While 92 million Americans are currently estimated to have some form of cardiovascular disease (CVD), nearly one-half of adults in the United States are projected to develop CVD by 2030, annually eclipsing all other causes of death for the past century (1). Coronary heart disease, which confers a plurality of CVD etiologies resulting in death, is exceptional in its ties to insufficient cholesterol transfer from peripheral sites to the liver and subsequent disposal (1, 2). As a mediator of inflammation, a source of peripheral cholesterol, and a cell type integral to the deposition of vascular fatty lesions, the macrophage has been cast to play a leading role in atherosclerosis. Each of these contributors to atherogenesis are regulated by the liver X receptor (LXR) (3). In PNAS, Muse et al. (4) manipulate ligand-induced LXR activities to accentuate a transcriptional program that promotes cholesterol efflux gene expression specifically in the macrophage.
The LXR family of nuclear receptors is implicated in a reciprocal coordination of gene products involved in lipid homeostasis and inflammation (3). While lipids bearing oxidative modifications not only underlie excessive neutral lipid accumulation and inflammatory gene expression, they also agonize LXR. Therefore, this nuclear receptor family is a uniquely suited drug target to mitigate vascular lesion development (5). LXR performs these functions by activating the transcription of cholesterol transporters (e.g., ATP-binding cassette transporters Abca1, Abcg1) and tolerogenic cytokines (e.g., interleukin-10), as well as preventing the removal of corepressor complexes from Toll-like receptor-activated regulatory elements, respectively (5–7). Synthetic LXR agonists, such as GW3965 and T0901317, have been instrumental in our appreciation for the breadth of LXR actions and atheroprotective activities in the macrophage in particular. These powerful LXR agonists came to represent an encouraging strategy for battling arterial plaque deposition.
However, a narrow focus on macrophages and mouse models of atherosclerosis obscures the pervasive impact of these potent synthetic compounds on activities in other essential sites of LXR expression and activity. While LXR may drive cholesterol efflux in macrophages (through ABCA1 and ABCG1), it further supports reverse cholesterol transport by enforcing cholesterol conversion to bile acids and excretion through ABCG5/8 in the liver (8). Unfortunately, synthetic ligand-induced LXR activity is not limited to reverse cholesterol transport in the macrophage, as genes promoting cholesterol efflux are up-regulated in other cell types, including hepatocytes (9). The futility of activating cholesterol efflux at both the source and sink is compounded by a concomitant activation of a lipogenic program, mediated by sterol regulatory element-binding protein (SREBP) (9). A coordinated activation of both fatty acid desaturase and synthase genes by downstream SREBP activity in the liver produces remarkable hepatic steatosis and dyslipidemia in mouse models and human patients (9, 10). Such an increase in neutral lipid deposition in the liver and diminishing high-density lipoprotein in circulation, respectively, renders LXR-targeting therapeutic strategies untenable.
A thorough examination of macrophages from hypercholesterolemic mice indicated an unexpectedly muted inflammatory state, compared with macrophages derived from healthy mice (6). While free cholesterol administration increases LXR-activating oxysterol levels and LXR target genes in macrophages, ablation of the major oxysterol-producing enzymes demonstrates that these cholesterol derivatives are only partially responsible, leading Glass and coworkers (6) to investigate supplementary mediators of compensatory LXR activity in the macrophage. They demonstrate that as macrophage oxysterol content increases with exogenous cholesterol levels, the expression of Dhcr24, which catalyzes a final step in the cholesterol biosynthetic Bloch pathway, is the most suppressed transcript by the hypercholesterolemic condition. Cellular desmosterol, a substrate of DHCR24, is accordingly increased in both macrophages from hypercholesterolemic mice and human atherosclerotic lesions (6). Desmosterol concentrations under these conditions exceed that of the well-characterized LXR-activating oxysterol levels by 50-fold, while desmosterol is itself an LXR agonist (6).
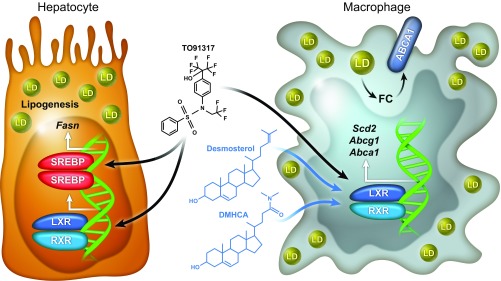
Muse et al. (4) demonstrate that, unlike the often-employed synthetic LXR agonists (i.e., GW3965, T0901317), desmosterol activates genes responsible for cholesterol export (e.g., Abca1, Abcg1), while lipogenic gene expression (e.g., fatty acid synthase, Fasn) remains unaffected (Fig. 1). In addition, this expression profile is restricted to macrophages from both humans and mice, sparing hepatocytes from a commensurate or futile expression of LXR targets.
Fig. 1.
Macrophage-restricted LXR agonists. Conventional synthetic LXR ligands, such as T091317 or GW3965, are exceptionally effective agonists, delivering systemic LXR target gene up-regulation. Desmosterol and similarly structured mimetics, such as DMHCA and MePipHCA, agonize LXR in macrophages. Similar to the accumulation of endogenous desmosterol, DMHCA and MePipHCA permit the efficient mobilization of cholesterol stored in macrophages through the ATP-binding cassette transporters ABCA1 and ABCG1. Importantly, these LXR-agonizing compounds, unlike T091317 or GW3965, avoid the concurrent activation of SREBP-initiated fatty acid biosynthesis.
Because macrophage desmosterol levels are already elevated in the presence of excessive circulating cholesterol levels, Muse et al. explored the utility of synthetic compounds that share chemical characteristics with desmosterol, such as a sterol backbone, to both mimic and supplement the transcriptional impact of desmosterol in the pathophysiological setting of hypercholesterolemia. They found that two such compounds, N,N-dimethyl-3β-hydroxycholenamide (DMHCA) and methylpiperidinyl-3β-hydroxycholenamide (MePipHCA), are potent activators of LXR target genes involved in cholesterol efflux (e.g., Abca1, Abcg1), while having no effect on the expression of lipogenic SREBP targets (e.g., Fasn) (4). In addition, like desmosterol, DMHCA and MePipHCA-stimulated transcriptional programs are restricted to human and murine macrophages as primary hepatocytes failed to follow suit.
Finally, Muse et al. (4) tested the efficacy of these desmosterol mimetics in vivo. Injection of DMHCA and MePipHCA up-regulates Abca1, but not Fasn in elicited macrophages, while failing to activate either gene in the liver. Interestingly, Kupffer cells, the resident macrophages of the liver, respond to DMHCA and MePipHCA similarly to thioglycollate-elicited macrophages, failing again to activate either gene in whole liver.
Synthetic LXR agonists, such as GW3965 and T0901317, have been important tools in furthering our understanding of generalized LXR action and, particularly, the integrative role of LXR in macrophage biology. However, the chemical structures of GW3965 and T0901317 are vastly different from endogenous LXR ligands and may obfuscate the more natural inclinations of pathophysiological LXR activities. Indeed, Muse et al. explore the divergent activation and roles for LXR in macrophages and hepatocytes and demonstrate that macrophage-specific synthetic LXR agonists such as DMHCA and MePipHCA appear to be a promising new class of atheroprotective agents.
Footnotes
The authors declare no conflict of interest.
See companion article on page E4680.
References
- 1.Benjamin EJ, et al. Heart disease and stroke statistics—2017 update: A report from the American Heart Association. Circulation. 2017;135:e146–e603, erratum (2017) 135:e646, and erratum (2017) 136:e196. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Emerging Risk Factors Collaboration, et al. Major lipids, apolipoproteins, and risk of vascular disease. JAMA. 2009;302:1993–2000. doi: 10.1001/jama.2009.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Joseph SB, Castrillo A, Laffitte BA, Mangelsdorf DJ, Tontonoz P. Reciprocal regulation of inflammation and lipid metabolism by liver X receptors. Nat Med. 2003;9:213–219. doi: 10.1038/nm820. [DOI] [PubMed] [Google Scholar]
- 4.Muse ED. Cell-specific discrimination of desmosterol and desmosterol mimetics confers selective regulation of LXR and SREBP in macrophages. Proc Natl Acad Sci USA. 2018;115:E4680–E4689. doi: 10.1073/pnas.1714518115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chawla A, et al. A PPAR gamma-LXR-ABCA1 pathway in macrophages is involved in cholesterol efflux and atherogenesis. Mol Cell. 2001;7:161–171. doi: 10.1016/s1097-2765(01)00164-2. [DOI] [PubMed] [Google Scholar]
- 6.Spann NJ, et al. Regulated accumulation of desmosterol integrates macrophage lipid metabolism and inflammatory responses. Cell. 2012;151:138–152. doi: 10.1016/j.cell.2012.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.A-González N, Castrillo A. Liver X receptors as regulators of macrophage inflammatory and metabolic pathways. Biochim Biophys Acta. 2011;1812:982–994. doi: 10.1016/j.bbadis.2010.12.015. [DOI] [PubMed] [Google Scholar]
- 8.Pfeifer T, et al. Synthetic LXR agonist suppresses endogenous cholesterol biosynthesis and efficiently lowers plasma cholesterol. Curr Pharm Biotechnol. 2011;12:285–292. doi: 10.2174/138920111794295774. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 9.Grefhorst A, et al. Stimulation of lipogenesis by pharmacological activation of the liver X receptor leads to production of large, triglyceride-rich very low density lipoprotein particles. J Biol Chem. 2002;277:34182–34190. doi: 10.1074/jbc.M204887200. [DOI] [PubMed] [Google Scholar]
- 10.Kirchgessner TG, et al. Beneficial and adverse effects of an LXR agonist on human lipid and lipoprotein metabolism and circulating neutrophils. Cell Metab. 2016;24:223–233. doi: 10.1016/j.cmet.2016.07.016. [DOI] [PubMed] [Google Scholar]