Abstract
Background
Angiotensin-(1-12) [Ang-(1-12)] is a chymase-dependent source for angiotensin II (Ang II) cardiac activity. The direct contractile effects of Ang-(1-12) in normal and heart failure (HF) remain to be demonstrated. We assessed the hypothesis that Ang-(1-12) may modulate [Ca2+]i regulation and alter cardiomyocyte contractility in normal and HF rats.
Methods and Results
We compared left ventricle (LV) myocyte contractile and calcium transient ([Ca2+]iT) responses to angiotensin peptides in 16 SD rats with isoproterenol-induced HF and 16 age-matched controls. In normal myocytes, versus baseline, Ang II (10−6 M) superfusion significantly increased myocyte contractility (dL/dtmax: 40%) and [Ca2+]iT (29%). Ang-(1-12) (4×10−6 M) caused similar increases in dL/dtmax (34%) and [Ca2+]iT (25%). Compared with normal myocytes, superfusion of Ang II and Ang-(1-12) in myocytes obtained from rats with isoproterenol-induced HF caused similar but significantly attenuated positive inotropic actions with about 42% to 50% less increases in dL/dtmax and [Ca2+]iT. Chymostatin abolished Ang-(1-12)-mediated effects in normal and HF myocytes. The presence of an inhibitory cAMP analog, Rp-cAMPS prevented Ang-(1-12)-induced inotropic effects in both normal and HF myocytes. Incubation of HF myocytes with pertussis toxin (PTX) further augmented Ang II-mediated contractility.
Conclusions
Ang-(1-12) stimulates cardiomyocyte contractile function and [Ca2+]iT in both normal and HF rats through a chymase mediated action. Altered inotropic responses to Ang-(1-12) and Ang II in HF myocytes are mediated through a cAMP-dependent mechanism that is coupled to both stimulatory G and inhibitory PTX-sensitive G proteins.
Keywords: Angiotensin-(1-12), Chymase, Heart failure, Cardiomyocyte, Contractility, Calcium transient
1. Introduction
Angiotensin-(1-12) [Ang-(1-12)], a newly identified member of the renin-angiotensin system (RAS) [1], is a chymase-dependent tissue substrate for angiotensin II (Ang II) production. Recent observations suggest that activation of this non-canonical chymase/Ang-(1-12) pathway may modulate cardiac function bypassing the inhibitory effects of RAS blockade with angiotensin converting enzyme (ACE) inhibitors and Ang II receptor blockers (ARBs).[2–4] However, the importance of this renin-independent mechanism as a source for Ang II paracrine/intracrine actions is not established, even though research from this laboratory has linked increased expression of a chymase-mediated Ang II formation from Ang-(1-12) to human left heart diseases [5] and the presence of resistant atrial fibrillation.[6] There are no previous studies of the direct cardiac effects of Ang-(1-12) independent of alterations in loading conditions and its role on intracellular Ca2+ mobilization in myocytes. There is a critical need to evaluate the role of a chymase-dependent Ang II action from Ang-(1-12) since recent in-depth analysis of landmark clinical trials with angiotensin converting enzyme (ACE) inhibitors or Ang II receptor blockers demonstrates a residual risk of cardiovascular events proportionally greater than their benefit.[4, 7] As reported by us previously, [4, 7] the limited efficacy of RAS inhibitors in major clinical trials may be accounted for a failure of these agents to directly reach the intracellular sites at which Ang II is formed and the fact that chymase, rather than ACE, is the predominant cardiac tissue Ang II-forming enzyme in humans. Characterization of Ang-(1-12) direct cardiac effects will be critical in targeting intracellular RAS signaling as a novel therapeutic strategy for patients with cardiac arrhythmias and HF.[8–13]
With this in mind, we assessed the hypothesis that Ang-(1-12) may modulate [Ca2+]i regulation and alter cardiomyocytes contractile performance via a chymase-mediated conversion to Ang II. We extended this hypothesis to include the demonstration of reduced Ang-(1-12) inotropic action and altered [Ca2+]i regulation in the presence of HF. Accordingly, we measured LV myocyte functional and [Ca2+]i transient ([Ca2+]iT) responses to Ang-(1-12) superfusion in normal and age-matched rats in which HF was induced by two subcutaneous injections of isoproterenol (ISO). This experimental HF model mimics many of the structural, functional, and hormonal changes of clinical HF. [12, 13]
2. Methods
2.1. Animal Model and Experimental Protocol
A detailed Methods section is available in the Online Supplement Materials.
This study was approved by the Wake Forest University School of Medicine Animal Care and Use Committee and conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication 8th edition, updated 2011).
Thirty-two male Sprague–Dawley rats weighing 200 ~ 250 g were randomly divided into normal control (n=16) and HF (n=16) groups. HF was induced by two subcutaneous injections of isoproterenol (ISO) spaced 24 h apart at a dose of 170 mg/kg. Rats injected with ISO were studied 2 months after the initial injection.
Briefly, calcium-tolerant, high-yield myocytes were obtained from both normal and HF rats as reported previously.[14–16] We simultaneously measured left ventricle (LV) myocyte systolic and diastolic function, and [Ca2+]i transient ([Ca2+]iT) responses before and after angiotensin peptides administration. The importance of a chymase-mediated pathway and cAMP-dependent mechanism on the modulation of cardiac functional response to Ang-(1-12) stimulation were determined using specific inhibitors (see below).
Functional performance and [Ca2+]i regulation was evaluated in myocytes randomly exposed to ISO (10−8 M), Ang II (10−6 M), Ang-(1-12) (4×10−6 M) alone or generated through incubation of the substrate (2×10−6 mol/L) with human recombinant chymase (Mixture; 10 μg/mL, incubated for 1 h at 37°C). The mixture solution served as an internal control; Ang II formation from Ang-(1-12) in the reaction mixture by chymase was analyzed by reverse-phase high-performance liquid chromatography (HPLC).[17] The Ang II product formation was identified by comparison of the retention time of synthetic Ang II standard peptide. The percent of Ang II formation from Ang-(1-12) substrate were analyzed with Shimadzu LC Solution (Kyoto, Japan) acquisition software. Nearly 85% of Ang-(1-12) parent substrate converted into Ang II by chymase (details presented in Supplemental Online Figure 1).
To gain an insight as to the mechanism associated with the inotropic effects of Ang-(1-12) in normal and HF, in a second series of experiments, myocytes were pretreated to inhibit either Ang II AT1 receptor with losartan (10−5 M, 30 min), chymase with chymostatin (8×10−5 M, 30 min), inhibitory G protein (Gi) with pertussis toxin (PTX) (2 μg/ml, 36°C, 5 h) or stimulatory G protein (Gs) with an inhibitory cAMP analog Rp-cAMPS (10−4 M, 36°C, 2 h), then followed by Ang-(1-12) or Ang II superfusion. Experimental conditions, such as the duration of incubation and peptide concentrations, were based on our pilot concentration-response studies and past reports by others and us. Percent shortening (SA), the maximum rate of shortening (dL/dtmax), and re-lengthening (dR/dtmax) were obtained as previously reported.[13, 14]
2.2. Statistical Analysis
All data are presented as mean ± SE or mean ± SD as indicated. Multiple comparisons were performed using analysis of variance. When a significant overall effect was present, intergroup comparisons were performed using a Bonferroni correction for multiple comparisons. Measurements of myocyte contraction and [Ca2+]iT were averaged from each animal and treated as a single data point. The mean differences in cell dynamics and indo-1-AM fluorescence ratios between groups were calculated. Significance was established as p<0.05.
3. Results
3.1. Verification of Experimental HF
The rat model of ISO-induced HF has been validated previously.[13, 18] The histological changes in the heart of ISO-treated rats resemble those of myocardial infarction in humans. [12] Consistently, all ISO-treated animals had unmistakable evidence of HF (anorexia, edema and pulmonary congestion). Although body weight was equivalent in control and HF rats (462 g versus 458 g), ISO-injected rats had significant increases in heart weight (1.87 g versus 1.56 g), calculated heart-to-body weight, and wet-lung-to-body weight ratios. Compared with normal controls, rats with ISO-induced HF showed decreases in stroke volume (SV), ejection fraction, and LV dP/dtmax and LV dP/dtmin while LV end-diastolic pressure increased 2.6-fold. The rate of LV relaxation slowed as indicated by a significant increase in the time constant of isovolumic LV pressure decay (τ, 61%; p <0.01). LV contractility decreased more than 40% as measured by the slopes of P-V relations of EES and MSW. The time constant of LV relaxation (τ, 13.9 vs. 9.2 msec) was increased by 51%. These findings documented the existence of established HF in this model.
In isolated cardiac myocytes, these abnormalities were accompanied by decreased myocyte contraction and relaxation, as indicated by decreased peak velocity of shortening (dL/dtmax) (43%) and peak velocity of re-lengthening (dR/dtmax) (42%). Peak systolic [Ca2+]iT was reduced (0.16 vs. 0.21), and the decline of [Ca2+]i was slower. The length of HF myocyte (HF: 147.7 ±9.5 μm vs Normal: 110.7 ±8.6 μm, p<0.01) and the length-width ratio (HF: 6.1 ± 0.9 % vs Normal: 4.3 ± 0.8%, p<0.01) were significantly increased, which suggests a remodeling of myocyte shape in HF rats. The depression in basal myocyte contraction and relaxation found in rats with ISO-induced HF was associated with a marked reduction in the ability of ISO to increase myocyte contractility (detailed changes on myocyte functional performance are presented as supplemental data of online Tables 1–2).
3.2. Effects of Ang II, Mixture and Ang-(1-12) on Myocyte Functional and [Ca2+]iT Responses
Freshly isolated myocytes from the LV of normal and HF rats responded with an increase in their contractile activity when the superfusion media contained either Ang II (10−6 mol/L), the solution in which Ang-(1-12) (2×10−6 mol/L) was incubated with human recombinant chymase (Mixture), or Ang-(1-12) alone. As illustrated in Figures 1 and 2, and online Table 2, exposure to Ang II, the Mixture, or Ang-(1-12), were characteristically associated with increases in myocyte contraction and relaxation accompanied with significant increases in [Ca2+]iT in both normal and HF LV myocytes (detailed changes are presented in online Table 2).
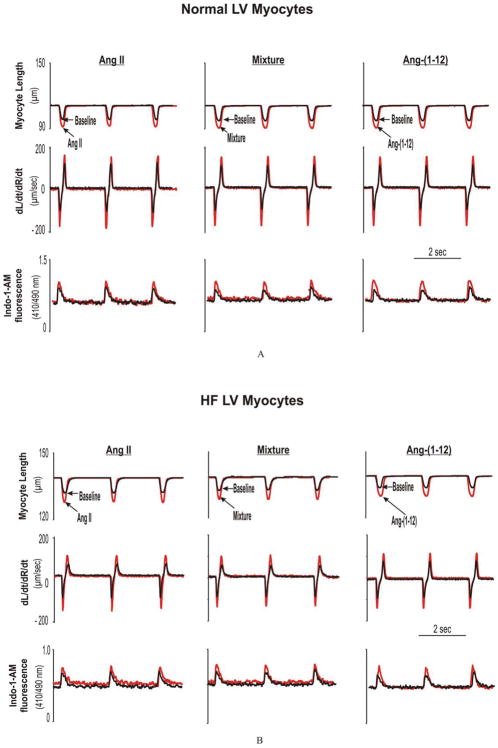
Figure 1.
Examples of LV myocyte functional responses to angiotensin peptides in normal (A) and HF (B). Fig 1A. Representative of superimposed traces of analog recordings from freshly-isolated LV myocytes obtained from one normal rat at baselines and after superfusion of Ang II, Mixture, and Ang-(1-12), respectively. Shown are myocyte percent of shortening (SA), peak velocity of shortening (dL/dtmax) and relengthening (dR/dtmax), and the peak [Ca2+]iT. Fig 1B. Representative of superimposed traces of analog recordings from freshly isolated LV myocytes obtained from one ISO-induced HF rat at baselines and after superfusion of Ang II, Mixture and Ang-(1-12), respectively.
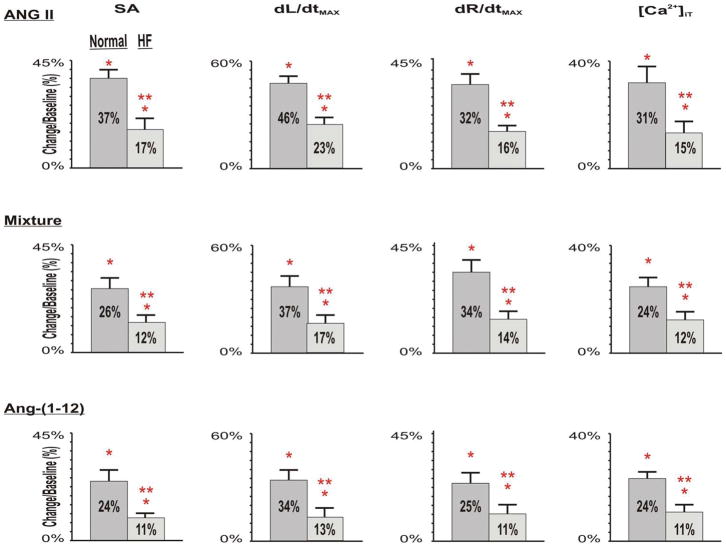
Figure 2.
Group means (± SD) of Ang-(1-12), Ang II, and Mixture-induced changes of myocyte contraction and relaxation (measured as SA, dL/dtmax, dR/dtmax) and [Ca2+]iT in normal control and HF rats. Compared with normal control at baselines, after HF, the basal myocyte contraction and relaxation and [Ca2+]iT all markedly reduced. Compared with normal myocytes in responses to angiotensin peptides, superfusions of Ang-(1-12), Ang II, and Mixture caused similar, but much less increases in SA, dL/dtmax, dR/dtmax and [Ca2+]iT in HF myocytes.
 Normal,
Normal,
 HF. * p<0.05, Ang peptides vs corresponding baselines; ** p<0.05, changes in HF vs changes in normals.
HF. * p<0.05, Ang peptides vs corresponding baselines; ** p<0.05, changes in HF vs changes in normals.
In normal myocytes, the changes in the contractile responses induced by Ang II, the mixture, and Ang-(1-12) were essentially equivalent in terms of dL/dtmax (Ang II: 185 ± 5 μm/sec; Mixture: 182 ± 5 μm/sec; Ang-(1-12): 174 ± 5 μm/sec) and dR/dtmax (Ang II: 141 ± 7 μm/sec; Mixture:142 ± 5 μm/sec; Ang-(1-12):136 ± 2 μm/sec). Peak systolic [Ca2+]iT changes in myocytes from normal rats averaged 0.31 ± 0.01 during superfusion with Ang II, 0.30 ± 0.01 following exposure to the Mixture, and 0.30 ± 0.01 after Ang-(1-12) application (p > 0.05).
Importantly, the magnitude of the responses to the three agents in HF myocytes was significantly reduced (Figure 1B and Supplemental Tables 2). In ISO-treated rats, peak Ang II-mediated increases in dL/dtmax averaged 91 ± 2 μm/sec, 89 ± 4 μm/sec in response to the addition of the Mixture, and 88 ± 1 μm/sec following Ang-(1-12) superfusion. The similar reductions in dR/dtmax found in HF myocytes during exposure to Ang II, the Mixture or Ang-(1-12) superfusion were associated with statistically significant decreases in peak systolic [Ca2+]iT when compared with the responses recorded in normal myocytes (Figure 2). In all conditions, the response induced by Ang-(1-12) began within 6 – 8 minutes after the addition of the peptide to the perfusate bathing isolated myocytes, whereas a comparative response to Ang II application was found within 3 – 4 minutes. These findings are consistent with a time-dependent conversion of Ang-(1-12) into Ang II.
3.3. Ang-(1-12)-Mediated Signal Pathways on Myocyte Functional Responses
To gain an insight as to the mechanism associated with the inotropic effects of Ang-(1-12), in four subsets series-studies, myocytes were pretreated with losartan, chymostatin or specific inhibitors to Gi or Gs proteins as described above.
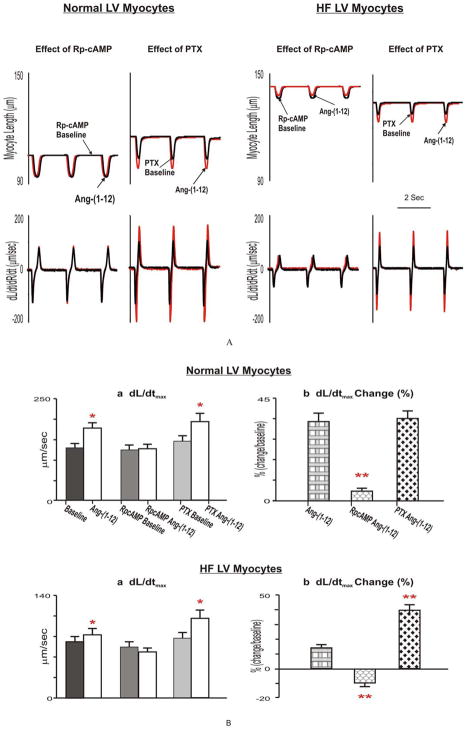
As shown in Figure 3, Ang-(1-12) inotropic responses were eliminated after pretreating myocytes with chymostatin. Similar observations were made following blockade of the AT1 receptor with losartan (data not shown) in both normal and HF myocytes. As displayed in Figure 4, pretreatment of myocytes with an inhibitory cAMP analog, Rp-cAMPS caused a slight, but not significant decrease in basal dL/dtMax and dR/dtMax. Ang-(1-12)-induced inotropic effects were nearly prevented in normal myocytes and markedly reduced in HF myocytes. Moreover, myocytes incubated with PTX showed no significant changes in basal dL/dtMax and dR/dtMax in normal and HF hearts. However, under this condition, both Ang-(1-12) and Ang II induced increases of dL/dtMax and dR/dtMax were augmented only in HF myocytes. As exhibited in Figure 4B, compared with HF and Ang-(1-12), in the presence of PTX, Ang-(1-12) increased dL/dt from 13% to 38% and dR/dt increased from 10% to 27%. Similar observations were obtained on Ang II (HFPTX Ang II: dL/dtMax, 41% vs HF Ang II: 19%, dR/dtMax, 29% vs 16%) (Figure 4).
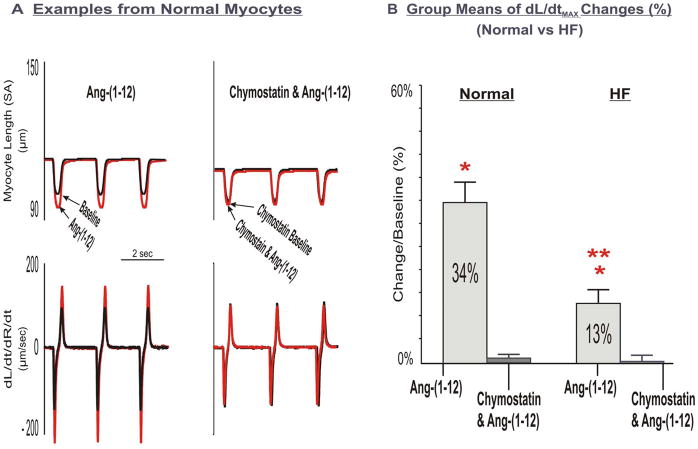
Figure 3.
Effects of chymostatin on LV myocyte functional responses to Ang-(1-12). Panel A: Examples of superimposed tracings of analog recordings of normal myocyte contractile function at baseline and after superfusion of Ang-(1-12) without and with incubation of chymostatin. Panel B: Group means of chymostatin on Ang-(1-12)-induced changes of LV myocyte contractility, dL/dtMax in normal and HF myocytes. Inhibition of chymase abolished myocyte Ang-(1-12)-stimulated contractile functional responses.
Figure 4.
Examples (Fig 4A) and group means (Fig 4B) of effects of Rp-cAMP and PTX on Ang-(1-12) induced changes to LV myocyte contractile functional performance in normal and HF. The Ang-(1-12)-induced inotropic effects were completely prevented in the presence of an inhibitory cAMP analog, Rp-cAMPS (10−4 M 2 h) in both normal and HF myocytes, but were further augmented only in HF after the incubation of myocytes with the Gi inhibitor, pertussis toxin (PTX, 2 μg/mL, 36°C, 5h). * p<0.05, Ang-(1-12) vs corresponding baselines. ** p<0.05, Ang-(1-12)-caused changes in pretreated myocytes vs Control Ang-(1-12)-resulted changes.
4. Discussion
We show Ang-(1-12) as a chymase-dependent substrate for generating Ang II inotropic activity in both normal and HF rats. The responses mediated by direct application of Ang-(1-12) to isolated myocytes are not different than those induced by Ang II and both are consistently similarly blunted in myocytes from rats with ISO-induced HF. Inhibition of Ang-(1-12) hydrolysis with chymostatin prevents Ang-(1-12) inotropic actions, a finding that denotes the presence of chymase in myocytes. Blockade of the Ang-(1-12) inotropic activity and intracellular Ca2+ mobilization with the AT1 receptor antagonist suggest that the substrate is converted into Ang II prior to exerting its activity. In keeping with a critical role of Ang II in modulating cardiac function, impaired contractility was documented for both Ang-(1-12) and Ang II in myocytes from rats with ISO-induced HF. The altered inotropic responses to Ang II and Ang-(1-12) in HF myocytes are likely mediated through a cAMP-dependent mechanism that is coupled to both stimulatory G and inhibitory PTX-sensitive G proteins.
The current study provides functional evidence for Ang-(1-12) as a key component of the cardiac RAS and as a source for direct Ang II formation in isolated cardiac myocytes. This interpretation is derived by the observed inotropic actions of Ang-(1-12), comparable to those produced by Ang II, and the blockade of the contractile response in the presence of either a chymase inhibitor or the AT1 receptor antagonist. The present data complements the finding that intracellular Ang-(1-12) increases the duration of the action potential followed by the generation of early after depolarizations in adult cardiac myocytes.[19] Previous studies demonstrate that Ang-(1-12) effects require conversion into Ang II.[6, 17, 20, 21] This conclusion derives from studies performed in the coronary vasculature [22] and the systemic circulation [1, 23] and in COS-7 or CHO cells transfected with AT1 receptors.[24] Extensive studies in human and rodent hearts demonstrate that chymase can directly generate Ang II from Ang-(1-12).[17, 21, 25] Chymase is found in cardiac interstitial spaces and the content of Ang II in LV interstitial fluid is suppressed in the presence of a chymase inhibitor. [20, 21, 26, 27]
What will happen when systemic Ang-(1-12) arrives at cardiac tissue? The inotropic activity of Ang-(1-12) in isolated myocytes perfused with a buffer solution implicates the presence in these freshly isolated myocytes of chymase and functional AT1 receptors in the plasma membrane, intracellular spaces or both. Previous studies suggested that mast cells and cardiac fibroblasts are the predominant source for chymase expression.[2, 26–28] On the other hand, chymase gene transcripts, chymase protein and enzymatic activity were reported in human and rodents cardiac myocytes.[2, 6, 10, 21, 28–31] The demonstration that Ang-(1-12) inotropic responses were blocked by chymostatin suggests the myocytes are a source for the conversion of Ang-(1-12) into Ang II. In keeping with this interpretation, we showed that intracellular delivery of chymostatin abolished the effect of intracellular Ang-(1-12) on the potassium current from rat cardiomyocytes. [19] It is possible that Ang-(1-12) interacts with membrane-bound chymase, leading to Ang II production. The Ang II generated from this alternate processing pathway may travel out of cell membrane and interact with AT1 receptors via autocrine/paracrine mechanism. [32]
Ang II is a cause of or a major contributor to the pathogenesis of heart disease. In the heart, increased Ang II activity from interstitial formation contributes to cardiac remodeling, arrhythmias, and fibrosis.[33–35]) We studied cardiomyocyte functional performance in freshly-isolated single myocytes from the LV in normal and HF rats, thereby removing potentially confounding effects of extra-cardiac factors that may influence contractility. Superfusion of Ang II produced direct inotropic effects on LV myocyte contractility and relaxation associated with increased peak systolic [Ca2+]iT. Compared with normal control myocytes, myocytes from HF rats demonstrated reduced basal myocyte contraction, relaxation and peak systolic [Ca2+]iT. Importantly, Ang II-stimulated myocyte functional and [Ca2+]iT response were significantly attenuated. The mechanism of Ang II inotropic effects have not been resolved and may be species dependent. Most investigators believe that inotropy is modulated by changes in intracellular calcium transients, which mediates excitation-contraction coupling.[36, 37] Others have suggested that an important effect of Ang II (in rabbit myocardium) is to increase the calcium-sensitivity of the myofilament, possibly by changing intracellular pH. [38] Ang II, through AT2 receptor stimulation and activation of protein tyrosine phosphatase, decreases T-type Ca2+ current, whereas in adrenal glomerulosa cells, Ang II, through AT1 receptor stimulation and activation of a Gi protein, increases T-type Ca2+ channel current.[39] Our findings indicate that Ang II-induced changes in [Ca2+]i regulation may be the primary driver of the altered cardiomyocyte force-generating capacity and relaxation changes of LV myocytes in both groups.
The significant increases induced by Ang-(1-12) or Ang II superfusion in dL/dtmax, dR/dtmax and [Ca2+]iT in freshly isolated myocytes from the heart of normal rats were markedly blunted in myocytes collected from HF rats. These findings demonstrated, for the first time, that HF alters cardiac contractile behavior to Ang-(1-12) and Ang II stimulation.
In this study, HF is associated with reduced inotropic responses to Ang II and Ang-(1-12). Why were the cardiac contractile responses to Ang II stimulation altered after HF? Factors such as a different Ang II AT1-mediated [Ca2+]i regulation, altered numbers of AT1 receptors, and changes in signal transductions might contribute to these changes. Past observations from cardiac structural changes showed that AT1 receptors are associate with the Gq and Gi families of GTP-binding proteins (G protein), leading to the activation of phospholipase C. Ang II cross-talks with several tyrosine kinases via AT1 receptors, including receptor tyrosine kinases. It is well known that Ang II–evoked signal transduction pathways differ among cell types. [18, 39, 40] We now report that Ang II and Ang-(1-12)–mediated contractile action couples to Gs in normal myocytes. In HF, Ang II and Ang-(1-12)-mediated contractile action couples to both Gs and Gi because pretreatment with PTX significantly enhanced Ang-(1-12)-mediated action in HF myocytes. In HF, the cAMP-dependent intracellular signal transduction system is disrupted and both Gs and Gi proteins are critically involved in ISO-induced HF.[18] There is an increase in the number and functional activity of inhibitory G protein, Gi. [41, 42] AT1 receptors and Gq may be down regulated, which may lead to a change for Ang II AT1 receptor coupling from Gs to Gi. This may exacerbate the dysfunctional Ca2+ homeostasis, which might account for the altered inotropic effect on myocardial contraction and relaxation that we observed after HF. Further studies are needed to characterize intracellular pathway coupling Ang II and Ang-(1-12) medicated myocardial functional performance.
In summary, direct assessment of contractile Ang-(1-12) actions in isolated myocytes in terms of both inotropism and intracellular Ca2+ fluxes show that its biological activity is primarily due to its conversion to Ang II via a chymase-mediated mechanism present in the membrane or the intracellular environment of these freshly isolated myocytes. ISO-induced HF is associated with blunting of the inotropic mechanisms in response to both Ang II and Ang-(1-12). The existence of a cellular system for the processing of Ang-(1-12) via chymase and the apparently rapid conversion of this alternate substrate to Ang II adds weight to our hypothesis that Ang-(1-12) is the functional source for intracellular Ang II actions.
5. Study limitations
Although pathologic changes in ISO-treated rats resemble those of myocardial infarction and ISO-induced HF mimics many structural, functional, and neurohormonal changes of clinical HF,[12, 43, 44] we cannot ascertain that these results would apply to clinical HF or HF from other causes, such as hypertrophic cardiomyopathy or volume overload. The Ang-(1-12) and Ang II doses used in these experiments are based on previous concentration-response studies.[36, 38, 45, 46] While it may be argued that these doses are higher than those reported in the circulation, it is now recognized that hormones in the tissue interstitium are present in the nanomolar range. Because Ang-(1-12) and Ang II are generated in rat myocardium [20, 47] and in the intact and failing human heart, [5, 6, 21] Ang-(1-12) tissue levels may exceed Ang-(1-12) plasma levels, in analogy to the situation with Ang II.
6. Conclusions
Ang-(1-12) stimulates LV myocyte contractile function and [Ca2+]iT in both normal and HF rats through a chymase mediated action. HF is associated with a reduced action of Ang-(1-12) and Ang II on myocyte contractility and [Ca2+]i regulation. Altered inotropic responses to Ang-(1-12) and Ang II in HF myocytes are mediated through a cAMP-dependent mechanism that is coupled to both stimulatory G and inhibitory PTX-sensitive G proteins.
This study confirms the importance of a chymase/Ang-(1-12) pathway on the modulation of cardiac function. These findings have important clinical implications because the current acceptance of ACE inhibitors as the foundation for HF management may need revision as new research including the one described here denotes the primacy of chymase as an Ang II tissue-generating hormone. Future therapeutic targets that block tissue or cellular Ang II synthesis via chymase-mediated pathway may be necessary to fully interrupt the effects of Ang II.
Supplementary Material
Highlights.
Ang-(1-12) as a chymase-dependent substrate for generating Ang II inotropic activity.
Ang-(1-12) stimulates myocyte contractile function and [Ca2+]iT in both normal and HF.
HF reduces Ang-(1-12) actions on myocyte contractility and [Ca2+]i regulation.
Altered Ang-(1-12) response in HF is mediated through a cAMP-dependent mechanism.
Attenuated cardiac effect of Ang-(1-12) in HF is coupled to both Gs and Gi proteins.
Acknowledgments
We gratefully acknowledge the computer programming of Ping Tan and the administrative support of Stacey Belton.
Footnotes
Grant Support: This study was supported by the National Institutes of Health grants [R01AG049770 (HJ Cheng); P01HL051952-21 (CM Ferrario); R01HL074318 (CP Cheng) and National Natural Science Foundation of China (81270252) (WM Li)
All authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and the discussed interpretation.
Abstract presented at American Heart Association Meeting
Disclosures: No conflicts of interest, financial or otherwise, are declared by the author (s)
Author contributions:
C.M.F., H.J.C., and C.P.C. conception and design of research as well as provided direction and supervision of the project; T. K. L., X.W.Z., Z. Z., S.A., J. V., and H.J.C performed experiments; T.K. L., X.W.Z., and C.P.C., analyzed data and the statistical analysis; T.K.L., X.W.Z., H.J.C., C.M.F., W.M. L., and C.P.C. interpreted results of experiments; T.K.L., and C.P.C. prepared figures; T.K.L., H.J.C., C.P. C., drafted manuscript; C.M.F., and C.P.C. edited and revised it critically for important intellectual content; T.K.L., X.W.Z., H.J.C., Z. Z., S.A., J. V., C.P.C and C.M.F. approved the final version of the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nagata S, Kato J, Sasaki K, Minamino N, Eto T, Kitamura K. Isolation and identification of proangiotensin-12, a possible component of the renin-angiotensin system. Biochem Biophys Res Commun. 2006;350:1026–31. doi: 10.1016/j.bbrc.2006.09.146. [DOI] [PubMed] [Google Scholar]
- 2.Ferrario CM, Ahmad S, Nagata S, Simington SW, Varagic J, Kon N, et al. An evolving story of angiotensin-II-forming pathways in rodents and humans. Clin Sci (Lond) 2014;126:461–9. doi: 10.1042/CS20130400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferrario CM, Ahmad S, Varagic J, Cheng CP, Groban L, Wang H, et al. Intracrine angiotensin II functions originate from noncanonical pathways in the human heart. Am J Physiol Heart Circ Physiol. 2016;311:H404–14. doi: 10.1152/ajpheart.00219.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reyes S, Varagic J, Ahmad S, VonCannon J, Kon ND, Wang H, et al. Novel Cardiac Intracrine Mechanisms Based on Ang-(1-12)/Chymase Axis Require a Revision of Therapeutic Approaches in Human Heart Disease. Curr Hypertens Rep. 2017;19:16. doi: 10.1007/s11906-017-0708-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nagata S, Varagic J, Kon ND, Wang H, Groban L, Simington SW, et al. Differential expression of the angiotensin-(1-12)/chymase axis in human atrial tissue. Ther Adv Cardiovasc Dis. 2015;9:168–80. doi: 10.1177/1753944715589717. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 6.Ahmad S, Simmons T, Varagic J, Moniwa N, Chappell MC, Ferrario CM. Chymase-dependent generation of angiotensin II from angiotensin-(1-12) in human atrial tissue. PLoS One. 2011;6:e28501. doi: 10.1371/journal.pone.0028501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferrario CM, Mullick AE. Renin angiotensin aldosterone inhibition in the treatment of cardiovascular disease. Pharmacol Res. 2017;125:57–71. doi: 10.1016/j.phrs.2017.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Mello WC. Local Renin Angiotensin Aldosterone Systems and Cardiovascular Diseases. Med Clin North Am. 2017;101:117–27. doi: 10.1016/j.mcna.2016.08.017. [DOI] [PubMed] [Google Scholar]
- 9.Dell’Italia LJ, Meng QC, Balcells E, Straeter-Knowlen IM, Hankes GH, Dillon R, et al. Increased ACE and chymase-like activity in cardiac tissue of dogs with chronic mitral regurgitation. Am J Physiol. 1995;269:H2065–73. doi: 10.1152/ajpheart.1995.269.6.H2065. [DOI] [PubMed] [Google Scholar]
- 10.Froogh G, Pinto JT, Le Y, Kandhi S, Aleligne Y, Huang A, et al. Chymase-dependent production of angiotensin II: an old enzyme in old hearts. Am J Physiol Heart Circ Physiol. 2017;312:H223–H31. doi: 10.1152/ajpheart.00534.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumar R, Thomas CM, Yong QC, Chen W, Baker KM. The intracrine renin-angiotensin system. Clin Sci (Lond) 2012;123:273–84. doi: 10.1042/CS20120089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Teerlink JR, Pfeffer JM, Pfeffer MA. Progressive ventricular remodeling in response to diffuse isoproterenol-induced myocardial necrosis in rats. Circ Res. 1994;75:105–13. doi: 10.1161/01.res.75.1.105. [DOI] [PubMed] [Google Scholar]
- 13.Zhang ZS, Cheng HJ, Onishi K, Ohte N, Wannenburg T, Cheng CP. Enhanced inhibition of L-type Ca2+ current by beta3-adrenergic stimulation in failing rat heart. J Pharmacol Exp Ther. 2005;315:1203–11. doi: 10.1124/jpet.105.089672. [DOI] [PubMed] [Google Scholar]
- 14.Morimoto A, Hasegawa H, Cheng HJ, Little WC, Cheng CP. Endogenous beta3-adrenoreceptor activation contributes to left ventricular and cardiomyocyte dysfunction in heart failure. Am J Physiol Heart Circ Physiol. 2004;286:H2425–33. doi: 10.1152/ajpheart.01045.2003. [DOI] [PubMed] [Google Scholar]
- 15.Shao Q, Cheng HJ, Callahan MF, Kitzman DW, Li WM, Cheng CP. Overexpression myocardial inducible nitric oxide synthase exacerbates cardiac dysfunction and beta-adrenergic desensitization in experimental hypothyroidism. Int J Cardiol. 2016;204:229–41. doi: 10.1016/j.ijcard.2015.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suzuki M, Ohte N, Wang ZM, Williams DL, Jr, Little WC, Cheng CP. Altered inotropic response of endothelin-1 in cardiomyocytes from rats with isoproterenol-induced cardiomyopathy. Cardiovasc Res. 1998;39:589–99. doi: 10.1016/s0008-6363(98)00166-7. [DOI] [PubMed] [Google Scholar]
- 17.Ahmad S, Varagic J, VonCannon JL, Groban L, Collawn JF, Dell’Italia LJ, et al. Primacy of cardiac chymase over angiotensin converting enzyme as an angiotensin-(1-12) metabolizing enzyme. Biochem Biophys Res Commun. 2016;478:559–64. doi: 10.1016/j.bbrc.2016.07.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou P, Cheng CP, Li T, Ferrario CM, Cheng HJ. Modulation of cardiac L-type Ca2+ current by angiotensin-(1-7): normal versus heart failure. Ther Adv Cardiovasc Dis. 2015;9:342–53. doi: 10.1177/1753944715587424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Mello WC, Dell’Itallia LJ, Varagic J, Ferrario CM. Intracellular angiotensin-(1-12) changes the electrical properties of intact cardiac muscle. Mol Cell Biochem. 2016;422:31–40. doi: 10.1007/s11010-016-2801-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahmad S, Varagic J, Westwood BM, Chappell MC, Ferrario CM. Uptake and metabolism of the novel peptide angiotensin-(1-12) by neonatal cardiac myocytes. PLoS One. 2011;6:e15759. doi: 10.1371/journal.pone.0015759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahmad S, Wei CC, Tallaj J, Dell’Italia LJ, Moniwa N, Varagic J, et al. Chymase mediates angiotensin-(1-12) metabolism in normal human hearts. J Am Soc Hypertens. 2013;7:128–36. doi: 10.1016/j.jash.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prosser HC, Forster ME, Richards AM, Pemberton CJ. Cardiac chymase converts rat proAngiotensin-12 (PA12) to angiotensin II: effects of PA12 upon cardiac haemodynamics. Cardiovasc Res. 2009;82:40–50. doi: 10.1093/cvr/cvp003. [DOI] [PubMed] [Google Scholar]
- 23.Nagata S, Kato J, Kuwasako K, Kitamura K. Plasma and tissue levels of proangiotensin-12 and components of the renin-angiotensin system (RAS) following low- or high-salt feeding in rats. Peptides. 2010;31:889–92. doi: 10.1016/j.peptides.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 24.Chan KH, Chen YH, Zhang Y, Wong YH, Dun NJ. Angiotensin-[1-12] interacts with angiotensin type I receptors. Neuropharmacology. 2014;81:267–73. doi: 10.1016/j.neuropharm.2013.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ahmad S, Varagic J, Groban L, Dell’Italia LJ, Nagata S, Kon ND, et al. Angiotensin-(1-12): a chymase-mediated cellular angiotensin II substrate. Curr Hypertens Rep. 2014;16:429. doi: 10.1007/s11906-014-0429-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fu L, Wei CC, Powell PC, Bradley WE, Ahmad S, Ferrario CM, et al. Increased fibroblast chymase production mediates procollagen autophagic digestion in volume overload. J Mol Cell Cardiol. 2016;92:1–9. doi: 10.1016/j.yjmcc.2016.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wei CC, Hase N, Inoue Y, Bradley EW, Yahiro E, Li M, et al. Mast cell chymase limits the cardiac efficacy of Ang I-converting enzyme inhibitor therapy in rodents. J Clin Invest. 2010;120:1229–39. doi: 10.1172/JCI39345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dell’Italia LJ, Meng QC, Balcells E, Wei CC, Palmer R, Hageman GR, et al. Compartmentalization of angiotensin II generation in the dog heart. Evidence for independent mechanisms in intravascular and interstitial spaces. J Clin Invest. 1997;100:253–8. doi: 10.1172/JCI119529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ahmad S, Sun X, Lin M, Varagic J, Zapata-Sudo G, Ferrario CM, et al. Blunting of estrogen modulation of cardiac cellular chymase/RAS activity and function in SHR. J Cell Physiol. 2018;233:3330–42. doi: 10.1002/jcp.26179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Husain A. The chymase-angiotensin system in humans. J Hypertens. 1993;11:1155–9. [PubMed] [Google Scholar]
- 31.Jessup JA, Trask AJ, Chappell MC, Nagata S, Kato J, Kitamura K, et al. Localization of the novel angiotensin peptide, angiotensin-(1-12), in heart and kidney of hypertensive and normotensive rats. Am J Physiol Heart Circ Physiol. 2008;294:H2614–8. doi: 10.1152/ajpheart.91521.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abadir PM, Walston JD, Carey RM. Subcellular characteristics of functional intracellular renin-angiotensin systems. Peptides. 2012;38:437–45. doi: 10.1016/j.peptides.2012.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kumar R, Singh VP, Baker KM. The intracellular renin-angiotensin system in the heart. Curr Hypertens Rep. 2009;11:104–10. doi: 10.1007/s11906-009-0020-y. [DOI] [PubMed] [Google Scholar]
- 34.Kurabayashi M, Yazaki Y. Downregulation of angiotensin II receptor type 1 in heart failure. A process of adaptation or deterioration? Circulation. 1997;95:1104–7. doi: 10.1161/01.cir.95.5.1104. [DOI] [PubMed] [Google Scholar]
- 35.Liao Y, Husain A. The chymase-angiotensin system in humans: biochemistry, molecular biology and potential role in cardiovascular diseases. Can J Cardiol. 1995;11(Suppl F):13F–9F. [PubMed] [Google Scholar]
- 36.Lindpaintner K, Ganten D. The cardiac renin-angiotensin system. An appraisal of present experimental and clinical evidence. Circ Res. 1991;68:905–21. doi: 10.1161/01.res.68.4.905. [DOI] [PubMed] [Google Scholar]
- 37.Lindpaintner K, Ganten D. Tissue renin-angiotensin systems and their modulation: the heart as a paradigm for new aspects of converting enzyme inhibition. Cardiology. 1991;79(Suppl 1):32–44. doi: 10.1159/000174905. [DOI] [PubMed] [Google Scholar]
- 38.Ikenouchi H, Barry WH, Bridge JH, Weinberg EO, Apstein CS, Lorell BH. Effects of angiotensin II on intracellular Ca2+ and pH in isolated beating rabbit hearts and myocytes loaded with the indicator indo-1. J Physiol. 1994;480(Pt 2):203–15. doi: 10.1113/jphysiol.1994.sp020353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.De Mello WC. Intracellular angiotensin II disrupts chemical communication and impairs metabolic cooperation between cardiac myocytes. Peptides. 2015;72:57–60. doi: 10.1016/j.peptides.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 40.De Mello WC, Ferrario CM, Jessup JA. Beneficial versus harmful effects of Angiotensin (1-7) on impulse propagation and cardiac arrhythmias in the failing heart. J Renin Angiotensin Aldosterone Syst. 2007;8:74–80. doi: 10.3317/jraas.2007.015. [DOI] [PubMed] [Google Scholar]
- 41.Ang R, Opel A, Tinker A. The Role of Inhibitory G Proteins and Regulators of G Protein Signaling in the in vivo Control of Heart Rate and Predisposition to Cardiac Arrhythmias. Front Physiol. 2012;3:96. doi: 10.3389/fphys.2012.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eschenhagen T, Mende U, Diederich M, Nose M, Schmitz W, Scholz H, et al. Long term beta-adrenoceptor-mediated up-regulation of Gi alpha and G(o) alpha mRNA levels and pertussis toxin-sensitive guanine nucleotide-binding proteins in rat heart. Mol Pharmacol. 1992;42:773–83. [PubMed] [Google Scholar]
- 43.Grimm D, Elsner D, Schunkert H, Pfeifer M, Griese D, Bruckschlegel G, et al. Development of heart failure following isoproterenol administration in the rat: role of the renin-angiotensin system. Cardiovasc Res. 1998;37:91–100. doi: 10.1016/s0008-6363(97)00212-5. [DOI] [PubMed] [Google Scholar]
- 44.Yeager JC, Iams SG. The hemodynamics of isoproterenol-induced cardiac failure in the rat. Circ Shock. 1981;8:151–63. [PubMed] [Google Scholar]
- 45.De Mello WC, Danser AH. Angiotensin II and the heart : on the intracrine renin-angiotensin system. Hypertension. 2000;35:1183–8. doi: 10.1161/01.hyp.35.6.1183. [DOI] [PubMed] [Google Scholar]
- 46.Lefroy DC, Crake T, Del Monte F, Vescovo G, Dalla Libera L, Harding S, et al. Angiotensin II and contraction of isolated myocytes from human, guinea pig, and infarcted rat hearts. Am J Physiol. 1996;270:H2060–9. doi: 10.1152/ajpheart.1996.270.6.H2060. [DOI] [PubMed] [Google Scholar]
- 47.Wang H, Jessup JA, Zhao Z, Da Silva J, Lin M, MacNamara LM, et al. Characterization of the cardiac renin angiotensin system in oophorectomized and estrogen-replete mRen2. Lewis rats. PLoS One. 2013;8:e76992. doi: 10.1371/journal.pone.0076992. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.