scholar]
- 4.Pinho SS, et al. E-cadherin and adherens-junctions stability in gastric carcinoma: Functional implications of glycosyltransferases involving N-glycan branching biosynthesis, N-acetylglucosaminyltransferases III and V. Biochim Biophys Acta. 2013;1830:2690–2700. doi: 10.1016/j.bbagen.2012.10.021. [DOI] [PubMed] [Google Scholar]
- 5.Pinho SS, Reis CA. Glycosylation in cancer: Mechanisms and clinical implications. Nat Rev Cancer. 2015;15:540–555. doi: 10.1038/nrc3982. [DOI] [PubMed] [Google Scholar]
- 6.Carvalho S, Reis CA, Pinho SS. Cadherins glycans in cancer: Sweet players in a bitter process. Trends Cancer. 2016;2:519–531. doi: 10.1016/j.trecan.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 7.Demetriou M, Granovsky M, Quaggin S, Dennis JW. Negative regulation of T-cell activation and autoimmunity by Mgat5 N-glycosylation. Nature. 2001;409:733–739. doi: 10.1038/35055582. [DOI] [PubMed] [Google Scholar]
- 8.Marth JD, Grewal PK. Mammalian glycosylation in immunity. Nat Rev Immunol. 2008;8:874–887. doi: 10.1038/nri2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen HL, Li CF, Grigorian A, Tian W, Demetriou M. T cell receptor signaling co-regulates multiple Golgi genes to enhance N-glycan branching. J Biol Chem. 2009;284:32454–32461. doi: 10.1074/jbc.M109.023630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morgan R, et al. N-acetylglucosaminyltransferase V (Mgat5)-mediated N-glycosylation negatively regulates Th1 cytokine production by T cells. J Immunol. 2004;173:7200–7208. doi: 10.4049/jimmunol.173.12.7200. [DOI] [PubMed] [Google Scholar]
- 11.Lee SU, et al. N-glycan processing deficiency promotes spontaneous inflammatory demyelination and neurodegeneration. J Biol Chem. 2007;282:33725–33734. doi: 10.1074/jbc.M704839200. [DOI] [PubMed] [Google Scholar]
- 12.Grigorian A, et al. N-acetylglucosamine inhibits T-helper 1 (Th1)/T-helper 17 (Th17) cell responses and treats experimental autoimmune encephalomyelitis. J Biol Chem. 2011;286:40133–40141. doi: 10.1074/jbc.M111.277814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grigorian A, et al. Control of T cell-mediated autoimmunity by metabolite flux to N-glycan biosynthesis. J Biol Chem. 2007;282:20027–20035. doi: 10.1074/jbc.M701890200. [DOI] [PubMed] [Google Scholar]
- 14.Zhou RW, et al. N-glycosylation bidirectionally extends the boundaries of thymocyte positive selection by decoupling Lck from Ca2+ signaling. Nat Immunol. 2014;15:1038–1045. doi: 10.1038/ni.3007. [DOI] [PubMed] [Google Scholar]
- 15.Araujo L, Khim P, Mkhikian H, Mortales CL, Demetriou M. Glycolysis and glutaminolysis cooperatively control T cell function by limiting metabolite supply to N-glycosylation. eLife. 2017;6:e21330. doi: 10.7554/eLife.21330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dias AM, et al. Dysregulation of T cell receptor N-glycosylation: A molecular mechanism involved in ulcerative colitis. Hum Mol Genet. 2014;23:2416–2427. doi: 10.1093/hmg/ddt632. [DOI] [PubMed] [Google Scholar]
- 17.Nakajima K, et al. Mass isotopomer analysis of metabolically labeled nucleotide sugars and N- and O-glycans for tracing nucleotide sugar metabolisms. Mol Cell Proteomics. 2013;12:2468–2480. doi: 10.1074/mcp.M112.027151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neurath MF. Cytokines in inflammatory bowel disease. Nat Rev Immunol. 2014;14:329–342. doi: 10.1038/nri3661. [DOI] [PubMed] [Google Scholar]
- 19.Wirtz S, Neufert C, Weigmann B, Neurath MF. Chemically induced mouse models of intestinal inflammation. Nat Protoc. 2007;2:541–546. doi: 10.1038/nprot.2007.41. [DOI] [PubMed] [Google Scholar]
- 20.Johansson ME. Mucus layers in inflammatory bowel disease. Inflamm Bowel Dis. 2014;20:2124–2131. doi: 10.1097/MIB.0000000000000117. [DOI] [PubMed] [Google Scholar]
- 21.Salvatore S, et al. A pilot study of N-acetyl glucosamine, a nutritional substrate for glycosaminoglycan synthesis, in paediatric chronic inflammatory bowel disease. Aliment Pharmacol Ther. 2000;14:1567–1579. doi: 10.1046/j.1365-2036.2000.00883.x. [DOI] [PubMed] [Google Scholar]
- 22.Lau KS, et al. Complex N-glycan number and degree of branching cooperate to regulate cell proliferation and differentiation. Cell. 2007;129:123–134. doi: 10.1016/j.cell.2007.01.049. [DOI] [PubMed] [Google Scholar]
- 23.Ryczko MC, et al. Metabolic reprogramming by hexosamine biosynthetic and Golgi N-glycan branching pathways. Sci Rep. 2016;6:23043. doi: 10.1038/srep23043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dennis JW, Brewer CF. Density-dependent lectin-glycan interactions as a paradigm for conditional regulation by posttranslational modifications. Mol Cell Proteomics. 2013;12:913–920. doi: 10.1074/mcp.R112.026989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stanley P, Taniguchi N, Aebi M. N-Glycans. In: Varki A, et al., editors. Essentials of Glycobiology. 3rd Ed. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 2015. pp. 99–111. [Google Scholar]
- 26.Shinzaki S, et al. N-acetylglucosaminyltransferase V exacerbates murine colitis with macrophage dysfunction and enhances colitic tumorigenesis. J Gastroenterol. 2016;51:357–369. doi: 10.1007/s00535-015-1119-3. [DOI] [PubMed] [Google Scholar]
- 27.D’Haens GR. Top-down therapy for IBD: Rationale and requisite evidence. Nat Rev Gastroenterol Hepatol. 2010;7:86–92. doi: 10.1038/nrgastro.2009.222. [DOI] [PubMed] [Google Scholar]
- 28.Plevy SE, Targan SR. Future therapeutic approaches for inflammatory bowel diseases. Gastroenterology. 2011;140:1838–1846. doi: 10.1053/j.gastro.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rabinovich GA, Toscano MA. Turning ‘sweet’ on immunity: Galectin-glycan interactions in immune tolerance and inflammation. Nat Rev Immunol. 2009;9:338–352. doi: 10.1038/nri2536. [DOI] [PubMed] [Google Scholar]
- 30.Fujino S, et al. Increased expression of interleukin 17 in inflammatory bowel disease. Gut. 2003;52:65–70. doi: 10.1136/gut.52.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Toscano MA, et al. Differential glycosylation of TH1, TH2 and TH-17 effector cells selectively regulates susceptibility to cell death. Nat Immunol. 2007;8:825–834. doi: 10.1038/ni1482. [DOI] [PubMed] [Google Scholar]
- 32.Maniati E, Potter P, Rogers NJ, Morley BJ. Control of apoptosis in autoimmunity. J Pathol. 2008;214:190–198. doi: 10.1002/path.2270. [DOI] [PubMed] [Google Scholar]
- 33.Santucci L, et al. Galectin-1 suppresses experimental colitis in mice. Gastroenterology. 2003;124:1381–1394. doi: 10.1016/s0016-5085(03)00267-1. [DOI] [PubMed] [Google Scholar]
- 34.Chen IJ, Chen HL, Demetriou M. Lateral compartmentalization of T cell receptor versus CD45 by galectin-N-glycan binding and microfilaments coordinate basal and activation signaling. J Biol Chem. 2007;282:35361–35372. doi: 10.1074/jbc.M706923200. [DOI] [PubMed] [Google Scholar]
- 35.Buck MD, O’Sullivan D, Pearce EL. T cell metabolism drives immunity. J Exp Med. 2015;212:1345–1360. doi: 10.1084/jem.20151159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gerriets VA, et al. Metabolic programming and PDHK1 control CD4+ T cell subsets and inflammation. J Clin Invest. 2015;125:194–207. doi: 10.1172/JCI76012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Orr SL, et al. A phenotype survey of 36 mutant mouse strains with gene-targeted defects in glycosyltransferases or glycan-binding proteins. Glycobiology. 2013;23:363–380. doi: 10.1093/glycob/cws150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mkhikian H, et al. Golgi self-correction generates bioequivalent glycans to preserve cellular homeostasis. eLife. 2016;5:e14814. doi: 10.7554/eLife.14814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kurimoto A, et al. The absence of core fucose up-regulates GnT-III and Wnt target genes: A possible mechanism for an adaptive response in terms of glycan function. J Biol Chem. 2014;289:11704–11714. doi: 10.1074/jbc.M113.502542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Levin RM, Krieger NN, Winzler RJ. Glucmsumine and acetylglucosamine tolerance in man. J Lab Clin Med. 1961;58:927–932. [PubMed] [Google Scholar]
- 41.Lee KY, et al. Subchronic toxicity study of dietary N-acetylglucosamine in F344 rats. Food Chem Toxicol. 2004;42:687–695. doi: 10.1016/j.fct.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 42.Dias AM, Almeida CR, Reis CA, Pinho SS. Studying T cells N-glycosylation by imaging flow cytometry. Methods Mol Biol. 2016;1389:167–176. doi: 10.1007/978-1-4939-3302-0_11. [DOI] [PubMed] [Google Scholar]
- 43.Oliveira L, et al. Deficits in endogenous adenosine formation by ecto-5′-nucleotidase/CD73 impair neuromuscular transmission and immune competence in experimental autoimmune myasthenia gravis. Mediators Inflamm. 2015;2015:460610. doi: 10.1155/2015/460610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li WP, Roth J. Expression of beta 1,6 branched asparagine-linked oligosaccharides in non-mitotic and non-migratory cells of normal human and rat tissues. Int J Cancer. 1997;71:483–490. doi: 10.1002/(sici)1097-0215(19970502)71:3<483::aid-ijc29>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 45.Takamatsu S, et al. N-acetylglucosaminyltransferase (GnT) assays using fluorescent oligosaccharide acceptor substrates: GnT-III, IV, V, and IX (GnT-Vb) Methods Mol Biol. 2013;1022:283–298. doi: 10.1007/978-1-62703-465-4_21. [DOI] [PubMed] [Google Scholar]
- 46.Teixeira L, et al. Immune response in the adipose tissue of lean mice infected with the protozoan parasite Neospora caninum. Immunology. 2015;145:242–257. doi: 10.1111/imm.12440. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.