Significance
Today, amino acids are primarily manufactured via microbial cultivation processes, which are costly, are time consuming, and require extensive separations processes. As an alternative, chemocatalytic approaches to produce amino acids from renewable feedstocks such as bio-based sugars could offer a rapid and potentially more efficient means of amino acid synthesis, but efforts to date have been limited by the development of facile chemistry and associated catalyst materials to selectively produce α-amino acids. In this work, various α-amino acids, including alanine, leucine, aspartic acid, and phenylalanine, were obtained from both biomass-derived α-hydroxyl acids and glucose. The route bridges plant-based biomass and proteinogenic α-amino acids, offering a chemical approach that is potentially superior to microbial cultivation processes.
Keywords: amino acids, α-hydroxyl acids, amination, catalysis, ruthenium
Abstract
Amino acids are the building blocks for protein biosynthesis and find use in myriad industrial applications including in food for humans, in animal feed, and as precursors for bio-based plastics, among others. However, the development of efficient chemical methods to convert abundant and renewable feedstocks into amino acids has been largely unsuccessful to date. To that end, here we report a heterogeneous catalyst that directly transforms lignocellulosic biomass-derived α-hydroxyl acids into α-amino acids, including alanine, leucine, valine, aspartic acid, and phenylalanine in high yields. The reaction follows a dehydrogenation-reductive amination pathway, with dehydrogenation as the rate-determining step. Ruthenium nanoparticles supported on carbon nanotubes (Ru/CNT) exhibit exceptional efficiency compared with catalysts based on other metals, due to the unique, reversible enhancement effect of NH3 on Ru in dehydrogenation. Based on the catalytic system, a two-step chemical process was designed to convert glucose into alanine in 43% yield, comparable with the well-established microbial cultivation process, and therefore, the present strategy enables a route for the production of amino acids from renewable feedstocks. Moreover, a conceptual process design employing membrane distillation to facilitate product purification is proposed and validated. Overall, this study offers a rapid and potentially more efficient chemical method to produce amino acids from woody biomass components.
As the basic building blocks of proteins, amino acids play an essential role in life and are widely used in food and feed supplements, as precursors to biodegradable plastics, pharmaceutical products, and elsewhere (1). Although the current production of amino acids mainly relies on microbial cultivation processes, the issues associated with the scale limitations of microbial processes, the strict need for sterile operating conditions, and the complexity of their separation have stimulated efforts to develop efficient chemical approaches to produce amino acids and their derivatives (2–5).
The Strecker reaction is a classical chemical approach to produce amino acids (6), which employs highly toxic cyanides as nitrogen sources and nonrenewable aldehydes. The selective oxidation of amino alcohols and/or aldehydes serves as another option (7). In this procedure, protection/deprotection strategies for amino groups and/or stoichiometric, hazardous oxidants are often involved. Furthermore, the nitrogen is provided by the substrates, which are not always easily accessible. To date, sustainable and generalizable approaches for the direct synthesis of amino acids from abundant and renewable feedstocks using NH3 are still quite rare.
The catalytic transformations of various biomass components, including polysaccharides, lignin, and their derivatives, into valuable oxygen-containing chemicals (8–15) have been intensively studied (16–21). One prominent example, among others, is the conversion of biomass to α-hydroxypropanoic acid (i.e., lactic acid) (22–26). Lanthanide-based catalysts afford ca. 90% lactic acid directly from cellulose under hydrothermal conditions (24), whereas barium hydroxide catalyzes the quantitative conversion of glucose into lactic acid at room temperature (26). Several other α-hydroxyl acids are also readily available from lignocellulosic biomass. Cellulose may be converted into glycolic acid via selective oxidation over heteropoly acids (27) and into phenyllactic acid biocatalytically (28). Erythrose, a tetrose, can be transformed to vinylglycolate in the presence of tin salts (29) and further to α-hydroxyl butyric acid via hydrogenation. Simple base treatment enables the isolation of p-coumaric acid from grass lignins (30), which can be converted to p-hydroxyphenyllactic acid by hydration.
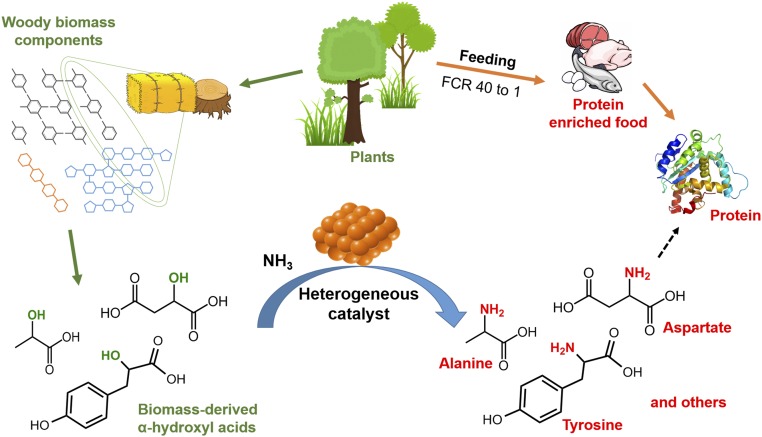
Because biomass-derived α-hydroxyl acids are readily accessible, we envisaged that the direct amination of these acids with ammonia could offer a promising and general method for amino acid synthesis (Fig. 1). Although homogeneous catalysis has proven success in producing primary amines from alcohols (31–33), recyclable heterogeneous catalysts are very limited, and the catalyst function is not well understood (34–37). Herein, we report an efficient heterogeneous catalytic system for the amination of multiple α-hydroxyl acids into corresponding amino acids using ammonia, including alanine, leucine, aspartic acid, and others in good to excellent yields. Based on the catalytic system, a chemical process transforming glucose to alanine via lactic acid as an intermediate was established, providing alanine yield higher than some of the best values obtained in microbial cultivation processes found in literature (38).
Fig. 1.
Catalytic transformation of biomass-derived α-hydroxyl acids into amino acids.
Results and Discussion
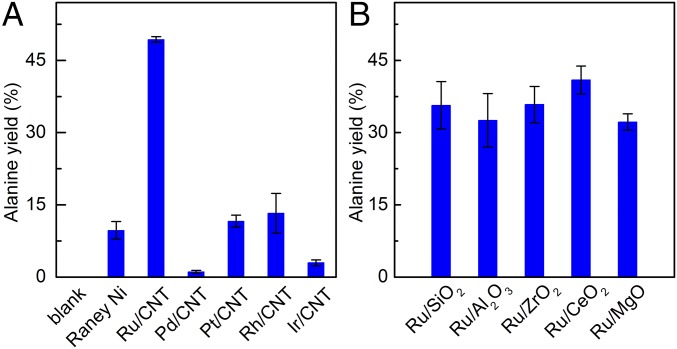
We first aimed to develop a robust amination catalyst and associated process on a model α-hydroxyl acid. Accordingly, amination of lactic acid was initially conducted over several noble metal nanoparticles (NPs) loaded on carbon nanotubes (CNT) and Raney Ni in a batch reactor. The catalysts were prepared by impregnation of metal precursors on CNT, followed by treatment in a H2/N2 (5%) atmosphere at 673 K to ensure complete reduction of the metals. X-ray diffraction (XRD) and transmission electron microscopy (TEM) confirm that the metal NPs are well dispersed on the support and possess similar sizes (SI Appendix, Figs. S1 and S2). Evaluation of the catalysts in lactic acid conversion in aqueous ammonia at 493 K for 2 h (Fig. 2A) shows that the Ru/CNT is the most effective, providing alanine in 49% yield. Rh/CNT, Pt/CNT, and Raney Ni afford alanine in yields of 14, 12, and 8%, respectively, whereas Pd/CNT and Ir/CNT were inactive. Ru NPs loaded on five other supports were further prepared and evaluated (Fig. 2B). The alanine yields were distributed in a narrow range between 32 and 38%, irrespective of the nature of support, i.e., whether it is acidic/basic, reducible/nonreducible, with the catalyst based on the CNT support remaining the most effective.
Fig. 2.
Catalytic conversion of lactic acid to alanine in a batch reactor. (A) Alanine yield with different metal catalysts. (B) Alanine yield across Ru-based catalysts on different supports. Reaction conditions: 0.5 mmol lactic acid, metal/substrate molar ratio = 0.025, 2.5 mL NH3H2O (25 wt %), 1 MPa H2, 493 K, 2 h. Error bars indicate SDs.
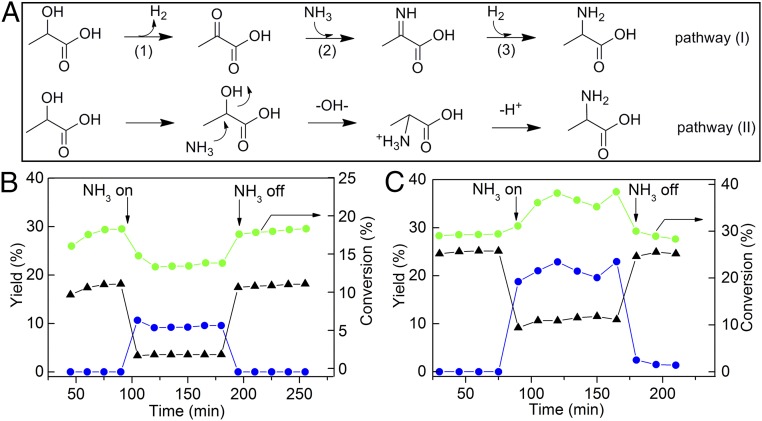
Fig. 3A shows two possible routes for alanine formation comprising indirect (pathway I) and direct (pathway II) pathways. The indirect path (I) includes the dehydrogenation to a ketone, the reaction of the ketone with ammonia to afford an imine, followed by hydrogenation (39), whereas the direct path (II) involves a SN2 substitution of the -OH group with an -NH2 group. If the absence of an α-H in the substrate does not affect the formation of the amino acid, the reaction should proceed via direct amination; otherwise, the indirect route will be dominant. We conducted the amination of α-hydroxyl isobutyric acid—a tertiary alcohol without an α-H—under various conditions (SI Appendix, Table S1). Amino acid formation was not observed in any test, suggesting that the α-H is crucial, which rules out the possibility of direct amination, i.e., pathway II. The indirect pathway (I) involves dehydrogenation and hydrogenation steps, and therefore, the concentration of hydrogen gas should influence the reaction. Indeed, a direct relationship between the hydrogen pressure and the alanine yield was observed in the pressure range tested (0–1 MPa) (SI Appendix, Table S2). Pathway I is reminiscent of the biosynthesis of various amino acids from glucose where pyruvate is often a key intermediate (1).
Fig. 3.
(A) Two possible reaction pathways for amination of lactic acid to alanine. (B) Dehydrogenation of isopropanol catalyzed by Pd/CNT and (C) Ru/CNT under a H2 atmosphere in a fixed-bed flow reactor. Green circles indicate isopropanol conversion, black triangles indicate acetone yield, and blue circles indicate isopropylamine yield. Reaction conditions: 50 mg catalyst, 2 μL/min isopropanol, 50 mL/min total flow rate, 473 K, 8 mL/min NH3 flow rate.
Following the indirect amination pathway (I), noble metals, in particular Pd and Pt, should show high efficiency because of their well-known ability in dehydrogenation/hydrogenation (40). However, our experiments show that only Ru-based catalysts are effective (Fig. 2A). We hypothesize that there are three possible origins for the unique performance of Ru: (i) the in situ formed α-keto acid could only be effectively converted to the amino acid by the Ru catalyst (steps 2 and 3 in pathway I); (ii) alanine decomposes over other metal catalysts under the reaction conditions; or (iii) the ability of other metal catalysts to perform the dehydrogenation step is suppressed in the presence of other reagents (e.g., NH3), whereas it is maintained or even enhanced by the Ru/CNT catalyst (step 1 in pathway I).
We first tested the reductive amination of pyruvic acid (41), a key dehydrogenation intermediate of lactic acid (SI Appendix, Table S3). All five noble metal catalysts (i.e., Ru, Pd, Pt, Rh, and Ir) exhibit similar activity and selectivity, showing that the transformation of dehydrogenated intermediates into amino acids can be achieved by all of the catalysts. We next compared the stability of alanine in the presence of the Pd/CNT and Ru/CNT (SI Appendix, Table S3). In a blank experiment, i.e., at 493 K for 2 h without catalyst, 17% of the alanine decomposed. The number only slightly increased to 20% with Pd/CNT, suggesting that the catalysts do not induce significant product decomposition. These experiments ruled out the hypotheses i and ii.
Consequently, isopropanol dehydrogenation on different catalysts was compared in a fixed-bed flow reactor. H2 was supplied in the reaction, mimicking the conditions used in the lactic acid amination, with NH3 gas switching on and off to evaluate its influence on dehydrogenation. In the absence of NH3, both catalysts were active: Ru/CNT yielded ca. 25% acetone, whereas Pd/CNT yielded ca. 20%. Surprisingly, turning on NH3 led to a significant decrease (∼25%) of isopropanol conversion with Pd/CNT (Fig. 3B) but a remarkable increase (∼30%) with Ru/CNT (Fig. 3C). Isopropyl amine and acetone were the two major products. The combined yields of the two with Ru/CNT were two times higher than with Pd/CNT, although the two catalysts exhibited comparable activities in the absence of NH3. Stopping the NH3 supply led to the recovery of both catalysts to their original activity and selectivity. These observations demonstrate a strong and reversible influence of NH3 on the dehydrogenation ability of Ru and Pd NPs; the former was enhanced, and the latter was suppressed. This effect has been further verified over commercial Ru and Pt catalysts (SI Appendix, Fig. S5), substantiating the unique activity enhancement of Ru catalysts by NH3. Note that the NH3 concentration in the batch reactor is much higher than in the flow reactor, and thus, the promotional effect of NH3 on Ru and the inhibition effects on other metals may be even more pronounced.
The activity of some Ru complexes for hydrogenation is remarkably enhanced with the addition of one equivalent of diamine because the ligand and the Ru metal work cooperatively to activate substrates (42). To test whether this promotional effect exists on the surface of Ru NPs, the hydrogenation of acetophenone was conducted with and without an ethylenediamine/KOH couple (SI Appendix, Table S4). In the presence of the two, acetophenone conversion was enhanced from 27 to 69% over the Ru/CNT catalyst. In contrast, no promotional effect on Pt/CNT and an inhibition effect on Pd/CNT were observed. As such, NH3 plausibly coordinates to the Ru surface to activate Ru by modifying its d-band center. NH3 also works synergistically with Ru to activate alcohol substrates by deprotonation of the OH group, in a similar manner to Ru homogeneous complexes (42).
The kinetic profile of the reaction with the Ru/CNT catalyst shows that the initial selectivity of alanine was ca. 70% (at 0.5 h) and only slightly decreases afterward (SI Appendix, Fig. S6). No pyruvic acid intermediate was detected, in agreement with the observation that the amination of the keto acid readily occurs. The dehydrogenation step is rate-determining and is favored at high temperatures; hence, a minimum temperature of 433 K is required (SI Appendix, Fig. S7).
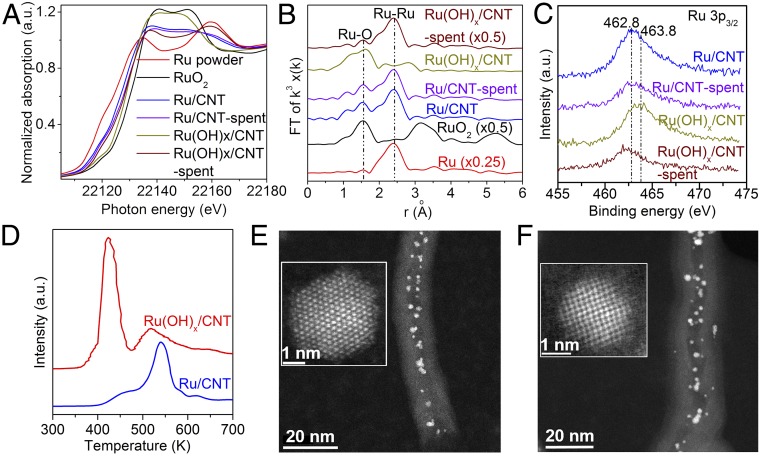
An Ru(OH)x/CNT catalyst with Ru in high-oxidation states was prepared for comparison. The Ru(OH)x/CNT catalyst resulted in slightly lower activity and selectivity to alanine than Ru/CNT catalyst (SI Appendix, Fig. S8). X-ray absorption near edge structure (XANES; Fig. 4A), extended X-ray absorption fine structure (EXAFS; Fig. 4B), X-ray photoelectron spectroscopy (XPS; Fig. 4C), and H2–temperature programmed reduction (H2-TPR; Fig. 4D) analysis suggested that Ru species on both fresh and spent Ru/CNT catalyst are mainly metallic with a small fraction of positive charges, whereas in situ reduction of Ru3+ into metallic Ru occurred on Ru(OH)x/CNT catalyst, providing strong evidence that Ru(0) is the key catalytic active species. Scanning transmission electron microscopy (STEM) micrographs showed uniformly dispersed Ru NPs on CNT (Fig. 4 E and F), which remained unchanged after reaction. However, Ru(OH)x/CNT did not survive under reaction conditions, and amorphously dispersed Ru species became Ru NPs (SI Appendix, Fig. S4). The in situ formed Ru NPs on Ru(OH)x/CNT exhibited a larger nanoparticle size than Ru/CNT, which is likely the reason for the lower catalytic activity and lower white line intensity in the XANES spectra.
Fig. 4.
(A) Ru K-edge XANES spectra of various catalysts. (B) Fourier transformed Ru K-edge EXAFS spectra for fresh and spent Ru/CNT and Ru(OH)x/CNT catalysts. (C) XPS spectra of Ru 3p for fresh and spent Ru/CNT, Ru(OH)x/CNT catalysts. (D) H2-TPR profile of Ru/CNT and Ru(OH)x/CNT. STEM images of (E) fresh Ru/CNT and (F) spent Ru/CNT.
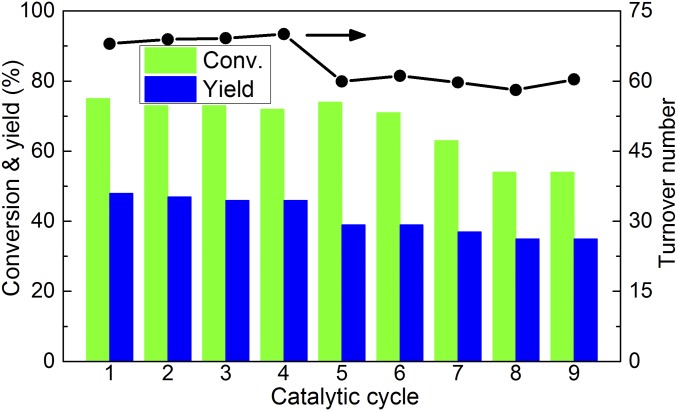
The stability of Ru/CNT was further tested with recycling experiments (Fig. 5). The performance of the Ru/CNT slightly decreased during repeated cycles: the alanine yield dropped from 48% in the first cycle to 35% in the ninth cycle. The activity loss was partially due to the loss of catalyst during repeated reactions (ca. 1 mg catalyst loss each round). After nine cycles, the accumulated turnover numbers reached 575 per surface Ru atom, considering a Ru dispersion of 24.3% based on H2 chemisorption experiments (SI Appendix, Fig. S3 and Table S5). Hot filtration experiments were conducted to identify the nature of the catalytically active species and the level of leached Ru in solution. The hot filtrate was inactive, suggesting the heterogeneous Ru NPs as the catalytically active species (SI Appendix, Fig. S6). Nevertheless, inductively coupled plasma mass spectrometry detected the existence of a small amount of soluble Ru species in the solution, corresponding to 0.37% of the total Ru on Ru/CNT.
Fig. 5.
Ru/CNT recycling test for catalytic conversion of lactic acid to alanine. Reaction conditions: 0.5 mmol lactic acid, 50 mg Ru/CNT (Ru loading 3 wt %), 2.5 mL NH3H2O (25 wt %), 1 MPa H2, 493 K, 2 h. Turnover number of each catalytic cycle is calculated based on Ru dispersion of fresh Ru/CNT catalyst and the total Ru in the system.
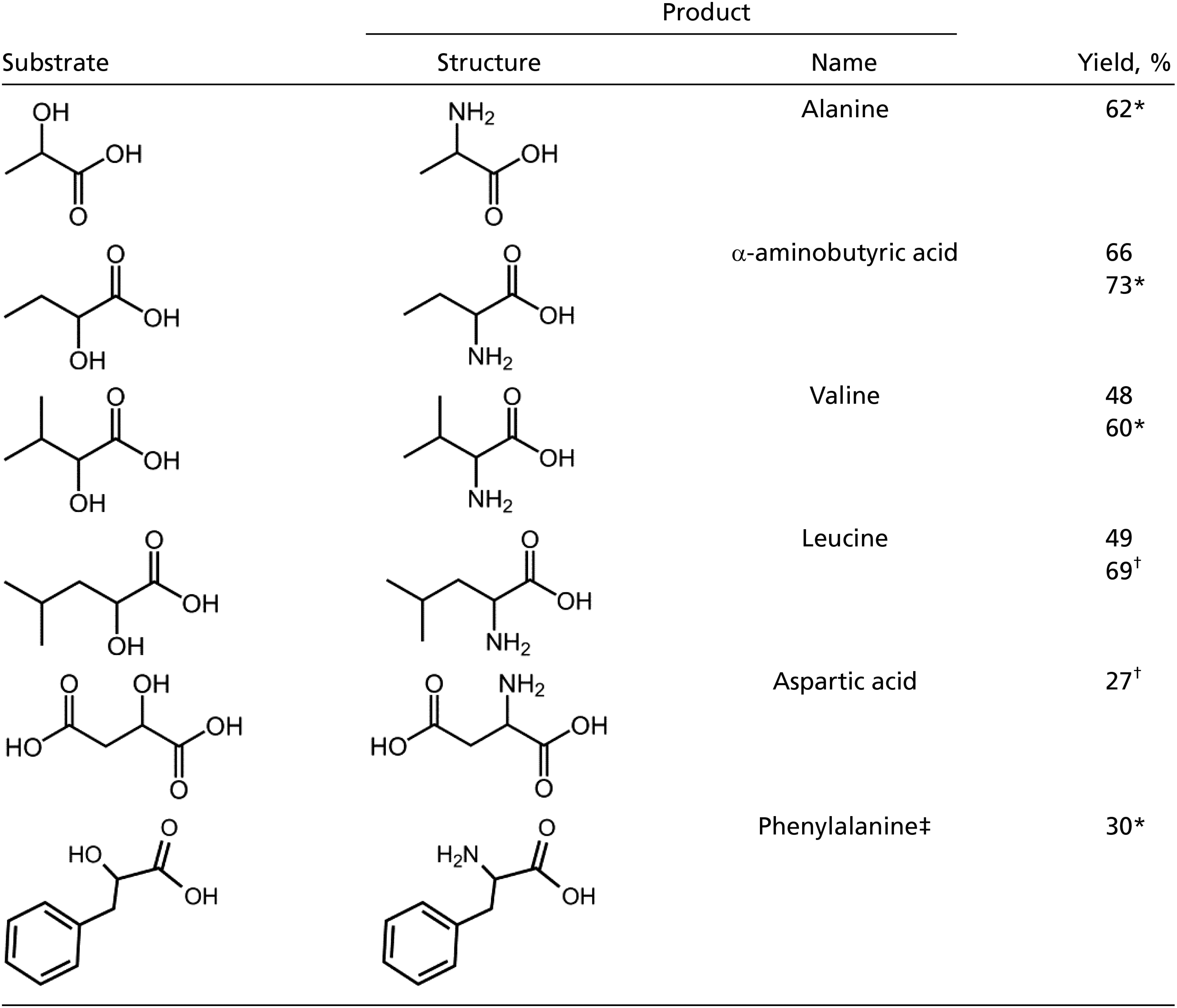
To further enhance the dehydrogenation capability of Ru, the Ru/CNT catalyst was modified by introducing a second metal component, and the reaction parameters were evaluated for lactic acid amination (SI Appendix, Tables S6–S8). Ni-doped Ru/CNT affords alanine in a yield of 62% under optimized reaction conditions (Table 1). We further attempted to produce alanine directly from glucose via a two-step process. Lactic acid was obtained in 75% yield from glucose at room temperature using Ba(OH)2 as the catalyst (26). The resulting solution was acidified, freeze-dried, and directly used in the amination step. An overall alanine yield of 43% was obtained (SI Appendix, Table S9), which is comparable with the well-established microbial cultivation process (38). Notably, the theoretical alanine yield from glucose in the chemical process is 100%, so that the yield can be further increased through catalyst and process optimization. In contrast, microbial cultivation process cannot reach 100% yield, because a substantial amount of glucose is consumed for cell growth. Moreover, production of certain amino acids, such as tyrosine, via microbial cultivation process is inefficient (43). In contrast, one can conceptually envisage quantitative transformation of p-coumarate into tyrosine from grass lignin in several chemical steps. As such, our route represents a competing chemical way for amino acid production with potentially improved yields and much higher space–time productivity.
Table 1.
Catalytic transformation of different biomass-derived α-hydroxyl acids to corresponding amino acids
 |
Reaction conditions: 0.5 mmol substrate, 50 mg Ru/CNT (Ru loading 3 wt %), 2.5 mL NH3H2O (25 wt %), 1 MPa H2, 493 K, 2 h.
Ru/CNT was modified with 10 wt % Ni.
KOH or NaOH (1 mmol) was added in the solution.
Ammonia was removed before derivatization.
Various amino acids were prepared from biomass-derived α-hydroxyl acids in aqueous ammonia (Table 1 and SI Appendix, Table S10). For example, α-aminobutyric acid, valine, and leucine were obtained from α-hydroxyl butyric acid, α-hydroxyl-3-methylbutyric acid, and α-hydroxyisocaproic acid, respectively. By modification of the Ru/CNT catalyst with 10 wt % nickel or simply by adding a small amount of base (e.g., NaOH and KOH) to promote dehydrogenation, the formation of amino acids was markedly enhanced. The yields of α-aminobutyric acid, valine, and leucine reached 73, 60, and 69%, respectively. The synthesis of aspartic acid was also achieved by amination of 2-hydroxysuccinic acid. The yield of 27% is lower than the other substrates tested, possibly due to the steric hindrance of the terminal acid group. In addition to aliphatic acids, the production of phenylalanine was achieved (30% yield) from 3-phenyllactic acid that is potentially derived from lignin. β-hydroxyl acids, such as 3-hydroxypropionic acid, were recently produced from sugar degradation (44), which afforded the starting materials for production of β-amino acids. We tested the transformation of β-hydroxybutyric acid and 3-hydroxypropionic acid in the amination system. Unlike α-hydroxyl acids, both of them afforded low yields toward corresponding amino acids (SI Appendix, Table S11), suggesting the current catalytic system is limited to producing α-amino acids.
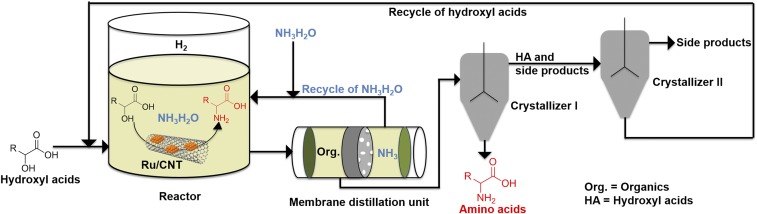
Prompted by the promising catalytic data, we conceived an integrated process comprising reaction, product purification, and reagent recycling (Fig. 6). Purification of the product is known to be the main cost driver in the amino acid production process (45), which also generates a significant amount of waste salts. Here we propose to utilize membrane distillation (MD) before crystallization to concentrate the amino acids and to recycle the aqueous ammonia solution. An MD-based system was constructed to experimentally demonstrate an initial proof of this concept, where the alanine feed solution was heated to 40 °C–46 °C at one side of the membrane, and the vaporized ammonia and water were collected at the other side by a cold stream of water at 12 °C. Results showed that alanine was maintained in the feed to near 100% retention, and the alanine concentration was increased from 0.020 g/mL to 0.077 g/mL. Compared with a conventional distillation process, the current approach is more energy efficient by consuming around two to three times less energy (SI Appendix, p. 13). In addition, the aqueous ammonia in the permeate can be recycled and reused. After membrane distillation, two crystallizers will be used to isolate amino acids and hydroxyl acids in the form of their ammonium salts (46).
Fig. 6.
A conceptual process diagram consists of a reactor, a membrane distillation unit, and two crystallizers.
Summary
In this work, we developed an efficient heterogeneous catalytic system for the synthesis of various amino acids from biomass-derived α-hydroxyl acids, in which dehydrogenation is rate-determining. Ru exhibits a unique enhancement effect of the dehydrogenation activity in the presence of NH3, resulting in superior performance for the reaction. The direct conversion of glucose into alanine is achieved following a simple, two-step chemical protocol. Effective concentration and separation of alanine from ammonia solution was demonstrated by a membrane distillation process. Our work demonstrates the feasibility of chemical transformation of lignocellulosic biomass components into amino acids and opens the way to the production of high-value proteins from agricultural wastes via chemical routes in the future.
Materials and Methods
Catalysts Synthesis.
The CNT-supported Ru nanoparticles were prepared by wet impregnation method. Typically, the CNT was added into the aqueous solution of RuCl3 and then subjected to stirring for 1 h at room temperature. After aging for 2 h, the water was evaporated at 373 K, and the solid product was reduced in H2/N2 (5%) gas at 673 K for 1 h. The CNT supported other metals (e.g., Pd, Pt, and Rh) and Ru loaded on different supports (e.g., Al2O3, SiO2, CeO2, ZrO2, and MgO) were prepared following a similar procedure. Ru(OH)x/CNT was obtained by a precipitation method, in which a diluted solution of NaOH (0.05 M) was slowly added into a mixture of solution of RuCl3 and CNT, followed by washing and drying. For all of the catalysts, the metal loadings were ∼3 wt %.
Catalytic Activity Evaluation.
The catalytic transformation of lactic acid and other biomass-derived acids was performed in an autoclave. For example, for the conversion of lactic acid, the Ru/CNT catalyst and lactic acid were added to the reactor that had been precharged with aqueous ammonia. After the introduction of H2 at a pressure of 1 MPa, the reactor was placed in a metal jacket wafer on an electronic hotplate (typically 493 K). After a fixed time, the reaction was quickly terminated by cooling the reactor to room temperature in cold water. The catalytic transformation of glucose to lactic acid was performed in a round-bottom flask with Schlenk line. Degassed water was added to the reactor that had been precharged with glucose and Ba(OH)2 under N2 atmosphere. The flask was put in an oil bath on an electronic stirrer. After a fixed time, the liquid product was neutralized with sulfuric acid and filtered to remove Ba2+. The filtrate was added equal mole of NaOH to prevent organic acids lose during freeze-dry. After freeze-drying, the products were acidified with HCl and used as the substrates in the amination step. The liquid products were analyzed by HPLC (Shimazu LC-20A) equipped with both RI and UV detectors. Glucose and hydroxyl acids were quantified on an Agilent Hi-Plex H column (7.7 × 300 mm, 8 µm) using a dilute H2SO4 aqueous solution as the mobile phase, whereas amino acids were analyzed on a Poroshell 120 EC-C8 column (4.6 × 100 mm) by using a precolumn derivatization method, where ortho-phthalaldehyde was used as the derivatization reagent. The dehydrogenation of isopropanol (IPA) over Ru and Pd catalysts was performed in a fixed bed flow reactor equipped with an online gas chromatography (GC, Agilent 7890A). A flame ionization detector (FID) and an Agilent Cyclodex-B column (30 m × 250 µm × 0.25 µm) were used to detect and analyze IPA and gas products.
Supplementary Material
Acknowledgments
We thank Ms. Kangjia Lu for providing the membrane distillation devices. This work was supported by the National University of Singapore Young Investigator award and the Ministry of Education, Singapore Tier-2 grant, respectively (R-279-000-462-112 and R-279-000-464-133), National Natural Science Foundation of China (Grants 91545203, 21690082, and 21473141), and the Fundamental Research Funds for the Central Universities (Grant 20720160029). SPring-8 is acknowledged for providing EXAFS and XANES analysis (Proposal 2017A1256). E.M.K., and G.T.B. thank the US Department of Energy (DOE) Energy Efficiency and Renewable Energy (EERE) Bioenergy Technologies Office (BETO) for funding under Contract DE-AC36-08GO28308 with National Renewable Energy Laboratory. The US Government retains and the publisher, by accepting the article for publication, acknowledges that the US Government retains a nonexclusive, paid up, irrevocable, worldwide license to publish or reproduce the published form of this work, or allow others to do so, for US Government purposes.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1800272115/-/DCSupplemental.
References
- 1.Tonouchi N, Ito H. Present global situation of amino acids in industry. In: Yokota A, Ikeda M, editors. Amino Acid Fermentation. Springer; Tokyo: 2017. pp. 3–14. [DOI] [PubMed] [Google Scholar]
- 2.Ogo S, Uehara K, Abura T, Fukuzumi S. pH-dependent chemoselective synthesis of α-amino acids. Reductive amination of α-keto acids with ammonia catalyzed by acid-stable iridium hydride complexes in water. J Am Chem Soc. 2004;126:3020–3021. doi: 10.1021/ja031633r. [DOI] [PubMed] [Google Scholar]
- 3.Zuend SJ, Coughlin MP, Lalonde MP, Jacobsen EN. Scaleable catalytic asymmetric Strecker syntheses of unnatural α-amino acids. Nature. 2009;461:968–970. doi: 10.1038/nature08484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang M, Imm S, Bähn S, Neumann H, Beller M. Synthesis of α-amino acid amides: Ruthenium-catalyzed amination of α-hydroxy amides. Angew Chem Int Ed Engl. 2011;50:11197–11201. doi: 10.1002/anie.201104309. [DOI] [PubMed] [Google Scholar]
- 5.Yan H, Suk Oh J, Lee J-W, Eui Song C. Scalable organocatalytic asymmetric Strecker reactions catalysed by a chiral cyanide generator. Nat Commun. 2012;3:1212. doi: 10.1038/ncomms2216. [DOI] [PubMed] [Google Scholar]
- 6.Shibasaki M, Kanai M, Mita T. The catalytic asymmetric strecker reaction. In: Overman LE, et al., editors. Organic Reactions. John Wiley; Hoboken, NJ: 2008. [Google Scholar]
- 7.Hu P, Ben-David Y, Milstein D. General synthesis of amino acid salts from amino alcohols and basic water liberating H2. J Am Chem Soc. 2016;138:6143–6146. doi: 10.1021/jacs.6b03488. [DOI] [PubMed] [Google Scholar]
- 8.Xing R, Qi W, Huber GW. Production of furfural and carboxylic acids from waste aqueous hemicellulose solutions from the pulp and paper and cellulosic ethanol industries. Energy Environ Sci. 2011;4:2193–2205. [Google Scholar]
- 9.de Clippel F, et al. Fast and selective sugar conversion to alkyl lactate and lactic acid with bifunctional carbon-silica catalysts. J Am Chem Soc. 2012;134:10089–10101. doi: 10.1021/ja301678w. [DOI] [PubMed] [Google Scholar]
- 10.Weingarten R, et al. Conversion of glucose into levulinic acid with solid metal(IV) phosphate catalysts. J Catal. 2013;304:123–134. [Google Scholar]
- 11.Dusselier M, Van Wouwe P, Dewaele A, Jacobs PA, Sels BF. Green chemistry. Shape-selective zeolite catalysis for bioplastics production. Science. 2015;349:78–80. doi: 10.1126/science.aaa7169. [DOI] [PubMed] [Google Scholar]
- 12.Wang Y, Lewis JD, Román-Leshkov Y. Synthesis of itaconic acid ester analogues via self-aldol condensation of ethyl pyruvate catalyzed by hafnium BEA zeolites. ACS Catal. 2016;6:2739–2744. [Google Scholar]
- 13.Anderson EM, et al. Reductive catalytic fractionation of corn stover lignin. ACS Sustain Chem Eng. 2016;4:6940–6950. [Google Scholar]
- 14.Zhang J, et al. Comparison of fast pyrolysis behavior of cornstover lignins isolated by different methods. ACS Sustain Chem Eng. 2017;5:5657–5661. [Google Scholar]
- 15.Choi YS, et al. Pyrolysis reaction networks for lignin model compounds: Unraveling thermal deconstruction of β-O-4 and α-O-4 compounds. Green Chem. 2016;18:1762–1773. [Google Scholar]
- 16.Clark JH. Chemistry goes green. Nat Chem. 2009;1:12–13. doi: 10.1038/nchem.146. [DOI] [PubMed] [Google Scholar]
- 17.Besson M, Gallezot P, Pinel C. Conversion of biomass into chemicals over metal catalysts. Chem Rev. 2014;114:1827–1870. doi: 10.1021/cr4002269. [DOI] [PubMed] [Google Scholar]
- 18.Li C, Zhao X, Wang A, Huber GW, Zhang T. Catalytic transformation of lignin for the production of chemicals and fuels. Chem Rev. 2015;115:11559–11624. doi: 10.1021/acs.chemrev.5b00155. [DOI] [PubMed] [Google Scholar]
- 19.Luque R, Triantafyllidis K. Valorization of lignocellulosic biomass. ChemCatChem. 2016;8:1422–1423. [Google Scholar]
- 20.Brun N, Hesemann P, Esposito D. Expanding the biomass derived chemical space. Chem Sci. 2017;8:4724–4738. doi: 10.1039/c7sc00936d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Key RE, Bozell JJ. Progress toward lignin valorization via selective catalytic technologies and the tailoring of biosynthetic pathways. ACS Sustain Chem Eng. 2016;4:5123–5135. [Google Scholar]
- 22.Holm MS, Saravanamurugan S, Taarning E. Conversion of sugars to lactic acid derivatives using heterogeneous zeotype catalysts. Science. 2010;328:602–605. doi: 10.1126/science.1183990. [DOI] [PubMed] [Google Scholar]
- 23.Wang Y, et al. Chemical synthesis of lactic acid from cellulose catalysed by lead(II) ions in water. Nat Commun. 2013;4:2141. doi: 10.1038/ncomms3141. [DOI] [PubMed] [Google Scholar]
- 24.Wang F-F, Liu C-L, Dong W-S. Highly efficient production of lactic acid from cellulose using lanthanide triflate catalysts. Green Chem. 2013;15:2091–2095. [Google Scholar]
- 25.Tang Z, et al. Transformation of cellulose and its derived carbohydrates into formic and lactic acids catalyzed by vanadyl cations. ChemSusChem. 2014;7:1557–1567. doi: 10.1002/cssc.201400150. [DOI] [PubMed] [Google Scholar]
- 26.Li L, Shen F, Smith RL, Qi X. Quantitative chemocatalytic production of lactic acid from glucose under anaerobic conditions at room temperature. Green Chem. 2017;19:76–81. [Google Scholar]
- 27.Zhang J, Liu X, Sun M, Ma X, Han Y. Direct conversion of cellulose to glycolic acid with a phosphomolybdic acid catalyst in a water medium. ACS Catal. 2012;2:1698–1702. [Google Scholar]
- 28.Kawaguchi H, et al. Simultaneous saccharification and fermentation of kraft pulp by recombinant Escherichia coli for phenyllactic acid production. Biochem Eng J. 2014;88:188–194. [Google Scholar]
- 29.Dusselier M, et al. Mechanistic insight into the conversion of tetrose sugars to novel α-hydroxy acid platform molecules. ChemCatChem. 2013;5:569–575. [Google Scholar]
- 30.Ralph J. Hydroxycinnamates in lignification. Phytochem Rev. 2009;9:65–83. [Google Scholar]
- 31.Gunanathan C, Milstein D. Selective synthesis of primary amines directly from alcohols and ammonia. Angew Chem Int Ed Engl. 2008;47:8661–8664. doi: 10.1002/anie.200803229. [DOI] [PubMed] [Google Scholar]
- 32.Pingen D, Müller C, Vogt D. Direct amination of secondary alcohols using ammonia. Angew Chem Int Ed Engl. 2010;49:8130–8133. doi: 10.1002/anie.201002583. [DOI] [PubMed] [Google Scholar]
- 33.Bähn S, et al. The catalytic amination of alcohols. ChemCatChem. 2011;3:1853–1864. [Google Scholar]
- 34.Ruiz D, et al. Direct amination of dodecanol with NH3 over heterogeneous catalysts. Catalyst screening and kinetic modelling. Chem Eng J. 2017;307:739–749. [Google Scholar]
- 35.Jagadeesh RV, et al. MOF-derived cobalt nanoparticles catalyze a general synthesis of amines. Science. 2017;358:326–332. doi: 10.1126/science.aan6245. [DOI] [PubMed] [Google Scholar]
- 36.Tomer A, et al. Facile preparation of Ni/Al2O3 catalytic formulations with the aid of cyclodextrin complexes: Towards highly active and robust catalysts for the direct amination of alcohols. J Catal. 2017;356:111–124. [Google Scholar]
- 37.Shimizu K-i, Imaiida N, Kon K, Hakim Siddiki SMA, Satsuma A. Heterogeneous Ni catalysts for N-alkylation of amines with alcohols. ACS Catal. 2013;3:998–1005. [Google Scholar]
- 38.Wada M, Narita K, Yokota A. Alanine production in an H+-ATPase- and lactate dehydrogenase-defective mutant of Escherichia coli expressing alanine dehydrogenase. Appl Microbiol Biotechnol. 2007;76:819–825. doi: 10.1007/s00253-007-1065-y. [DOI] [PubMed] [Google Scholar]
- 39.Watson AJ, Williams JM. Chemistry. The give and take of alcohol activation. Science. 2010;329:635–636. doi: 10.1126/science.1191843. [DOI] [PubMed] [Google Scholar]
- 40. Anonymous (2008) Handbook of Heterogeneous Catalysis, eds Ertl G, Knözinger H, Schüth F, Weitkamp J (Wiley-VCH, Weinheim, Germany), 2nd Ed.
- 41.Liang G, et al. Production of primary amines by reductive amination of biomass-derived aldehydes/ketones. Angew Chem Int Ed Engl. 2017;56:3050–3054. doi: 10.1002/anie.201610964. [DOI] [PubMed] [Google Scholar]
- 42.Noyori R. Asymmetric catalysis: Science and opportunities (Nobel lecture) Angew Chem Int Ed Engl. 2002;41:2008–2022. [PubMed] [Google Scholar]
- 43.Santos CN, Xiao W, Stephanopoulos G. Rational, combinatorial, and genomic approaches for engineering L-tyrosine production in Escherichia coli. Proc Natl Acad Sci USA. 2012;109:13538–13543. doi: 10.1073/pnas.1206346109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Karp EM, et al. Quantification of acidic compounds in complex biomass-derived streams. Green Chem. 2016;18:4750–4760. [Google Scholar]
- 45.D’Este M, Alvarado-Morales M, Angelidaki I. Amino acids production focusing on fermentation technologies—A review. Biotechnol Adv. 2017;36:14–25. doi: 10.1016/j.biotechadv.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 46.Kirwan DJ, Orella CJ. 11—Crystallization in the pharmaceutical and bioprocessing industries. In: Myerson AS, editor. Handbook of Industrial Crystallization. Butterworth-Heinemann; Woburn, MA: 2002. pp. 249–266. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.