Abstract
The aim of this study was to evaluate the pattern of protein expression of the steroid receptor isoforms of nuclear progesterone receptors (PGR) A and B, and estrogen receptors (ESR1 and 2) in utero-placental compartments during early pregnancy. Utero-placental tissues were collected from days 14-30 (n=4 ewes/day), and uterine tissues were collected from non-pregnant ewes on day 10 after estrus (n=4). Cross sections of formalin-fixed and paraffin embedded tissues were immunofluorescently stained to detect PGRAB, PGRB, ESR1 and ESR2, followed by image generation of entire cross-sections of uterine and utero-placental tissues, confocal imaging of individual uterine and utero-placental compartments, and image and statistical analyses. PGRAB, PGRB, ESR1 and ESR2 were detected in several compartments of uterine and utero-placental tissues. Quantitative image analysis of staining intensity demonstrated that compared to non-pregnant controls 1) expression of PGRAB and PGRB was less in luminal epithelium and endometrial glands from day 14-16 till 30; 2) PGRAB expression tended to be greater in endometrial and myometrial blood vessels on days 28 and/or 30; 3) PGRB expression in myometrum was lower on days 16 and 28; 4) ESR1 in endometrial stroma was lower in all days of pregnancy; 5) ESR2 expression was similar in all compartments and not affected by pregnancy stage; and 6) in FM, expression of steroid receptors was similar. Thus, we have demonstrated spatial and temporal expression of nuclear PGR and ESR isoforms in utero-placental compartments during early pregnancy.
Keywords: Sheep, early pregnancy, placenta, steroid receptors, immunofluorescence
Graphical abstract
1. Introduction
Early pregnancy is marked by several events such as implantation, placentation and initiation of fetal and placental growth, which are critical for establishment of pregnancy [1,2]. Around 30-50% of embryonic loss occurs during early pregnancy [3]. Among various factors, an unsynchronized uterine environment due to an altered function and/or production of steroid hormones, progesterone (P4) and estradiol-17β (E2), can contribute to embryo loss during early pregnancy. Thus, steroids, major regulators of utero-placental development and function, are recognized as critical factors determining successful pregnancy establishment and maintenance [4-7].
Progesterone and E2 act via their steroid receptors PGR and ESR, respectively [7-9]. Nuclear PGR has two protein isoforms PGRA (81 kDa) and PGRB (116 kDa). Both isoforms are encoded by a single gene as a result of transcription from two alternative promoters and translation initiation at two different initiation codons [8]. On the other hand, ESR1 and ESR2 are products of two distinct genes [10]. In ovine uterine tissues, estrogens increase the expression of their own receptors as well as PGR [11-13]. In contrast, P4 downregulates the expression of PGR and ESR in uterine tissues both in non-pregnant and pregnant ewes, and also in other species [11,14-18].
The spatio-temporal expression of PGR and ESR in uterine and utero-placental tissues, and in fetal membranes (FM) has been demonstrated for several species such as mice, cows, horses, pigs, sheep and primates from early to late pregnancy [19-22]. We have recently demonstrated that mRNA expression of PGR and ESR changes in ovine utero-placental tissues during early pregnancy [1]. However, despite their potential importance, limited studies have evaluated the localization and expression of PGR and ESR isoforms protein in utero-placenta during early pregnancy.
We hypothesized that localization and expression of steroid receptors protein would change in a time- and compartment-dependent manner in utero-placental tissues during early pregnancy. To test this hypothesis, present study was designed to 1) immunolocalize and 2) evaluate expression level (based on immunofluorescent staining and intensity of fluorescence determined using image analysis) of steroid receptor isoform proteins, PGRAB, PGRB, ESR1 and ESR2, in utero-placental compartments including luminal epithelium (LE), endometrial glands (EG) and stroma (ES), endometrial and myometrial blood vessels (EBV and MBV, respectively) and myometrium (Myo) on days 14-30 of early pregnancy after natural mating, and 3) compare steroid receptor expression during pregnancy to non-pregnant ewes.
2. Materials and Methods
2.1. Animals and experimental design
The Institutional Animal Care and Use Committee at NDSU approved all animal procedures in this study. The experimental design, tissues collection and processing has been described in detail before [1,23,24]. Briefly, 24 h after CIDR removal, ewes were exposed to a fertile ram and allowed to breed. Gravid uteri were obtained from the ewes (n = 4/day) on days 14, 16, 18, 20, 24, 26, 28, and 30 after mating, and from non-pregnant (day 10 after estrus; mid-luteal phase; n = 4) controls. Days 14-30 of early pregnancy were chosen as they encompass the period of implantation and placentation (i.e., from early attachment through establishment of the placenta) as well as the critical period for pregnancy establishment and embryo loss [23,24]. Cross sections (approximately 0.5-cm thick) of the entire gravid uterus including fetal membranes (FM, chorioallantois) were fixed by immersion in formalin and then embedded in paraffin.
2.2 Immunofluorescent staining
Immunohistochemistry was performed as described before [23,24]. Briefly, tissues were sectioned at 3 μm, mounted onto slides and de-paraffinized in xylene and graded alcohol. Tissue sections underwent antigen recovery by boiling in 0.01 M citric buffer followed by cooling to room temperature and followed by incubation with a specific primary antibody and then with a secondary antibody. Table 1 presents the source and dilution of primary and fluorescently-labeled secondary antibodies for all antigens immunodetected, and incubation conditions. Tissue sections were then stained with DAPI (Biotium; Fremont, CA, USA) for nuclear detection. For control staining the primary antibody was replaced with mouse or rabbit IgG.
Table 1.
Antibodies used to detect PGRAB, PGRB, ESR1 and ESR2 by immunofluorescence in the uterus from non-pregnant and utero-placenta from early pregnancy
| Antigen | 1° antibody source, dilution and incubation time | 2° antibody source, dilution and incubation time |
|---|---|---|
| PGRAB | mouse monoclonal, PR Ab-8 (Clone h PRa 2+ h PRa 3), Cat # MS-298-P0, Thermo Scientific, Fremont, CA; 1:40; overnight, 4°C | CF633 (fluorescent dye) goat anti-mouse IgG, Cat # 20250-1, 0.5 mL, Biotium, Fremont, CA; 1:250;1 hour, Room temperature |
| PGRB | mouse monoclonal, PR Ab-2 (Clone h PRa 2), Cat # MS-192-P0, Thermo Scientific, Fremont, CA; 1:40; overnight, 4°C | CF633 (fluorescent dye) goat anti- mouse IgG, Cat # 20250-1, 0.5 mL, Biotium, Fremont, CA; 1:250;1 hour, Room temperature |
| ESR1 | rabbit polyclonal, Cat # SC-7207, Santa Cruz Biotechnology, Dallas, TX; 1:50; overnight, 4°C | CF633 (fluorescent dye) goat anti-rabbit IgG, Cat # 202122-1, 0.5 mL, Biotium, Fremont, CA; 1:250; 1 hour, Room temperature |
| ESR2 | Rabbit polyclonal, Cat # sc-8974, Santa Cruz Biotechnology, Dallas, TX; 1:25; overnight, 4°C | CF633 (fluorescent dye) goat anti-rabbit IgG, Cat # 202122-1, 0.5 mL, Biotium, Fremont, CA; 1:250;1 hour, Room temperature |
2.3. Image generation and analysis
Images of entire cross sections of the stained utero-placental tissues (2 sections/animal) were generated using the MosaiX option of Zeiss Axiovision Imaging software and a Zeiss AxioImager M2 (Zeiss Inc., Thornwood, NY). Confocal images of individual compartments (n=4-6) including FM, LE, EG, ES, EBV, MBV and Myo were generated using a Zeiss AxioObserver Z1 microscope (Zeiss Inc). Image analyses (Image-Pro Plus, Ver. 5.0; Media Cybernetics, Inc., Silver Spring, MD) were conducted to determine the fluorescence intensity of PGRAB, PGRB, ESR1 and ESR2 in the compartments listed above. Then, the average value for each compartment and each sheep was calculated for statistical analysis.
2.4. Statistical Analysis
Data were analyzed using the general linear models (GLM) procedure of SAS, with the main effect of day of pregnancy and/or estrous cycle, and “an animal” was treated as experimental unit. When the F-test was significant (P < 0.05), differences between specific means were evaluated by using the least significant difference test [25]. For regression analysis, PROC REG of SAS was used. The data is presented as means ± SEM, and as regression equations.
3. Results
3.1. Distribution of steroid receptors in utero-placental and uterine tissues
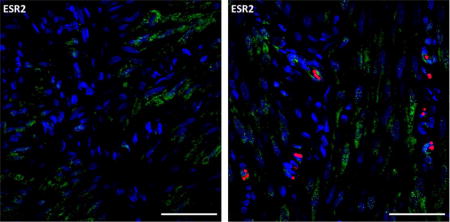
PGRAB, PGRB, ESR1 and ESR2 proteins were immunodetected in uterine and utero-placental tissues from non-pregnant and pregnant ewes (Fig. 1). Strong staining of PGRAB and PGRB was detected in LE, EG, ES and Myo of uterine (non-pregnant), and weak staining was detected in utero-placental (pregnant) compartments (Fig. 1). Overall, the pattern of staining of ESR1 and ESR2 was relatively similar in uterine and utero-placental tissues (Fig. 1). In uterine tissues, strong staining of ESR1 was detected in deep EG and weaker in luminal (superficial) EG, LE, ES and Myo, and in utero-placental tissues staining was stronger in luminal EG than deep EG, strong in LE and ES, and weaker in Myo (Fig. 1). ESR2 staining was detected in all compartments of uterine and utero-placental tissues (Fig. 1). In the FM, PGRAB, ESR1 and ESR2, but not PGRB, were detected (Fig. 2). The intensity of fluorescence of PGRAB, ESR1 and ESR2 in FM was similar throughout all stages of early pregnancy (data not shown).
Fig. 1.
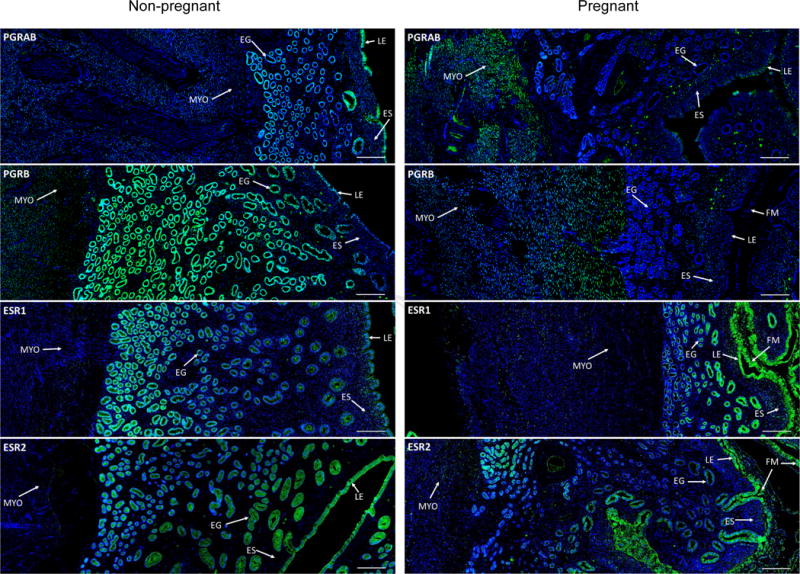
Representative images of PGRAB, PGRB, ESR1 and ESR2 protein expression in uterine (non-pregnant; left column) and utero-placental (pregnant, days 20-26; right column) tissues. Green color indicates positive receptor staining (arrows) which is present in fetal membrane (FM), luminal epithelium (LE), endometrial glands (EG), endometrial stroma (ES) and myometrium (Myo). Blue color is DAPI nuclear counterstaining. Note positive staining in all uterine and utero-placental compartments, and, overall, stronger staining in non-pregnant than pregnant ewes. Size bar is 200 μm for all images.
Fig. 2.

Representative images of PGRAB, ESR1 and ESR2 protein expression in fetal membranes (FM) on day 30 of pregnancy. Green color indicates positive staining, red color indicates red blood cell auto-fluorescence, and blue color indicates cell nuclei stained with DAPI. DAPI control demonstrates a lack of positive staining when primary antibody was omitted. LE, luminal epithelium, ES, endometrial stroma. Note positive PGRAB, ESR1 and ESR2 staining in FM, and other compartments (e.g., LE and ES). Size bar is 50 μm for all images.
3.2. Expression of steroid receptors in LE, ES, EG, EBV and MBV
In LE, PGRAB were localized to nuclei and cytoplasm whereas PGRB were localized primarily to cell nuclei (Fig. 3). The staining of PGRAB and PGRB was strong in LE of uterine tissues but was weak in utero-placental tissues (Fig. 3). Image analysis demonstrated that in LE, intensity of PGRAB staining was less (P<0.001) on days 16-30 of pregnancy than in non-pregnant animals (Fig. 4A) and PGRB was less (P<0.0001) in pregnant than non-pregnant ewes (Fig. 4B). Regression analysis of PGRAB expression demonstrated cubic pattern of changes (P<0.01) from non-pregnant stage to day 30 of pregnancy (Table 2). ESR1 was immunolocalized to cytoplasm and nuclei in LE (Fig. 3), and intensity of staining was similar in uterine and utero-placental tissues (data not shown). A weak ESR2 cytoplasmic staining was detected in LE, which was similar, both in non-pregnant and pregnant tissues (data not shown).
Fig. 3.

Representative images of PGRAB, PGRB and ESR1 protein expression in luminal epithelium (LE) and endometrial stroma (ES) in non-pregnant (left column) and pregnant (right column) ewes. Green color indicates positive staining, red color indicates red blood cell auto-fluorescence, and blue color indicates cell nuclei stained with DAPI. Note stronger PGRAB and PGRB staining in LE in non-pregnant than pregnant ewes. Size bar is 50 μm for all images.
Fig. 4.

Expression of PGRAB and PGRB proteins in luminal epithelium (LE) as determined by immunofluorescence and image analysis based on staining intensity. (A) PGRAB protein expression in LE (a,bP=0.1), (B) PGRB protein expression in LE (a,b,cP<0.003) and (C) Expression of ESR1 protein in ES (a,b,cP<0.03). Data are expressed as fold change compared with NP arbitrarily set as 1. Values ± SEM with different superscripts are significantly different.
Table 2.
Regression analysis of steroid receptor protein expression by immunofluorescence and image analysis in utero-placental compartments from early pregnancy
| Protein | Compartments | Regression Type | P value | R2 | Equation |
|---|---|---|---|---|---|
| PGRAB | LE | Quadratic | 0.0051 | 0.9409 | 0.0248x2−0.3028x+1.305 |
| PGRAB | EG | Cubic | <0.0001 | 0.9216 | 0.02x3+0.0039x2−0.2592x+1.3122 |
| PGRAB | EBV | Quadratic | 0.0004 | 0.8845 | 0.0206x2−0.1447x+1.0838 |
| PGRB | MYO | Quadratic | <0.0001 | 0.3324 | 0.0094 x2−0.1237x+0.9552 |
| ESR1 | ES | Quadratic | <0.02 | 0.4409 | 0.0121x2−0.1224x+1.0243 |
In ES, PGRAB, PGRB, and ESR2 were detected in cell nuclei (Fig. 3), and intensity of staining was similar in uterine and in utero-placental tissues (data not shown). In ES, ESR1 was detected in cell nuclei (Fig. 3), and intensity of staining was lower (P<0.003) on day 16 compared to days 14, 20 and 26-30 of pregnancy, and to non-pregnant controls (Fig. 4C). Regression analysis demonstrated quadratic pattern of changes (P<0.02) of ESR1 expression in ES from non-pregnant stage to day 30 of pregnancy (Table 2).
In EG, PGRAB and PGRB were detected in cell nuclei in uterus and utero-placental tissues (Fig. 5). For PGRAB, the intensity of staining was greater (P<0.0002) in non-pregnant animals and on day 14 of pregnancy when compared to days 16-28 of pregnancy (Fig. 6A). The intensity of staining of PGRB was greater (P<0.05) in non-pregnant ewes and on day 14 of pregnancy when compared to any other days of pregnancy (Fig. 6B). Regression analysis demonstrated cubic pattern of changes (P<0.0001) of PGRAB expression in EG from non-pregnant stage to day 30 of pregnancy (Table 2). In EG, ESR1 and ESR2 were detected in cell nuclei and cytoplasm in both uterine and utero-placental tissues (Fig. 1), and the intensity of ESR1 and ESR2 staining in EG was similar in non-pregnant and pregnant ewes (data not shown).
Fig. 5.
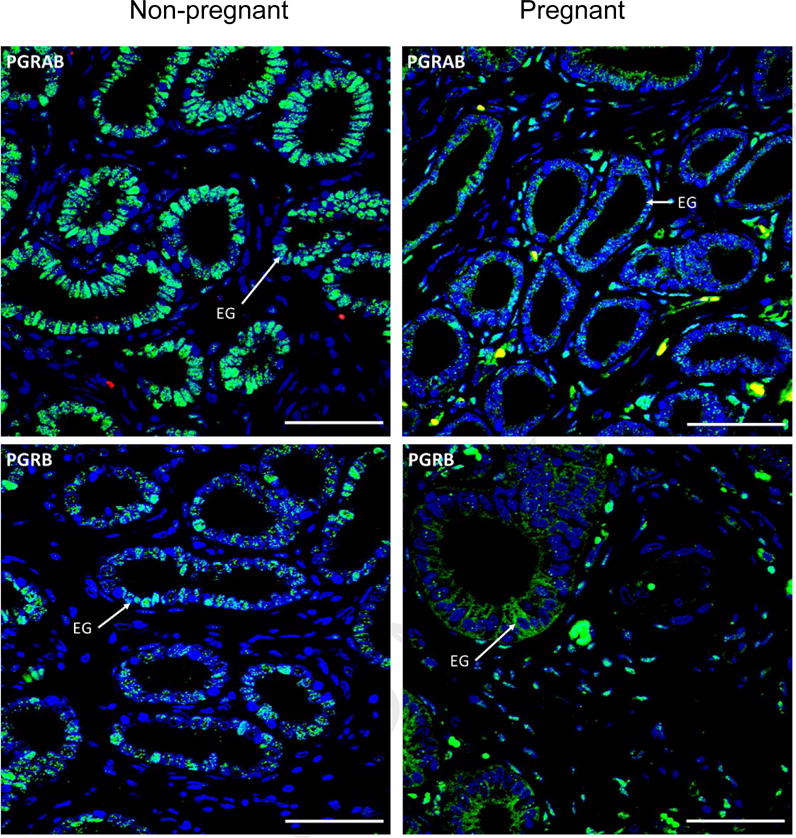
Representative images of PGRAB, and PGRB protein expression in endometrial glands (EG) in non-pregnant (left column) and pregnant (right column) ewes. Green color indicates positive receptor staining, red color indicates red blood cell auto-fluorescence, and blue color indicates cell nuclei stained with DAPI. Note positive PGRAB and PRB staining in EG (arrows), and stronger staining in non-pregnant than pregnant ewes. Size bar is 50 μm for all images.
Fig. 6.

Expression of PGRAB and PGRB proteins in endometrial glands (EG) as determined by immunofluorescence and image analysis based on staining intensity. (A) PGRAB protein expression in EG (a,b,cP<0.05) and (B) PRB protein expression in EG (a,bP<0.05). Data are expressed as fold change compared with NP arbitrarily set as 1. Values ± SEM with different superscripts are significantly different.
In EBV, PGRAB was expressed in endothelium in utero-placental but not in uterine tissues (Fig. 7). Intensity of PGRAB staining was greater (P<0.02-0.09) on days 28 and 30 than in any other stages of pregnancy or non-pregnant controls (Fig 8A). Regression analysis demonstrated cubic pattern of changes (P<0.0004) of PGRAB expression in EBV from non-pregnant stage to day 30 of pregnancy (Table 2). In MBV, PGRAB was expressed in the endothelium and intensity of staining was greatest (P<0.05) on day 30 of pregnancy when compared to non-pregnant ewes and other days of pregnancy (Fig. 8B). ESR1 was detected in EBV, and level of expression was similar for all stages evaluated (Fig. 7). PGRB and ESR2 were not detected in EBV or MBV.
Fig. 7.

Representative images of PGRAB and ESR1 protein expression in endometrial blood vessels (EBV) in non-pregnant (left column) and pregnant (right column) ewes. Green color indicates positive receptor staining, red/yellow color indicates red blood cell auto-fluorescence, and blue color indicates cell nuclei stained with DAPI. RBC, red blood cells. Note positive PGRAB and ESR1 staining in endothelium of EBV. Size bar is 50 μm for all images.
Fig. 8.

Expression of PGRAB protein in (A) endometrial (EBV) and (B) myometrial (MBV) blood vessels as determined by immunofluorescence and image analysis based on staining intensity. Data are expressed as fold change compared with NP control arbitrarily set as 1. a,b,cP<0.02 and *P<0.09; values ± SEM with different superscripts are significantly different.
In Myo, PGRAB, PGRB, ESR1 and ESR2 were expressed in cell nuclei in uterine and utero-placental tissues (Fig. 9). Intensity of PGRB staining was less (P<0.0001) on days 16 and 28 of pregnancy than in non-pregnant controls (Fig 10). Expression of PGRAB, ESR1 and ESR2 in Myo remained constant throughout the days of pregnancy, and was similar to non-pregnant controls (data not shown).
Fig. 9.

Representative images of PGRAB, PGRB, ESR1 and ERS2 protein expression in myometrium (Myo) in uterine (non-pregnant; left column) and utero-placental (pregnant; right column) tissues. Green color indicates positive receptor staining, and blue color indicates cell nuclei stained with DAPI. Note positive PGRAB, PGRB and ESR1 staining in myometrial cells. Size bar is 50 μm for all images.
Fig. 10.
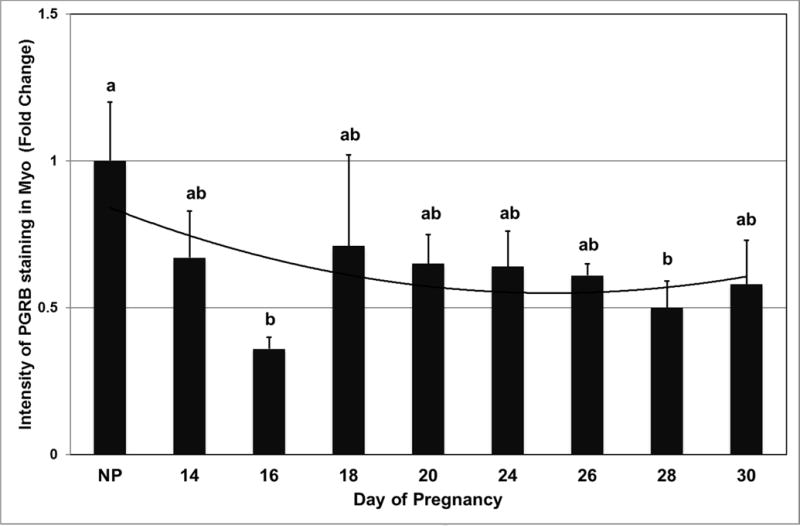
Expression of PGRB protein in Myo as determined by immunofluorescence and image analysis based on staining intensity. Data are expressed as fold change compared with NP control arbitrarily set as 1. a,bP<0.05; values ± SEM with different superscripts are significantly different.
4. Discussion
This study has demonstrated the differential localization and expression of the steroid receptor proteins in utero-placental compartments during early pregnancy after natural mating, and differences in steroid receptor expression in uterine vs. utero-placental tissues in sheep. Steroid receptor proteins are expressed in FM and in several compartments including LE, ES, EG, EBV, MBV and Myo, and their expression in selected compartments changed during early pregnancy, and differed in pregnant compared to non-pregnant ewes in this experiment. Localization and level of expression of steroid receptors (both at the protein and mRNA levels) in placenta have been studied in several species including sheep, cow, and humans during early pregnancy [1,11,14,16,26-30].
We have observed a decrease in expression of PGRAB and PGRB in LE, ES, EG and Myo as pregnancy progressed with the exception of the endothelium of the EBV and MBV where the expression of PGRAB increased on days 26-30 of pregnancy. Furthermore, expression of ESR1 and ESR2 remained constant in all compartments throughout pregnancy with the exception ESR1 in ES where lowest expression was observed on day 16 of pregnancy. Changes in the expression of ESR and PGR in utero-placental compartments have been reported in several species [1,11,14,16,26-30]. In sheep, expression of PGR and ESR protein and mRNA was low or absent in LE and luminal (shallow/superficial) EG between day 13 to 25 of pregnancy, and maintained at detectable and relatively similar levels in ES and deep EG from day 11 to 25 of pregnancy [11]. Furthermore, expression of PGR protein was low in luminal EG and not detected in LE, deep EG and ES, and expression of ESR protein was detected in ES, deep EG and Myo but not in LE or luminal EG on days 14 and 21 of pregency in sheep [31]. Our previous data have demonstrated that the mRNA expression of the nuclear PGR in ovine caruncular (CAR) tissues and FM decreased from days 18-22 to 30, and ESR in CAR decreased from day 16 to 30 and in FM from day 20 to 30 of pregnancy [1]. The discrepancies in pattern of expression of steroid receptors at protein and mRNA level are likely due to a fact that proteins were evaluated in specific uterine compartments, but mRNA in homogenized tissues that contained all compartments. In addition, complex and separate regulatory mechanisms control transcription and translation, stability and half-lives, and therefore, the rates of production or expression of mRNA and proteins differ substantially in mammalian cells [32].
In bovine utero-placenta, expression of PGR protein was detected in ES and Myo but not in LE or EG, and in the ES was less on days 18 and 21 in comparison to days 16, 24 and 30 of pregnancy [29]. Moreover, PGRAB and PGRB protein were expressed in LE, EG and ES, and PGRAB mRNA and protein expression in LE and EG was lower on days 13 and 16 than 5 and 7 of pregnancy in cows [16]. In cows, ESR1 mRNA and protein were detected in LE, EG and ES, and expression was greater on days 12-14 than18 of pregnancy [31]. In humans, PGR protein was detected in the EG, ES, smooth muscles, pericytes and endothelial cells of decidualized endometrium, and in Myo at first trimester of pregnancy [26]. Moreover, PGR protein was expressed in ES, endometrial and myometrial smooth muscle cells and spiral artery wall cells but not LE or EG, and PGR expression has remained constant in endometrium throughout human pregnancy [27]. In the same study, ESR expression was detected in ES, spiral arteries and myometrial smooth muscle cells that gradually decreased during first three months of human pregnancy to undetectable levels [27]. These results indicate that the spatio-temporal changes in the localization and levels of expression of PGR and ESR in utero-placental tissues appear in several species and underline the involvement of PGR and ESR in regulating utero-placental functions. Some differences concerning steroid receptor protein localization and expression among above discussed results from a few studies are likely due to detection method, antibody source and specificity, and breed. In fact, we have used very sensitive immunofluorescent detection and modern computerized image analysis, but earlier studies used colorimetric detection and subjective evaluation of staining intensity.
In our study, PGRAB, ESR1 and ESR2 were detected in FM, and level of expression was similar throughout early pregnancy. In fact, the expression of sex steroid receptors in FM and early embryos was demonstrated for several species including mice, cows, sheep, rhesus macaques and humans [1,19,20,33-35]. In sheep FM, mRNA expression of PGR was greater on days 20-22 than on days 24-30, and ESR1 mRNA was greater on day 20 than other days of early pregnancy [1]. In the mouse, ESR mRNA was detected in oocytes, embryos and blastocyst, and PGR mRNA in blastocyst indicating functional role of ESR and PGR during early embryonic development [19]. In cows, PGR mRNA was detected in the oocytes and at all stages of early embryonic development except in the morula stage; the relative abundance was highest in the immature oocytes and lowest at 2-4 cell and 16 cell stages [35]. In rhesus macaques, PGR protein and mRNA were detected in amnion epithelial, mesenchymal cells and in chorion during mid to late-pregnancy (days 80-100 to 130-145), and PGR protein expression in amnion was undetectable at term [20]. Since the functional significance of sex steroid receptors in FM during early and later stages of pregnancy is not fully clear, future studies should be pursued to determine the influence of steroids on various aspects of conceptus development such as cell proliferation, vascular development, gene expression, and other processes. Based on results discussed above, we hypothesize that sex steroids exert direct effects on conceptus development via the steroid receptors present on FM.
Luminal epithelium is the first area of contact between the embryo and the maternal placenta [36]. Though PGR is critical for the process of implantation [37,38], decrease in the levels of PGR protein and/or mRNA expression during early pregnancy has been reported in several species [39, 40]. In our study, we have observed a decline in the expression of PGR A and B protein in LE as early as on days 14-16 onwards of pregnancy. In other studies using sheep as a model, PGR and ESR protein and mRNA were not detected [11,31]. In mice and hamsters, decrease in PGR protein and mRNA in LE occurs after embryo attachment and before decidualization [39]. In LE, PGR plays a role in downregulating the expression of apical glycoproteins such as mucin-1, which is anti-adhesive and a barrier to implantation and has to be downregulated before embryo implantation occurs [10,41]. In humans, increased PGR expression in LE during pregnancy is associated with luteal phase defect and subsequent pregnancy loss [42,43]. Failure of PGR downregulation is associated with aberrant avb3 integrin expression, which is critical for implantation and is a marker of endometrial receptivity [42,43]. These observations emphasize that decreased PGR expression in LE during early pregnancy is physiologically crucial for the establishment and maintenance of pregnancy.
We have observed a lower expression of ESR1 protein in ES during early pregnancy compared to non-pregnant controls. However the expression of PGRAB, PGRB and ESR2 in ES were constant in uterine and utero-placental tissues throughout early pregnancy. A major role of the stroma is to mediate steroid actions during growth and development of several adult tissues including endometrium [44]. For example, in the human endometrium during proliferative phase of menstrual cycle, progesterone suppression of an epithelial specific protein (matrix metalloproteinases of stromelysin family) has been shown to be mediated by the stroma [45]. Moreover, endometrial stroma secretes growth factors such as hepatocyte growth factor and fibroblast growth factors -7 and -10, which are steroid responsive and may mediate epithelial-mesenchymal interactions which are important for establishment and maintenance of pregnancy [36]. We speculate that the expression of steroid receptors in ES is required for placental development and function. However further research is warranted to determine the role of steroid receptors in the regulation of ES functions.
Endometrial glands are known to secrete histotroph, which contains enzymes, cytokines, growth factors, ions and hormones, glucose, transport proteins, adhesion molecules and others [26]. Besides being the first available nutrient for the conceptus, histotroph also promote cell division, proliferation, morphogenesis and differentiation [46-48]. Histotroph’s components such as leukemia inhibitory factor (LIF), calcitonin, osteopontin and others mediate conceptus-maternal interactions and implantation [46,49,50]. We have observed a decline in the expression of PGR in EG as pregnancy progressed although the levels of ESR1 and ESR2 did not change. This downregulation might be due to high circulating levels of P4 in ewes [51] which is known to be important for histotrophic secretions [52].
The pattern of utero-placental vascular development during early pregnancy is well established in sheep as well as in other species [2,23,53-55]. Previous studies from our and other laboratories have demonstrated increased angiogenesis, angiogenic factor expression and uterine blood flow to the pregnant uterus in sheep [23,24,56]. We have observed significant increase in PGR protein expression in EBV and MBV on days 28-30 of pregnancy, while ESR1 and ESR2 expression was constant in the utero-placental blood vessels. Our study confirms previously obtained results where both ESR and PGR were detected in EBV and MBV in ovine placenta during early pregnancy and were absent in uterine tissues from non-pregnant sheep [28]. Estrogens regulate blood flow to the pregnant uterus, and the expression of ESR1 (but not ESR2) is significantly increased in ovine uterine arteries during pregnancy [57]. Intravenous administration of E2 to chronically ovariectomized ewes resulted in a 6-10 fold increase in uterine blood flow within 90 minutes, and when administered chronically mimicked the physiological symptoms of pregnancy such as increased cardiac output and blood volume [58]. Estradiol-17β is involved in the regulation of placental angiogenesis, placental expression of angiogenic factors and nitric oxide synthase (NOS) in several species [46,59]. Progesterone and/or E2 administration to ovariectomized sheep demonstrated increase in endothelial NOS protein expression in uterine artery endothelium [56] and mRNA expression for several angiogenic factors in the uterus [60]. These results indicate that the expression of PGR and ESR in EBV and MBV may be essential for utero-placental vascular development and function, and increased placental blood flow during early pregnancy.
A sustained expression of PGRAB, ESR1 and/or ESR2 in the Myo in non-pregnant as well as pregnant uterus has been observed by us and others [11,31]. In our study, there was a sharp decrease in the expression of the PGRB on days 16 and 28 of pregnancy but expression was similar to non-pregnant uterus on other days of pregnancy. The presence of PGRAB in Myo ensures myometrial quiescence throughout early pregnancy whereas ESR expression may be directed towards maintenance of PGR expression [28]. However, the additional studies are needed to determine the importance of steroid receptors for myometrial functions during early pregnancy.
The strength of our study is that we have localized PGR and ESR using a sensitive immunofluorescent detection system, and quantified the protein expression of steroid receptors using modern computerized image analysis techniques to determine level of expression in selected uterine and utero-placental compartments. Furthermore, our detection system allowed for identification of low level of protein expression. In addition, a focus of our study was on early stages of pregnancy (which corroborates with first trimester in humans), which is critical for the establishment and maintenance of pregnancy, and also characterized by high embryo loss [61,62]. Thus, from one hand we confirmed a portion of already published results but on the other hand we were able to demonstrate that steroid receptors were expressed at low level that was undetectable in older studies [11,31].
4.1. Conclusions
In summary, we have demonstrated that steroid receptors PGR A and B, ESR1 and/or ESR2 are expressed in several compartments of uterus and utero-placenta, level of expression and distribution of these receptors differ between non-pregnant and pregnant ewes, and that expression of selected receptor changes in several compartments as pregnancy progresses. Thus, this experiment further supports existing data concerning the critical role of the sex steroid receptors in utero-placental development and function during early pregnancy as implied by their differential protein expression pattern in different compartments. Furthermore, these data can serve as a baseline for comparisons to results of placental steroid receptor expression in compromised pregnancies. We hypothesize that in the future, steroid receptors can be used as biomarkers of placental function during early pregnancy in physiological and pathological conditions.
Highlights.
Steroid receptors’ proteins are differentially distributed in utero-placental compartments
Expression of selected receptors change in several compartments as early pregnancy progresses
Pattern of placental steroid receptor expression in normal pregnancy can serve as a baseline for comparisons to compromised pregnancies
Acknowledgments
This project was partially supported by funds from grant #2007-01215 from the National Research Initiative of the U.S. Department of Agriculture and fully supported by grant #R03HD076073 from the National Institutes of Health, as well as by hutch funds from the North Dakota Agricultural Experiment Station to Anna T Grazul-Bilska and Lawrence P Reynolds. The authors would like to thank Dr. Sheri Dorsam for laboratory assistance, and Jim Kirsch, Terry Skunberg, the staff at NDSU Animal Nutrition and Physiology Center, and undergraduate students for assistance with animal care and handling.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Reynolds LP, Haring JS, Johnson ML, Ashley RL, Redmer DA, Borowicz PP, et al. Placental development during early pregnancy in sheep: estrogen and progesterone receptor messenger RNA expression in pregnancies derived from in vivo-produced and in vitro-produced embryos. Domest Anim Endocrinol. 2015;53:60–9. doi: 10.1016/j.domaniend.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 2.Bairagi S, Quinn KE, Crane AR, Ashley RL, Borowicz PP, Caton JS, et al. Maternal environment and placental vascularization in small ruminants. Theriogenology. 2016;86:288–305. doi: 10.1016/j.theriogenology.2016.04.042. [DOI] [PubMed] [Google Scholar]
- 3.Reynolds LP, Borowicz PP, Palmieri C, Grazul-Bilska AT. Placental vascular defects in compromised pregnancies: effects of assisted reproductive technologies and other maternal stressors. Adv Exp Med Biol. 2014;814:193–204. doi: 10.1007/978-1-4939-1031-1_17. [DOI] [PubMed] [Google Scholar]
- 4.Wilmut I, Sales DI, Ashworth CJ. Maternal and embryonic factors associated with prenatal loss in mammals. J Reprod Fertil. 1986;76:851–64. doi: 10.1530/jrf.0.0760851. [DOI] [PubMed] [Google Scholar]
- 5.Smith GC. First trimester origins of fetal growth impairment. Semin Perinatol. 2004;28:41–50. doi: 10.1053/j.semperi.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 6.Rai R, Regan L. Recurrent miscarriage. Lancet. 2006;368:601–11. doi: 10.1016/S0140-6736(06)69204-0. [DOI] [PubMed] [Google Scholar]
- 7.Binder AK, Winuthayanon W, Hewill SC. Steroid receptors in the uterus and ovary. In: Plant TM, Zeleznik AJ, editors. The Knobil and Neill’s Physiology of Reproduction. 4. New York, NY: Academic Press as an imprint of Elsevier; 2015. pp. 1099–193. [Google Scholar]
- 8.Conneely OM, Lydon JP. Progesterone receptors in reproduction: functional impact of the A and B isoforms. Steroids. 2000;65:571–7. doi: 10.1016/s0039-128x(00)00115-x. [DOI] [PubMed] [Google Scholar]
- 9.Muramatsu M, Inoue S. Estrogen receptors: how do they control reproductive and nonreproductive functions? Biochem Biophys Res Commun. 2000;270:1–10. doi: 10.1006/bbrc.2000.2214. [DOI] [PubMed] [Google Scholar]
- 10.Carson DD, DeSouza MM, Kardon R, Zhou X, Lagow E, Julian J. Mucin expression and function in the female reproductive tract. Hum Reprod Update. 1998;4:459–64. doi: 10.1093/humupd/4.5.459. [DOI] [PubMed] [Google Scholar]
- 11.Spencer TE, Bazer FW. Temporal and spatial alterations in uterine estrogen receptor and progesterone receptor gene expression during the estrous cycle and early pregnancy in the ewe. Biol Reprod. 1995;53:1527–43. doi: 10.1095/biolreprod53.6.1527. [DOI] [PubMed] [Google Scholar]
- 12.Wathes DC, Mann GE, Payne JH, Riley PR, Stevenson KR, Lamming GE. Regulation of oxytocin, oestradiol and progesterone receptor concentrations in different uterine regions by oestradiol, progesterone and oxytocin in ovariectomized ewes. J Endocrinol. 1996;151:375–93. doi: 10.1677/joe.0.1510375. [DOI] [PubMed] [Google Scholar]
- 13.Ing NH, Tornesi MB. Estradiol up-regulates estrogen receptor and progesterone receptor gene expression in specific ovine uterine cells. Biol Reprod. 1997;56:1205–15. doi: 10.1095/biolreprod56.5.1205. [DOI] [PubMed] [Google Scholar]
- 14.Meikle A, Tasende C, Sosa C, Garófalo EG. The role of sex steroid receptors in sheep female reproductive physiology. Reprod Fertil Dev. 2004;16:385–94. doi: 10.10371/RD04036. [DOI] [PubMed] [Google Scholar]
- 15.Spencer TE, Johnson GA, Bazer FW, Burghardt RC, Palmarini M. Pregnancy recognition and conceptus implantation in domestic ruminants: roles of progesterone, interferons and endogenous retroviruses. Reprod Fertil Dev. 2007;19:65–78. doi: 10.1071/rd06102. [DOI] [PubMed] [Google Scholar]
- 16.Okumu LA, Forde N, Fahey AG, Fitzpatrick E, Roche JF, Crowe MA, et al. The effect of elevated progesterone and pregnancy status on mRNA expression and localisation of progesterone and oestrogen receptors in the bovine uterus. Reproduction. 2010;140:143–53. doi: 10.1530/REP-10-0113. [DOI] [PubMed] [Google Scholar]
- 17.Tibbetts TA, Mendoza-Meneses M, O’Malley BW, Conneely OM. Mutual and intercompartmental regulation of estrogen receptor and progesterone receptor expression in the mouse uterus. Biol Reprod. 1998;59:1143–52. doi: 10.1095/biolreprod59.5.1143. [DOI] [PubMed] [Google Scholar]
- 18.Dorniak P, Bazer FW, Spencer TE. Physiology and Endocrinology Symposium: biological role of interferon tau in endometrial function and conceptus elongation. J Anim Sci. 2013;91:1627–38. doi: 10.2527/jas.2012-5845. [DOI] [PubMed] [Google Scholar]
- 19.Hou Q, Gorski J. Estrogen receptor and progesterone receptor genes are expressed differentially in mouse embryos during preimplantation development. Proc Natl Acad Sci U S A. 1993;90:9460–4. doi: 10.1073/pnas.90.20.9460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haluska GJ, Wells TR, Hirst JJ, Brenner RM, Sadowsky DW, Novy MJ. Progesterone receptor localization and isoforms in myometrium, decidua, and fetal membranes from rhesus macaques: evidence for functional progesterone withdrawal at parturition. J Soc Gynecol Investig. 2002;9:125–36. [PubMed] [Google Scholar]
- 21.Goldman S, Weiss A, Almalah I, Shalev E. Progesterone receptor expression in human decidua and fetal membranes before and after contractions: possible mechanism for functional progesterone withdrawal. Mol Hum Reprod. 2005;11:269–77. doi: 10.1093/molehr/gah161. [DOI] [PubMed] [Google Scholar]
- 22.Grazul-Bilska AT, Thammasiri J, Kraisoon A, Reyaz A, Bass CS, Kaminski SL, et al. Expression of progesterone receptor protein in the ovine uterus during the estrous cycle: Effects of nutrition, arginine and FSH. Theriogenology. 2017;108:7–15. doi: 10.1016/j.theriogenology.2017.11.008. [DOI] [PubMed] [Google Scholar]
- 23.Grazul-Bilska AT, Borowicz PP, Johnson ML, Minten MA, Bilski JJ, Wroblewski R, et al. Placental development during early pregnancy in sheep: vascular growth and expression of angiogenic factors in maternal placenta. Reproduction. 2010;140:165–74. doi: 10.1530/REP-09-0548. [DOI] [PubMed] [Google Scholar]
- 24.Grazul-Bilska AT, Johnson ML, Borowicz PP, Minten M, Bilski JJ, Wroblewski R, et al. Placental development during early pregnancy in sheep: cell proliferation, global methylation, and angiogenesis in the fetal placenta. Reproduction. 2011;141:529–40. doi: 10.1530/REP-10-0505. [DOI] [PubMed] [Google Scholar]
- 25.Cody RP, Smith JK. Applied statistics and the SAS programming language. 4. Upper Saddle River, N.J.: Prentice Hall; 1997. [Google Scholar]
- 26.Wang JD, Fu Y, Shi WL, Zhu PD, Cheng J, Qiao GM, et al. Immunohistochemical localization of progesterone receptor in human decidua of early pregnancy. Hum Reprod. 1992;7:123–7. doi: 10.1093/oxfordjournals.humrep.a137545. [DOI] [PubMed] [Google Scholar]
- 27.Perrot-Applanat M, Deng M, Fernandez H, Lelaidier C, Meduri G, Bouchard P. Immunohistochemical localization of estradiol and progesterone receptors in human uterus throughout pregnancy: expression in endometrial blood vessels. J Clin Endocrinol Metab. 1994;78:216–24. doi: 10.1210/jcem.78.1.8288707. [DOI] [PubMed] [Google Scholar]
- 28.Zheng J, Johnson ML, Redmer DA, Reynolds LP. Estrogen and progesterone receptors, cell proliferation, and c-fos expression in the ovine uterus during early pregnancy. Endocrinology. 1996;137:340–8. doi: 10.1210/endo.137.1.8536633. [DOI] [PubMed] [Google Scholar]
- 29.Kimmins S, MacLaren LA. Oestrous cycle and pregnancy effects on the distribution of oestrogen and progesterone receptors in bovine endometrium. Placenta. 2001;22:742–8. doi: 10.1053/plac.2001.0708. [DOI] [PubMed] [Google Scholar]
- 30.Robinson RS, Mann GE, Lamming GE, Wathes DC. Expression of oxytocin, oestrogen and progesterone receptors in uterine biopsy samples throughout the oestrous cycle and early pregnancy in cows. Reproduction. 2001;122:965–79. [PubMed] [Google Scholar]
- 31.Wathes DC, Hamon M. Localization of oestradiol, progesterone and oxytocin receptors in the uterus during the oestrous cycle and early pregnancy of the ewe. J Endocrinol. 1993;138:479–92. doi: 10.1677/joe.0.1380479. [DOI] [PubMed] [Google Scholar]
- 32.Schwanhäusser B, Busse D, Li N, Dittmar G, Schuchhardt J, Wolf J, et al. Global quantification of mammalian gene expression control. Nature. 2011;473:337–42. doi: 10.1038/nature10098. [DOI] [PubMed] [Google Scholar]
- 33.Gorski J, Hou Q. Embryonic estrogen receptors: do they have a physiological function? Environ Health Perspect. 1995;103(Suppl 7):69–72. doi: 10.1289/ehp.95103s769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ying C, Yang YC, Hong WF, Cheng WT, Hsu WL. Progesterone receptor gene expression in preimplantation pig embryos. Eur J Endocrinol. 2000;143:697–703. doi: 10.1530/eje.0.1430697. [DOI] [PubMed] [Google Scholar]
- 35.Clemente M, de La Fuente J, Fair T, Al Naib A, Gutierrez-Adan A, Roche JF, et al. Progesterone and conceptus elongation in cattle: a direct effect on the embryo or an indirect effect via the endometrium? Reproduction. 2009;138:507–17. doi: 10.1530/REP-09-0152. [DOI] [PubMed] [Google Scholar]
- 36.Spencer TE, Bazer FW. Biology of progesterone action during pregnancy recognition and maintenance of pregnancy. Front Biosci. 2002;7:d1879–98. doi: 10.2741/spencer. [DOI] [PubMed] [Google Scholar]
- 37.Wetendorf M, DeMayo FJ. Progesterone receptor signaling in the initiation of pregnancy and preservation of a healthy uterus. Int J Dev Biol. 2014;58:95–106. doi: 10.1387/ijdb.140069mw. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wetendorf M, Wu SP, Wang X, Creighton CJ, Wang T, Lanz RB, et al. Decreased epithelial progesterone receptor A at the window of receptivity is required for preparation of the endometrium for embryo attachment. Biol Reprod. 2017;96:313–26. doi: 10.1095/biolreprod.116.144410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Diao H, Paria BC, Xiao S, Ye X. Temporal expression pattern of progesterone receptor in the uterine luminal epithelium suggests its requirement during early events of implantation. Fertil Steril. 2011;95:2087–93. doi: 10.1016/j.fertnstert.2011.01.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Press MF, Udove JA, Greene GL. Progesterone receptor distribution in the human endometrium. Analysis using monoclonal antibodies to the human progesterone receptor. Am J Pathol. 1988;131:112–24. [PMC free article] [PubMed] [Google Scholar]
- 41.DeSouza MM, Surveyor GA, Price RE, Julian J, Kardon R, Zhou X, et al. MUC1/episialin: a critical barrier in the female reproductive tract. J Reprod Immunol. 1999;45:127–58. doi: 10.1016/s0165-0378(99)00046-7. [DOI] [PubMed] [Google Scholar]
- 42.Lessey BA, Yeh I, Castelbaum AJ, Fritz MA, Ilesanmi AO, Korzeniowski P, et al. Endometrial progesterone receptors and markers of uterine receptivity in the window of implantation. Fertil Steril. 1996;65:477–83. [PubMed] [Google Scholar]
- 43.Lessey BA. Assessment of endometrial receptivity. Fertil Steril. 2011;96:522–9. doi: 10.1016/j.fertnstert.2011.07.1095. [DOI] [PubMed] [Google Scholar]
- 44.Osteen KG, Rodgers WH, Gaire M, Hargrove JT, Gorstein F, Matrisian LM. Stromal-epithelial interaction mediates steroidal regulation of metalloproteinase expression in human endometrium. Proc Natl Acad Sci U S A. 1994;91:10129–33. doi: 10.1073/pnas.91.21.10129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bruner KL, Rodgers WH, Gold LI, Korc M, Hargrove JT, Matrisian LM, et al. Transforming growth factor beta mediates the progesterone suppression of an epithelial metalloproteinase by adjacent stroma in the human endometrium. Proc Natl Acad Sci U S A. 1995;92:7362–6. doi: 10.1073/pnas.92.16.7362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fazleabas AT, Hild-Petito S, Verhage HG. Secretory proteins and growth factors of the baboon (Papio anubis) uterus: potential roles in pregnancy. Cell Biol Int. 1994;18:1145–53. doi: 10.1006/cbir.1994.1041. [DOI] [PubMed] [Google Scholar]
- 47.Martal J, Chêne N, Camous S, Huynh L, Lantier F, Hermier P, et al. Recent developments and potentialities for reducing embryo mortality in ruminants: the role of IFN-tau and other cytokines in early pregnancy. Reprod Fertil Dev. 1997;9:355–80. doi: 10.1071/r96083. [DOI] [PubMed] [Google Scholar]
- 48.Burton GJ, Watson AL, Hempstock J, Skepper JN, Jauniaux E. Uterine glands provide histiotrophic nutrition for the human fetus during the first trimester of pregnancy. J Clin Endocrinol Metab. 2002;87:2954–9. doi: 10.1210/jcem.87.6.8563. [DOI] [PubMed] [Google Scholar]
- 49.Stewart CL, Kaspar P, Brunet LJ, Bhatt H, Gadi I, Köntgen F, et al. Blastocyst implantation depends on maternal expression of leukaemia inhibitory factor. Nature. 1992;359:76–9. doi: 10.1038/359076a0. [DOI] [PubMed] [Google Scholar]
- 50.Zhu LJ, Bagchi MK, Bagchi IC. Attenuation of calcitonin gene expression in pregnant rat uterus leads to a block in embryonic implantation. Endocrinology. 1998;139:330–9. doi: 10.1210/endo.139.1.5707. [DOI] [PubMed] [Google Scholar]
- 51.Chang K, Zhang Lubo. Review article: steroid hormones and uterine vascular adaptation to pregnancy. Reprod Sci. 2008;15:336–48. doi: 10.1177/1933719108317975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Spencer TE, Bazer FW. Uterine and placental factors regulating conceptus growth in domestic animals. J Anim Sci. 2004;82(E-Suppl):E4–13. doi: 10.2527/2004.8213_supplE4x. [DOI] [PubMed] [Google Scholar]
- 53.Pfarrer C, Ebert B, Miglino MA, Klisch K, Leiser R. The three-dimensional feto-maternal vascular interrelationship during early bovine placental development: a scanning electron microscopical study. J Anat. 2001;198:591–602. doi: 10.1046/j.1469-7580.2001.19850591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sherer DM, Abulafia O. Angiogenesis during implantation, and placental and early embryonic development. Placenta. 2001;22:1–13. doi: 10.1053/plac.2000.0588. [DOI] [PubMed] [Google Scholar]
- 55.Reynolds LP, Borowicz PP, Caton JS, Vonnahme KA, Luther JS, Buchanan DS, et al. Uteroplacental vascular development and placental function: an update. Int J Dev Biol. 2010;54:355–66. doi: 10.1387/ijdb.082799lr. [DOI] [PubMed] [Google Scholar]
- 56.Rupnow HL, Phernetton TM, Shaw CE, Modrick ML, Bird IM, Magness RR. Endothelial vasodilator production by uterine and systemic arteries. VII. Estrogen and progesterone effects on eNOS. Am J Physiol Heart Circ Physiol. 2001;280:H1699–705. doi: 10.1152/ajpheart.2001.280.4.H1699. [DOI] [PubMed] [Google Scholar]
- 57.Liao WX, Magness RR, Chen DB. Expression of estrogen receptors-alpha and -beta in the pregnant ovine uterine artery endothelial cells in vivo and in vitro. Biol Reprod. 2005;72:530–7. doi: 10.1095/biolreprod.104.035949. [DOI] [PubMed] [Google Scholar]
- 58.Magness RR, Parker CR, Rosenfeld CR. Systemic and uterine responses to chronic infusion of estradiol-17 beta. Am J Physiol. 1993;265:E690–8. doi: 10.1152/ajpendo.1993.265.5.E690. [DOI] [PubMed] [Google Scholar]
- 59.Tan J, Paria BC, Dey SK, Das SK. Differential uterine expression of estrogen and progesterone receptors correlates with uterine preparation for implantation and decidualization in the mouse. Endocrinology. 1999;140:5310–21. doi: 10.1210/endo.140.11.7148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Johnson ML, Grazul-Bilska AT, Redmer DA, Reynolds LP. Effects of estradiol-17beta on expression of mRNA for seven angiogenic factors and their receptors in the endometrium of ovariectomized (OVX) ewes. Endocrine. 2006;30:333–42. doi: 10.1007/s12020-006-0012-5. [DOI] [PubMed] [Google Scholar]
- 61.Goldstein SR. Embryonic death in early pregnancy: a new look at the first trimester. Obstet Gynecol. 1994;84:294–7. [PubMed] [Google Scholar]
- 62.Macklon NS, Geraedts JP, Fauser BC. Conception to ongoing pregnancy: the ‘black box’ of early pregnancy loss. Hum Reprod Update. 2002;8:333–43. doi: 10.1093/humupd/8.4.333. [DOI] [PubMed] [Google Scholar]


