Abstract
Social neuroscience has traditionally focused on the functionality of gray matter regions, ignoring the critical role played by axonal fiber pathways in supporting complex social processes. In this paper, we argue that research on white matter is essential for understanding a range of topics in social neuroscience such as face processing, theory of mind, empathy, and imitation, as well as clinical disorders defined by aberrant social behavior such as prosopagnosia, autism, and schizophrenia. We provide practical advice on how best to carry out these studies which ultimately, will substantially deepen our understanding of the neurobiological basis of social behavior.
Keywords: white matter, diffusion-weighted imaging, structural connectivity, social cognition, social neuroscience, social disorder
The Historical Neglect of the Structural Connectome
Social neuroscience is a rapidly growing field that is providing considerable insight into brain mechanisms of social cognition, social behavior, and social disorders. However, the history of this field shows an overwhelming emphasis on the functionality of gray matter, with a relative disregard of white matter. Students are taught to identify sulcal-gyral landmarks that denote gray matter regions, but the identification of white matter structures (see Glossary) is usually neglected. Similarly, studies of individuals with focal brain lesions due to stroke or traumatic brain injury often show involvement of white matter tissue in brain scans, but rarely is white matter damage an explicit focus of the discussion, and in some cases, its involvement in the symptomatology is discounted (Box 1). Nevertheless, few would deny the importance of white matter for human cognition and behavior, as it plays a vital role in communications between cortical areas [1]. Studies of human white matter provide key insights into the organization of brain systems and the functions they perform. Several structures (e.g. optic tracts, corticospinal tract, fornix, and arcuate fasciculus) have been well characterized for vision, sensorimotor processing, episodic memory, and language [2–5]. However, current knowledge about the specific white matter tracts underlying social cognition is very limited.
Box 1. The Neglect of White Matter in Social Neuroscience.
Just as H.M. arguably became the index case for the neuroscience of memory, Phineas Gage serves as the index case for the neuroscience of social cognition. Gage suffered a severe traumatic brain injury when an iron rod was driven through his skull, destroying much of his frontal lobe and dramatically changing his personality and social behavior. This notable case appears in every textbook of social neuroscience, and illustrates the critical role of the frontal cortex in human personality and social cognition.
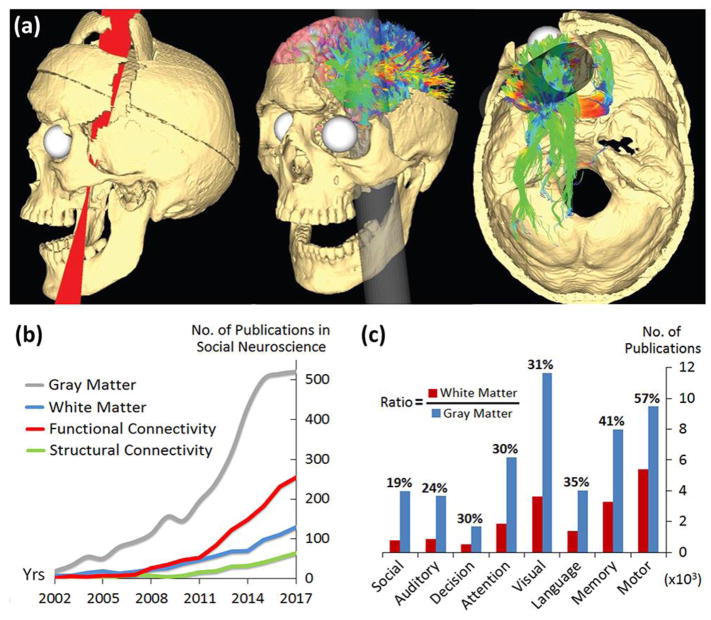
The amount of damage to Gage’s frontal cortical gray matter has been systematically assessed by over 15 studies. Recently, however, a research team turned their attention to how the iron tamping rod may have damaged critical white matter tracts [110]. While 4% of Gage’s frontal cortex was intersected by the rod’s passage, 11% of frontal white matter was found to be damaged (Figure I-a, adapted from [110]). Fasciculi that were severely impacted include the uncinate fasciculus, cingulum bundle, and superior longitudinal fasciculus. This suggests that, loss of white matter connectivity between the frontal lobe and the rest of the brain may have compromised the surviving brain networks and their functions. This extensive white matter network destruction might be a key contributor to Gage’s long-term social behavioral changes. Clearly, the significance of white matter for social cognition has been profoundly underestimated since the beginning of social neuroscience.
Even with the advent of magnetic resonance imaging (MRI) that provides an unprecedented capacity for noninvasively measuring diverse aspects of brain structure, research on white matter appears to lag for social neuroscientists. We examined the last 15 years of publications in PubMed (Figure I-b) and found that there are four times more research studies focused on gray matter (e.g. functional MRI) than on white matter (e.g. diffusion MRI) in social neuroscience. Even though the field in the past decade has ardently embraced connectomics and network methods, research studying structural connectivity of the social brain is still heavily outnumbered by studies of functional connectivity. In addition, compared to other fields in cognitive neuroscience, social neuroscience has the smallest ratio between the size of white matter and gray matter literature (19%), much smaller than neuroscience of language (35%), memory (41%) and motor function (57%) (Figure I-c). Thus, structural connectivity has been excessively overlooked by social neuroscientists.
Figures I-a is courtesy of John Darrell Van Horn, USC Mark and Mary Stevens Neuroimaging and Informatics Institute, University of Southern California
This issue becomes even more pressing as the field of social neuroscience is increasingly moving away from a strict modular view of the brain and toward an appreciation of circuits and networks (i.e. “connectomics”) [6]. Although early research attempted to locate social cognitive processes in individual brain regions, such as medial prefrontal cortex and temporo-parietal junction, there is a growing consensus that social processes and behavior emerge from interactions across distributed areas of the so-called “social brain networks” (see Figure 1a) [7]. Correspondingly, this network-based approach features a variety of brain connectivity analyses examining the interconnections between social brain areas, such as functional connectivity during resting state and effective connectivity during social tasks. Again, these methods are solely based on gray matter co-activation (i.e. BOLD signal). However, the presence of anatomical connectivity is a prerequisite for the existence of functional networks and the ensuing rapid, efficient interactions [8], thus the properties of structural connectivity determine the properties of functional connectivity to a great extent [9]. Some social disorders, such as developmental prosopagnosia, appear to be mainly caused by damage to white matter tracts between social brain regions, as functional activation within gray matter regions remains relatively intact [10]. Therefore, our understanding of social cognition and social dysfunction will be incomplete until we understand the structural connectome of the social brain.
Figure 1.
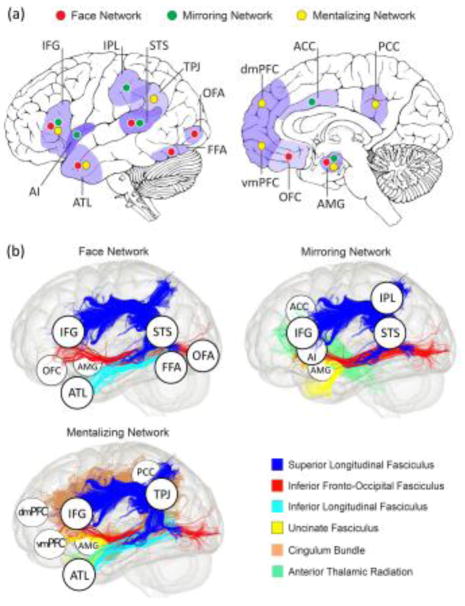
Gray matter and white matter structures that constitute core social brain networks. (a) A depiction of gray matter regions that are highly activated when people perform face perception, imitation/empathy, and mentalizing tasks in an MRI scanner [7,55]. (b) Major white matter bundles associated with each social brain network are depicted. They form the overall architecture of interconnections between social brain regions depicted in (a). Disruption of these fiber tracts (either by brain damage or neuromodulation) can lead to a range of social deficits, such as prosopagnosia [2], impaired empathy and emotion recognition skills [45,104], and mind-blindness [79,80,94,105]. For simplicity and clarity, not all gray and white matter regions/tracts implicated in each network are shown. ACC, anterior cingulate cortex; AI, anterior insula; AMG, amygdala; ATL, anterior temporal lobe; dMPFC, dorsomedial prefrontal cortex; FFA, fusiform face area; IFG, inferior frontal gyrus; IPL, inferior parietal lobule; OFC, orbitofrontal cortex; OFA, occipital face area; PCC, posterior cingulate cortex; STS, superior temporal sulcus; TPJ, temporo-parietal junction; vMPFC, ventromedial prefrontal cortex. This figure is adapted from [22].
Why White Matter Matters for Social Cognition
Relatively to body size, the human brain is greatly enlarged compared to that of other mammals. One putative force behind this expansion is the increased social complexity and boosted social skills found in humans (i.e. the ‘social brain hypothesis’) [11]. Larger brains require longer fibers to communicate between distant cortical areas, and evolutionarily the volume of white matter containing long axons increased disproportionally faster than the volume of the gray matter containing cell bodies, dendrites, and axons for local information processing [12]. That’s why in humans, white matter makes up over half of the whole cerebral volume, a far greater proportion than is found in other animals [1].
The putative ‘social brain’ comprises a collection of large-scale networks of diverse brain regions encompassing all five cerebral lobes and both hemispheres (Figure 1a), as well as portions of the cerebellum [13]. Operation and coordination of such highly-distributed brain areas would be impossible without extensive white matter tracts for long-range transmission of communication, and this neuroanatomical infrastructure might be more crucial for social cognition than other cognitive functions that are underpinned by a single or a set of geographically clustered brain structures (e.g. the hippocampus and medial temporal lobe structures for episodic memory). The downside of such widespread connections for social cognition is that it is very vulnerable to brain injuries and diseases. Even minor white matter abnormalities can lead to social dysfunction [14,15]. Therefore, it is not surprising that sociocognitive deficits are the most common symptoms observed across a wide range of psychiatric and neurological disorders [7,16].
From the perspective of information-processing, social interaction is computationally demanding [17]. Our social environment is highly complex, dynamic, and unpredictable. Consequently, adaptive social behavior and efficient communication are contingent upon rapid implementation of multiple skills such as face processing, language, action observation and execution, mind-reading, and decision-making. Given the inherently multimodal, highly interactive, reciprocal and real-time nature of social interaction, there is substantial pressure to integrate large amount of mental processes as efficiently as possible; therefore, social cognition requires considerable amounts of myelinated fiber bundles across the whole brain to facilitate information integration [7]. Patients with demyelinating disorders often exhibit severe social difficulties [18,19].
What We Will Gain by Measuring Structural Connectivity
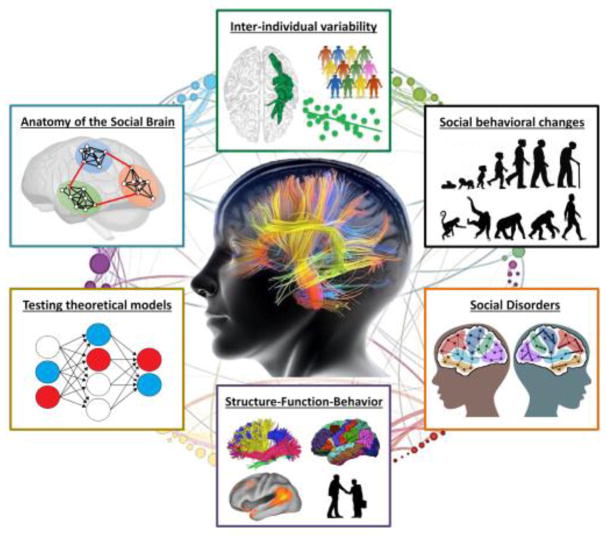
Analysis of white matter complements the analysis of gray matter, as well as provides unique insights into the neurobiology of social cognition (Key Figure). First, in-vivo imaging of white matter using diffusion-weighted MRI makes it possible to virtually dissect the brain [8] and explore patterns of fiber projections between social brain areas [20,21]. Preliminary white matter mapping has elucidated a set of tracts scaffolding major social brain networks such as face, mirroring, and mentalizing systems (Figure 1b) [22]. When combined with task fMRI, diffusion tractography can delicately trace the specific fiber pathway for a particular social process, such as gaze perception [23] and imitation [24].
Key Figure.
The advantages of examining white matter for social neuroscience. When compared to functional approaches (e.g. fMRI, EEG, MEG, TMS), studying white matter allows for several unique insights (described in a clockwise manner). First, white matter mapping can reveal network-level anatomy, functioning, plasticity and compensation of the social brain. Second, white matter characteristics, distinct from gray matter properties and genetic factors, can be effectively used to account for remarkable inter-individual variability of social skills and behavior. Third, white matter research can unravel the structural correlates of social behavioral changes during development, aging, disease, and across species. Fourth, our understanding of social disorders such as prosopagnosia, autism and schizophrenia will benefit significantly from investigation of white matter; structural connectivity can be treated as a legitimate and reliable biomarker for clinical diagnosis and therapy. Fifth, collecting multimodal brain data including functional and diffusion imaging enable us to infer “structure-function” relationships that subserve social cognition and behavior. Finally, clarifying white matter pathways in the social brain can be used to guide the construction of new neurobiologically realistic models for social processing as well as to validate existing theories in social neuroscience.
Second, a hallmark of human social behavior is the remarkable variance between individuals. A number of studies have begun to understand such inter-individual variability by examining differences in white matter macro- and microstructure [25]. White matter characteristics have been successfully used to account for diversity in personality traits [26,27], self-processes [28,29], intergroup bias [30], and social network size [31], and to predict personal social skills such as face recognition ability [32], social memory [33,34], empathy [35,36], and emotional intelligence [37]. In some instances, associations between white matter indices and social impairments can make superior predictions than measures of regional gray matter in clinical populations [38,39].
Third, research on white matter can play a key role in unraveling the structural correlates of social behavior changes during development [40,41], aging [42,43], disease [44–46], training/intervention [47], and across social contexts [48,49]. It has been consistently found that maturation or deterioration of white matter throughout the lifespan shapes the development or decline in social skills [25,40,50]. In addition, white matter changes can be associated with phylogeny of social cognition. For example, cross-species comparison of white matter structures has helped to reveal the anatomical signature of certain evolved social skills, such as imitation [51], theory of mind (ToM) [52], cooperation [53] and self-recognition [54]. It is worth mentioning that white matter imaging techniques (see Box 2) are relatively easy to carry out for special populations who may not tolerate long scan times or challenging tasks.
Box 2. Techniques to Study Human White Matter.
A common misperception is that white matter connections have a binary nature like electrical cables: either they are connected and functioning or they are disconnected. We now know that it is much more nuanced, with a fiber pathway’s size, shape and density (also known as macrostructure) as well as its integrity, connectivity strength, and degree of myelination (also known as microstructure) contributing to the functional profile of white matter connections.
A wealth of neuroanatomical tools has been developed to measure these macro- and microstructural properties. Histological tracing techniques are the gold standard for measuring white matter connections. They provide extremely detailed, precise local axonal connectivity information with low false positive rates, but are restricted to human postmortem or non-human animal research [8]. Another powerful, but invasive tool is direct electrical stimulation during awake neurosurgery. This neuromodulation technique provides real-time causal investigations into the functional role of a particular tract [111].
MRI techniques allow us to measure brain-wide structural connections in vivo, in addition to the potential for parallel acquisition of other types of data that can be related to or predicted by white matter connectivity. Three methods are commonly used: structural MRI (sMRI), diffusion-weighted MRI (dMRI), and quantitative MRI (qMRI). sMRI collects conventional T1/T2-weighted images to visualize and evaluate macroscopic features of white matter at high resolution, which is useful for anatomical morphometry and detection of white matter abnormalities and damage for clinical diagnosis. dMRI measures water diffusivity to indirectly infer the macrostructural organization and microstructural properties of white matter tissue and has been the most powerful tool for identifying and characterizing long-range fiber pathways [2,73]. qMRI (e.g. magnetization transfer, T1/T2 relaxometry) can directly measure the properties and composition of complex tissue matrix around axons (e.g. glia, myelin basic protein, chemical metabolites) and provides information related to the physiology (e.g. metabolism) and pathology (e.g. inflammation) of white matter tissue substrates, which may ultimately affect brain connectivity [72]. MRI techniques are valuable because they are non-invasive. However a fundamental limitation of MRI-based techniques is the indirect nature of their measurements on white matter [92]. Since all estimates are based on water diffusivity, dMRI techniques provides only computational models of white matter structures and properties with many theoretical assumptions about the local fiber bundle geometry that have not been fully validated by the ground truth (e.g. tracing studies or simulated brain data). This makes dMRI error-prone [103] and highly dependent on data quality, the chosen diffusion model, and the analysis methods [22,112].
The brain’s structural connectome can be linked to behavior by correlating macro and microstructural differences with behavior. Because of this, white matter research requires a sufficiently large sample size to robustly reveal the relationship between fiber tracts and behavior, as well as carefully conceived behavioral tasks that maximize both within-subject reliability and between-subject variation.
Fourth, our understanding of social disorders can significantly benefit from investigations of white matter. Multiple psychiatric and neurological disorders are defined by primary impairment in social cognition (e.g. developmental prosopagnosia, autism, behavioral-variant frontotemporal dementia, social phobia, antisocial personality disorder) [7,55]. As already mentioned above, the network-based approach has prompted clinical researchers to consider connectivity rather than focal gray matter damage when interpreting disorders [56], and the health of white matter connections has already been found to be an important factor underlying many social disorders [1,7]. For example, autism has long been conceptualized as a developmental ‘disconnection syndrome’ [57] and one of the most accepted brain “signatures” is reduced white matter integrity of long-range fiber tracts [14,15]. This altered structural connectivity in the autistic brain is tightly associated with impaired social functions such as ToM, emotion recognition, empathy, and social communication [58–61]. The extant literature suggests that aberrant white matter structure and organization have a strong genetic basis, thus structural connectivity can be treated as a heritable biomarker for clinical diagnosis and therapy [25,62,63]. In addition, by linking anatomical characteristics to clinical phenotypes (e.g. neuropsychological standardized tests), one can investigate the impact of brain pathology on social behavior via white matter alterations [64–66] or study the etiology of social disorders [10,14,15]. Different from classical disconnection syndromes with white matter damage typically occurring to a particular site or tract (e.g. agenesis of corpus callosum, conduction aphasia) [67], certain social disorders could implicate a diffuse pattern of white matter abnormality encompassing a wider range of tracts [68,69] and complicated conditions (e.g. hypo- and hyper-connectivity co-exist) [70]. Even different symptoms in a social disorder could correspond to disparate tracts. For example, imitation and social communication deficits in autism are associated with the integrity of superior longitudinal fasciculus [61,71] whereas emotional recognition and ToM deficits in autism are associated with the cingulum and inferior longitudinal fasciculus respectively [59]. With the ongoing development of white matter imaging techniques [72,73], more subtle microstructural properties and changes can be detected in each disorder, making white matter approaches particularly fruitful for studying network-level anatomy, functioning, plasticity, and compensation in the social brain [7].
Fifth, since structural and functional networks are mutually interdependent, studying white matter enables us to infer “structure-function” relationships that subserve social cognition and behavior. Take face processing as an example. Structural maturation of white matter tracts during early development is necessary for functional specialization of the face network to emerge. It has been found that increasing myelination in face pathways promotes the propagation of the neural signal throughout the face network, thereby enhancing the functional characteristics of the nodes within the network [40]. In addition, the function that a social brain region serves is often dynamic, and changes across networks [74]; even for the same region, different sub-areas might belong to different anatomical networks and thus play different functional roles [75]. In fact, we can use white matter connectivity profiles to identify boundaries of functionally distinct brain areas (i.e. tractography-based parcellation). This “connectional localizer” approach [8] has been successfully employed in functional segmentation of multiple social brain areas such as medial prefrontal cortex and temporo-parietal junction [75,76]. When combined with powerful machine learning algorithms, white matter connectivity fingerprints can even predict idiosyncratic functional responses to social stimuli [77].
Finally, structural connectivity research opens up new opportunities to testing current theories in social neuroscience [20,35,36,78–80]. Theories that are most amenable to this endeavor are ones that propose some sort of ordered information processing. One prominent example is Haxby’s model of face processing [81]. This model postulates a hierarchical structure for the core face system such that occipital face area (OFA) is a gateway to two independent and parallel processing streams: one in the fusiform face area (FFA), and one along the superior temporal sulcus (STS). Recent white matter research challenges this hierarchy by suggesting that face processing does not proceed strictly in sequence but rather in a parallel and interactive fashion. Several direct fiber pathways have been found between early visual cortices and FFA/STS face-area, without any mediation by the OFA [20,21]; the STS appears to be somewhat isolated from the rest of the system as there are no direct connections between the OFA/FFA and the STS [20,21]. In addition, redundant fiber bundles have been discovered within the face system for resilience in brain injury/disease [78] and abundant connections exist between face and non-face areas for bottom-up and top-down processing [82]. These new findings suggest that Haxby’s model is overly simplistic and requires revision [78,83]. Overall, research on structural connectivity can be leveraged for theory validation as well as a stepping-stone for discovery of new psychological and neurocognitive principles of social processing.
Future Directions
We have listed many benefits of studying structural connectivity in social neuroscience. However, investigators entering this field should be mindful of certain methodological and theoretical challenges. The most significant challenge is that diffusion imaging techniques are imperfect and multiple factors (e.g. data acquisition, preprocessing pipeline, diffusion modeling) can influence its reliability and reproducibility (see [22] for best practice in diffusion imaging data collection and analyses). Here we discuss three conceptual recommendations for social neuroscientists.
Recommendation 1. Examine Structural Connectivity at a High Granularity
One fundamental endeavor in social neuroscience is to delineate the functional parcellation within each social brain area. In a similar vein, one important future direction will be to investigate the fine-grained functional segmentation within each social white matter tract. For example, the literature has associated the inferior longitudinal fasciculus (ILF) with face processing, empathy, emotion recognition, and mentalizing [22]. This seemingly nonspecific role of the ILF in a variety of social processes may not be surprising, considering that the ILF is a large tract reaching up to 15cm in length and that different fiber bundles enter and exit the fasciculus at various positions, so not all bundles traverse the full length of the tract [84]. As such, the properties of any given tract may vary systematically along the trajectory of the fascicle [85]. Indeed, several studies have reported that the anterior portion of the ILF is associated with face memory whereas the middle and posterior portions are associated with scene memory [32]. While the entire ILF is critical for face recognition [2], a ventral branch between FFA and amygdala [86] and a dorsal branch between STS and inferior parietal lobule [51] may be responsible for emotion discrimination and imitation, respectively. These findings all suggest the existence of segregated segments or sub-bundles within a social white matter tract, each specialized for distinct social processing [32,34].
To study social white matter at a finer scale, future research needs to take several practical steps. First, prior studies chose to extract and analyze white matter characteristics across the entire tract. Such large-scale approach is crude and limits our ability to examine how white matter relates to functional regions or relays information specific to a social process. More recently, researchers have begun to quantify tissue properties systematically across different positions to reveal variations within a specific tract [85]. This approach should be adopted more because it provides much richer information about white matter function or dysfunction that are not obvious from the mean measures. Second, instead of using traditional anatomical ways to define entire fasciculi [87], researchers should consider utilizing functional regions of interest as seeds in tractography to identify the sub-tracts of large fasciculi that are specific to particular social processes [23,24]. These functionally-defined tracts will provide a more detailed and refined picture of fiber organization within large fasciculi. Finally, advanced imaging techniques such as diffusion spectrum imaging and Q-Ball Imaging [73] coupled with sophisticated analyses such as multivariate pattern analysis [88], computational and statistical modelling [89,90], and machine learning algorithms [91] will be tremendously important for elucidating the social structural connectome at a finer scale.
Recommendation 2. Leverage Multimodal Neuroimaging Methods
Collecting multimodal brain data from the same individual using different neuroimaging methods has recently become a standard in human neuroscience research. Several large-scale coordinated brain projects (e.g. Human Connectome Project, UK Biobank, and Autism Brain Imaging Data Exchange) provide notably high quality structural and diffusion MRI data, resting-state and social-task-based fMRI, and social behavioral measures from a large sample of healthy and clinical adults. These excellent multimodal resources can be exploited by social neuroscientists to unveil new properties of the human connectome.
In general, different white matter imaging techniques (e.g. structural, diffusion, and quantitative MRI) can be systematically combined to reveal more fine-grained features of fiber microstructures (e.g. axon density, diameter, and myelin sheath thickness) [73,92], and different anatomical characteristics (e.g., cortical folding, cytoarchitecture, structural connectivity) can be jointly evaluated with regard to social functions and behavior [78,88,93]. For some clinical populations (e.g. patients with gliomas), white matter imaging can also be combined with axonal stimulation technique (see direct electrical stimulation in Box 2) to scrutinize the causal role (i.e. critical versus participatory) of a particular tract for social functions [94].
Similarly, white matter and gray matter methods can be complementarily integrated to provide a more complete description of brain connectivity underpinning social cognition [23,29,58,86,95]. Each imaging modality provides a different view of social neural networks, and data fusion capitalizes on the strengths of each imaging modality as well as their interrelationships. We can use functional responses or connectivity to infer information regarding anatomical connections [96] or use structural connectivity to guide the construction of neurobiologically realistic models of functional connectivity during computational modeling [97]. Since functional connectivity is not affected by complex trajectory patterns (e.g. crossing fibers) or algorithm parameters (e.g. curvature and stopping criterion), it may allow us to indirectly validate the accuracy of tractography measures [8]. When tractography is combined with computational modeling of functional data, one might even be able to reveal the directionality (afferent or efferent) and function (inhibitory or excitatory) of white matter pathways in the social brain [98].
Multimodal data fusion also has promising clinical applications [99]. Joint biomarkers may be effective at discriminating social disorders [100] and predicting their treatment outcomes [39]. By examining structural–functional coupling in patients with disconnection syndrome (e.g. autism, schizophrenia), we can test whether social neural network disconnections arise from loss of white matter connectivity or from a purely functional reorganization based on synaptic alterations [101]. Ultimately, by exploring correspondence between structural and functional connectivity, a multimodal approach provides invaluable insights into the organization of social brain networks (e.g. neural integration vs segregation), neural origins of social behavior (e.g. which type of connectivity is mostly behaviorally-relevant), and etiology of social disorders (e.g. synaptic-level vs fiber-level disconnection).
Recommendation 3. Examine Functional Specificity of Social White Matter Tracts
Once you’ve decided how to gather your high resolution structural connectivity data, it is important to consider whether your behavioral tasks are sufficient for understanding functional specificity. You will want to know if the tracts or sub-tracts that you identify are specifically involved in social behavior and if so, what particular aspect of social behavior? The challenge is that social processes are interdependent and multifaceted, and we are far from having an agreed-upon taxonomy to guide our experimental design [102]. A behavioral task usually taps into multiple social constructs, making them difficult to disentangle when linking with white matter characteristics. Consider for example, a typical face emotion recognition task. Performing this task involves face perception, sensorimotor simulation, and retrieval of socioemotional conceptual knowledge. If this is your only task, it will be difficult to disentangle which subprocess contributes to tract-behavior correlations [45]. In addition, if non-social tasks are not included in the protocol, it will be impossible to discern whether the observed effect is socially specific or domain-general. It is thus ideal to employ multiple measurements per study to probe the targeted social process. By using a variety of stimuli (e.g. cartoons or stories), modalities (e.g. visual or auditory), paradigms (e.g. implicit or explicit) [79], task difficulties [50], or methods (e.g. self-report, or neurophysiological tool), future studies will be able to clarify the specificity versus generality of any given white matter tract in a particular social process.
Concluding Remarks
White matter has long been neglected in social neuroscience. Even with the advent of in vivo imaging techniques, the white matter foundation of many social functions remains unexplored or has received a cursory examination at most. We have only limited knowledge of how social brain areas are structurally inter-connected, which white matter structures and properties contribute to individual variability and behavioral changes of social functions, and what specific roles white matter dysfunction plays in social disorders. Although there are several challenges for studying social white matter, such as imperfect imaging techniques [22,103] and lack of a standard taxonomy to well define social constructs, we believe an exhaustive investigation of the social brain connectome (see Outstanding Questions) will deeply benefit our understanding of the neurobiological basis of social cognition, social behavior, and social disorders.
Outstanding Questions Box.
For any pair of social brain areas, what is the relationship between their structural connectivity, functional connectivity while at rest, and effective connectivity during social tasks? Which connectivity measure is most relevant for social behavior, and the best predictor for social disorders?
What is the timing of developmental milestones for social white matter networks (e.g. formation of anatomical hub, emergence and maturation of structural connectivity)? For a specific social function, do different sets of white matter fibers become involved during different stages of development? Do social brain regions that are wired together also functionally develop and later, decline together? When are the critical periods of developmental vulnerability for each social white matter pathway?
What’s the short-term and long-term plasticity of structural connectivity in the social brain? How does social experience (e.g. childhood deprivation, adolescent ostracism, divorce, widowhood) and social environment (e.g. socioeconomic status, cultural contexts) influence structural connectivity?
Are white matter abnormalities the cause or the consequence of each social disorder? What is the biological cause of white matter abnormality in social ‘disconnection syndromes’— synaptic alterations, loss of myelin, axonal loss, glial dysfunction, or neuronal death?
Present literature has focused almost exclusively on large, major fasciculi. But what’s the functional role of short-range white matter for social cognition, and how their abnormalities contribute to social disorders? What is the relationship between short-range and long-range connections in the social brain?
Prior research suggests that the cerebellum is important for social cognition and certain types of cerebellar pathology can cause social deficits such as Schmahmann’s syndrome. What is the role of cerebral-cerebellar structural connectivity in social processing?
Highlights.
Historically, social neuroscience research has adopted a topological (brain regions) rather than a hodological (neural pathways) approach. However the field is currently moving away from modularism to connectomics.
White matter structures have been well characterized for non-social cognition, but our current knowledge about white matter tracts underlying social cognition is limited.
Healthy white matter is essential for social information processing. Minor white matter abnormalities can cause severe social impairments.
Methods used to measure gray matter and white matter (e.g. functional and diffusion MRI) can be complementarily integrated to gain a more complete understanding of the neurobiology of social cognition. Multimodal imaging of the social brain connectome is the future of social neuroscience
Acknowledgments
This work was supported by an NIH grant to I. Olson [RO1 MH091113]. We thank Antonia F. de C. Hamilton, Nora Newcombe, and Athanasia Metoki for helpful comments on earlier versions of the manuscript. We would also like to thank two anonymous reviewers for extremely helpful comments on earlier versions of this manuscript.
Glossary Box
- Brain connectivity analyses
analysis of the pattern of anatomical links (“structural connectivity”) or statistical dependencies (“functional connectivity”) or causal interactions (“effective connectivity”) between distinct units within a nervous system.
- Diffusion-weighted MRI (dMRI)
dMRI measures the random motion or diffusion of water molecules, which are restricted by tissue microstructure. When this microstructure is more organized, such as in white matter, water diffusion is anisotropic, in that diffusion is less hindered parallel than perpendicular to white matter fibers. Thus, by measuring the orientational dependence of water diffusion, dMRI infers the microstructure and properties of surrounding white matter tissue.
- Diffusion tractography
(also known as fiber tract reconstruction): a dMRI method used to identify and visualize a continuous 3-dimensional trajectory of white matter fibers by sequentially piecing together the estimates of fiber orientation from the directionality of individual voxels.
- Disconnection syndrome
a general term for neurological symptoms caused by damage to communication pathways, via lesions to association fibers or commissural fibers, independent of any lesions to the cortex. A well-known example of this is callosal syndrome, or split-brain, caused by surgically splitting the corpus callosum.
- Short-range white matter
(also referred to as U-shaped fibers): the fiber tracts connecting adjacent gyri. In contrast to long-range fiber bundles located in deep white matter, short-range fibers are typically located in superficial white matter. Short-range connections are thought to be predominant in both structural and functional networks [106] and they comprise >85% of the whole human connectome [8,73,107]. Many social functions are associated with superficial/regional white matter [22], and in some instances, short-range fibers predict social behavior better than long-range fibers [108,109]. One challenge for the field is that we lack tools to reliably measure short-range white matter [108].
- Social brain networks
a set of functional networks or circuits involved in social processing
- White matter integrity
in dMRI literature, changes or differences in diffusion metrics are often interpreted as changes or differences in the “integrity” of white matter microstructure (or, in the opposite way, as structural damage, decline or degeneration). This implies that some aspect of the white matter microstructure (e.g. myelination, axonal density) is abnormal or damaged. An extensively used measure of white matter integrity is fractional anisotropy.
- White matter structures
Adjacent neuronal cell bodies often project axons that course through the brain together, arriving at a similar destination. These bundles, called fascicles (Latin for bunch), are sometimes grouped into larger structures called tracts. A large tract can include many fascicles (e.g. superior longitudinal fasciculus (SLF) I, SLF II, and SLF III). Fascicles can be estimated by tractography algorithms from diffusion imaging data. The computational representation of a fascicle is called a streamline. The complete set of streamlines generated by whole-brain tractography is called a connectome.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Douglas Fields R. White matter in learning, cognition and psychiatric disorders. Trends Neurosci. 2008;31:361–370. doi: 10.1016/j.tins.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rokem A, et al. The visual white matter: The application of diffusion MRI and fiber tractography to vision science. J Vis. 2017;17:1–30. doi: 10.1167/17.2.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ciccarelli O, et al. Diffusion-based tractography in neurological disorders: concepts, applications, and future developments. Lancet Neurol. 2008;7:715–727. doi: 10.1016/S1474-4422(08)70163-7. [DOI] [PubMed] [Google Scholar]
- 4.Thomas AG, et al. The fornix in health and disease: an imaging review. RadioGraphics. 2011;31:1107–1121. doi: 10.1148/rg.314105729. [DOI] [PubMed] [Google Scholar]
- 5.Friederici AD. Chapter 10 – White-matter pathways for speech and language processing. Handbook of Clinical Neurology. 2015;129:177–186. doi: 10.1016/B978-0-444-62630-1.00010-X. [DOI] [PubMed] [Google Scholar]
- 6.Bassett DS, Sporns O. Network neuroscience. Nat Neurosci. 2017;20:353–364. doi: 10.1038/nn.4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kennedy DP, Adolphs R. The social brain in psychiatric and neurological disorders. Trends Cogn Sci. 2012;16:559–572. doi: 10.1016/j.tics.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jbabdi S, et al. Measuring macroscopic brain connections in vivo. Nat Neurosci. 2015;18:1546–1555. doi: 10.1038/nn.4134. [DOI] [PubMed] [Google Scholar]
- 9.Bullmore E, Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci. 2009;10:312–312. doi: 10.1038/nrn2575. [DOI] [PubMed] [Google Scholar]
- 10.Thomas C, et al. Reduced structural connectivity in ventral visual cortex in congenital prosopagnosia. Nat Neurosci. 2009;12:29–31. doi: 10.1038/nn.2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dunbar RIM. The social brain hypothesis and its implications for social evolution. Ann Hum Biol. 2009;36:562–572. doi: 10.1080/03014460902960289. [DOI] [PubMed] [Google Scholar]
- 12.Zhang K, Sejnowski TJ. A universal scaling law between gray matter and white matter of cerebral cortex. Proc Natl Acad Sci U S A. 2000;97:5621–5626. doi: 10.1073/pnas.090504197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Overwalle F, et al. Social cognition and the cerebellum: A meta-analytic connectivity analysis. Hum Brain Mapp. 2015;36:5137–5154. doi: 10.1002/hbm.23002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Travers BG, et al. Diffusion Tensor Imaging in Autism Spectrum Disorder: A Review. Autism Res. 2012;5:289–313. doi: 10.1002/aur.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ameis SH, Catani M. Altered white matter connectivity as a neural substrate for social impairment in Autism Spectrum Disorder. Cortex. 2015;62:158–181. doi: 10.1016/j.cortex.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 16.Cotter J, et al. Social cognitive dysfunction as a clinical marker: a systematic review of meta-analyses across 30 clinical conditions. Neurosci Biobehav Rev. 2018;84:92–99. doi: 10.1016/j.neubiorev.2017.11.014. [DOI] [PubMed] [Google Scholar]
- 17.Adolphs R. The neurobiology of social cognition. Curr Opin Neurobiol. 2001;11:231–239. doi: 10.1016/s0959-4388(00)00202-6. [DOI] [PubMed] [Google Scholar]
- 18.Bora E, et al. Social cognition in multiple sclerosis: a meta-analysis. Neuropsychol Rev. 2016;26:160–172. doi: 10.1007/s11065-016-9320-6. [DOI] [PubMed] [Google Scholar]
- 19.Chalah MA, Ayache SS. Deficits in Social Cognition: An Unveiled Signature of Multiple Sclerosis. J Int Neuropsychol Soc. 2017;23:266–286. doi: 10.1017/S1355617716001156. [DOI] [PubMed] [Google Scholar]
- 20.Gschwind M, et al. White-matter connectivity between face-responsive regions in the human brain. Cereb Cortex. 2012;22:1564–1576. doi: 10.1093/cercor/bhr226. [DOI] [PubMed] [Google Scholar]
- 21.Pyles JA, et al. Explicating the face perception network with white matter connectivity. PLoS One. 2013;8:1–12. doi: 10.1371/journal.pone.0061611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Y, et al. White matter pathways and social cognition. Neurosci Biobehav Rev. doi: 10.1016/j.neubiorev.2018.04.015. (accepted pending minor revisions) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ethofer T, et al. Processing social aspects of human gaze: A combined fMRI-DTI study. Neuroimage. 2011;55:411–419. doi: 10.1016/j.neuroimage.2010.11.033. [DOI] [PubMed] [Google Scholar]
- 24.Hamzei F, et al. The dual-loop model and the human mirror neuron system: An exploratory combined fMRI and DTI study of the inferior frontal gyrus. Cereb Cortex. 2016;26:2215–2224. doi: 10.1093/cercor/bhv066. [DOI] [PubMed] [Google Scholar]
- 25.Johansen-Berg H. Behavioural relevance of variation in white matter microstructure. Curr Opin Neurol. 2010 doi: 10.1097/WCO.0b013e32833b7631. [DOI] [PubMed] [Google Scholar]
- 26.Cohen MX, et al. Connectivity-based segregation of the human striatum predicts personality characteristics. Nat Neurosci. 2008;12:32–34. doi: 10.1038/nn.2228. [DOI] [PubMed] [Google Scholar]
- 27.Xu J, Potenza MN. White matter integrity and five-factor personality measures in healthy adults. Neuroimage. 2012;59:800–807. doi: 10.1016/j.neuroimage.2011.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang S, et al. A voxel-based morphometry study of regional gray and white matter correlate of self-disclosure. Soc Neurosci. 2014;9:495–503. doi: 10.1080/17470919.2014.925502. [DOI] [PubMed] [Google Scholar]
- 29.Chavez RS, Heatherton TF. Multimodal frontostriatal connectivity underlies individual differences in self-esteem. Soc Cogn Affect Neurosci. 2015;10:364–370. doi: 10.1093/scan/nsu063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baumgartner T, et al. Neuroanatomy of intergroup bias: A white matter microstructure study of individual differences. Neuroimage. 2015;122:345–354. doi: 10.1016/j.neuroimage.2015.08.011. [DOI] [PubMed] [Google Scholar]
- 31.Hampton WH, et al. Neural connections foster social connections: a diffusion-weighted imaging study of social networks. Soc Cogn Affect Neurosci. 2016;11:721–727. doi: 10.1093/scan/nsv153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tavor I, et al. Separate parts of occipito-temporal white matter fibers are associated with recognition of faces and places. Neuroimage. 2014;86:123–130. doi: 10.1016/j.neuroimage.2013.07.085. [DOI] [PubMed] [Google Scholar]
- 33.Unger A, et al. Variation in white matter connectivity predicts the ability to remember faces and discriminate their emotions. J Int Neuropsychol Soc. 2016;22:180–190. doi: 10.1017/S1355617715001009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Metoki A, et al. Never forget a name: white matter connectivity predicts person memory. Brain Struct Funct. 2017;222:4187–4201. doi: 10.1007/s00429-017-1458-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takeuchi H, et al. White matter structures associated with empathizing and systemizing in young adults. Neuroimage. 2013;77:222–236. doi: 10.1016/j.neuroimage.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 36.Parkinson C, Wheatley T. Relating anatomical and social connectivity: White matter microstructure predicts emotional empathy. Cereb Cortex. 2014;24:614–625. doi: 10.1093/cercor/bhs347. [DOI] [PubMed] [Google Scholar]
- 37.Pisner DA, et al. Highways of the emotional intellect: white matter microstructural correlates of an ability-based measure of emotional intelligence. Soc Neurosci. 2017;12:253–267. doi: 10.1080/17470919.2016.1176600. [DOI] [PubMed] [Google Scholar]
- 38.Downey LE, et al. White matter tract signatures of impaired social cognition in frontotemporal lobar degeneration. NeuroImage Clin. 2015;8:640–651. doi: 10.1016/j.nicl.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Whitfield-Gabrieli S, et al. Brain connectomics predict response to treatment in social anxiety disorder. Mol Psychiatry. 2016;21:680–685. doi: 10.1038/mp.2015.109. [DOI] [PubMed] [Google Scholar]
- 40.Scherf KS, et al. Emerging structure-function relations in the developing face processing system. Cereb Cortex. 2014;24:2964–2980. doi: 10.1093/cercor/bht152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grosse Wiesmann C, et al. White matter maturation is associated with the emergence of Theory of Mind in early childhood. Nat Commun. 2017;8:14692. doi: 10.1038/ncomms14692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Charlton RA, et al. Theory of mind associations with other cognitive functions and brain imaging in normal aging. Psychol Aging. 2009;24:338–348. doi: 10.1037/a0015225. [DOI] [PubMed] [Google Scholar]
- 43.Cabinio M, et al. Mind-reading ability and structural connectivity changes in aging. Front Psychol. 2015;6:1–10. doi: 10.3389/fpsyg.2015.01808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baggio HC, et al. Structural correlates of facial emotion recognition deficits in Parkinson’s disease patients. Neuropsychologia. 2012;50:2121–2128. doi: 10.1016/j.neuropsychologia.2012.05.020. [DOI] [PubMed] [Google Scholar]
- 45.Philippi CL, et al. Damage to association fiber tracts impairs recognition of the facial expression of emotion. J Neurosci. 2009;29:15089–15099. doi: 10.1523/JNEUROSCI.0796-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rigon A, et al. Frontal and temporal structural connectivity is associated with social communication impairment following traumatic brain injury. J Int Neuropsychol Soc. 2016;22:705–716. doi: 10.1017/S1355617716000539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thomas C, Baker CI. Teaching an adult brain new tricks: A critical review of evidence for training-dependent structural plasticity in humans. Neuroimage. 2013;73:225–236. doi: 10.1016/j.neuroimage.2012.03.069. [DOI] [PubMed] [Google Scholar]
- 48.Mount CW, Monje M. Wrapped to adapt: experience-dependent myelination. Neuron. 2017;95:743–756. doi: 10.1016/j.neuron.2017.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nakagawa S, et al. White matter structures associated with loneliness in young adults. Sci Rep. 2015;5:17001. doi: 10.1038/srep17001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thomas C, et al. Reduction in white matter connectivity, revealed by diffusion tensor imaging, may account for age-related changes in face perception. J Cogn Neurosci. 2008;20:268–284. doi: 10.1162/jocn.2008.20025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hecht EE, et al. Process versus product in social learning: Comparative diffusion tensor imaging of neural systems for action execution-observation matching in macaques, chimpanzees, and humans. Cereb Cortex. 2013;23:1014–1024. doi: 10.1093/cercor/bhs097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mars RB, et al. On the relationship between the “default mode network” and the “social brain”. Front Hum Neurosci. 2012;6:1–9. doi: 10.3389/fnhum.2012.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rilling JK, et al. Differences between chimpanzees and bonobos in neural systems supporting social cognition. Soc Cogn Affect Neurosci. 2012;7:369–379. doi: 10.1093/scan/nsr017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hecht EE, et al. A neuroanatomical predictor of mirror self-recognition in chimpanzees. Soc Cogn Affect Neurosci. 2017;12:37–48. doi: 10.1093/scan/nsw159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Barak B, Feng G. Neurobiology of social behavior abnormalities in autism and Williams syndrome. Nat Neurosci. 2016;19:647–655. doi: 10.1038/nn.4276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gleichgerrcht E, et al. Connectome-based lesion-symptom mapping (CLSM): A novel approach to map neurological function. NeuroImage Clin. 2017;16:461–467. doi: 10.1016/j.nicl.2017.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Geschwind DH, Levitt P. Autism spectrum disorders: developmental disconnection syndromes. Curr Opin Neurobiol. 2007;17:103–111. doi: 10.1016/j.conb.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 58.Kana RK, et al. Functional brain networks and white matter underlying theory-of-mind in autism. Soc Cogn Affect Neurosci. 2014;9:98–105. doi: 10.1093/scan/nss106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li Y, et al. A fiber tractography study of social-emotional related fiber tracts in children and adolescents with autism spectrum disorder. Neurosci Bull. 2017;33:722–730. doi: 10.1007/s12264-017-0155-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mueller S, et al. Convergent Findings of Altered Functional and Structural Brain Connectivity in Individuals with High Functioning Autism: A Multimodal MRI Study. PLoS One. 2013:8. doi: 10.1371/journal.pone.0067329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lo YC, et al. Reduced tract integrity of the model for social communication is a neural substrate of social communication deficits in autism spectrum disorder. J Child Psychol Psychiatry Allied Discip. 2017;58:576–585. doi: 10.1111/jcpp.12641. [DOI] [PubMed] [Google Scholar]
- 62.Jansen AG, et al. What Twin Studies Tell Us About the Heritability of Brain Development, Morphology, and Function: A Review. Neuropsychol Rev. 2015;25:27–46. doi: 10.1007/s11065-015-9278-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dopper EGP, et al. Structural and functional brain connectivity in presymptomatic familial frontotemporal dementia. Neurology. 2013;80:814–823. doi: 10.1212/WNL.0b013e31828407bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Levin HS, et al. Mental State Attributions and Diffusion Tensor Imaging After Traumatic Brain Injury in Children. Dev Neuropsychol. 2011;36:273–287. doi: 10.1080/87565641.2010.549885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mike A, et al. Disconnection mechanism and regional cortical atrophy contribute to impaired processing of facial expressions and theory of mind in multiple sclerosis: A structural MRI study. PLoS One. 2013:8. doi: 10.1371/journal.pone.0082422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jalbrzikowski M, et al. Altered white matter microstructure is associated with social cognition and psychotic symptoms in 22q11.2 microdeletion syndrome. Front Behav Neurosci. 2014;8:1–18. doi: 10.3389/fnbeh.2014.00393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Catani M, Ffytche DH. The rises and falls of disconnection syndromes. Brain. 2005;128:2224–2239. doi: 10.1093/brain/awh622. [DOI] [PubMed] [Google Scholar]
- 68.Waller R, et al. White-matter tract abnormalities and antisocial behavior: A systematic review of diffusion tensor imaging studies across development. NeuroImage Clin. 2017;14:201–215. doi: 10.1016/j.nicl.2017.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Batista S, et al. Disconnection as a mechanism for social cognition impairment in multiple sclerosis. Neurology. 2017;89:38–45. doi: 10.1212/WNL.0000000000004060. [DOI] [PubMed] [Google Scholar]
- 70.Solso S, et al. Diffusion tensor imaging provides evidence of possible axonal overconnectivity in frontal lobes in autism spectrum disorder toddlers. Biol Psychiatry. 2016;79:676–684. doi: 10.1016/j.biopsych.2015.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fishman I, et al. Reduced integration and differentiation of the imitation network in autism: A combined functional connectivity magnetic resonance imaging and diffusion-weighted imaging study. Ann Neurol. 2015;78:958–969. doi: 10.1002/ana.24533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Alexander AL, et al. Characterization of Cerebral White Matter Properties Using Quantitative Magnetic Resonance Imaging Stains. Brain Connect. 2011;1:423–446. doi: 10.1089/brain.2011.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wandell BA. Clarifying Human White Matter. Annu Rev Neurosci. 2016;39:103–128. doi: 10.1146/annurev-neuro-070815-013815. [DOI] [PubMed] [Google Scholar]
- 74.Yang DY-J, et al. An integrative neural model of social perception, action observation, and theory of mind. Neurosci Biobehav Rev. 2015;51:263–275. doi: 10.1016/j.neubiorev.2015.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mars RB, et al. Connectivity-based subdivisions of the human right “temporoparietal junction area”: Evidence for different areas participating in different cortical networks. Cereb Cortex. 2012;22:1894–1903. doi: 10.1093/cercor/bhr268. [DOI] [PubMed] [Google Scholar]
- 76.Sallet J, et al. The Organization of Dorsal Frontal Cortex in Humans and Macaques. J Neurosci. 2013;33:12255–12274. doi: 10.1523/JNEUROSCI.5108-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Saygin ZM, et al. Anatomical connectivity patterns predict face selectivity in the fusiform gyrus. Nat Neurosci. 2011;15:321–327. doi: 10.1038/nn.3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Grill-Spector K, et al. The Functional Neuroanatomy of Human Face Perception. Annu Rev Vis Sci. 2017;3:167–196. doi: 10.1146/annurev-vision-102016-061214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Herbet G, et al. Inferring a dual-stream model of mentalizing from associative white matter fibres disconnection. Brain. 2014;137:944–959. doi: 10.1093/brain/awt370. [DOI] [PubMed] [Google Scholar]
- 80.Herbet G, et al. A disconnection account of subjective empathy impairments in diffuse low-grade glioma patients. Neuropsychologia. 2015;70:165–176. doi: 10.1016/j.neuropsychologia.2015.02.015. [DOI] [PubMed] [Google Scholar]
- 81.Haxby JV, et al. The distributed human neural system for face perception. Trends Cogn Sci. 2000;4:223–233. doi: 10.1016/s1364-6613(00)01482-0. [DOI] [PubMed] [Google Scholar]
- 82.Kay KN, Yeatman JD. Bottom-up and top-down computations in word- and face-selective cortex. Elife. 2017;6:1–29. doi: 10.7554/eLife.22341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Duchaine B, Yovel G. A Revised Neural Framework for Face Processing. Annu Rev Vis Sci. 2015;1:393–416. doi: 10.1146/annurev-vision-082114-035518. [DOI] [PubMed] [Google Scholar]
- 84.Latini F, et al. Segmentation of the inferior longitudinal fasciculus in the human brain: A white matter dissection and diffusion tensor tractography study. Brain Res. 2017;1675:102–115. doi: 10.1016/j.brainres.2017.09.005. [DOI] [PubMed] [Google Scholar]
- 85.Yeatman JD, et al. Tract profiles of white matter properties: automating fiber-tract quantification. PLoS One. 2012:7. doi: 10.1371/journal.pone.0049790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Marstaller L, et al. Individual differences in structural and functional connectivity predict speed of emotion discrimination. Cortex. 2016;85:65–74. doi: 10.1016/j.cortex.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 87.Catani M, Thiebaut de Schotten M. A diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex. 2008;44:1105–1132. doi: 10.1016/j.cortex.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 88.Karnath HO, et al. Mapping human brain lesions and their functional consequences. Neuroimage. 2018;165:180–189. doi: 10.1016/j.neuroimage.2017.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Takemura H, et al. Ensemble Tractography. PLoS Comput Biol. 2016;12:1–22. doi: 10.1371/journal.pcbi.1004692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.O’Donnell LJ, et al. Advances in computational and statistical diffusion MRI. NMR Biomed. 2017 doi: 10.1002/nbm.3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang F, et al. Whole brain white matter connectivity analysis using machine learning: An application to autism. Neuroimage. 2017 doi: 10.1016/j.neuroimage.2017.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lerch JP, et al. Studying neuroanatomy using MRI. Nat Neurosci. 2017;20:314–326. doi: 10.1038/nn.4501. [DOI] [PubMed] [Google Scholar]
- 93.Libero LE, et al. Multimodal neuroimaging based classification of autism spectrum disorder using anatomical, neurochemical, and white matter correlates. Cortex. 2015;66:46–59. doi: 10.1016/j.cortex.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yordanova YN, et al. Neural pathways subserving face-based mentalizing. Brain Struct Funct. 2017;0:1–19. doi: 10.1007/s00429-017-1388-0. [DOI] [PubMed] [Google Scholar]
- 95.Bjørnebekk A, et al. Neuronal correlates of the five factor model (FFM) of human personality: Multimodal imaging in a large healthy sample. Neuroimage. 2013;65:194–208. doi: 10.1016/j.neuroimage.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 96.Deco G, et al. Identification of optimal structural connectivity using functional connectivity and neural modeling. J Neurosci. 2014;34:7910–7916. doi: 10.1523/JNEUROSCI.4423-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Stephan KE, et al. Bayesian model selection for group studies. Neuroimage. 2009;46:1004–17. doi: 10.1016/j.neuroimage.2009.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Park HJ, Friston K. Structural and functional brain networks: from connections to cognition. Science. 2013;342:1238411. doi: 10.1126/science.1238411. [DOI] [PubMed] [Google Scholar]
- 99.Meng X, et al. Predicting individualized clinical measures by a generalized prediction framework and multimodal fusion of MRI data. Neuroimage. 2017;145:218–229. doi: 10.1016/j.neuroimage.2016.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Arbabshirani MR, et al. Single subject prediction of brain disorders in neuroimaging: Promises and pitfalls. Neuroimage. 2017;145:137–165. doi: 10.1016/j.neuroimage.2016.02.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Alexander-Bloch AF. Disconnectionism in Biological Psychiatry. Biol Psychiatry. 2017;82:e75–e77. doi: 10.1016/j.biopsych.2017.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Happé F, et al. The structure of social cognition: in(ter)dependence of sociocognitive processes. Annu Rev Psychol. 2017;68:243–267. doi: 10.1146/annurev-psych-010416-044046. [DOI] [PubMed] [Google Scholar]
- 103.Maier-Hein KH, et al. The challenge of mapping the human connectome based on diffusion tractography. Nat Commun. 2017;8:1349. doi: 10.1038/s41467-017-01285-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Oishi K, et al. Critical role of the right uncinate fasciculus in emotional empathy. Ann Neurol. 2015;77:68–74. doi: 10.1002/ana.24300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Herbet G, et al. Interfering with the neural activity of mirror-related frontal areas impairs mentalistic inferences. Brain Struct Funct. 2015;220:2159–2169. doi: 10.1007/s00429-014-0777-x. [DOI] [PubMed] [Google Scholar]
- 106.Ouyang M, et al. Short-range connections in the developmental connectome during typical and atypical brain maturation. Neurosci Biobehav Rev. 2017;83:109–122. doi: 10.1016/j.neubiorev.2017.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Schuz A, Braitenberg V. The human cortical white matter: quantitative aspects of cortico-cortical long-range connectivity. In: Shuez A, Miller R, editors. Cortical Areas: Unity and Diversity. Taylor & Francis; London: 2002. pp. 377–384. [Google Scholar]
- 108.Gomez J, et al. Functionally defined white matter reveals segregated pathways in human ventral temporal cortex associated with category-specific processing. Neuron. 2015;85:216–228. doi: 10.1016/j.neuron.2014.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Song S, et al. Local but not long-range microstructural differences of the ventral temporal cortex in developmental prosopagnosia. Neuropsychologia. 2015;78:195–206. doi: 10.1016/j.neuropsychologia.2015.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.van Horn JD, et al. Mapping connectivity damage in the case of phineas gage. PLoS One. 2012;7:e37454. doi: 10.1371/journal.pone.0037454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Duffau H. Stimulation mapping of white matter tracts to study brain functional connectivity. Nat Rev Neurol. 2015;11:255–265. doi: 10.1038/nrneurol.2015.51. [DOI] [PubMed] [Google Scholar]
- 112.Jones DK, et al. White matter integrity, fiber count, and other fallacies: The do’s and don’ts of diffusion MRI. Neuroimage. 2013;73:239–254. doi: 10.1016/j.neuroimage.2012.06.081. [DOI] [PubMed] [Google Scholar]