Significance
The efficacy of the influenza vaccine against the predominant influenza strain appears to be relatively low during this 2017–2018 season. Our analyses demonstrate the substantial effect of even low-efficacy vaccines in averting infections, hospitalizations, and particularly deaths. Our results also demonstrate that the health burden resulting from influenza is more sensitive to changes to vaccination coverage than to changes to vaccine efficacy. We further determined the uptake distribution of the 140 million doses available that would maximize the effectiveness of vaccination. Our results inform current public health policies and underscore the importance of influenza vaccination.
Keywords: mathematical model, age structured, vaccination, DALY, hospitalization
Abstract
The efficacy of influenza vaccines varies from one year to the next, with efficacy during the 2017–2018 season anticipated to be lower than usual. However, the impact of low-efficacy vaccines at the population level and their optimal age-specific distribution have yet to be ascertained. Applying an optimization algorithm to a mathematical model of influenza transmission and vaccination in the United States, we determined the optimal age-specific uptake of low-efficacy vaccine that would minimize incidence, hospitalization, mortality, and disability-adjusted life-years (DALYs), respectively. We found that even relatively low-efficacy influenza vaccines can be highly impactful, particularly when vaccine uptake is optimally distributed across age groups. As vaccine efficacy declines, the optimal distribution of vaccine uptake shifts toward the elderly to minimize mortality and DALYs. Health practitioner encouragement and concerted recruitment efforts are required to achieve optimal coverage among target age groups, thereby minimizing influenza morbidity and mortality for the population overall.
A century since the 1918 influenza pandemic killed an estimated 50–100 million people, influenza remains a global threat. Influenza causes 9.2–35.6 million infections, 140,000–710,000 hospitalizations, and 12,000–56,000 deaths every year in the United States alone (1). The rapid evolution of influenza antigens requires annual reformulation of the vaccine. Exacerbating this natural antigenic evolution, viral adaptation may occur within the chicken eggs used in the manufacture of the inactivated vaccine (2). In the current 2017–2018 influenza season, such adaptation has reduced the efficacy against H3N2 (3), the strain thus far dominating the US epidemic (4). The lower vaccine efficacy is expected to elevate attack rates, as observed in Australia (3, 5). Compounding the epidemiologic effects of low efficacy, widespread media attention regarding the lower efficacy may discourage vaccination uptake. Early reports from the 2017–2018 season indicate that both mortality and hospitalizations have been more than twice those reported at a comparable stage of the influenza epidemiological trajectory during the 2016–2017 season (6).
Randomized controlled trials are the gold standard for measuring vaccine efficacy in terms of the direct individual-level protection conferred. Mathematical modeling provides a complementary approach through which transmission dynamics can be simulated and population-level effectiveness of vaccination programs quantified in terms of key outcome measures. Further, parameter search procedures can be applied with mathematical models to evaluate a vast range of potential vaccination programs across multidimensional programmatic options. This approach has been used to determine optimal age-specific uptake for highly efficacious influenza vaccines (7). However, optimized uptake of relatively low-efficacy influenza vaccines has not yet been considered despite a range in efficacy during the last decade of from 19% to 60%, with a mean of 45% (8).
We use an age-structured dynamic model of influenza transmission and vaccination to evaluate the effect of influenza vaccines with relatively low efficacy, which we define as below the 45% mean. To identify socially optimal vaccine uptake for low-efficacy influenza vaccines, we applied an optimization algorithm to our model. We consider both impact and optimal uptake in terms of minimizing incidence, hospitalizations, deaths, and disability-adjusted life-years (DALYs). DALYs measure disease burden by capturing both morbidity and mortality, in which a single DALY represents 1 lost year of healthy life (9). Our results indicate that as efficacy declines, optimal uptake to minimize mortality and DALYs shifts some doses from school-age children and young adults, who have disproportionately high transmission rates, to the elderly, who are at greater risk for severe clinical outcomes. We further show that even for vaccines with lower efficacy, optimal uptake is projected to substantially reduce incidence, hospitalizations, deaths, and DALYs compared with projections under typical US vaccine uptake.
Results
We first simulated epidemiological trajectories projected under age-specific vaccination coverages that are typical in the United States. We then considered the optimal uptake of 140 million doses (the average number of doses that have been delivered annually over the five seasons spanning 2012–2017), equivalent to a coverage of 43%. Epidemiological outcomes of infections, hospitalizations, deaths, and DALYs averted were compared with no vaccination. Specifically, in the absence of vaccination, about 77 million infections, 470,000 hospitalizations, and 130,000 deaths would be expected during an influenza season.
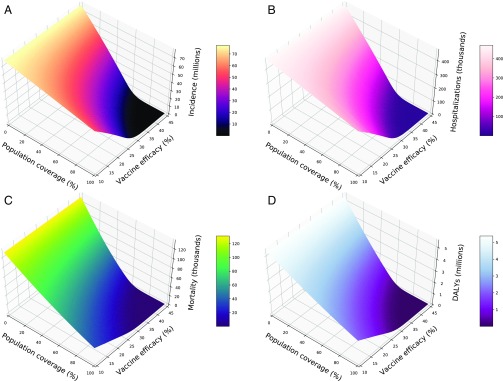
Compared with no vaccination, we found that typical uptake of even a low-efficacy vaccine can significantly avert infections, hospitalizations, deaths, and DALYs (Fig. 1). For example, an overall coverage of 43% with a 20% efficacy vaccine is projected to avert 20.99 million infections, 129,701 hospitalizations, 61,812 deaths, and 2.22 million DALYs. Expanding coverage to 50% with the same 20% efficacy vaccine would further reduce influenza burden by 3.63 million infections, 21,987 hospitalizations, 8,479 deaths, and 319,921 DALYs. All epidemiological outcomes, particularly mortality and DALYs, are more sensitive to changes in coverage than to changes in efficacy (Fig. 1 and SI Appendix, Fig. S2). For example, with a constant efficacy of 40%, a drop in coverage from 40% to 20% is projected to result in 39,738 more deaths. In contrast, a drop in efficacy across the same range at a constant 40% coverage would lead to 28,343 additional deaths.
Fig. 1.
Population impact of vaccination on (A) incidence, (B) hospitalizations, (C) mortality, and (D) DALYs when doses of a low-efficacy vaccine are distributed according to age-specific uptake typical in the United States.
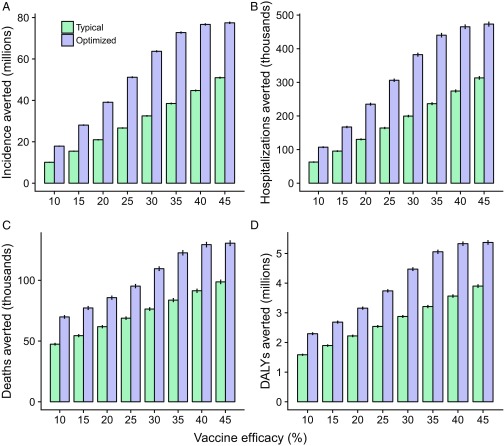
When optimally allocated, vaccines with efficacies as low as 20% or even 10% can markedly reduce influenza morbidity and mortality beyond typical age-specific vaccine uptake. Specifically, 140 million doses of a 20% efficacy vaccine could avert 39.10 million infections, 235,059 hospitalizations, 85,734 deaths, and 3.16 million DALYs when optimized according to the respective objectives (Fig. 2). Compared with reductions under typical vaccine uptake, these projections correspond to a 86% greater effect on incidence, 80% on hospitalizations, 39% on mortality, and 42% on DALYs.
Fig. 2.
Comparisons of the impact of low-efficacy influenza vaccines between optimal age-stratified uptake of 140 million vaccine doses and typical age-specific vaccine uptake. Uptake was optimized to avert (A) incidence, (B) hospitalizations, (C) deaths, and (D) overall disease burden, measured by DALYs. Error bars represent SEM.
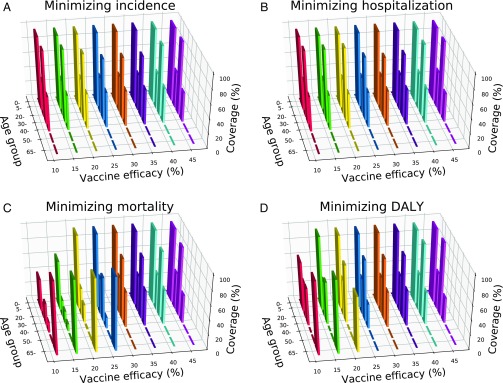
Across the low-efficacy range from 10% to 45%, incidence and hospitalizations can be minimized by prioritizing vaccination of school-age children (5–19 y) and young adults (30–39 y; Fig. 3 A and B), the groups to which most transmission is attributable. At an efficacy of 20%, for example, uptake of 62.18 million of the available 140 million doses by school-age children and 39.30 million doses by young adults minimizes incidence. Likewise, uptake of 62.18 million and 42.56 million doses by the two age groups minimizes hospitalizations (SI Appendix, Fig. S3). This strategy similarly minimizes mortality for more efficacious vaccines (Fig. 3C).
Fig. 3.
Optimal age-stratified coverage of 140 million doses of a low-efficacy vaccine to minimize (A) incidence, (B) hospitalizations, (C) mortality, and (D) overall disease burden, measured in Disability Adjusted Life Years (DALYs). Bar widths are proportional to the size of each age group. Bar heights correspond to the median of 1,000 optimization runs.
For efficacies below 30%, vaccination of school-age children remains a priority to minimize mortality (Fig. 3C). As efficacy declines, however, some vaccine uptake shifts from young adults to the elderly (>65 y). At an efficacy of 45%, for example, optimal uptake calls for 94% coverage in young adults. At an efficacy of 20%, in contrast, optimal coverage in young adults is reduced to 25%, with a simultaneous boost in the elderly.
We further optimized uptake to minimize DALYs, the combined measure of influenza morbidity and mortality. Above an efficacy of 25%, vaccination priorities include school-age children and young adults, consistent with the other outcome measures (Fig. 3D). As efficacy is reduced, some vaccine uptake shifts away from young adults to the elderly. At an efficacy of 45%, for example, uptake of 42.56 million doses is optimal for young adults (SI Appendix, Fig. S3). When vaccine efficacy is 20%, the target uptake for young adults drops to 33.60 million doses, with a simultaneous increase in the optimal uptake for the elderly to 37.43 million doses. This shift is less pronounced than that observed when minimizing mortality is the exclusive objective. To minimize mortality, the elderly would optimally receive an additional 11.81 million doses of a 20% efficacy vaccine compared with the uptake optimal to minimize DALYs (SI Appendix, Fig. S3).
Discussion
Viral evolution and annual vaccine reformulation generate variable influenza vaccine efficacy across seasons (2, 10). Given the relatively low efficacy anticipated during the 2017–2018 influenza season, there is concern that rates of infection will be significantly elevated. Our results demonstrate that even a low-efficacy influenza vaccine under age-specific vaccine uptake typical in the United States can avert considerable incidence, hospitalizations, mortality, and DALYs, particularly if high coverage can be achieved. We further find that mortality and overall health burden are more sensitive to changes in coverage than to comparable changes in efficacy. This result highlights how reduced motivation to vaccinate could present a greater danger than low vaccine efficacy itself.
Given current uptake, extensive coverage of the US population remains a distant goal. To help bolster vaccination impact, public health officials may target vaccination campaigns and outreach to specific groups, with the goal of minimizing adverse consequences for the population overall. To identify effective targets, we determined the optimal distribution of the 140 million vaccine doses typically available in the United States. Our results indicate that optimized distribution of even a relatively low-efficacy vaccine can avert millions of infections and DALYs, as well as thousands of hospitalizations and deaths, beyond reductions garnered by the age-specific pattern of uptake typical in the United States.
At vaccine efficacies above 30%, transmission reduction is the most effective approach to minimizing all measures of influenza morbidity and mortality. Targeting school-age children and young adults, both age groups with disproportionately high transmission rates, confers indirect protection throughout the population. The elderly are at heightened risk for both hospitalization and death, but immunological response to influenza vaccines tends to be weaker in this age class. As a consequence, protection via herd immunity is more effective than direct protection when vaccine efficacy is above 30%. This finding is consistent with our previous analyses (7). As referenced, our prior modeling predictions prompted a randomized trial (11) and field studies (12), both of which confirmed the substantial community-level effect of vaccinating children. Citing the randomized trial (11), the Advisory Committee on Immunization Practices began recommending influenza vaccination for children (13). Since then, local public health agencies initiated school-located programs, demonstrated to be epidemiologically effective and economically sustainable (14–16). These implementations highlight the translation of modeling analyses to improve preventative health. However, neither modeling nor empirical studies had been conducted to evaluate population-level effectiveness of strategies deploying low-efficacy influenza vaccines.
As efficacy declines, optimal strategies diverge depending on the objective of the vaccination program. To minimize both incidence and hospitalizations, targeting school-age children and young adults (30–39 y) remains the most effective strategy across the efficacy range considered. To minimize mortality, the potential for vaccination-mediated herd immunity to provide indirect protection is diminished as efficacy declines. As a consequence, the optimal approach to minimize mortality shifts toward direct vaccination of the elderly because of their greater case fatality rate. Optimization to avert DALYs is accomplished by an uptake distribution intermediate to those under the exclusive incidence, hospitalization, and mortality objectives. Specifically, the shift toward direct vaccination of the elderly occurs below an efficacy of 25% compared with 30% for minimizing mortality. This intermediate uptake distribution to minimize DALYs is consistent with the composite nature of the DALY measure, which takes into account both morbidity and mortality caused by a disease (17).
In most of the scenarios examined, vaccination in school-age children and in adults aged 30–39 y should be prioritized. However, uptake among adults younger than 50 y has been as low as 33% during the last 5 y spanning the 2012–2013 to 2016–2017 seasons. Increasing vaccination uptake among this group is likely to be challenging because of their comparatively lower personal protective benefit. Nonetheless, it has been found that altruism can be harnessed to boost vaccination uptake by emphasizing the protective role of young adults with regard to their children, parents, and other elderly family members (18–21). Health provider recommendation, email reminders from health maintenance organizations, workplace incentivization, and opt-out scheduling of vaccination appointments are also influential in promoting vaccination (22–25). These efforts can be augmented by improving the accessibility of vaccination; for instance, through active recruitment at convenient locations such as pharmacies, retail establishments, community centers, workplaces, and schools (14, 16, 25, 26). When herd immunity is anticipated to be inadequate to protect the elderly, our results highlight the importance of making an extra effort to encourage vaccination uptake in this age class. Complementary to such efforts would be administering vaccines with efficacy augmented by adjuvants or high dosage (27). Augmented vaccines are recommended for the elderly by the Canadian National Advisory Committee on Immunization (28), but supplies are limited in the United States and are not preferentially recommended by the Advisory Committee on Immunization Practices (29).
Until an efficacious universal influenza vaccine is developed, responsive vaccination policies based on projected efficacy will be fundamental to minimize influenza morbidity and mortality. Given that optimal uptake is relatively robust for vaccine efficacies above 25%, early indications of unusually low efficacy are integral to an adaptive coverage strategy. The influenza season in the South Hemisphere, which occurs about 6 mo before that in the Northern Hemisphere, serves as a source for preliminary estimates of vaccine efficacy. In the 2017–2018 season, for example, concern about low efficacy initially arose from observations in Australia (3, 5). Early estimates may also derive from phylogenetic analysis of strains selected for vaccine production and the influenza strains with rising incidence during late summer in the United States (30). Likewise, sequence comparisons of the vaccine viruses before and after replication in the eggs used in manufacture could help identify egg adaptation in antigenic regions that could degrade efficacy (10).
Our results demonstrate that vaccines can have substantial epidemiological impact even when efficacy is low. The lower the reproductive number of an infectious disease, the more sensitive its epidemic size is to small changes in vaccination coverage. As a consequence, even a vaccine with low efficacy can have an appreciable effect on influenza incidence and population-level burden. Furthermore, in all scenarios considered, an optimized distribution of vaccines would substantially improve outcomes compared with the typical age-specific vaccination uptake in the United States.
Methods
To optimize age-specific uptake of low-efficacy influenza vaccines, defined as vaccines with an efficacy below the empirical mean of 45% (31), we extended our previous model of influenza transmission and vaccination (7). Our extended model was stratified into seven age groups: 0–4, 5–19, 20–29, 30–39, 40–49, 50–65, and 65 years and older, parameterized from US census data. Influenza transmission was simulated within and between these age groups, using a dynamic SEIR (susceptible, exposed, infected, removed) framework that integrates an empirically determined contact network (32). Specifically, people susceptible to influenza had a risk for exposure that depended on the prevalence of infections at a given time in the epidemiological trajectory, age-specific contact mixing (32), and per contact transmission rate (β). We used the incidence, vaccine efficacy, and vaccination coverage for each year from 2010–2011 to 2015–2016 reported by the Centers for Disease Control and Prevention (31, 33, 34) to calculate the annual R0 values (SI Appendix, Table S2). The median R0 for these years was 1.222, to which we calibrated our model. We also took into account the age-specific risks for clinical outcomes, including hospitalization and death. For example, very young children have an elevated risk for hospitalization, whereas the elderly have greater risks for both hospitalization and death.
The probability that a vaccinated individual would be protected from infection depended on baseline vaccine efficacy, κ, and relative age-specific immunocompetency to mount a protective response, . Thus, the number of vaccinated people for whom the vaccine does not take, and who are consequently susceptible to influenza infection, was calculated as , where is total number of vaccinated people in age group a. We also took into account that if a vaccinated individual of a specific age is infected, the hospitalization and case fatality rate is lower than if an unvaccinated individual of the same age is infected. Distributions of these age-specific vaccine efficacy parameters were parameterized from a meta-analysis of case control studies and randomized trials (35, 36). Other epidemiological, immunological, and clinical parameters were also derived from empirical distributions (SI Appendix, Table S3). From these distributions, we drew sets of values to parameterize 1,000 simulations for each vaccine efficacy considered.
We first evaluated the population effect of vaccination when doses are distributed according to typical age-specific uptake in the United States. Typical age-specific vaccine uptake in the United States was estimated by averaging empirical data during the last 5 y (37). We computed impact with regard to four outcomes: incidence, hospitalizations, mortality, and DALYs.
DALYs correspond to the sum of years lived with disability and years of life lost from each infection and death, respectively. Years of life lost quantify the gap between age at death and life expectancy (38). Life expectancies for each age group were derived from Centers for Disease Control and Prevention life tables (39). For years lived with disability, the disability weight assigned to influenza by the Global Burden of Disease study was applied to uncomplicated cases (40). We considered four age-specific clinical complications (SI Appendix, Table S4): acute respiratory distress syndrome, pneumonia, otitis media, and acute otitis media leading to long-term hearing impairment (41, 42). Patients with acute respiratory distress syndrome and pneumonia were considered likely to be hospitalized. Lifelong disability was associated with acute respiratory distress syndrome, and a 2-wk disability was associated with pneumonia (43). Other hospitalized patients were assigned the disability weight corresponding to severe respiratory infection (40). Otitis media was assumed to be an outpatient complication, with a probability of long-term hearing impairment (41, 42).
We used an iterative parameter search procedure (SI Appendix, SI Methods) to determine the optimal age-specific distribution of 140 million vaccine doses, the number available averaged over the last five seasons from 2011–2012 to 2016–2017 (44) and corresponding to 43% overall coverage. Minimization of four health outcomes was evaluated, each independently: incidence, hospitalizations, mortality, and DALYs. We compared the epidemiological effectiveness of optimal uptake to typical age-specific vaccine uptake in the United States, with the typical population coverage of 43%. Further methodological detail is provided in the SI Appendix.
Supplementary Material
Acknowledgments
P.S., J.M., M.C.F., and A.P.G. were supported by National Institutes of Health Grants U01 GM105627 and U01 GM087719.
Footnotes
The authors declare no conflict of interest.
Data deposition: The replication data reported in this paper have been deposited in the Harvard Dataverse, https://dataverse.harvard.edu (available at https://doi.org/10.7910/DVN/LB6CFZ).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1802479115/-/DCSupplemental.
References
- 1.Centers for Disease Control and Prevention 2017 Disease burden of influenza. Available at https://www.cdc.gov/flu/about/disease/burden.htm. Accessed December 7, 2017.
- 2.Zost SJ, et al. Contemporary H3N2 influenza viruses have a glycosylation site that alters binding of antibodies elicited by egg-adapted vaccine strains. Proc Natl Acad Sci USA. 2017;114:12578–12583. doi: 10.1073/pnas.1712377114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sullivan SG, et al. Low interim influenza vaccine effectiveness, Australia, 1 May to 24 September 2017. Euro Surveill. 2017;22 doi: 10.2807/1560-7917.ES.2017.22.43.17-00707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention 2017 2017-2018 influenza season week 49 ending December 9, 2017. FluView: A Weekly Influenza Surveillance Report Prepared by the Influenza Division. Available at https://www.cdc.gov/flu/weekly/weeklyarchives2017-2018/Week49.htm. Accessed December 27, 2017.
- 5.Paules CI, Sullivan SG, Subbarao K, Fauci AS. Chasing seasonal influenza. The need for a universal influenza vaccine. N Engl J Med. 2017;378:7–9. doi: 10.1056/NEJMp1714916. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention; Seasonal Influenza (Flu) 2017 Weekly US influenza surveillance report. Available at https://www.cdc.gov/flu/weekly/index.htm. Accessed December 31, 2017.
- 7.Medlock J, Galvani AP. Optimizing influenza vaccine distribution. Science. 2009;325:1705–1708. doi: 10.1126/science.1175570. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention Seasonal Influenza (Flu) 2017 Seasonal influenza vaccine effectiveness 2005–2017. Available at https://www.cdc.gov/flu/professionals/vaccination/effectiveness-studies.htm. Accessed December 31, 2017.
- 9.GBD 2016 Mortality Collaborators Global, regional, and national under-5 mortality, adult mortality, age-specific mortality, and life expectancy, 1970–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1084–1150. doi: 10.1016/S0140-6736(17)31833-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Skowronski DM, et al. Low 2012-13 influenza vaccine effectiveness associated with mutation in the egg-adapted H3N2 vaccine strain not antigenic drift in circulating viruses. PLoS One. 2014;9:e92153. doi: 10.1371/journal.pone.0092153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Loeb M, et al. Effect of influenza vaccination of children on infection rates in Hutterite communities: A randomized trial. JAMA. 2010;303:943–950. doi: 10.1001/jama.2010.250. [DOI] [PubMed] [Google Scholar]
- 12.Pannaraj PS, et al. School-located influenza vaccination decreases laboratory-confirmed influenza and improves school attendance. Clin Infect Dis. 2014;59:325–332. doi: 10.1093/cid/ciu340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fiore AE, et al. Centers for Disease Control and Prevention (CDC) Prevention and control of influenza with vaccines: Recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Recomm Rep. 2010;59:1–62. [PubMed] [Google Scholar]
- 14.Tran CH, et al. School-located influenza vaccination reduces community risk for influenza and influenza-like illness emergency care visits. PLoS One. 2014;9:e114479. doi: 10.1371/journal.pone.0114479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoo B-K, et al. Cost effectiveness analysis of Year 2 of an elementary school-located influenza vaccination program-Results from a randomized controlled trial. BMC Health Serv Res. 2015;15:511. doi: 10.1186/s12913-015-1169-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tran CH, et al. Sustainability of school-located influenza vaccination programs in Florida. Vaccine. 2016;34:2737–2744. doi: 10.1016/j.vaccine.2016.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sassi F. Calculating QALYs, comparing QALY and DALY calculations. Health Policy Plan. 2006;21:402–408. doi: 10.1093/heapol/czl018. [DOI] [PubMed] [Google Scholar]
- 18.Shim E, Chapman GB, Townsend JP, Galvani AP. The influence of altruism on influenza vaccination decisions. J R Soc Interface. 2012;9:2234–2243. doi: 10.1098/rsif.2012.0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chapman GB, et al. Using game theory to examine incentives in influenza vaccination behavior. Psychol Sci. 2012;23:1008–1015. doi: 10.1177/0956797612437606. [DOI] [PubMed] [Google Scholar]
- 20.Taylor E, et al. Cross-cultural household influence on vaccination decisions. Med Decis Making. 2016;36:844–853. doi: 10.1177/0272989X15591007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li M, Taylor EG, Atkins KE, Chapman GB, Galvani AP. Stimulating influenza vaccination via prosocial motives. PLoS One. 2016;11:e0159780. doi: 10.1371/journal.pone.0159780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dexter PR, Perkins SM, Maharry KS, Jones K, McDonald CJ. Inpatient computer-based standing orders vs physician reminders to increase influenza and pneumococcal vaccination rates: A randomized trial. JAMA. 2004;292:2366–2371. doi: 10.1001/jama.292.19.2366. [DOI] [PubMed] [Google Scholar]
- 23.Chapman GB, Li M, Colby H, Yoon H. Opting in vs opting out of influenza vaccination. JAMA. 2010;304:43–44. doi: 10.1001/jama.2010.892. [DOI] [PubMed] [Google Scholar]
- 24.Opel DJ, et al. The influence of provider communication behaviors on parental vaccine acceptance and visit experience. Am J Public Health. 2015;105:1998–2004. doi: 10.2105/AJPH.2014.302425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nowalk MP, et al. Improving influenza vaccination rates in the workplace: A randomized trial. Am J Prev Med. 2010;38:237–246. doi: 10.1016/j.amepre.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 26.Chun GJ, Sautter JM, Patterson BJ, McGhan WF. Diffusion of pharmacy-based influenza vaccination over time in the United States. Am J Public Health. 2016;106:1099–1100. doi: 10.2105/AJPH.2016.303142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DiazGranados CA, et al. Efficacy of high-dose versus standard-dose influenza vaccine in older adults. N Engl J Med. 2014;371:635–645. doi: 10.1056/NEJMoa1315727. [DOI] [PubMed] [Google Scholar]
- 28.Public Health Agency of Canada 2017 Canadian immunization guide chapter on influenza and statement on seasonal influenza vaccine for 2017–2018. Available at https://www.canada.ca/en/public-health/services/publications/healthy-living/canadian-immunization-guide-statement-seasonal-influenza-vaccine-2017-2018.html. Accessed March 21, 2018.
- 29.Grohskopf LA, et al. Prevention and control of seasonal influenza with vaccines: Recommendations of the Advisory Committee on Immunization Practices–United States, 2017-18 influenza season. MMWR Recomm Rep. 2017;66:1–20. doi: 10.15585/mmwr.rr6602a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Veljkovic V, et al. Evolution of 2014/15 H3N2 influenza viruses circulating in US: Consequences for vaccine effectiveness and possible new pandemic. Front Microbiol. 2015;6:1456. doi: 10.3389/fmicb.2015.01456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seasonal Influenza (Flu) CDC 2018 Seasonal influenza vaccine effectiveness 2005–2018. Available at https://www.cdc.gov/flu/professionals/vaccination/effectiveness-studies.htm. Accessed March 5, 2018.
- 32.Mossong J, et al. Social contacts and mixing patterns relevant to the spread of infectious diseases. PLoS Med. 2008;5:e74. doi: 10.1371/journal.pmed.0050074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.FluVaxView Seasonal Influenza (Flu) CDC 2017 National early-season flu vaccination coverage United States, November 2017. Available at https://www.cdc.gov/flu/fluvaxview/nifs-estimates-nov2017.htm. Accessed March 5, 2018.
- 34.Seasonal Influenza (Flu) CDC 2016 Estimated influenza illnesses, medical visits, hospitalizations, and deaths averted by vaccination in the United States. Available at https://www.cdc.gov/flu/about/disease/2015-16.htm. Accessed March 5, 2018.
- 35.Gross PA, Hermogenes AW, Sacks HS, Lau J, Levandowski RA. The efficacy of influenza vaccine in elderly persons. A meta-analysis and review of the literature. Ann Intern Med. 1995;123:518–527. doi: 10.7326/0003-4819-123-7-199510010-00008. [DOI] [PubMed] [Google Scholar]
- 36.Nichol KL. The efficacy, effectiveness and cost-effectiveness of inactivated influenza virus vaccines. Vaccine. 2003;21:1769–1775. doi: 10.1016/s0264-410x(03)00070-7. [DOI] [PubMed] [Google Scholar]
- 37.Centers for Disease Control and Prevention 2017 Flu vaccination coverage, United States, 2016-17 influenza season. Available at https://www.cdc.gov/flu/fluvaxview/coverage-1617estimates.htm. Accessed December 14, 2017.
- 38.GBD 2015 DALYs and HALE Collaborators Global, regional, and national disability-adjusted life-years (DALYs) for 315 diseases and injuries and healthy life expectancy (HALE), 1990-2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1603–1658. doi: 10.1016/S0140-6736(16)31460-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arias E, Heron M, Xu J. United States life tables, 2014. Natl Vital Stat Rep. 2017;66:1–64. [PubMed] [Google Scholar]
- 40.Global Health Data Exchange 2016 Global Burden of Disease Study 2016 (GBD 2016) disability weights. Available at ghdx.healthdata.org/record/global-burden-disease-study-2016-gbd-2016-disability-weights. Accessed January 19, 2018.
- 41.Meier CR, Napalkov PN, Wegmüller Y, Jefferson T, Jick H. Population-based study on incidence, risk factors, clinical complications and drug utilisation associated with influenza in the United Kingdom. Eur J Clin Microbiol Infect Dis. 2000;19:834–842. doi: 10.1007/s100960000376. [DOI] [PubMed] [Google Scholar]
- 42.Plass D, et al. The disease burden of hepatitis B, influenza, measles and salmonellosis in Germany: First results of the burden of communicable diseases in Europe study. Epidemiol Infect. 2014;142:2024–2035. doi: 10.1017/S0950268813003312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Niessen LW, et al. Comparative impact assessment of child pneumonia interventions. Bull World Health Organ. 2009;87:472–480. doi: 10.2471/BLT.08.050872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Centers for Disease Control and Prevention 2017 Historical reference of seasonal influenza vaccine doses distributed. Available at https://www.cdc.gov/flu/professionals/vaccination/vaccinesupply.htm. Accessed December 12, 2017.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.