Abstract
Background
Prostate-specific antigen (PSA) screening reduces prostate cancer deaths but leads to harm from overdiagnosis and overtreatment.
Objective
To determine the long-term risk of prostate cancer mortality using kallikrein blood markers measured at baseline in a large population of healthy men to identify men with low risk for prostate cancer death.
Design, setting, participants
Study based on the Malmö Diet and Cancer cohort enrolling 11 506 unscreened men aged 45–73 yr during 1991–1996, providing cryopreserved blood at enrollment and followed without PSA screening to December 31, 2014. We measured four kallikrein markers in the blood of 1223 prostate cancer cases and 3028 controls.
Outcome measurements and statistical analysis
Prostate cancer death (n = 317) by PSA and a prespecified statistical model based on the levels of four kallikrein markers.
Results and limitations
Baseline PSA predicted prostate cancer death with a concordance index of 0.86. In men with elevated PSA (≥2.0 ng/ml), predictive accuracy was enhanced by the four-kallikrein panel compared with PSA (0.80 vs 0.73; improvement 0.07; 95% confidence interval 0.04, 0.10). Nearly half of men aged 60+ yr with elevated PSA had a four-kallikrein panel score of <7.5%, translating into 1.7% risk of prostate cancer death at 15 yr—a similar estimate to that of a man with a PSA of 1.6 ng/ml. Men with a four-kallikrein panel score of ≥7.5% had a 13% risk of prostate cancer death at 15 yr.
Conclusions
A prespecified statistical model based on four kallikrein markers (commercially available as the 4Kscore) reclassified many men with modestly elevated PSA, to have a low long-term risk of prostate cancer death. Men with elevated PSA but low scores from the four-kallikrein panel can be monitored rather than being subject to biopsy.
Patient summary
Men with elevated prostate-specific antigen (PSA) are often referred for prostate biopsy. However, men with elevated PSA but low scores from the four-kallikrein panel can be monitored rather than being subject to biopsy.
Keywords: Prostate-specific antigen, Kallikrein, Screening
1. Introduction
There is clear randomized trial evidence that prostate-specific antigen (PSA) screening reduces prostate cancer mortality [1]. However, PSA has modest specificity for aggressive prostate cancer [2–4], with the result that many men must be screened, biopsied, and diagnosed to prevent one death [1].
There have been extensive efforts to improve the specificity of PSA, including longitudinal change in PSA levels (“PSA velocity” [5]), the ratio of free-to-total PSA [6], and more recently developed markers such as PHI [7], PCA3 [8], or TMPRSS2-ERG [6]. Based on the theory that the enzymatic action of PSA is critical to its function as a biomarker in the blood, we developed a panel of four kallikrein markers in the blood. We have demonstrated in multiple studies that a statistical model based on this panel enhances the specificity of PSA for Gleason score 7 (GGG2) or higher prostate cancer on prostate biopsy [9–12]. Following a prospective, independent, multicenter validation study in the USA [13], the biomarker panel has been made commercially available by OPKO Inc. as the 4Kscore.
One limitation of our prior studies, similar to most other studies of markers for prostate cancer detection, is the use of biopsy outcome as the end point. Gleason grade on biopsy is a surrogate end point and does not have a 1:1 association with prostate cancer morbidity or mortality: a biopsy may miss a GGG2 or higher (high-grade) cancer or detect a cancer that, while seemingly aggressive, would never become apparent to the patient during the course of his natural life. As such, we have an interest in determining whether the four-kallikrein panel can predict the true clinical outcome of prostate cancer morbidity and mortality. We have previously shown that in a largely unscreened population, the four-kallikrein panel can discriminate men with elevated PSA who later developed distant prostate cancer metastases from those who had no evidence of metastases by 10–15 yr [14]. Here, we attempt to replicate the findings of this prior study in a large independent population-based cohort of unscreened men with longer follow-up so that the performance of the four-kallikrein panel for the critical outcome of prostate cancer death can be determined.
2. Patients and methods
2.1. Patient population
The Malmö Diet and Cancer (MDC) project is a large, prospective, observational, population-based study of the relationship between diet and incident cancer that has been described previously [15,16]. In brief, 11 506 men living in Malmö, Sweden, born between 1923 and 1945, participated and provided an anticoagulated blood plasma sample at enrolment during 1991–1996. The Swedish Cancer Registry, the National Prostate Cancer Registry of the Southern Region [17,18], and Swedish National Cause of Death Registry, previously shown to provide highly accurate data, were used to identify men subsequently diagnosed with prostate cancer, along with diagnostic data, as of December 31, 2014. Cause of death was obtained from the Causes of Death Registry at the National Board of Health and Welfare in Sweden, a reliable source of the cause of death from prostate cancer [19]. A personal identity number, unique for every Swedish citizen, was used to track and merge data from the different registries. Rates of PSA screening in this cohort were initially very low, but have increased during the last years of follow-up [20]. Data on follow-up for prostate cancer diagnosis and death from prostate cancer in the MDC cohort were collected until December 31, 2014. Overall, 1476 men were diagnosed with and 317 died from prostate cancer. The kallikrein panel markers were measured for 291 of the prostate cancer deaths and 1223 diagnoses. There were 3028 men without prostate cancer whose kallikrein panel markers were also measured. Details on the selection of men for marker measurement has been reported previously [21]; additional details can also be found in the Supplemental material.
2.2. Laboratory methods
We measured four kallikrein markers—human kallikrein-related peptidase 2 (hK2) and total, free, and intact PSA—in cryopreserved (below −70°C) heparin anticoagulated blood plasma from cases and controls. All laboratory analyses were conducted blind to outcome and case-control status. We measured total and free PSA with the dual-label DELFIA ProStatus assay (PerkinElmer, Turku, Finland) [22], calibrated against the World Health Organization (WHO) 96/670 (PSA-WHO) and WHO 68/668 (free PSA-WHO) standards, in previously unthawed cryopreserved heparin anticoagulated blood plasma. Intact PSA and hK2 were measured using F(ab′)2 fragments of the monoclonal capture antibodies to reduce the frequency of nonspecific assay interference [23].
2.3. Statistical methods
Our primary aim was to assess the ability of a prespecified model using the four kallikrein markers to predict long-term risk of prostate cancer mortality. Total PSA, intact PSA, free PSA, and hK2 from controls who were not selected as a part of the case-control subset were imputed using predictive mean matching across 10 multiple imputation sets. Analytic results were combined from these 10 sets using Rubin’s methods [24].
The four-kallikrein panel of markers were combined as previously described into a prespecified prediction model that provides the risk of Gleason score 7 (GGG2) or higher (high-grade) cancer on prostate biopsy [10]. The model was developed utilizing data from 4765 men who underwent a 10-core biopsy without a prior negative prostate biopsy and had a PSA of >3 ng/ml, who were enrolled in the Prostate Testing for Cancer and Treatment (ProtecT) study. The model included total PSA, free PSA, intact PSA, hK2, and age.
Discrimination of the four-kallikrein panel was assessed in the MDC cohort with Harrell’s concordance index (c-index). The kallikrein panel has previously been shown to be useful among men with elevated PSA and is designed as a reflex test to improve the specificity of PSA [10,11,13,14,25]. our primary focus is the performance of the panel among men with elevated PSA levels. As the markers are subfractions of PSA occurring at about 1–10% of the level of total PSA, the precision at which these markers can be measured in men with total PSAs below median (≈1 ng/ml) is impaired compared with those with higher PSAs, and, therefore, our primary focus is the performance of the panel among men with elevated PSA levels. The discrimination of the panel of four kallikrein markers was compared with total PSA alone, and with another model using total PSA, free PSA, and patient age. This second prespecified model was generated using the same cohort as that used for the full four-kallikrein panel model [10]. Bootstrap resampling was utilized to estimate the 95% confidence intervals (CIs) for the difference in discrimination between the base models and the full four-kallikrein panel model. To assess whether the discrimination of PSA, the four-kallikrein panel, or the difference between the two varied by age, meta-analytic methods were used. The cohort was broken into five age subgroups: 45–54, 55–59, 60–64, 65–59, and 70–73 yr. The discrimination (or difference in discrimination) was estimated in each subgroup, and heterogeneity was assessed using Cochran’s Q. As men with low PSA are not typically considered for biopsy, primary analyses of the four-kallikrein panel were conducted in the subgroup of men with elevated PSAs. Sensitivity analyses were conducted to assess whether the improvement in discrimination of the kallikrein panel over total PSA alone was modified when men with the highest PSA levels were excluded. Analyses were repeated excluding men with PSA >25, 10, and 4 ng/ml.
Kaplan-Meier methods were used to estimate probability of prostate cancer death from time of blood draw. To obtain risk estimates of death from prostate cancer at specific PSA levels, Cox proportional hazards regression was used. PSA was modeled with restricted cubic splines to account for the nonlinear relationship with prostate cancer death. Survival analyses were performed separately for 6243 men aged 45–59 yr, for 5263 men aged 60–73 yr, and for all 11 506 men aged 45–73 yr at baseline. Statistical analyses were completed using Stata 13.1 (StataCorp, College Station, TX, USA).
3. Results
Tables 1 and 2 show the number of men in the cohort, and the number of men who were subsequently diagnosed with prostate cancer and died from prostate cancer by December 31, 2014. There were 317 prostate cancer deaths, with 5605 men being followed for more than 20 yr without this event and a median follow-up of 21 yr among survivors.
Table 1.
Cohort characteristics showing the number of men with four kallikrein panel measurements by age and prostate cancer status
| Age group | Total N | Prostate cancer death | Prostate cancer death, kallikrein panel | Prostate cancer | Prostate cancer, kallikrein panel | Control, kallikrein panel | Control, no kallikrein panel |
|---|---|---|---|---|---|---|---|
| All men | 11 506 | 317 | 291 | 1476 | 1223 | 3028 | 7002 |
| Age <60 yr | 6243 | 72 | 64 | 696 | 521 | 1275 | 4272 |
| Age ≥60 yr | 5263 | 245 | 227 | 780 | 702 | 1753 | 2730 |
Table 2.
Centile of PSA (ng/ml) by age and case control status
| Cohort | Group | 10% | 25% | 50% | 75% | 80% | 90% |
|---|---|---|---|---|---|---|---|
| Age ≥60 yr | All men | 0.4 | 0.7 | 1.3 | 2.7 | 3.1 | 4.9 |
| Prostate cancer death | 1.1 | 2.1 | 4.3 | 8.8 | 11.9 | 21.9 | |
| Prostate cancer | 1.0 | 1.8 | 3.4 | 7.2 | 8.1 | 13.4 | |
| Non–prostate cancer control | 0.4 | 0.7 | 1.2 | 2.2 | 2.6 | 3.7 | |
| Non–prostate cancer imputed | 0.4 | 0.6 | 1.1 | 2.2 | 2.6 | 3.6 | |
| Age <60 yr | All men | 0.3 | 0.5 | 0.8 | 1.3 | 1.5 | 2.2 |
| Prostate cancer death | 0.6 | 1.4 | 2.5 | 5.1 | 6.1 | 8.0 | |
| Prostate cancer | 0.7 | 0.9 | 1.5 | 2.6 | 3.0 | 4.7 | |
| Non–prostate cancer control | 0.3 | 0.5 | 0.7 | 1.2 | 1.3 | 1.9 | |
| Non–prostate cancer imputed | 0.3 | 0.5 | 0.7 | 1.2 | 1.3 | 1.8 |
PSA = prostate-specific antigen.
Baseline PSA predicted prostate cancer death with a c-index of 0.862 (95% CI 842, 887) among all men aged 45–73 yr. Table 3 shows the improvement in discrimination of the kallikrein panel over PSA alone. As expected, the kallikrein markers did not improve discrimination over PSA among all men. The panel, however, improved upon PSA alone among men with elevated PSA. In men with PSA ≥1.0 ng/ml, predictive accuracy of prostate cancer death was significantly enhanced by the four-kallikrein panel at baseline compared with PSA alone, with the c-index increasing by 0.039 (95% CI 0.012, 0.062) from 0.800 to 0.840. The improvement in discrimination of the four-kallikrein panel increased monotonically with the PSA threshold. A c-index improvement of 0.067 (95% CI 0.040, 0.102) from 0.735 to 0.803 was observed in men with PSA ≥2.0 ng/ml at baseline (Table 3). Moreover, the four-kallikrein panel had higher discrimination than the model including age, PSA, and free PSA in this same subset of men (c-index of 0.767 for age, PSA, and free PSA; improvement 0.036; 95% CI 0.021, 0.052; Supplementary Table 5), demonstrating the predictive value of the two additional kallikrein markers (intact PSA and hK2). We did not find evidence that the discrimination of PSA, the four-kallikrein panel, or the difference in discrimination between the two varied by age (p = 0.4, 0.9, and 0.4, respectively; Supplementary Fig. 1).
Table 3.
Discrimination of PSA (ng/ml), age with free and total PSA, and age with the four kallikrein panel for predicting death from prostate cancer
| PSA subgroup | N | Prostate cancer death N | Model | C-index | Difference from PSA alone | 95% CI |
|---|---|---|---|---|---|---|
| PSA < 1.0 | 5734 | 28 | Total PSA | 0.628 | – | – |
| Age + free and total PSA | 0.625 | –0.003 | –0.163, 0.160 | |||
| Kallikrein panel | 0.683 | 0.055 | –0.091, 0.191 | |||
| PSA ≥ 0.0 | 11 506 | 317 | Total PSA | 0.862 | – | – |
| Age + free and total PSA | 0.819 | –0.043 | –0.069, –0.016 | |||
| Kallikrein panel | 0.864 | 0.003 | –0.020, 0.023 | |||
| PSA ≥ 1.0 | 5772 | 289 | Total PSA | 0.800 | – | – |
| Age + free and total PSA | 0.806 | 0.005 | –0.024, 0.033 | |||
| Kallikrein panel | 0.840 | 0.039 | 0.012, 0.062 | |||
| PSA ≥ 1.5 | 3561 | 267 | Total PSA | 0.747 | – | – |
| Age + free and total PSA | 0.782 | 0.035 | 0.002, 0.069 | |||
| Kallikrein panel | 0.818 | 0.071 | 0.042, 0.102 | |||
| PSA ≥ 1.8 | 2888 | 250 | Total PSA | 0.730 | – | – |
| Age + free and total PSA | 0.766 | 0.036 | 0.004, 0.069 | |||
| Kallikrein panel | 0.800 | 0.070 | 0.040, 0.103 | |||
| PSA ≥ 2.0 | 2556 | 233 | Total PSA | 0.735 | – | – |
| Age + free and total PSA | 0.767 | 0.031 | 0.001, 0.065 | |||
| Kallikrein panel | 0.803 | 0.067 | 0.040, 0.102 | |||
| PSA ≥ 3.0 | 1455 | 184 | Total PSA | 0.720 | – | – |
| Age + free and total PSA | 0.750 | 0.029 | 0.000, 0.060 | |||
| Kallikrein panel | 0.785 | 0.065 | 0.034, 0.099 | |||
| PSA ≥ 3.5 | 1152 | 174 | Total PSA | 0.685 | – | – |
| Age + free and total PSA | 0.741 | 0.056 | 0.021, 0.090 | |||
| Kallikrein panel | 0.775 | 0.090 | 0.053, 0.125 |
CI = confidence interval; PSA = prostate-specific antigen.
When men with PSA >25 were excluded, the estimated improvement in discrimination for the kallikrein panel over PSA along was 0.078. Excluding men with PSA >10, the improvement was 0.093, and it was 0.123 after excluding men with PSA >4. These results show that the improvement of the kallikrein panel over PSA along is robust when men with the highest PSAs are excluded from the analyses.
Among the 5263 men providing blood at age 60–73 yr, 245 died from prostate cancer leading to a 15-yr risk of prostate cancer death of 3.1% (2.6–3.7%). The 15-yr risk of prostate cancer death more than doubles to 7.7% (95% CI 6.4%, 9.2%) among those with baseline PSA ≥2.0 ng/ml (65th PSA percentile; Fig. 1, Table 4, and Supplementary Table 3). Among these men, 46% had a four-kallikrein panel risk score of <7.5%, with low 10- and 15-yr risks of prostate cancer death (0.55% [95% CI 0.16–1.5%] and 1.7% [95% CI 0.85–3.1%], respectively). By contrast, the 15-yr risk of death is 11.6% (95% CI 9.6%, 13.9%) for a man with PSA ≥2.0 ng/ml and four-kallikrein panel risk ≥7.5% at age 60–73 yr. When using age, free PSA, and total PSA to reclassify men with elevated PSA as low risk using the same 7.5% risk threshold, the 10- and 15-yr risks of prostate cancer death are larger than those when using the kallikrein panel (1.2% and 2.4%, respectively). At 10 yr, the risk of prostate cancer death is more than double in the low-risk group identified by age, and total and free PSA compared with the low-risk group identified by the kallikrein panel. Results were similar for longer follow-up.
Fig. 1.
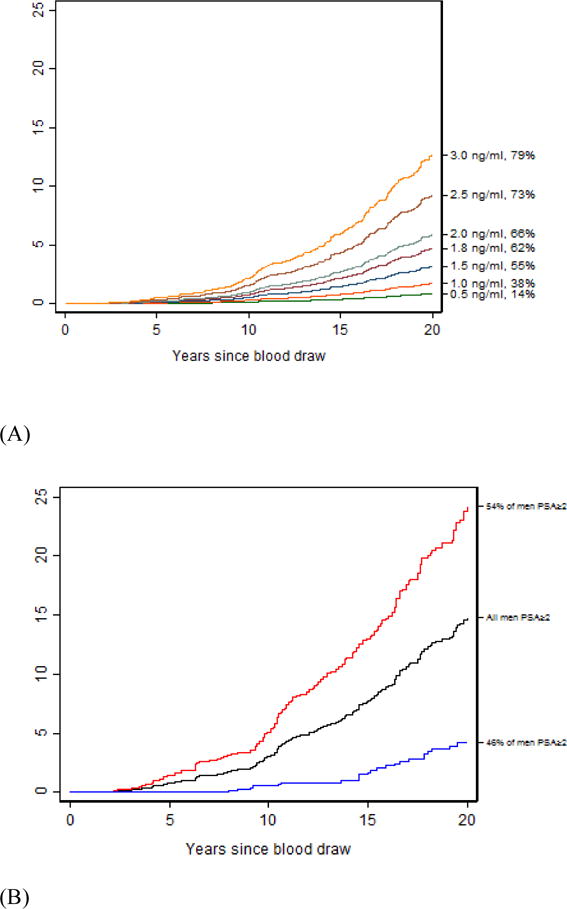
Risk of death from prostate cancer among men 60 yr and older. (A) Risk of death from prostate cancer at varying PSA (ng/ml) levels at baseline (PSA and PSA percentile on right y axis). (B) Risk of death from prostate cancer among men providing blood at age 60–73 yr who had PSA ≥2.0 ng/ml, split by four kallikrein panel risk at 7.5% at baseline. The black line indicates the risk of prostate cancer death among all men with baseline PSA ≥2.0 ng/ml, red line indicates the risk of prostate cancer death among men with PSA ≥2.0 ng/ml and four kallikrein panel score ≥7.5% at baseline, and blue line indicates risk of prostate cancer death among men with PSA ≥2.0 ng/ml and four kallikrein panel score <7.5% at baseline. PSA = prostate specific antigen.
Table 4.
Risk of prostate cancer death within PSA subgroups split by risk from the four kallikrein panel
| N | Prostate cancer death | Reclassified | 5-yr risk | 10-yr risk | 15-yr risk | 20-yr risk | |
|---|---|---|---|---|---|---|---|
| Men providing a blood sample at age 60–73 yr | |||||||
| PSA ≥ 2.0 ng/ml | 1825 | 191 | 0.80 (0.45–1.35) | 3.00 (2.22–3.95) | 7.73 (6.39–9.23) | 14.80 (12.72–17.04) | |
| Panel risk | |||||||
| <6.0% | 664 | 13 | 36% | 0 (NA) | 0.17 (0.01–1.22) | 1.04 (0.35–2.49) | 3.33 (1.72–5.79) |
| ≥6.0% | 1160 | 178 | 64% | 1.27 (0.70–2.13) | 4.64 (3.42–6.11) | 11.61 (9.56–13.86) | 21.49 (18.39–24.76) |
| <7.5% | 842 | 24 | 46% | 0 (NA) | 0.55 (0.16–1.45) | 1.70 (0.85–3.05) | 4.24 (2.64–6.40) |
| ≥7.5% | 983 | 167 | 54% | 1.49 (0.82–2.51) | 5.12 (3.74–6.80) | 13.00 (10.65–15.59) | 24.21 (20.62–27.98) |
| <10% | 1075 | 37 | 59% | 0 (NA) | 0.86 (0.38–1.71) | 2.16 (1.28–3.41) | 5.12 (3.54–7.09) |
| ≥10% | 750 | 154 | 41% | 1.92 (1.06–3.23) | 5.99 (4.30–8.06) | 15.59 (12.69–18.76) | 28.87 (24.41–33.48) |
| Men providing a blood sample at age 45–59 yr | |||||||
| PSA ≥ 1.5 ng/ml | 1184 | 53 | 0 (NA) | 0.45 (0.16–1.09) | 2.32 (1.49–3.44) | 4.86 (3.57–6.43) | |
| Panel risk | |||||||
| <6.0% | 858 | 22 | 73% | 0 (NA) | 0 (NA) | 0.97 (0.40–2.03) | 2.63 (1.56–4.17) |
| ≥6.0% | 326 | 31 | 27% | 0 (NA) | 1.63 (0.54–3.86) | 5.77 (3.33–9.14) | 10.70 (7.11–15.11) |
| <7.5% | 962 | 28 | 82% | 0 (NA) | 0.22 (0.04–0.89) | 1.21 (0.59–2.24) | 3.11 (1.99–4.62) |
| ≥7.5% | 222 | 25 | 18% | 0 (NA) | 1.43 (0.31–4.37) | 6.87 (3.70–11.36) | 12.16 (7.62–17.83) |
| <10% | 1056 | 32 | 89% | 0 (NA) | 0.20 (0.03–0.81) | 1.44 (0.77–2.46) | 3.30 (2.19–4.76) |
| ≥10% | 128 | 21 | 11% | 0 (NA) | 2.41 (0.50–7.29) | 8.89 (4.32–15.52) | 16.32 (9.68–24.47) |
NA = not available; PSA = prostate-specific antigen.
Among the 6243 men aged 45–59 yr with a baseline PSA of ≥1.5 ng/ml (81st PSA percentile), the 15- and 20-yr risks of prostate cancer death are 2.3% and 4.9%. Eighty-two percent of the men aged 45–59 yr with a baseline PSA of ≥1.5 ng/ml have a four-kallikrein panel risk of <7.5%, with a low risk of death at 15 yr (1.2%; 95% CI 0.6%, 2.2%) and 20 yr (3.1%; 95% CI 2.0%, 4.6%). By contrast, 15-yr (6.9%; 95% CI 3.7%, 11.3%) and 20-yr (12.2%; 95% CI 7.6%, 17.8%) risks of death are about four- to six-fold higher in a man with PSA ≥1.5 ng/ml and four-kallikrein panel risk of ≥7.5%.
4. Discussion
A prespecified model based on four kallikrein markers (total PSA, free PSA, intact PSA, and hK2) increases the predictive discrimination for 15–20-yr risk of prostate cancer death among a population-based cohort of men aged 45–73 yr with elevated PSA. This is the first report where the four-kallikrein panel has shown to be able to predict the long-term risk of death from prostate cancer.
PSA exhibited excellent predictive discrimination for prostate cancer death with a c-index for all men of 0.86. Among men with elevated PSA, the four-kallikrein panel enhanced the discrimination of PSA alone, with the improvement increasing with PSA thresholds. For example, among men with PSA ≥2 ng/ml, PSA had a c-index of 0.74 (note that this is lower than the value of 0.86 for all men, as it excludes men with low PSA and low risk). Adding age and free PSA to total PSA increased the discrimination to 0.77; the full four-kallikrein panel increased the discrimination to 0.80 (95% CI for difference 0.040, 0.102). The improvement in the discrimination among men with elevated PSA suggests that the four-kallikrein panel would be beneficial as a reflex test after PSA measurement.
Among men with modestly elevated PSA, the four-kallikrein panel is able to identify a subset of men who are at low risk of dying from prostate cancer. For example, a man who has had a PSA of >2 ng/ml may consider prostate biopsy. Our findings suggest that such a man should be spared from biopsy if he has a low risk from the four-kallikrein panel. If, for instance, he has a four-kallikrein panel score below 7.5%, his risk of prostate cancer death is equivalent to that of a man with a PSA of 1.6 ng/ml, well below the population mean risk at 15 yr (3.1%; such a man would not be considered for prostate biopsy; Supplementary Table 2). Similar results were observed among younger men, although the difference in risk reclassification was not as large. For example, younger men aged 45–59 yr with a PSA of ≥1.5 ng/ml and a kallikrein risk score below 7.5% have a 15-yr risk of prostate cancer death of 1.2%. While the reclassified risk is not below the low population average of 0.5%, it is much lower compared with men with kallikrein risk above 7.5% by a factor of nearly 6.
These results support the inclusion of the kallikrein panel in prostate cancer guidelines [26,27]. Our finding that men who have PSA <1 ng/ml have an extremely low risk of prostate cancer mortality (Fig. 2A), even at 15–20 yr of follow-up, supports guideline recommendations that the frequency of screening be individualized by PSA level, with men who have low PSA recommended to have much less frequent screens at 5–10-yr intervals. Most guidelines [27,28] recommend that screening ends, or is greatly restricted, for men aged over 70 yr. The data presented in Figure 1A, demonstrating a low risk of prostate cancer death at 20 yr for older men with PSA <2–3 ng/ml, provide evidence for this recommendation. This is because men with lower PSA would likely die of other causes by the age of 90 yr (ie, 20-yr follow-up for a 70-yr-old man) before any substantial risk of prostate cancer mortality. While our results suggest that it might be justified to continue follow-up of a 70-yr-old man with a PSA of 2–3 ng/ml, this should likely be considered clinical follow-up of an abnormal value, rather than screening per se. Our study confirms the findings of many previous observational studies showing that PSA is strongly predictive of the long-term risk of clinically diagnosed prostate cancer and prostate cancer death [29]. For instance, in a prior study from the Malmö cohort, we found that 90% of prostate cancer deaths by the age of 85 yr occur in men with PSA ≥2 ng/ml at the age of 60 yr [2]. We also replicated the findings of Stattin et al [14] who reported that the kallikrein markers improved the prediction of the long-term risk of metastasis in men with elevated PSA followed for many years without screening, where they report that the kallikrein panel can be used as a reflex test to aid in biopsy decision making. Our findings support the many studies of the four-kallikrein panel in biopsy settings [30]. In the key validation study leading to commercialization of the 4Kscore, Parekh et al [13] reported that the panel had an area under the curve of 0.82 for high-grade disease, compared with 0.74 for a standard risk calculator. The association between the panel and prostate cancer mortality, reported here, suggest that the findings of Parekh et al [13] are not weakened by the use of biopsy outcome as an end point.
Fig. 2.
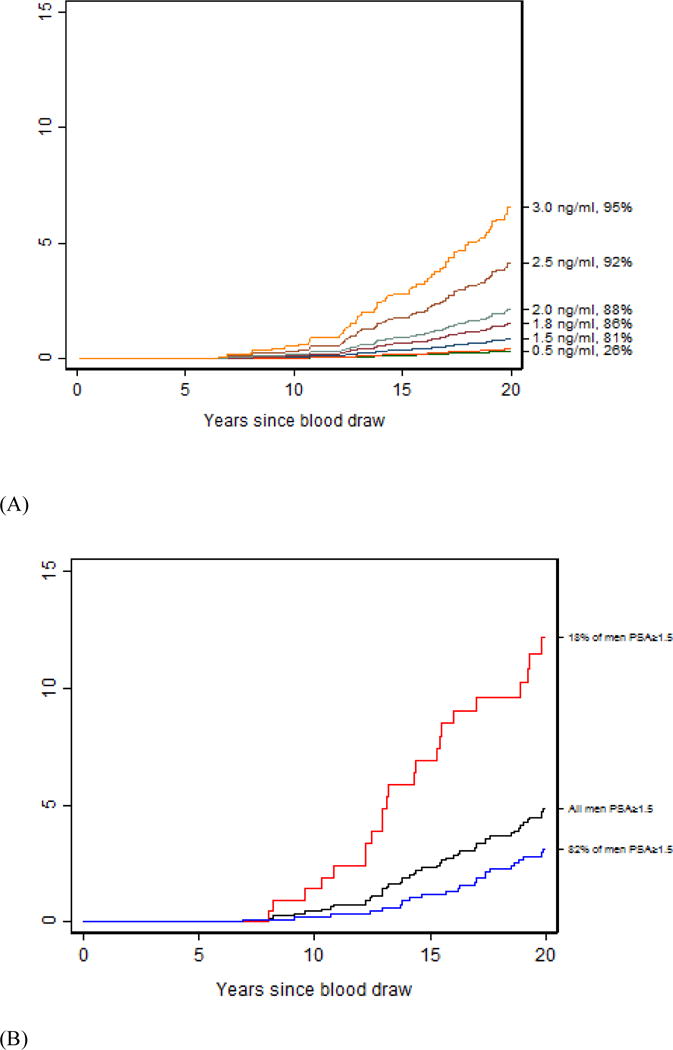
Risk of death from prostate cancer among men 59 yr and younger. (A) Risk of death from prostate cancer at varying levels of PSA at baseline (PSA and PSA percentile on right y axis). (B) Risk of death from prostate cancer among men providing blood at age 45–59 yr who had PSA ≥1.5 ng/ml, split by four kallikrein panel risk at 7.5% at baseline. The black line indicates the risk of prostate cancer death among all men with baseline PSA ≥1.5 ng/ml, red line indicates the risk of prostate cancer death among men with PSA ≥1.5 ng/ml and four kallikrein panel score ≥7.5% at baseline, and blue line indicates the risk of prostate cancer death among the men with PSA ≥1.5 ng/ml and four kallikrein panel score <7.5% at baseline. PSA = prostate specific antigen.
While most of the prostate cancer cases diagnosed in this cohort were found clinically outside of PSA screening, the use of PSA screening has increased in Sweden in recent years, leading to a rise in the number of screen-detected prostate cancer cases. PSA screening in the cohort would have the effect of increasing the discrimination of PSA for prostate cancer diagnosis (due to an ascertainment bias) but decreasing discrimination for mortality, due to what would have otherwise been fatal cases being detected by PSA screening at a curable stage. That said, PSA screening was not used in Sweden until the mid-1990s, and not widely disseminated until the 2000s [20], and therefore, the impact of PSA screening is unlikely to have an important effect on our key estimate for the marginal improvement in predictive discrimination associated with the four-kallikrein panel for predicting death from prostate cancer. Further details on opportunistic PSA testing are found in the Supplementary material. A second limitation is that the cohort is predominantly Caucasian. However, there are clear data that the utility of the four-kallikrein panel is comparable in African Americans [12].
5. Conclusions
In a large population-based cohort of healthy unscreened men, the four-kallikrein panel reclassified many men with elevated PSA, considered at an increased risk of prostate cancer death by PSA alone, to have a low 20-yr risk of prostate cancer death. The results from this study build upon prior reports of the utility of the kallikrein markers to predict aggressive cancer on biopsy and the development of distant metastases up to 15 yr subsequently. Men with elevated PSA but low scores from the four-kallikrein panel can be monitored rather than being subject to biopsy.
Supplementary Material
Acknowledgments
Funding/Support and role of the sponsor: This work was supported in parts by grants from National Cancer Institute (R01CA160816, R01 CA175491, P50-CA92629, and P30-CA008748), the Sidney Kimmel Center for Prostate and Urologic Cancers, and David H. Koch through the Prostate Cancer Foundation, the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre Program in UK, the Swedish Cancer Society (project no. 14-0722), and the Swedish Research Council (VR-MH project no. 2016-02974).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions: Hans G. Lilja had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Sjoberg, Vickers, Dahlin, Lilja.
Acquisition of data: Vickers, Dahlin, Ulmert, Lilja.
Analysis and interpretation of data: Sjoberg, Vickers, Assel, Poon, Lilja.
Drafting of the manuscript: Sjoberg, Vickers, Lilja.
Critical revision of the manuscript for important intellectual content: Sjoberg, Vickers, Assel, Dahlin, Poon, Ulmert, Lilja.
Statistical analysis: Sjoberg, Vickers, Assel, Lilja.
Obtaining funding: Vickers, Lilja.
Administrative, technical, or material support: Dahlin, Lilja.
Supervision: Vickers, Lilja.
Other: None.
Financial disclosures: Hans G. Lilja certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Hans Lilja holds patents for free PSA, hK2, and intact PSA assays, and is named, along with Andrew J. Vickers, on a patent for a statistical method to detect prostate cancer. The marker assay patents and the patent for the statistical model have been licensed and commercialized as the 4Kscore by OPKO Diagnostics. Drs. Vickers and Lilja receive royalties from sales of this test. Additionally, Dr. Lilja owns stock and Dr. Vickers owns stock options in OPKO.
References
- 1.Schroder FH, Hugosson J, Roobol MJ, et al. Screening and prostate cancer mortality: results of the European Randomised Study of Screening for Prostate Cancer (ERSPC) at 13 years of follow-up. Lancet. 2014;384:2027–35. doi: 10.1016/S0140-6736(14)60525-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vickers AJ, Cronin AM, Bjork T, et al. Prostate specific antigen concentration at age 60 and death or metastasis from prostate cancer: case-control study. BMJ. 2010;341:c4521. doi: 10.1136/bmj.c4521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vickers AJ, Ulmert D, Sjoberg DD, et al. Strategy for detection of prostate cancer based on relation between prostate specific antigen at age 40-55 and long term risk of metastasis: case-control study. BMJ. 2013;346:f2023. doi: 10.1136/bmj.f2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Preston MA, Batista JL, Wilson KM, et al. Baseline prostate-specific antigen levels in midlife predict lethal prostate cancer. J Clin Oncol. 2016;34:2705–11. doi: 10.1200/JCO.2016.66.7527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vickers AJ, Till C, Tangen CM, Lilja H, Thompson IM. An empirical evaluation of guidelines on prostate-specific antigen velocity in prostate cancer detection. J Natl Cancer Inst. 2011;103:462–9. doi: 10.1093/jnci/djr028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tomlins SA, Aubin SM, Siddiqui J, et al. Urine TMPRSS2:ERG fusion transcript stratifies prostate cancer risk in men with elevated serum PSA. Sci Transl Med. 2011;3:94ra72. doi: 10.1126/scitranslmed.3001970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Catalona WJ, Partin AW, Sanda MG, et al. A multicenter study of [-2]pro-prostate specific antigen combined with prostate specific antigen and free prostate specific antigen for prostate cancer detection in the 2.0 to 10.0 ng/ml prostate specific antigen range. J Urol. 2011;185:1650–5. doi: 10.1016/j.juro.2010.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Groskopf J, Aubin SM, Deras IL, et al. APTIMA PCA3 molecular urine test: development of a method to aid in the diagnosis of prostate cancer. Clin Chem. 2006;52:1089–95. doi: 10.1373/clinchem.2005.063289. [DOI] [PubMed] [Google Scholar]
- 9.Vickers A, Cronin A, Roobol M, et al. Reducing unnecessary biopsy during prostate cancer screening using a four-kallikrein panel: an independent replication. J Clin Oncol. 2010;28:2493–8. doi: 10.1200/JCO.2009.24.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bryant RJ, Sjoberg DD, Vickers AJ, et al. Predicting high-grade cancer at ten-core prostate biopsy using four kallikrein markers measured in blood in the ProtecT study. J Natl Cancer Inst. 2015;107 doi: 10.1093/jnci/djv095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Braun K, Sjoberg DD, Vickers AJ, Lilja H, Bjartell AS. A four-kallikrein panel predicts high-grade cancer on biopsy: independent validation in a community cohort. Eur Urol. 2016;69:505–11. doi: 10.1016/j.eururo.2015.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim EH, Andriole GL, Crawford ED, et al. Detection of high grade prostate cancer among PLCO participants using a prespecified 4-kallikrein marker panel. J Urol. 2017;197:1041–7. doi: 10.1016/j.juro.2016.10.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parekh DJ, Punnen S, Sjoberg DD, et al. A multi-institutional prospective trial in the USA confirms that the 4Kscore accurately identifies men with high-grade prostate cancer. Eur Urol. 2015;68:464–70. doi: 10.1016/j.eururo.2014.10.021. [DOI] [PubMed] [Google Scholar]
- 14.Stattin P, Vickers AJ, Sjoberg DD, et al. Improving the specificity of screening for lethal prostate cancer using prostate-specific antigen and a panel of kallikrein markers: a nested case-control study. Eur Urol. 2015;68:207–13. doi: 10.1016/j.eururo.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pero RW, Olsson A, Berglund G, Janzon L, Larsson SA, Elmståhl S. The Malmo biological bank. J Intern Med. 1993;233:63–7. doi: 10.1111/j.1365-2796.1993.tb00650.x. [DOI] [PubMed] [Google Scholar]
- 16.Manjer J, Carlsson S, Elmstahl S, et al. The Malmo Diet and Cancer Study: representativity, cancer incidence and mortality in participants and non-participants. Eur J Cancer Prev. 2001;10:489–99. doi: 10.1097/00008469-200112000-00003. [DOI] [PubMed] [Google Scholar]
- 17.Sandblom G, Dufmats M, Olsson M, Varenhorst E. Validity of a population-based cancer register in Sweden–an assessment of data reproducibility in the South-East Region Prostate Cancer Register. Scand J Urol Nephrol. 2003;37:112–9. doi: 10.1080/00365590310008839. [DOI] [PubMed] [Google Scholar]
- 18.Van Hemelrijck M, Wigertz A, Sandin F, et al. Cohort profile: the National Prostate Cancer Register of Sweden and Prostate Cancer data Base Sweden 2.0. Int J Epidemiol. 2013;42:956–67. doi: 10.1093/ije/dys068. [DOI] [PubMed] [Google Scholar]
- 19.Fall K, Stromberg F, Rosell J, Andrèn O, Varenhorst E, South-East Region Prostate Cancer Group Reliability of death certificates in prostate cancer patients. Scand J Urol Nephrol. 2008;42:352–7. doi: 10.1080/00365590802078583. [DOI] [PubMed] [Google Scholar]
- 20.Stattin P, Carlsson S, Holmstrom B, et al. Prostate cancer mortality in areas with high and low prostate cancer incidence. J Natl Cancer Inst. 2014;106:dju007. doi: 10.1093/jnci/dju007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lilja H, Ulmert D, Bjork T, et al. Long-term prediction of prostate cancer up to 25 years before diagnosis of prostate cancer using prostate kallikreins measured at age 44 to 50 years. J Clin Oncol. 2007;25:431–6. doi: 10.1200/JCO.2006.06.9351. [DOI] [PubMed] [Google Scholar]
- 22.Mitrunen K, Pettersson K, Piironen T, Björk T, Lilja H, Lövgren T. Dual-label one-step immunoassay for simultaneous measurement of free and total prostate-specific antigen concentrations and ratios in serum. Clin Chem. 1995;41:1115–20. [PubMed] [Google Scholar]
- 23.Vaisanen V, Peltola MT, Lilja H, Nurmi M, Pettersson K. Intact free prostate-specific antigen and free and total human glandular kallikrein 2. Elimination of assay interference by enzymatic digestion of antibodies to F(ab′)2 fragments. Anal Chem. 2006;78:7809–15. doi: 10.1021/ac061201+. [DOI] [PubMed] [Google Scholar]
- 24.Rubin DB. Multiple imputation for nonresponse in surveys. New York, NY: John Wiley & Sons; 2004. [Google Scholar]
- 25.Benchikh A, Savage C, Cronin A, et al. A panel of kallikrein markers can predict outcome of prostate biopsy following clinical work-up: an independent validation study from the European Randomized Study of Prostate Cancer screening, France. BMC Cancer. 2010;10:635. doi: 10.1186/1471-2407-10-635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carroll PR, Parsons JK, Andriole G, et al. NCCN guidelines insights: prostate cancer early detection, version 2.2016. J Natl Compr Canc Netw. 2016;14:509–19. doi: 10.6004/jnccn.2016.0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vickers AJ, Eastham JA, Scardino PT, Lilja H. The Memorial Sloan Kettering Cancer Center recommendations for prostate cancer screening. Urology. 2016;91:12–8. doi: 10.1016/j.urology.2015.12.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carter HB, Albertsen PC, Barry MJ, et al. Early detection of prostate cancer: AUA guideline. J Urol. 2013;190:419–26. doi: 10.1016/j.juro.2013.04.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Loeb S, Carter HB, Catalona WJ, Moul JW, Schroder FH. Baseline prostate-specific antigen testing at a young age. Eur Urol. 2012;61:1–7. doi: 10.1016/j.eururo.2011.07.067. [DOI] [PubMed] [Google Scholar]
- 30.McDonald ML, Parsons JK. 4-Kallikrein test and kallikrein markers in prostate cancer screening. Urol Clin North Am. 2016;43:39–46. doi: 10.1016/j.ucl.2015.08.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


