Organisms in seasonal environments are known to adjust their phenology in response to climate change (1, 2), that is, they change their schedules of seasonal occurrence and annual life-history events. In particular, the advancement of spring emergence and activities is one of the strongest and best-documented ecological responses to climate change (1, 3, 4). As the rate of advancement varies between species, for example at different trophic levels, the occurrence of interacting species may become asynchronous, altering or disrupting the ecological interactions—a phenomenon referred to as the match–mismatch hypothesis (4, 5). While many studies have documented changes in phenological synchrony, with various effects on the focal species’ populations (4, 6, 7), we still lack a general picture of how widespread changes we see in phenological synchrony and how they affect ecological communities. In PNAS, Kharouba et al. (8) address this topic in a meta-analysis on 54 pairs of interacting species. The phenologies of the studied species advanced with an average rate of ca. 4 d per decade. Importantly, phenological synchrony of the species pairs was changing at a rapid and accelerating pace, with an average of 6.1 d per decade, either toward more or toward less synchrony. This change was approximately 10 times faster compared with what happened before 1981, which was used as a baseline in this study.
Climate change is recognized as a major threat to global biodiversity (9). Changes in phenological synchrony have raised serious concerns about adverse population-level consequences. Insectivorous birds and their prey (caterpillars) have for a long time been a model system for studying match–mismatch. In birds there is correlative evidence for species with increasing phenological mismatch showing more negative population trends (7, 10, 11). Despite these results, a wide range of patterns have been reported, for example evidence for increasing synchrony (12). Several recent studies that have reported trends of decreasing synchrony found no relevant adverse effects on vital rates or population dynamics (13–15), suggesting all populations may not be as vulnerable as feared. Interestingly, in their meta-analysis Kharouba et al. (8) detected no consistent average direction in whether the phenology of two interacting species shifted further apart or closer together. Acknowledging this does not necessarily imply a purely random pattern, they suggested that a future research challenge is improving our predictive ability of the direction of change in phenological synchrony. While the direction is certainly relevant for the ecological interactions, it is only one of several parameters in a complex story and it may not alone provide sufficient answers to questions such as the following. (i) Do the changes in synchrony depend on interacting species, or are they mainly driven by abiotic cues? (ii) Are the changes adaptive or nonadaptive? (iii) What do changes in synchrony imply for species’ vital rates and population trends? (iv) How should we revise conservation priorities in the light of species’ vulnerability to phenological mismatch?
As discussed by Kharouba et al. (8), a number of confounding variables are likely affecting the degree of synchrony change, suggesting, for example, that the degree of specialism in the interactions may be important. Also, the type of interaction must be crucial for determining what kind of shifts we might expect. In resource–consumer (predator–prey) systems, clearly, the consumer (predator) should benefit from better synchrony, while the resource (prey) should benefit from less synchrony, and hence a temporal release from predation pressure. Strong mutualistic interactions would be expected to track their previously already synchronous phenology. Likewise, strong competition or other antagonistic interactions should favor asynchrony.
Temperature, in combination with a range of other abiotic variables, is of crucial importance for determining optimal phenology. The phenology of an interacting species is in the best case only one of several predictors, or possible cues, determining phenology. However, even if we disregard the cues and assume that organisms have perfect knowledge about what is best for them, the question of phenological synchrony is still complex. In the literature both positive and negative relationships have been demonstrated between phenological change and population growth or vital rates (16). In a theoretical study, Jonzén et al. (17) studied the optimal advancement of phenology of migratory birds in response to climate change, accounting for variation in food distribution, competition for territories, and the risk of mortality. According to their results, the optimal advancement is always less than the food peak shift (i.e., we should expect the birds to lag behind their food peaks). Moreover, they suggested that the large variety of responses found in nature is indeed expected, even if the birds would have perfect knowledge of what to do. Johansson et al. (18) expanded on this topic, investigating ecological and evolutionary aspects of the problem in the light of life-history theory, evolutionary game theory, and population dynamic models. They confirmed that the optimal changes in synchrony can be surprisingly complex and diverse; for example, they may or may not be toward more synchronous phenology. Further, they argued that the evolutionary optimal strategy, favored by natural selection, is not necessarily optimal in terms of maximal population growth, for example due to density-dependent effects (13, 14, 18). Obviously, the population consequences are a completely different question from whether changes in synchrony are adaptive. Empirical case studies on great tits (Parus major) (14) and willow warblers (Phylloscopus trochilus) (15) in Western Europe echo these theoretical results, showing that phenology affects individual fitness but not population growth.
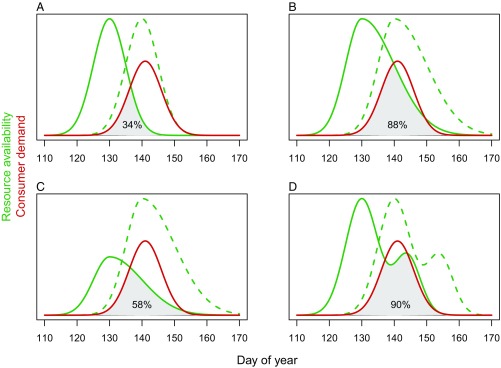
A prerequisite for quantitatively studying phenological effects on ecological interactions is a rigorous definition of phenological mismatch and a straightforward practical way to measure it. While most of the match–mismatch literature measures the time differences between the peaks of interacting species as the focal parameter, or uses an early phase of occurrence as the reference point instead of the peak (8), these do not fully capture all aspects of interaction. The shapes and locations of the whole phenological distributions are largely relevant for determining the potential strength of interaction (Fig. 1). For example, in resource–consumer systems, it is important to assess the availability of a resource throughout the period in relation to the consumer’s demand. Naturally, the general abundance or population size of the resource strongly affects availability and should not be disregarded when studying effects of phenological synchrony (4). In a study on willow tits (Poecile montanus) in Finland (12), nestling survival was affected by synchrony with the food peak, but also by the height of the peak and the interaction between these two. Also, the finding that mismatch has the largest effects in the most seasonal habitats (19) reflects the importance of the width of the resource distribution. Considering that excess food may not matter too much for the consumer, an intuitive and parsimonious focal quantity from the consumer point of view could be the intersecting area of the curves describing resource availability and consumer demand, divided with the total consumer demand (Fig. 1). Given regularly sampled data on both aspects throughout the season, such a quantity can be calculated from fitted phenological functions (20), reducing the dimensionality of the problem and facilitating inclusion in, for example, ecological or evolutionary models (16, 18).
Fig. 1.
Changes in phenological synchrony, defined as the temporal delay in early or peak phases of occurrence, may have very different effects on consumer–resource systems depending on a number of other features of the full phenological distributions. In the illustrated examples, the original phenology of resource availability (green dashed line; peak at day 140) has advanced with 10 d (green solid line; peak at day 130), while the consumer demand has stayed unchanged (red solid line; peak at day 141). Assuming no benefits of resource surplus, the intersecting area of the resource and consumer curves (gray shaded area) divided with total consumer demand (area under red curve) is a parsimonious parameter for measuring phenological match (the percentage reported). In the classical scenario (A), where the resource availability and consumer demand are symmetric and narrow, phenological asynchrony strongly affects resource supply. In a wide and right-skewed distribution of resource availability, asynchrony has a much smaller effect (B), and changes in resource population abundance may be much more important (C). Food supply may show multiple peaks, for example corresponding to the phenology of several prey species (D), leading to a situation similar to that described in B.
The apparent commonness and accelerating speed of change in phenological synchrony among interacting species (8) suggests important implications for the strength of ecological interactions. Instead of focusing future efforts solely on phenological synchrony, such as the time elapsed between the peaks of two interacting species, we may try to measure the strength of interaction by integrating information throughout the season, simultaneously accounting for the full pattern of phenology and abundance. This should help us answer fundamental questions relating to phenological synchrony. We still do not know whether the changes occurring are adaptive or merely random. Independently of that question, we need to address how changes in phenology and phenological synchrony affect the viability of natural populations, and whether conservation priorities should be adjusted accordingly.
Footnotes
The author declares no conflict of interest.
See companion article on page 5211.
References
- 1.Walther G-R, et al. Ecological responses to recent climate change. Nature. 2002;416:389–395. doi: 10.1038/416389a. [DOI] [PubMed] [Google Scholar]
- 2.Stenseth NC, et al. Ecological effects of climate fluctuations. Science. 2002;297:1292–1296. doi: 10.1126/science.1071281. [DOI] [PubMed] [Google Scholar]
- 3.Knudsen E, et al. Challenging claims in the study of migratory birds and climate change. Biol Rev Camb Philos Soc. 2011;86:928–946. doi: 10.1111/j.1469-185X.2011.00179.x. [DOI] [PubMed] [Google Scholar]
- 4.Durant JM, Hjermann DØ, Ottersen G, Stenseth NC. Climate and the match or mismatch between predator requirements and resource availability. Clim Res. 2007;33:271–283. [Google Scholar]
- 5.Cushing DH. The regularity of the spawning season of some fishes. ICES J Mar Sci. 1969;33:81–92. [Google Scholar]
- 6.Visser ME, Both C, Lambrechts MM. Global climate change leads to mistimed avian reproduction. Adv Ecol Res. 2004;35:89–110. [Google Scholar]
- 7.Jones T, Cresswell W. The phenology mismatch hypothesis: Are declines of migrant birds linked to uneven global climate change? J Anim Ecol. 2010;79:98–108. doi: 10.1111/j.1365-2656.2009.01610.x. [DOI] [PubMed] [Google Scholar]
- 8.Kharouba HM, et al. Global shifts in the phenological synchrony of species interactions over recent decades. Proc Natl Acad Sci USA. 2018;115:5211–5216. doi: 10.1073/pnas.1714511115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dawson TP, Jackson ST, House JI, Prentice IC, Mace GM. Beyond predictions: Biodiversity conservation in a changing climate. Science. 2011;332:53–58. doi: 10.1126/science.1200303. [DOI] [PubMed] [Google Scholar]
- 10.Møller AP, Rubolini D, Lehikoinen E. Populations of migratory bird species that did not show a phenological response to climate change are declining. Proc Natl Acad Sci USA. 2008;105:16195–16200. doi: 10.1073/pnas.0803825105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saino N, et al. Climate warming, ecological mismatch at arrival and population decline in migratory birds. Proc Biol Sci. 2011;278:835–842. doi: 10.1098/rspb.2010.1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vatka E, Orell M, Rytkönen S. Warming climate advances breeding and improves synchrony of food demand and food availability in a boreal passerine. Glob Change Biol. 2011;17:3002–3009. [Google Scholar]
- 13.Reed TE, Grøtan V, Jenouvrier S, Sæther BE, Visser ME. Population growth in a wild bird is buffered against phenological mismatch. Science. 2013;340:488–491. doi: 10.1126/science.1232870. [DOI] [PubMed] [Google Scholar]
- 14.Reed TE, Jenouvrier S, Visser ME. Phenological mismatch strongly affects individual fitness but not population demography in a woodland passerine. J Anim Ecol. 2013;82:131–144. doi: 10.1111/j.1365-2656.2012.02020.x. [DOI] [PubMed] [Google Scholar]
- 15.Morrison CA, Robinson RA, Clark JA, Leech DI, Gill JA. Season-long consequences of shifts in timing of breeding for productivity in Willow Warblers, Phylloscopus trochilus. Bird Study. 2015;62:161–169. [Google Scholar]
- 16.Ramula S, Johansson J, Lindén A, Jonzén N. Linking phenological shifts to demographic change. Clim Res. 2015;63:135–144. [Google Scholar]
- 17.Jonzén N, Hedenström A, Lundberg P. Climate change and the optimal arrival of migratory birds. Proc Biol Sci. 2007;274:269–274. doi: 10.1098/rspb.2006.3719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johansson J, Kristensen NP, Nilsson J-Å, Jonzén N. The eco-evolutionary consequences of interspecific phenological asynchrony – A theoretical perspective. Oikos. 2015;124:102–112. [Google Scholar]
- 19.Both C, et al. Avian population consequences of climate change are most severe for long-distance migrants in seasonal habitats. Proc Biol Sci. 2010;277:1259–1266. doi: 10.1098/rspb.2009.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lindén A, Meller K, Knape J. An empirical comparison of models for the phenology of bird migration. J Avian Biol. 2017;48:255–265. [Google Scholar]