Significance
The biosynthesis and mechanism of action of nitrogenase, an enzyme that converts dinitrogen to ammonia under ambient conditions, are problems of prominent significance in metallobiochemistry. Because the active centers of the enzyme are metal–ligand clusters, it is feasible that they are attainable by synthesis and as such are primary goals in the field of biomimetic inorganic chemistry. Here we present a ligand metathesis strategy utilizing the periodic near-identity of molybdenum and tungsten when incorporated into analogous compounds. The approach provides a pathway for constructing heterometal heteroleptic Fe–S clusters of presumed relevance to the active site. Based on cubane-type stereochemistry, clusters have been prepared allowing alterations in structure and ligand binding, and inclusion of a light core atom.
Keywords: ligand metathesis, tungsten–iron–sulfur clusters, cubane-type clusters, heteroleptic clusters, FeMo cofactor
Abstract
Molybdenum-dependent nitrogenases catalyze the transformation of dinitrogen into ammonia under ambient conditions. The active site (FeMo cofactor) is the structurally and electronically complex weak-field metal cluster [MoFe7S9C] built of Fe4S3 and MoFe3S3C portions connected by three sulfur bridges and containing an interstitial carbon atom centered in an Fe6 trigonal prism. Chemical synthesis of this cluster is a major challenge in biomimetic inorganic chemistry. One synthetic approach of core ligand metathesis has been developed based on the design and synthesis of unprecedented incomplete ([(Tp*)WFe2S3Q3]−) and complete ([(Tp*)WFe3S3Q4]2−) cubane-type clusters containing bridging halide (Q = halide). These clusters are achieved by template-assisted assembly in the presence of sodium benzophenone ketyl reductant; products are controlled by reaction stoichiometry. Incomplete cubane clusters are subject to a variety of metathesis reactions resulting in substitution of a μ2-bridging ligand with other bridges such as N3−, MeO−, and EtS−. Reactions of complete cubanes with Me3SiN3 and S8 undergo a redox metathesis process and lead to core ligand displacement and formation of [(Tp*)WFe3S3(μ3-Q)Cl3]− (Q = Me3SiN2−, S2−). This work affords entry to a wide variety of heteroleptic clusters derivable from incomplete and complete cubanes; examples are provided. Among these is the cluster [(Tp*)WFe3S3(μ3-NSiMe3)Cl3]−, one of the very few instances of a synthetic Fe–S cluster containing a light atom (C, N, O) in the core, which constitutes a close mimic of the [MoFe3S3C] fragment in FeMo cofactor. Superposition of them and comparison of metric information disclose a clear structural relationship [Tp* = tris(3,5-dimethyl-1-pyrazolyl)hydroborate(1−)].
Molybdenum-dependent nitrogenases catalyze the conversion of dinitrogen to ammonia under ambient conditions and contain the weak-field iron–molybdenum cofactor (FeMo-co) cluster bearing the [MoFe7S9C] core at the catalytic site. The complex physicochemical properties of this cluster free in solution and bound in the FeMo protein component of the enzyme are slowly yielding to interpretation by a broad array of physical and theoretical methods. Additional significant information would include definition of the biosynthetic pathway of FeMo-co cluster formation and methods of its nonbiological chemical synthesis. We and others are engaged in the latter challenge, having demonstrated that many types of homometallic (Fe–S) and heterometallic (M–Fe–S) clusters are synthetically accessible (1–5).
The cofactor presents cubane-type MoFe3S3 and Fe4S3 subclusters bridged together by three µ2-S atoms (6, 7) and an interstitial μ6-C atom centered in a trigonal prismatic Fe6 arrangement (8–10). Such features are absent in all known synthetic homo- and heterometallic Fe–S species. Light elements (carbon, nitrogen, oxygen) are very rarely found in synthetic cores and rational synthetic strategies to include them are decidedly scarce. Bridging interactions within the cluster core are nearly always introduced by direct synthesis, which raises significant challenges in accessing FeMo-co analogs with heteroleptic cores. The invariable iron-bound S2−/RS− (R = alkyl or aryl) core ligands in most cases account for difficulties in incorporating light elements into the core. Therefore, it is highly desirable to develop new synthetic methodologies toward this end.
In seeking new cluster types potentially suitable for elaboration into cofactor-related species, we utilize previously unreported complete (3, 7) and incomplete (2, 4, 5) cubane-type clusters [(Tp*)WFe2,3S3Q3,4]z (Fig. 1). The tridentate ligand Tp* = tris(3,5-dimethyl-1-pyrazolyl)hydroborate(1−) blocks reaction at the tungsten site, Q is a variable bridging and/or terminal ligand, and z is the cluster charge. These clusters have been synthesized with a core ligand metathesis strategy based on halide-bridged cores constructed by template-assisted assembly. Here we utilize the general isostructural principle for isovalent M = Mo and W molecules (11) recently demonstrated at length with various M–Fe–S cluster types (12), and note that the molybdenum analog of 1 is unknown (13).
Fig. 1.
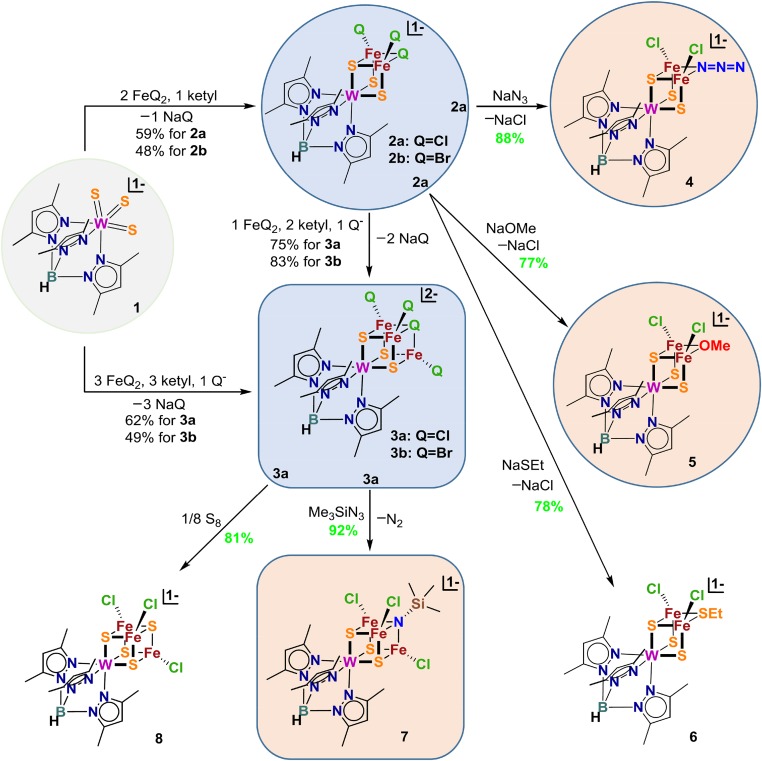
Depiction of the synthesis of clusters: formation of incomplete and complete cubanes 2ab and 3ab from the mononuclear precursor 1, incomplete cubanes 4–6 by ligand substitution of 2a, and the complete cubanes 7 and 8 by redox metathesis of 3a, as well as the conversion from 2ab to 3ab (ketyl = Na+(Ph2CO•)−). All clusters were isolated as Et4N+ salts (except for 2b as the [Fe(DMF)6]2+ salt). Compounds in blue circles and 6 are incomplete cubane series and those in squares and 8 are complete cubanes. Compounds highlighted in light blue are clusters with halide bridge and those in light orange are clusters incorporating light atoms (N/O).
Results and Discussion
Clusters 2–8 are schematically depicted together with reactions initiated from precursor 1 which functions as a pyramidal WS3 template (Fig. 1). When reduced below the WVI oxidation level under anaerobic conditions in N,N-dimethylformamide (DMF) solutions, the template-bound FeII, III forms clusters in incomplete or complete cubane modes, which are of considerable interest in the assembly of species relevant to the native cluster. Sulfur atoms derive entirely from 1. Precursors 2 and 3 are readily formed (48–62%) by controlling stoichiometries of reactions (Eqs. 1–3; Q = Cl, Br) with sodium benzophenone ketyl as reductant. Incomplete and complete cubane clusters 4–8 with heteroligated cores were obtained (77–92%) by ligand metathesis reactions based on clusters 2 and 3. Cluster structures 2–5 and 7 were determined by X-ray single-crystal diffraction. Crystallographic data and metric parameters are collected in Supporting Information. Structures are set out in Figs. 2–4, including selected data on ligand binding.
Fig. 2.
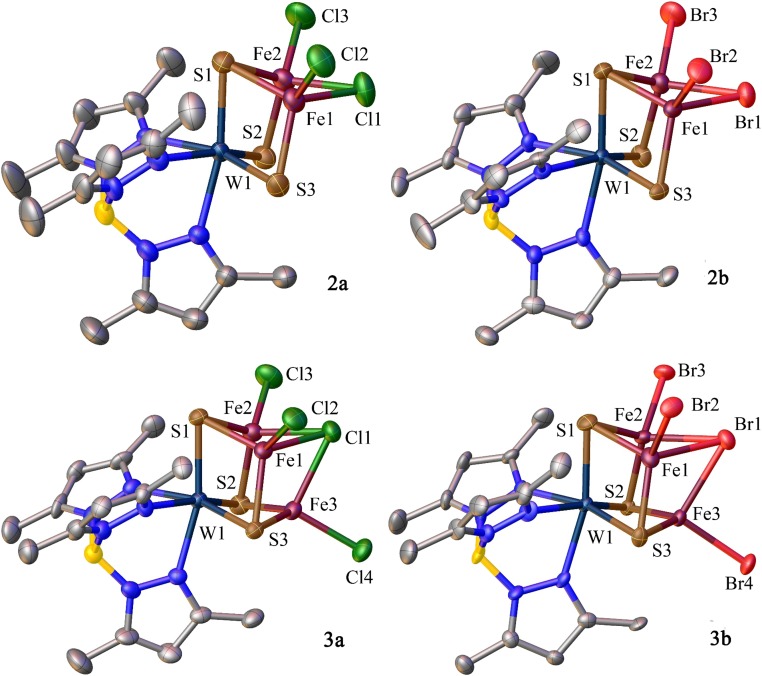
Structures of [(Tp*)WFe2S3(μ2-Q)Q2]− [Q = Cl (2a), Br (2b)] and [(Tp*)WFe3S3(μ3-Q)Q3]2− [Q = Cl (3a), Br (3b)]. In this and other figures atoms are shown at 50% thermal ellipsoids probability and hydrogen atoms are omitted for clarity. Selected bond metrics (mean, Å or deg): 2a, Fe-Cl1 2.365(3), Fe1-Cl1-Fe2 67.57(7); 2b, Fe-Br1 2.518(2), Fe1-Br1-Fe2 62.83(4); 3a, Fe-Cl1 2.495(3), Fe-Cl1-Fe 63.27(9); 3b, Fe-Br1 2.622(2), Fe-Br1-Fe 59.75(6).
Fig. 4.
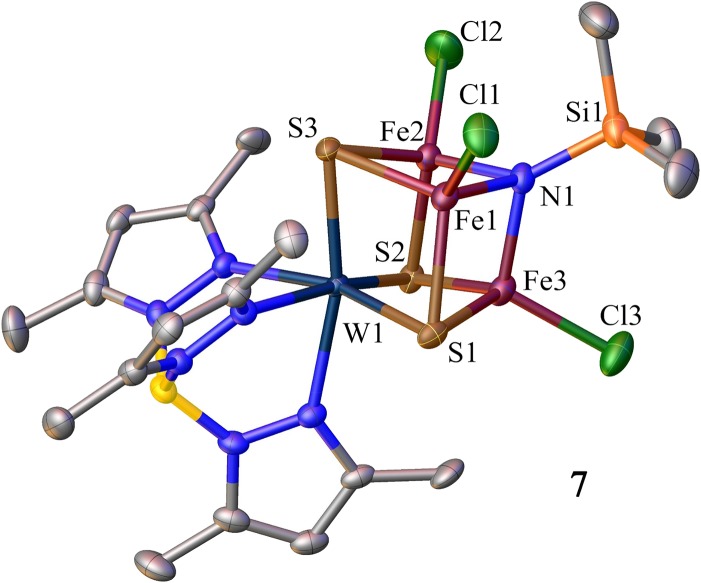
Structure of [(Tp*)WFe3S3(μ3-NSiMe3)Cl3]− (7). Selected metric features (mean, Å, deg): Fe-N1.936(3), Si-N 1.749(4), Fe-Cl 2.221(2), Fe-N-Si 129.1(2).
The strategy of template-assisted assembly utilized by Majumdar and Holm (14) has demonstrated the feasibility of incorporating selenium atoms into cubane-type M–Fe–S (M = Mo or W) cores, in which bridges between iron atoms are introduced independently in addition to the M-bound sulfide ligand. We have thus designed the syntheses eliminating the usage of an additional S/Se source in the assembly process and expecting halide ligand to coordinately bridge the iron atoms in the cubane-type clusters, with [(Tp*)WS3]− as template. Clusters 2a/2b and 3a/3b have been obtained as a result. For comparison, we have also examined the possibility of using [(Tp)MoS5]− as the template (See Scheme S1 in Supporting Information for structure), but in all cases examined, the free sulfide generated during the assembly process occupies the bridging site to form the [MoFe3S4]z core. Its presence in the reaction system impedes halide coordination and deactivates the template.
Incomplete cubanes 2a and 2b, of an unusual structural form, are readily recognized by vacant metal sites whereas the complete clusters 3a and 3b have the full cubane [WFe3S3Q] core (Q = Cl or Br) (Fig. 2). Isostructural clusters 2a and 2b are prepared by direct reaction 1 and contain one Fe–Q–Fe bridge, an unusual feature in Fe–S clusters. Complete cubanes 3a/3b were obtained by two methods, reactions 2 and 3, whose products have μ3-bridging and terminal halide ligands. The first of these is direct synthesis with a stoichiometry intended to produce a reduced core [WFe3S3(μ3-Q)]2+. In reaction 3, clusters 3a and 3b were obtained by conversion of preisolated clusters 2a and 2b generated in reaction 1, in 75% and 83% yield, respectively. Direct synthesis gave a better yield of complete cubane clusters compared with the total yield through the preisolation pathway.
| [1] |
| [2] |
| [3] |
Structural analysis of 2a reveals that the mean Fe–Cl (bridging) bond length of 2.365(3) Å is much longer than the average Fe–Cl (terminal) bond distances at 2.230(3) Å. And in 3a, the mean Fe–Cl (bridging) contact of 2.495(3) Å is significantly longer compared with average Fe–Cl (terminal) distances at 2.284(4) Å. The longer Fe–Cl (bridging) contacts indicate that the bridging Cl sites are likely to be more susceptible to substitution than terminal sites. Therefore, a rational ligand metathesis strategy is envisioned utilizing these halide-bridged clusters as precursors for heteroligated cores.
| [4] |
| [5] |
| [6] |
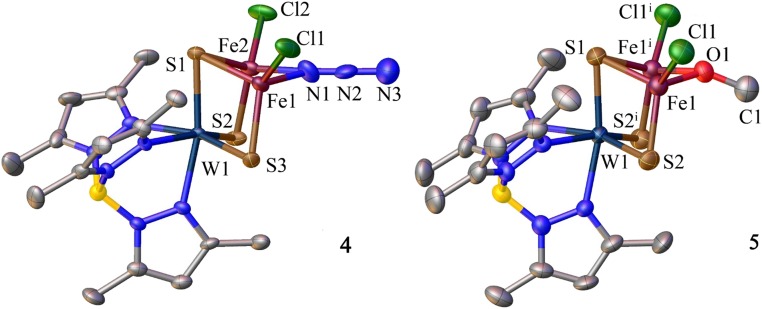
The metathesis strategy was first examined over the incomplete cubane-type cluster [(Tp*)WFe2S3(μ2-Cl)Cl2]−, resulting in displacement of the μ2-bridge from chloride (2a) to azide (4), MeO− (5), and EtS− (6) by suitable variation of ligands Q− in reaction 4 (Figs. 1 and 3). When the metathesis strategy is applied to the complete cubane cluster [(Tp*)WFe3S3(μ3-Cl)Cl3]2− (3a), these ligands failed in direct substitution reactions. Usage of oxidative reactants Me3SiN3 and S8 triggers redox metathesis reactions 5 and 6 to form [(Tp*)WFe3S3(μ3-Q)Cl3]− [Q = Me3SiN2− (7) and S2− (8); Figs. 1 and 4]. A favorable feature of these reactions is high yield. As one example, equimolar Me3SiN3 converts 3a to cluster 7 with the complete [WFe3S3(μ3-N)] cubane core and retention of terminal chloride in 92% yield. The metathesis strategy should be amenable to extension with a variety of ligand types, including formation of the heterobridges Fe-(μ2,3-Q)-Fe.
Fig. 3.
Structures of [(Tp*)WFe2S3(μ2-Q)Cl2]− [Q = N3 (4), OMe (5)]. Selected metric features (mean, Å, deg): 4, Fe-N1 2.045(8), N1-N2 1.079(9), N2-N3 1.22(1), Fe1-N1-Fe2 80.2(3), N1-N2-N3 178.8(8); 5, Fe1-O1 1.964(4), O1-C1 1.381(9), Fe1-O1-Fe1i 84.3(2). Symmetry code: i = +x, −y + 1/2, +z.
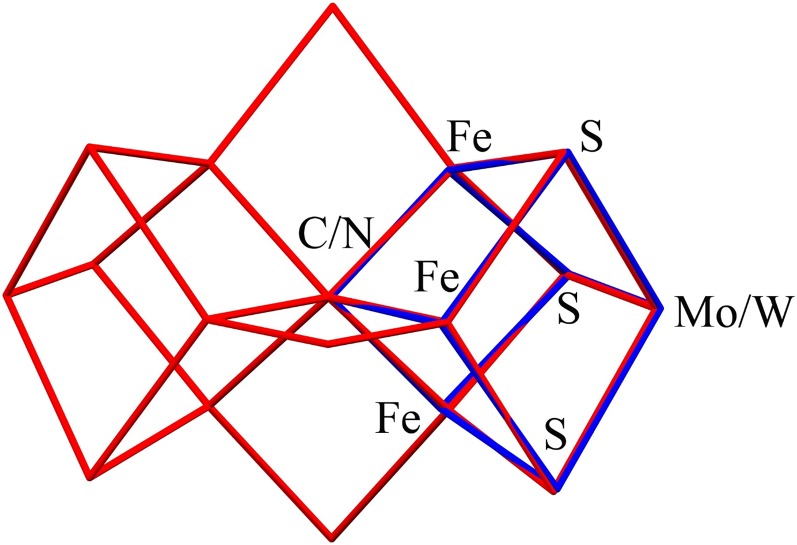
The [WFe3S3N] core in 7 demonstrates a highly close topological mimicking of the [MoFe3S3C] fragment of FeMo-co, as shown by least-squares structural superposition of them with very good agreement (Fig. 5). The mean Fe-N/C distances are 1.963(3)/2.00 Å and the root-mean-square deviation of fit in core atom positions is 0.0535. The fitting procedure for FeMo-co and another synthetic cubane give nearly the same result (4, 15).
Fig. 5.
X-ray structural superposition of the [WFe3S3N] core in 7 (blue lines) and the [MoFe3S3C] portion of FeMo-co (red lines). Central atom X = N or C. For further details on this type of structural comparison, see refs.4 and 15.
In assessing these reactions, it is observed that only a very limited number of light-atom bridging ligands C, N, O have been incorporated into Fe–S or M–Fe–S cubane-type structures (4). Bridging interactions nearly always are supported by μ2–4-S connectivities formed in direct synthesis. Other than clusters originating in this work, the heteroligand μ3-RN2− is known only in the cubane series [Fe4(NtBu)nS4-nCl4]z whose members are obtained from condensation reactions of FeIII amides (15). In very recent work, we have prepared additional heteroligand and heterometal clusters with nuclearities above those of individual cubanes. In view of this development and the proposed biosynthetic modular pathways of clusters leading to FeMo-co and the PN-type cluster of nitrogenase (16–18), chemical synthesis is a goal of increasing import in a full interpretation of this enzyme.
Materials and Methods
All reactions and manipulations were performed on standard Schlenk lines or in glove box under the atmosphere of dry dinitrogen. Physical measurements and characterizations were also carried out under the protection of dry dinitrogen. All reagents used were anhydrous either commercially available or purified by conventional methods. See Supporting Information for details.
Supplementary Material
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (21671104), Jiangsu Key Technology R&D Program (BE2016010), the program of Jiangsu Specially Appointed Professor, the Major Program of the Natural Science Research of Jiangsu Higher Education Institutions of China (17KJA430010 and 15KJA350002), the Priority Academic Program Development of Jiangsu Higher Educational Institutions, and the Foundation of Jiangsu Collaborative Innovation Center of Biomedical Functional Materials. Research at Harvard University was supported by NIH Grant 28856.
Footnotes
The authors declare no conflict of interest.
Data deposition: The atomic coordinates and structure factors have been deposited in the Cambridge Structural Database (CSD) of the Cambridge Crystallographic Data Centre (CCDC), https://www.ccdc.cam.ac.uk/structures/ [CSD reference nos. 1587148 (2a), 1587149 (2b), 1587150 (3a), 1587151 (3b), 1587152 (5), 1587153 (4), and 1587154 (7)].
See Commentary on page 5054.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1801025115/-/DCSupplemental.
References
- 1.Lee SC, Holm RH. The clusters of nitrogenase: Synthetic methodology in the construction of weak-field clusters. Chem Rev. 2004;104:1135–1158. doi: 10.1021/cr0206216. [DOI] [PubMed] [Google Scholar]
- 2.Lee SC, Lo W, Holm RH. Developments in the biomimetic chemistry of cubane-type and higher nuclearity iron-sulfur clusters. Chem Rev. 2014;114:3579–3600. doi: 10.1021/cr4004067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holm RH, Lo W. Structural conversions of synthetic and protein-bound iron-sulfur clusters. Chem Rev. 2016;116:13685–13713. doi: 10.1021/acs.chemrev.6b00276. [DOI] [PubMed] [Google Scholar]
- 4.Lee SC. Synthetic models of the nitrogenase clusters. In: Hille R, Schulzke C, Kirk ML, editors. Molybdenum and Tungsten Enzymes: Bioinorganic Chemistry. Royal Society of Chemistry; Cambridge: 2017. pp. 130–165. [Google Scholar]
- 5.Ohki Y. Synthetic analogues of the active sites of nitrogenase and [NiFe] hydrogenases. Bull Chem Soc Jpn. 2014;87:1–19. [Google Scholar]
- 6.Peters JW, et al. Redox-dependent structural changes in the nitrogenase P-cluster. Biochemistry. 1997;36:1181–1187. doi: 10.1021/bi9626665. [DOI] [PubMed] [Google Scholar]
- 7.Mayer SM, Lawson DM, Gormal CA, Roe SM, Smith BE. New insights into structure-function relationships in nitrogenase: A 1.6 A resolution X-ray crystallographic study of Klebsiella pneumoniae MoFe-protein. J Mol Biol. 1999;292:871–891. doi: 10.1006/jmbi.1999.3107. [DOI] [PubMed] [Google Scholar]
- 8.Spatzal T, et al. Evidence for interstitial carbon in nitrogenase FeMo cofactor. Science. 2011;334:940. doi: 10.1126/science.1214025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lancaster KM, et al. X-ray emission spectroscopy evidences a central carbon in the nitrogenase iron-molybdenum cofactor. Science. 2011;334:974–977. doi: 10.1126/science.1206445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu Y, Ribbe MW. Nitrogenases-A tale of carbon atom(s) Angew Chem Int Ed Engl. 2016;55:8216–8226. doi: 10.1002/anie.201600010. [DOI] [PubMed] [Google Scholar]
- 11.Holm RH, Solomon EI, Majumdar A, Tenderholt A. Comparative molecular chemistry of molybdenum and tungsten and its relation to hydroxylase and oxotransferase enzymes. Coord Chem Rev. 2011;255:993–1015. [Google Scholar]
- 12.Zheng B, Chen X-D, Zheng SL, Holm RH. Selenium as a structural surrogate of sulfur: Template-assisted assembly of five types of tungsten-iron-sulfur/selenium clusters and the structural fate of chalcogenide reactants. J Am Chem Soc. 2012;134:6479–6490. doi: 10.1021/ja3010539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seino H, et al. Preparation of mononuclear tungsten tris(sulfido) and molybdenum sulfido-tetrasulfido complexes with hydridotris(pyrazolyl)borate coligand and conversion of the former into sulfido-bridged bimetallic complex having Pt(μ-S)2WS core. Inorg Chem. 2001;40:1677–1682. doi: 10.1021/ic0008823. [DOI] [PubMed] [Google Scholar]
- 14.Majumdar A, Holm RH. Specific incorporation of chalcogenide bridge atoms in molybdenum/tungsten-iron-sulfur single cubane clusters. Inorg Chem. 2011;50:11242–11251. doi: 10.1021/ic2018117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen X-D, Zhang W, Duncan JS, Lee SC. Iron-amide-sulfide and iron-imide-sulfide clusters: Heteroligated core environments relevant to the nitrogenase FeMo cofactor. Inorg Chem. 2012;51:12891–12904. doi: 10.1021/ic301868m. [DOI] [PubMed] [Google Scholar]
- 16.Hu Y, Ribbe MW. Biosynthesis of metalloclusters. Annu Rev Biochem. 2016;85:455–483. doi: 10.1146/annurev-biochem-060614-034108. [DOI] [PubMed] [Google Scholar]
- 17.Sickerman NS, Ribbe MW, Hu Y. Nitrogenase cofactor assembly: An elemental inventory. Acc Chem Res. 2017;50:2834–2841. doi: 10.1021/acs.accounts.7b00417. [DOI] [PubMed] [Google Scholar]
- 18.Sickerman NS, Hu Y, Ribbe MW. Iron-sulfur cluster enzymes. Methods Enzymol Part A. 2017;595:261–302. doi: 10.1016/bs.mie.2017.07.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.