Significance
Elevated atmospheric N deposition threatens ecosystem health through eutrophication in terrestrial ecosystems, but little is known about consequences of N deposition in N-rich tropical ecosystems. We added several levels of N to an N-rich tropical forest and monitored plant growth dynamics, forest nutrient status, plant water use, and water losses from the ecosystem for a decade. We found that plants can acclimate and maintain nutrient balance by altering hydrological cycling. These results demonstrate that while elevated N deposition to already N-rich tropical forests may have minor effects on forest growth, it can exert a detectable influence on hydrological dynamics. Reduced runoff may threaten water supply in rapidly developing tropical regions.
Keywords: plant acclimation, nutrient balance, soil acidification, transpiration, water use strategy
Abstract
Anthropogenic nitrogen (N) deposition has accelerated terrestrial N cycling at regional and global scales, causing nutrient imbalance in many natural and seminatural ecosystems. How added N affects ecosystems where N is already abundant, and how plants acclimate to chronic N deposition in such circumstances, remains poorly understood. Here, we conducted an experiment employing a decade of N additions to examine ecosystem responses and plant acclimation to added N in an N-rich tropical forest. We found that N additions accelerated soil acidification and reduced biologically available cations (especially Ca and Mg) in soils, but plants maintained foliar nutrient supply at least in part by increasing transpiration while decreasing soil water leaching below the rooting zone. We suggest a hypothesis that cation-deficient plants can adjust to elevated N deposition by increasing transpiration and thereby maintaining nutrient balance. This result suggests that long-term elevated N deposition can alter hydrological cycling in N-rich forest ecosystems.
Acclimation is a key to plant survival in changing environments, as it allows plants to adjust morphologically, anatomically, and/or physiologically to changes in their environment (1). Mechanisms underlying acclimation of plants to environmental stress have been investigated under global change scenarios, including studies focused on mineral nutrition (2, 3). Morphological adjustments and symbioses can help plants acquire and retain nutrients under conditions of low nutrient availability (4, 5).
Currently, anthropogenic emissions of reactive N (Nr) and subsequent deposition have at least doubled natural N sources, and they will continue to increase in the future (6). In N-limited ecosystems, low-level N deposition can play a beneficial role in tree growth and carbon uptake (7). With progressive N saturation, elevated N deposition can threaten ecosystem health through acidification and eutrophication, and lead to soil cation depletion and plant nutrient imbalance (8–12). A few studies have shown that interactions exist between nitrogen (7, 13) or cation supply (14) and water use strategy. For example, a metaanalysis on temperate forests (mostly low in N) demonstrated that intrinsic water use efficiency (iWUE) generally increased in response to increased N applications (7). At the Hubbard Brook Experimental Forest, Green et al. (14) found that Ca amendment significantly increased annual evapotranspiration, thus decreasing water flowing from that forest. However, many tropical forests are naturally rich in N (15–17), and it is unclear how prolonged N addition affects the dynamics of such forests (18, 19).
In this study, we explore how an N-rich tropical forest is changed by long-term high N inputs, and how plants maintain their nutrient balance under these conditions. Specifically, we hypothesize that long-term N additions acidify soils and deplete biologically available soil calcium (Ca) and magnesium (Mg); and we explore whether and, if so, how plants can acclimate to acidification and nutrient depletion. Here, we experimentally test these hypotheses through N additions to a tropical forest.
In 2002, we chose an N-rich lowland tropical forest at the Dinghushan Biosphere Reserve in southern China. That site was naturally high in N, as are many lowland tropical forests, and it had already been affected by anthropogenic N deposition. There are four main indicators of high N status in this forest as follows.
i) High N cycling rate: Studying the N accumulation and cycling of various organs of 44 plant individuals belonging to 20 species, Mo et al. (20) found high recycling coefficient (0.8) and short turnover time (5.9 y), reflecting characters of fast N cycling. Also, rates of decomposition of litter in this mature forest were high in comparison with the values reported for temperate ecosystems and were similar to those of other tropical ecosystems (21).
ii) High plant N status: Mo et al. (22) further found plants were richer in N but poorer in P compared with those in the other forests, and suggested P may limit plant productivity in this forest.
iii) Apparent isotopic fractionation: We observed substantial 15N enrichment in soils of this forest, suggesting high N availability (23, 24).
iv) Continuing large leaching losses of N (25, 26): The large loss of deposited N further reflected N saturation in this site (27). Through N additions, we moved the forest more rapidly in a direction that it was already going (see more information in Materials and Methods). In this study, we report results from a decade of experimental N applications.
Results and Discussion
We found that long-term N addition had no effects on foliar N contents and foliar cations (e.g., Ca and Mg; Table 1 and SI Appendix, Table S1), photosynthesis, litterfall production, or plant annual growth rate (SI Appendix, Fig. S1), in contrast to previous studies in N-limited ecosystems (19, 28).
Table 1.
Effects of long-term N addition on foliar N and metal element contents of dominant tree plants in the mature tropical forest
| Species/N level | N, mg⋅g−1 | K+, mg⋅g−1 | Na+, mg⋅g−1 | Ca2+, mg⋅g−1 | Mg2+, mg⋅g−1 | Al3+, mg⋅g−1 |
| Castanopsis chinensis | ||||||
| Control | 15.83 ± 0.36 | 2.78 ± 0.11 | 0.21 ± 0.04 | 1.89 ± 0.21 | 0.61 ± 0.04 | 0.28 ± 0.04 |
| Low N | 15.44 ± 0.27 | 2.98 ± 0.17 | 0.20 ± 0.05 | 1.82 ± 0.14 | 0.59 ± 0.01 | 0.31 ± 0.07 |
| Medium N | 16.63 ± 0.46 | 2.80 ± 0.09 | 0.23 ± 0.02 | 1.80 ± 0.10 | 0.61 ± 0.07 | 0.31 ± 0.03 |
| High N | 16.49 ± 1.20 | 2.61 ± 0.18 | 0.18 ± 0.01 | 1.75 ± 0.13 | 0.53 ± 0.04 | 0.31 ± 0.08 |
| Machilus chinensis | ||||||
| Control | 15.42 ± 0.12 | 3.34 ± 0.79 | 0.36 ± 0.01 | 3.32 ± 0.41 | 0.39 ± 0.02 | 0.029 ± 0.002 |
| Low N | 15.16 ± 0.24 | 3.61 ± 0.62 | 0.41 ± 0.01 | 3.40 ± 0.24 | 0.38 ± 0.06 | 0.031 ± 0.002 |
| Medium N | 15.41 ± 0.56 | 3.73 ± 0.42 | 0.34 ± 0.01 | 2.88 ± 0.46 | 0.35 ± 0.02 | 0.027 ± 0.003 |
| High N | 15.98 ± 0.41 | 3.06 ± 0.14 | 0.28 ± 0.06 | 2.69 ± 0.12 | 0.33 ± 0.02 | 0.032 ± 0.001 |
| Schima superba | ||||||
| Control | 16.28 ± 0.47 | 5.15 ± 0.35 | 0.11 ± 0.02 | 4.60 ± 0.24 | 1.54 ± 0.10 | 0.41 ± 0.01 |
| Low N | 16.78 ± 0.22 | 5.03 ± 0.23 | 0.12 ± 0.02 | 4.65 ± 0.31 | 1.39 ± 0.09 | 0.46 ± 0.05 |
| Medium N | 17.35 ± 0.52 | 4.56 ± 0.25 | 0.12 ± 0.02 | 4.39 ± 0.19 | 1.32 ± 0.01 | 0.47 ± 0.02 |
| High N | 16.91 ± 0.79 | 4.62 ± 0.03 | 0.08 ± 0.01 | 3.85 ± 0.25 | 1.26 ± 0.06 | 0.47 ± 0.01 |
| Cryptocarya chinensis | ||||||
| Control | 19.02 ± 0.61 | 3.89 ± 0.23 | 0.19 ± 0.03 | 2.12 ± 0.16 | 0.46 ± 0.01 | 0.11 ± 0.02 |
| Low N | 19.16 ± 0.34 | 3.15 ± 0.30 | 0.15 ± 0.01 | 2.31 ± 0.11 | 0.52 ± 0.04 | 0.11 ± 0.02 |
| Medium N | 19.03 ± 1.28 | 3.25 ± 0.39 | 0.14 ± 0.01 | 1.77 ± 0.04 | 0.40 ± 0.06 | 0.12 ± 0.01 |
| High N | 19.90 ± 0.23 | 3.69 ± 0.35 | 0.20 ± 0.01 | 2.03 ± 0.18 | 0.43 ± 0.02 | 0.14 ± 0.01 |
| Syzygium rehderianum | ||||||
| Control | 13.36 ± 0.19 | 4.98 ± 0.26 | 0.19 ± 0.03 | 3.75 ± 0.22 | 1.62 ± 0.03 | 0.07 ± 0.01 |
| Low N | 13.51 ± 0.63 | 4.52 ± 0.29 | 0.16 ± 0.04 | 3.64 ± 0.43 | 1.50 ± 0.08 | 0.06 ± 0.01 |
| Medium N | 14.08 ± 0.22 | 4.99 ± 0.65 | 0.14 ± 0.01 | 3.63 ± 0.10 | 1.34 ± 0.05 | 0.06 ± 0.00 |
| High N | 13.74 ± 0.20 | 4.33 ± 0.32 | 0.19 ± 0.03 | 3.27 ± 0.28 | 1.35 ± 0.09 | 0.05 ± 0.01 |
| Acmena acuminatissima | ||||||
| Control | 19.11 ± 0.71 | 4.16 ± 0.42 | 0.38 ± 0.06 | 4.09 ± 0.38 | 0.64 ± 0.01 | 0.16 ± 0.01 |
| Low N | 19.86 ± 1.14 | 4.23 ± 0.82 | 0.29 ± 0.08 | 4.13 ± 0.60 | 0.69 ± 0.09 | 0.14 ± 0.02 |
| Medium N | 19.39 ± 0.76 | 3.55 ± 0.33 | 0.27 ± 0.04 | 3.40 ± 0.06 | 0.52 ± 0.04 | 0.16 ± 0.01 |
| High N | 20.03 ± 0.10 | 3.45 ± 0.25 | 0.28 ± 0.03 | 3.25 ± 0.09 | 0.57 ± 0.04 | 0.16 ± 0.03 |
Note: We commonly selected two trees for each species per plot. Values are mean ± SE (n = 3 for all, except n = 2 for Machilus chinensis in the low-N treatment); element contents were the average values between the seventh and ninth sampling year. There were no significant differences between controls and N treatments (P > 0.05; Tukey’s HSD test).
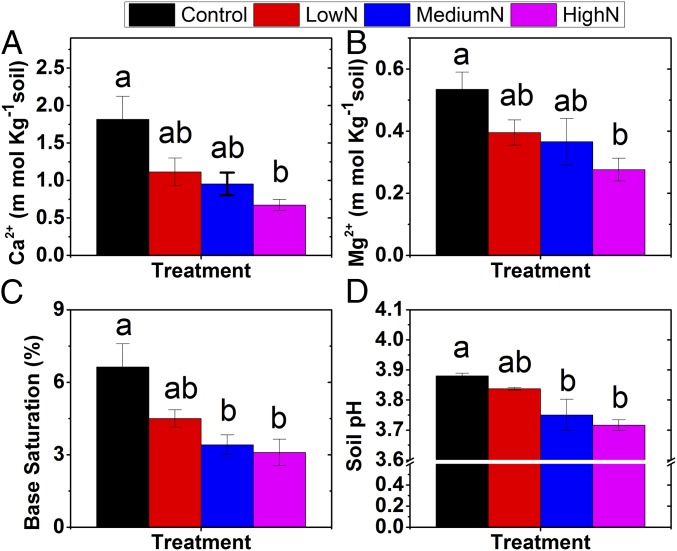
However, we found that added N acidified soils and reduced biologically available concentrations of Ca and Mg in soil (Fig. 1 and SI Appendix, Fig. S2), an expected consequence of N deposition in excess of the forest’s capacity to absorb it. Principal-component analysis further showed no significant relationships between soil Ca and foliar Ca as the first principal component (SI Appendix, Tables S2 and S3). Accordingly, we conclude that, in this forest, trees were able to acclimate to soil cation depletion, making the response of foliar nutrient contents to N treatment partially independent of soil acidification and cation depletion. What mechanisms could offset Ca and Mg depletion in this N-rich forest?
Fig. 1.
Effects of long-term N addition on soil-exchangeable Ca2+ (A) and Mg2+ (B), base saturation (C), and pH (D) in the mature tropical forest of southern China. The different lowercase letters indicate significant differences at P < 0.05 level (Tukey’s HSD test) among N treatment levels. Values are mean with SE.
We identify three potential (and nonexclusive) mechanisms that could cause the observed results. First, added N could enhance the rate of transpiration, potentially increasing the uptake of passively acquired nutrients like Ca and Mg. Second, an increase in transpiration could be driven by lower levels of exchangeable cations in the N-fertilized plots. Third, N additions could suppress the abundance and activity of mycorrhizal fungi, as has been observed for ectomycorrhizae in N-limited ecosystems (29). A reduction in mycorrhizae could enhance cation uptake if cation mobility through soil is more rapid than that through fungal hyphae under equivalent nutrient supply. Of our six focal species, three (Castanopsis chinensis, Acmena acuminatissima, and Syzygium rehderianum) are ectomycorrhizal, while three (Machilus chinensis, Schima superba, and Cryptocarya chinensis) have arbuscular mycorrhizae (30).
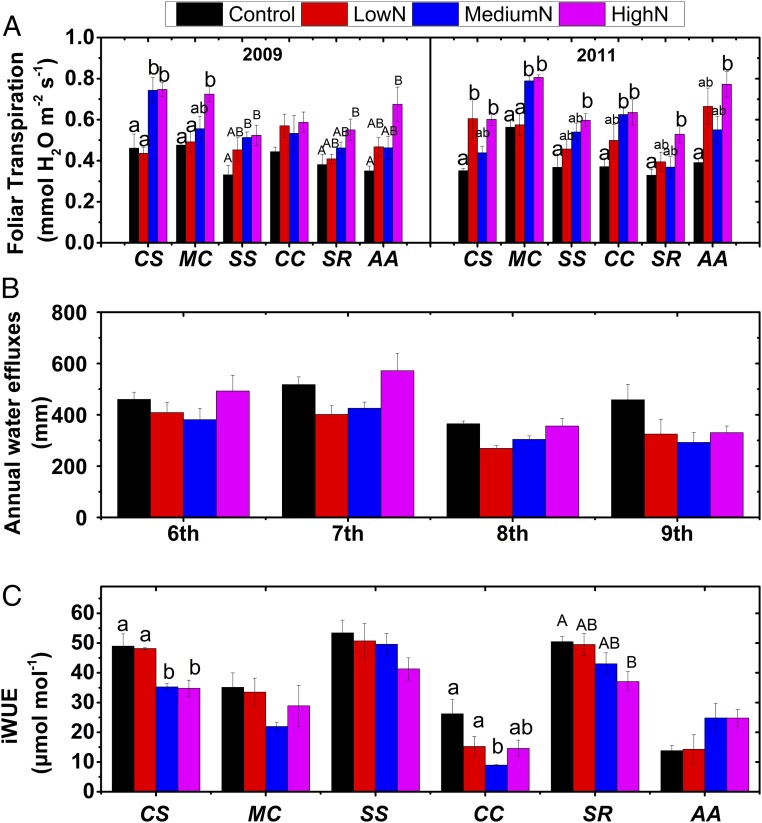
Our observations clearly show that transpiration was enhanced in the fertilized plots, as demonstrated by greater instantaneous rates of transpiration (Fig. 2A), with overall average increases of 25%, 37%, and 59%, in the low-N, medium-N, and high-N treatments, respectively. Similarly, long-term N additions greatly decreased water efflux below the bulk of the rooting zone (Fig. 2B and SI Appendix, Fig. S3). For the relative monthly water efflux, linear regression analysis showed that there were significantly decreasing trends for water loss via drainage across all sampling data for all three N treatments, especially in the high-N treatment. We further found that foliar iWUE was generally decreased by elevated N additions (SI Appendix, Fig. S4), especially for the canopy trees of Castanopsis chinensis and Cryptocarya chinensis (Fig. 2C). By increasing water uptake, enhanced transpiration can lower groundwater levels (31) and reduce streamflow in forested catchments (14). In a forest watershed near our research site at the Dinghushan reserve that is also subject to increasing N deposition, Li et al. (32) found that although total annual precipitation changed little from 1978 to 2010, soil moisture decreased significantly, coupled with increased evapotranspiration especially in rain-free days. Using specimens from 1947 to 2014 in three subtropical forests, Huang et al. (13) found that long-term N deposition linked to reduced water use efficiency (WUE) in forests with low phosphorus availability, supporting our findings in this study, although outside an experimental context, it is difficult to separate the contribution of N additions from other climate change factors such as warming.
Fig. 2.
Effects of long-term N addition on foliar transpiration of dominant tree species (A), annual water flux below the primary rooting zones (B), and foliar intrinsic water use efficiency (iWUE) (C). Notes: (i) The different lowercase letters indicate significant differences at P < 0.05 levels among N treatment levels, and the different uppercase letters indicate significant differences at P < 0.1 levels, and no letters indicate no significant differences among N treatment levels (Tukey’s HSD test). Values are mean with SE. AA, Acmena acuminatissima; CC, Cryptocarya chinensis; CS, Castanopsis chinensis; MC, Machilus chinensis; SR, Syzygium rehderianum; SS, Schima superba. (ii) For annual water flux, there are significant differences at P < 0.05 level between controls and N treatment plots in the eighth and ninth year by using an independent-samples t test.
Enhanced transpiration could increase the upward movement of dissolved solutes in the xylem, and thus enhance nutrient delivery through mass flow from soil solution to root surface (33). The role of transpiration in driving acquisition of passively acquired nutrients like Ca is clear (34–37). Cramer et al. (34) found that plants can respond to nutrient limitation by varying transpiration-driven mass flow of nutrients, indicating plant traits may interact with the edaphic environment to determine “functional nutrient availability.” Studying tropical tree and liana seedlings, Cernusak et al. (35) found transpiration could play an important role in modulating nutrient uptake by delivering nutrients to root surfaces through mass flow. Also, the very low mobility of Ca within phloem often leads to Ca deficiencies in new plant tissues, and long-distance transport of Ca within plants is predominantly apoplastic flux, which is dependent on transpiration rate (36), making transpiration-driven fluxes from soils the main source to support new growth. Our information cannot separate whether the increased transpiration we observed is driven directly by the addition of N or indirectly by depletion of soil cations. We suspect that soil cations represent the most important and proximate control, because tropical forests growing on soils that already are enriched in N are unlikely to have faced acclimation to much greater N supply in their evolutionary past—but acclimation to low levels of soil cations has been a frequent challenge.
While elevated transpiration clearly contributes to the patterns in foliar cations that we observe, we cannot rule out the possibility that symbiotic mycorrhizae play a role (29). On this site, it is reported that long-term N additions had no effect on the relative abundance of soil fungal phospholipid-derived fatty acids, but decreased microbial biomass (38). An earlier study showed decreases both in microbial biomass and fine root biomass under elevated N additions (39). Hence, long-term high N inputs could reduce the importance of nutrient uptake through mycorrhizal symbiosis—and, for a passively acquired nutrient like Ca, potentially increase the rate of uptake through roots.
In summary, our findings suggest that elevated N deposition to already N-rich tropical forests may have minor effects on forest growth but can exert a profound influence on hydrological dynamics, including the possibility that groundwater and ultimately streamflow may decrease in many lowland tropical forests under the scenarios of long-term high N deposition. In addition, we find that the changes in foliar Ca and Mg contents, which are required to detect a significant difference under N treatments, varied with plant species and cations (SI Appendix, Table S4), indicating potential thresholds on the lack of change in foliar cations related to plant acclimation strategy. The proposed acclimation strategy, however, is worth testing in broad regions of the world where excess N deposition is a present and future concern. If it is a widespread feature of N-rich tropical forests, continued development by present pathways could have substantial effects on municipal water supply in tropical regions.
Materials and Methods
Study Site.
This study was conducted at the Dinghushan Biosphere Reserve of southern China (112°10′ E, 23°10′ N). The reserve has a monsoon climate and is in a subtropical/tropical moist forest life zone (40). The annual average precipitation was 1,748 mm from 2002 to 2012, mainly concentrated from April to September (SI Appendix, Fig. S5). Annual mean relative humidity is 80%. Mean annual temperature is 21.9 °C. The reserve has been experiencing high atmospheric N deposition in precipitation (e.g., commonly >30 kg N⋅ha−1⋅y−1) at least since 1990s (25, 41). In 2009–2010, total wet N deposition was 34.4 kg N⋅ha−1⋅y−1 (41), and total dry N deposition was 14.2 kg N⋅ha−1⋅y−1 (42).
We established our research site in a monsoon evergreen broadleaf forest (mature forest), belonging to a regional climax type, at elevations between 250 and 300 m above sea level. The forest has been protected from disturbances related to land use for >400 y (43) and supports a rich assemblage of plant species, most of which are natives of tropical and subtropical China, including Castanopsis chinensis Hance, Schima superba Chardn. and Champ., Cryptocarya chinensis (Hance) Hemsl., Machilus chinensis (Champ. Ex Benth.) Hemsl., Syzygium rehderianum Merr. and Perry, and Acmena acuminatissima (Blume) Merr. and Perry, six species that together represent up to 80% of total biomass. Canopy closure is typically above 95% (44). Understory layers are mainly woody species, with dominant species being Cryptocarya concinna and Randia canthioides. Soils in the study site are lateritic red earths (Oxisols) formed from sandstone, commonly with soil depth greater than 60 cm (45). The soil bulk density is about 0.98 g/cm3, and the soil total C and N are 3.2% and 0.25%, respectively, in the 0–10 mineral soil layers. The study site is an acid-sensitive ecosystem, with poor soil-buffering capacity (see more soil information in ref. 46).
This forest can be regarded as a reasonably representative N-rich tropical forest, because it is high in N, as are many lowland tropical forests (see more information in the Introduction). Whereas net primary productivity and net ecosystem productivity in many temperate ecosystems are N-limited, considerable evidence suggests that biological activity in many humid lowland tropical forests, especially those on highly weathered soils, is not limited by N but rather by some other nutrient (e.g., P or Ca) (15–17). These tropical forests often have high soil N availability and rapid rates of N cycling (18, 47).
Experimental Treatments.
We established long-term research plots in 2002. Nitrogen amendments were initiated in July 2003, with four rates used: control (0 N added), low N (50 kg N⋅ha−1⋅y−1), medium N (100 kg N⋅ha−1⋅y−1), and high N (150 kg N⋅ha−1⋅y−1). These were based on present atmospheric N deposition rates and their expected increase in the future due to the rapid development of agricultural and industrial activities (6, 48). Considering the potentially cumulative effects of low rates of N additions, our study with high rates of N addition may be useful for predicting long-term effects of chronic low rates of N deposition. In total, there were 12 10 × 20-m plots surrounded by unfertilized buffer strips of at least 10 m wide, with treatments replicated in triplicate and randomly assigned. To exclude roots growing outside the treatment area, we built concrete and plastic barriers around each plot to the depth of the parent rock while establishing the research plots. Sample plots have been fertilized monthly with NH4NO3 since July 2003. At each N application, NH4NO3 fertilizer was weighed, mixed with 20 L of water, and sprayed onto the floor of these plots using a backpack sprayer. The control plot received equal volumes (20 L) of water without fertilizer. The NH4NO3 solution concentrations were about 0, 0.3, 0.6, and 0.9 mol N⋅L−1 for the control, low-N, medium-N, and high-N plots, respectively, at each application.
Soil Water Sampling.
Soil water sampling began 5 y after the initial experimental N application and was collected from all plots at a 20-cm soil depth, chosen because the 0- to 20-cm soil layer represents the dominant rooting zone, where 72% of the fine and small root biomass (<5 mm) and 68% of total root biomass are located (49). Two zero tension tray lysimeters (755 cm2 per tray) per plot were installed in May 2003. Each lysimeter was connected to a 10-L bottle using slope to facilitate water flow into lysimeters. Water samples were taken between July 2008 and June 2012, after each rain event. For all soil water samples, water volume was recorded and composited within a plot on the date of collection. We obtained precipitation and air temperature data from a weather station located within the reserve (SI Appendix, Fig. S5).
Photosynthetic Measurements.
Six dominant tree species in the canopy (Castanopsis chinensis, Machilus chinensis, Schima superba, Cryptocarya chinensis) and subcanopy (Syzygium rehderianum, Acmena acuminatissim) were chosen for measurements at each plot. These species commonly occurred in each plot, except Machilus chinensis was absent from one low-N treatment plot. We made sure that at least one tree was sampled for each species per plot. If there were more than two individuals for one given species per plot, we selected just two plants for sampling. For the canopy tree species (Castanopsis chinensis, Machilus chinensis, Schima superba, Cryptocarya chinensis), the diameter at breast height (DBH) (1.3 m aboveground) was generally above 8 cm. For the subcanopy trees, the DBH was more than 2.5 cm. Branches of 60–100 cm in length and 8–12 mm in diameter were sampled from the middle canopy using pruning shears, with the branch bottom immediately placed into plastic bottles containing distilled water. Photosynthetic parameters were measured on fully developed mature leaves using a LI-COR 6400 photosynthesis system (LI-COR) in the midmorning during the first 10 d of September 2009 and September 2011 (7 and 9 y following initial N application, respectively). We measured light-saturated photosynthetic rate (Pmax) and related parameters such as foliar transpiration and stomatal conductance at a light intensity of 1,000 μmol⋅m−2⋅s−1 maintained by a red-blue LED light source, a chamber temperature of 25 °C (approximating ambient air temperature in the forest), at near-ambient humidity, and with a reference CO2 concentration of 390 µmol⋅mol−1. Leaves were placed in cuvettes for at least 2 min, after which stable gas exchange values were recorded every 15 s for at least 1 min. A preliminary experiment showed that (i) excision had no systematic effect on the measured rates of photosynthesis or stomatal conductance during the measuring period, and (ii) a 1,000 μmol⋅m−2⋅s−1 photosynthetic photon flux density was sufficient to saturate photosynthesis.
Foliar and Soil Sampling and Laboratory Analysis.
Foliar sampling was carried out following photosynthetic measurements. Subsamples were dried at 70 °C for more than 48 h and then ground to a fine powder with a vibrating sample mill. Ground tissue was acid digested (H2SO4-HClO4) and analyzed for cation (e.g., K+, Na+, Ca2+, Mg2+, and Al3+) concentrations using an ICP optical emission spectrometer (Perkin-Elmer). We used an elemental analyzer (Vario Isotope cube Elemental Analyzer; Elementar) to analyze foliar N content.
Soils were collected for pH and cation measurements after 7 y of treatment. Three cores (5-cm diameter × 20-cm deep) were randomly collected from each plot. The soils were sieved (2 mm) to remove roots and stones, and mixed thoroughly by hand. Soil pH values were measured with soil:water of 1:2.5 using a glass electrode. The soils were extracted with 0.1 mol/L BaCl2 (50:1, solution:soil), and exchangeable cations (K+, Na+, Ca2+, Mg2+, Al3+, Fe3+) were analyzed using an ICP optical emission spectrometer (Perkin-Elmer). Exchangeable H+ was determined by titration with NaOH. Base saturation was calculated by dividing the sum of the charge equivalents of the exchangeable base cations by total cation exchangeable capacity.
Stable Isotope Analysis.
To evaluate long-term WUE, leaves of the six tree species were sampled in July 2014, and δ13C analyses were performed. Isotope abundances were expressed using the δ notation in per mille (‰) relative to the international standards:
| [1] |
where Rsample is the molar fraction of the 13C/12C ratio of the sample, and Rstandard that of the International Atomic Energy Agency standards Vienna Pee Dee Belemnite. δ13C was measured by isotope ratio mass spectrometry (IsoPrime100; Elementar Vario Isotope cube).
Beginning with raw δ13C measurements, Eqs. 2–4 were used to calculate iWUE. Eq. 2 was used to calculate changes in C isotopic discrimination (Δ) relative to the source (atmospheric CO2):
| [2] |
where δ13Ca and δ13Cp denote the δ13C values of atmospheric CO2 and plant tissue, respectively.
Following the reference of Farquhar et al. (50), carbon discrimination during CO2 fixation of C3 plants is linearly related to the ratio of intercellular to atmospheric CO2 concentration (ci/ca) by the following equation:
| [3] |
where a refers to the fraction from diffusion through stomata (4.4‰), and b is fractionation by ribulose-1,5-bisphosphate carboxylase/oxygenase enzyme during carboxylation (27‰).
Finally, Eq. 4 relates these parameters to iWUE of the plants by using the scaling factor 1.6, which is the ratio of diffusivity of water vapor to CO2:
| [4] |
where A is net photosynthesis and gs is stomatal conductance. The δ13Cair and the CO2 concentrations in parts per million were obtained from the Global Monitoring Division of the Earth System Research Laboratory at Mauna Loa Observatory (National Oceanic and Atmospheric Administration, www.esrl.noaa.gov/gmd/index.html; accessed on Jan. 20, 2018).
Forest Tree Growth and Litterfall Sampling.
In June 2003, before the start of N addition, all trees with DBH ≥ 1 cm in all of the plots were tagged, numbered, and identified to species, and their height and DBH were measured. Since then, all trees with DBH ≥ 1 cm were measured again in July each year. The biomass of each tree was estimated based on species-specific biomass equations obtained from Wen et al. (51). The annual relative growth rate was calculated as the total biomass at the following years divided by the biomass at the first year in the given plots, respectively.
To collect litterfall, we randomly placed two litter traps (1 × 1 m) with 1-mm mesh in each plot about 0.5 m above the ground surface. Traps were emptied monthly during the year. Litterfall was separated into three components: leaves, small woody material (branches and bark), and miscellaneous (mainly reproductive parts).
Data Analyses.
Repeated-measure ANOVA was performed to examine the overall effects of N treatments on soil water efflux, plant relative growth rate, and litterfall during the study period. Principal-component analysis with varimax rotation was conducted on foliar elements and soil exchangeable cations and chemical properties to reduce the complexity of the dataset for further analysis. One-way ANOVA with Tukey’s honest significant difference (Tukey’s HSD) test was performed to determine the effects of N treatment on plant photosynthetic parameters, foliar element chemistry, and soil properties. We conducted independent-sample t tests to compare differences of response variables between trees in control plots, and executed the planned contrast analysis to test differences between control plots and N treatment plots. For foliar cations, Wilcoxon rank sum test was also used to test the differences between control plots and N treatment plots. Relative measures of soil water efflux were calculated as the plot average for the specified period divided by the average soil water efflux in the control over the same period. In addition, we used a general linear model to analyze the trends of relative water efflux with sequential sampling data for three N treatment levels. In addition, we calculated the effect sizes in foliar cation contents required to detect a significant difference under N treatments. All analyses were conducted using SPSS 14.0 for Windows (SPSS). Statistical significant differences were set with values of P < 0.05, unless otherwise stated. All data met the assumptions of the tests.
Supplementary Material
Acknowledgments
We thank invaluable comments from Prof. James N. Galloway, Dr. Kabir Peay, and Y.L.’s EcoLab. We also appreciate Dinghushan Forest Ecosystem Research Station for the support in the field work, and Ms. Xiaoping Pan, and Mr. Hui Mo, Lijie Deng, and Jingbin Liang for their skillful assistance in laboratory and field work. This study was funded by the National Natural Science Foundation of China (41731176, 31370498, and 31770523), the National Basic Research Program of China (2014CB954400), and Youth Innovation Promotion Association, Chinese Academy of Sciences (2015287).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1720777115/-/DCSupplemental.
References
- 1.Leimu R, Fischer M. A meta-analysis of local adaptation in plants. PLoS One. 2008;3:e4010. doi: 10.1371/journal.pone.0004010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Han WX, Fang JY, Reich PB, Ian Woodward F, Wang ZH. Biogeography and variability of eleven mineral elements in plant leaves across gradients of climate, soil and plant functional type in China. Ecol Lett. 2011;14:788–796. doi: 10.1111/j.1461-0248.2011.01641.x. [DOI] [PubMed] [Google Scholar]
- 3.Sardans J, Peñuelas J. The role of plants in the effects of global change on nutrient availability and stoichiometry in the plant-soil system. Plant Physiol. 2012;160:1741–1761. doi: 10.1104/pp.112.208785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mclaughlin SB, Wimmer R. Calcium physiology and terrestrial ecosystem processes. New Phytol. 1999;142:373–417. [Google Scholar]
- 5.Chapin FS, III, Matson PA, Vitousek P. Principles of Terrestrial Ecosystem Ecology. Springer Science and Business Media; New York: 2011. [Google Scholar]
- 6.Galloway JN, et al. Nitrogen cycles: Past, present, and future. Biogeochemistry. 2004;70:153–226. [Google Scholar]
- 7.Guerrieri R, et al. The legacy of enhanced N and S deposition as revealed by the combined analysis of δ13C, δ18O and δ15N in tree rings. Glob Change Biol. 2011;17:1946–1962. [Google Scholar]
- 8.Vitousek PM, et al. Human alteration of the global nitrogen cycle: Sources and consequences. Ecol Appl. 1997;7:737–750. [Google Scholar]
- 9.Gruber N, Galloway JN. An Earth-system perspective of the global nitrogen cycle. Nature. 2008;451:293–296. doi: 10.1038/nature06592. [DOI] [PubMed] [Google Scholar]
- 10.Bobbink R, et al. Global assessment of nitrogen deposition effects on terrestrial plant diversity: A synthesis. Ecol Appl. 2010;20:30–59. doi: 10.1890/08-1140.1. [DOI] [PubMed] [Google Scholar]
- 11.Lu X, Mao Q, Gilliam FS, Luo Y, Mo J. Nitrogen deposition contributes to soil acidification in tropical ecosystems. Glob Change Biol. 2014;20:3790–3801. doi: 10.1111/gcb.12665. [DOI] [PubMed] [Google Scholar]
- 12.Högberg P, Fan H, Quist M, Binkley D, Tamm CO. Tree growth and soil acidification in response to 30 years of experimental nitrogen loading on boreal forest. Glob Change Biol. 2006;12:489–499. [Google Scholar]
- 13.Huang Z, et al. Long-term nitrogen deposition linked to reduced water use efficiency in forests with low phosphorus availability. New Phytol. 2016;210:431–442. doi: 10.1111/nph.13785. [DOI] [PubMed] [Google Scholar]
- 14.Green MB, et al. Decreased water flowing from a forest amended with calcium silicate. Proc Natl Acad Sci USA. 2013;110:5999–6003. doi: 10.1073/pnas.1302445110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vitousek PM, Sanford RL., Jr Nutrient cycling in moist tropical forest. Annu Rev Ecol Syst. 1986;17:137–167. [Google Scholar]
- 16.Hedin LO, Brookshire EJ, Menge D, Barron AR. The nitrogen paradox in tropical forest ecosystems. Annu Rev Ecol Evol Syst. 2009;40:613–635. [Google Scholar]
- 17.Brookshire EJ, Hedin LO, Newbold JD, Sigman DM, Jackson JK. Sustained losses of bioavailable nitrogen from montane tropical forests. Nat Geosci. 2012;5:123–126. [Google Scholar]
- 18.Matson PA, McDowell WH, Townsend AR, Vitousek PM. The globalization of N deposition: Ecosystem consequences in tropical environments. Biogeochemistry. 1999;46:67–83. [Google Scholar]
- 19.Niu S, et al. Global patterns and substrate-based mechanisms of the terrestrial nitrogen cycle. Ecol Lett. 2016;19:697–709. doi: 10.1111/ele.12591. [DOI] [PubMed] [Google Scholar]
- 20.Mo J, Ding M, Zhang Z, Yi W. Nitrogen accumulation and cycling in a monsoon evergreen broad-leafed forest-the Cryptocarya concinna, Lindera chunii community of Dinghushan. Acta Phytoecol Sin. 1994;18:140–146. [Google Scholar]
- 21.Mo J, Brown S, Xue J, Fang Y, Li Z. Response of litter decomposition to simulated N deposition in disturbed, rehabilitated and mature forests in subtropical China. Plant Soil. 2006;282:135–151. [Google Scholar]
- 22.Mo J, Zhang D, Huang Z, Yu Q, Kong G. Distribution pattern of nutrient elements in plants of Dinghushan lower subtropical evergreen broad-leaved forest. Redai Yaredai Zhiwu Xuebao. 2000;8:198–206. [Google Scholar]
- 23.Martinelli L, et al. Nitrogen stable isotopic composition of leaves and soil: Tropical versus temperate forests. Biogeochemistry. 1999;46:45–65. [Google Scholar]
- 24.Koba K, et al. δ15N of soil N and plants in a N-saturated, subtropical forest of southern China. Rapid Commun Mass Spectrom. 2010;24:2499–2506. doi: 10.1002/rcm.4648. [DOI] [PubMed] [Google Scholar]
- 25.Huang Z, Ding M, Zhang Z, Yi W. The hydrological processes and nitrogen dynamics in a monsoon evergreen broad-leafed forest of Dinghushan. Acta Phytoecol Sin. 1994;18:194–199. [Google Scholar]
- 26.Fang YT, Gundersen P, Mo J, Zhu W. Input and output of dissolved organic and inorganic nitrogen in subtropical forests of South China under high air pollution. Biogeosciences. 2008;5:339–352. [Google Scholar]
- 27.Gurmesa GA, et al. High retention of 15N-labeled nitrogen deposition in a nitrogen saturated old-growth tropical forest. Glob Change Biol. 2016;22:3608–3620. doi: 10.1111/gcb.13327. [DOI] [PubMed] [Google Scholar]
- 28.Aber J, et al. Nitrogen saturation in temperate forest ecosystems. Bioscience. 1998;48:921–934. [Google Scholar]
- 29.Lilleskov EA, Hobbie EA, Horton TR. Conservation of ectomycorrhizal fungi: Exploring the linkages between functional and taxonomic responses to anthropogenic N deposition. Fungal Ecol. 2011;4:174–183. [Google Scholar]
- 30.Niu JQ. An investigation on mycorrhiza from Dinghushan. Trop Subtrop For Ecosyst. 1990;6:40–45. [Google Scholar]
- 31.Engel V, Jobbágy EG, Stieglitz M, Williams M, Jackson RB. Hydrological consequences of Eucalyptus afforestation in the Argentine Pampas. Water Resour Res. 2005;41:W10409. [Google Scholar]
- 32.Li R, et al. Are functional traits a good predictor of global change impacts on tree species abundance dynamics in a subtropical forest? Ecol Lett. 2015;18:1181–1189. doi: 10.1111/ele.12497. [DOI] [PubMed] [Google Scholar]
- 33.Tanner W, Beevers H. Transpiration, a prerequisite for long-distance transport of minerals in plants? Proc Natl Acad Sci USA. 2001;98:9443–9447. doi: 10.1073/pnas.161279898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cramer MD, Hoffmann V, Verboom GA. Nutrient availability moderates transpiration in Ehrharta calycina. New Phytol. 2008;179:1048–1057. doi: 10.1111/j.1469-8137.2008.02510.x. [DOI] [PubMed] [Google Scholar]
- 35.Cernusak LA, Winter K, Turner BL. Transpiration modulates phosphorus acquisition in tropical tree seedlings. Tree Physiol. 2011;31:878–885. doi: 10.1093/treephys/tpr077. [DOI] [PubMed] [Google Scholar]
- 36.Gilliham M, et al. Calcium delivery and storage in plant leaves: Exploring the link with water flow. J Exp Bot. 2011;62:2233–2250. doi: 10.1093/jxb/err111. [DOI] [PubMed] [Google Scholar]
- 37.Matimati I, Verboom GA, Cramer MD. Nitrogen regulation of transpiration controls mass-flow acquisition of nutrients. J Exp Bot. 2014;65:159–168. doi: 10.1093/jxb/ert367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang C, et al. Responses of soil microbial community to continuous experimental nitrogen additions for 13 years in a nitrogen-rich tropical forest. Soil Biol Biochem. 2018;121:103–112. [Google Scholar]
- 39.Mo J, et al. Nitrogen addition reduces soil respiration in a mature tropical forest in southern China. Glob Change Biol. 2008;14:403–412. [Google Scholar]
- 40.Holdridge LR. Life Zone Ecology. Tropical Science Center; San Jose, Costa Rica: 1967. [Google Scholar]
- 41.Lu X, et al. Long-term nitrogen addition decreases carbon leaching in a nitrogen-rich forest ecosystem. Biogeosciences. 2013;10:3931–3941. [Google Scholar]
- 42.Long XJ. 2010. Analysis of factors affecting the characteristics of atmospheric organic acids and nitrogen deposition: A case study over DingHu Mountain and Guang Zhou City. Master’s thesis (Sun Yat-sen University, Guangzhou, China), pp 79–80.
- 43.Shen CD, et al. 14C measurement of forest soils in Dinghushan Biosphere Reserve. Chin Sci Bull. 1999;44:251–256. [Google Scholar]
- 44.Lu X, Mo J, Gilliam FS, Zhou G, Fang Y. Effects of experimental nitrogen additions on plant diversity in an old-growth tropical forest. Glob Change Biol. 2010;16:2688–2700. [Google Scholar]
- 45.Mo J, Brown S, Peng S, Kong G. Nitrogen availability in disturbed, rehabilitated and mature forests of tropical China. For Ecol Manage. 2003;175:573–583. [Google Scholar]
- 46.Lu X, et al. Divergent responses of soil buffering capacity to long-term N deposition in three typical tropical forests with different land-use history. Environ Sci Technol. 2015;49:4072–4080. doi: 10.1021/es5047233. [DOI] [PubMed] [Google Scholar]
- 47.Corre MD, Veldkamp E, Arnold J, Wright SJ. Impact of elevated N input on soil N cycling and losses in old-growth lowland and montane forests in Panama. Ecology. 2010;91:1715–1729. doi: 10.1890/09-0274.1. [DOI] [PubMed] [Google Scholar]
- 48.Lü C, Tian H. Spatial and temporal patterns of nitrogen deposition in China: Synthesis of observational data. J Geophys Res. 2007;112:D22S05. [Google Scholar]
- 49.Wen DZ, Wei P, Kong GH, Ye WH. Production and turnover rate of fine roots in two lower subtropical forest sites at Dinghushan. Acta Phytoecol Sin. 1999;23:361–369. [Google Scholar]
- 50.Farquhar GD, Oleary MH, Berry JA. On the relationship between carbon isotope discrimination and the inter-cellular arbon-dioxide concentration in leaves. Aust J Plant Physiol. 1982;9:121–137. [Google Scholar]
- 51.Wen D, Wei P, Kong G, Zhang Q, Huang Z. Biomass study of the community of Castanopsis chinessis + Cryptocarya concinna + Schima superby in a southern China reserve. Acta Ecol Sin. 1997;17:497–504. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.