Abstract
OBJECTIVES
Anomalous aortic origin of the coronary artery(AAOCA) from the opposite sinus of Valsalva is a rare cardiac anomaly associated with sudden cardiac death(SCD). Single center studies describe surgical repair as safe, though medium and long-term effects on symptoms and risk of SCD remain unknown. We sought to describe outcomes of surgical repair of AAOCA.
METHODS
We reviewed institutional records for patients who underwent AAOCA repair, 2001–2016, at two affiliated institutions. Patients with associated heart disease were excluded.
RESULTS
In total, 60 patients underwent AAOCA repair. Half of patients(n=30) had an anomalous left coronary artery arising from the right sinus of Valsalva and half had an anomalous right. Median age at surgery was 15.4 years(IQR 11.9–17.9yrs;range 4mos–68yrs). Most common presenting symptoms were chest pain(n=38;63%) and shortness of breath(n=17;28%); aborted sudden cardiac death was the presenting symptom in 4(7%) patients. Follow-up data were available for 54(90%) patients over a median of 1.6years. Of 53 patients with symptoms at presentation, 34(64%) had complete resolution post-operatively. Postoperative mild or greater aortic insufficiency was present in 8(17%) patients and moderate supravalvar aortic stenosis in 1(2%). One patient required aortic valve replacement for aortic insufficiency. Two patients required reoperation for coronary stenosis at 3mos and 6years postoperatively.
CONCLUSIONS
Surgical repair of AAOCA is generally safe and adverse events are rare. Restenosis, and even sudden cardiac events, can occur and long-term surveillance is critical. Multi-institutional collaboration is vital to identify at risk sub-populations and refine current recommendations for long-term management.
Introduction
Anomalous aortic origin of a coronary artery (AAOCA) arising from the opposite sinus of Valsalva is a rare cardiac anomaly but is the second most common cause of sudden cardiac death (SCD) in young competitive athletes.1–5 The incidence of AAOCA is reported to be between 0.1–0.7%, with an anomalous right coronary artery arising from the left sinus of Valsalva (ARCA) reported more commonly than an anomalous left coronary (ALCA).6–8
The goal of surgical AAOCA repair is to eliminate the risk of SCD. Single center studies have demonstrated that surgical repair is generally safe with short-term improvement in symptoms of ischemia after repair.9–15 While a few studies have demonstrated persistent symptoms and/or signs of new ischemia post-operatively,11,15,16 data on medium- and long-term outcomes for these patients are limited, and the residual risk of SCD remains unknown.
The purpose of this analysis is to describe outcomes after surgical repair of isolated AAOCA at two cardiac centers where the same surgical team operates.
Methods
Study Population
We performed a retrospective cohort study of patients undergoing initial surgical repair of AAOCA from 2001–2016 at NewYork-Presbyterian/Columbia University Medical Center (CUMC) and NewYork-Presbyterian/Weill Cornell Medical Center (WCMC). Patients were identified through our institutional surgical database. Attending cardiothoracic surgeons at NewYork-Presbyterian operate at both campuses and report to the STS database as a single center. All patients with isolated AAOCA operated on by one of our congenital heart surgeons were included, regardless of age. Patients with associated congenital heart defects were excluded.
Perioperative management and long-term follow-up
The historical practice at CUMC and WCMC has been to refer all ALCA patients, regardless of symptoms, as well as ARCA patients demonstrating symptoms and/or objective findings of ischemia for surgical repair. The decision to repair asymptomatic ARCA patients has been debated and has remained at the discretion of each cardiologist and cardiothoracic surgeon, in consultation with the patient and family. Repair is typically performed by unroofing of the septum between the aortic lumen and intramural portion of the coronary. In a small subset of patients with short intramural segments, coronary translocation was performed at the discretion of the surgeon. If the intramural portion of the coronary was at or below the level of the aortic commissure, takedown of the tip of the commissure was performed. In those patients who required takedown of the aortic commissure, resuspension was attempted in all cases.
During the study period, no standardized protocol existed for routine pre- or post-operative follow-up or testing, and testing was obtained at the discretion of the referring cardiologist and operating surgeon.
Data collection
We collected baseline data on patient demographics, indications for surgery and type of repair. We examined perioperative and post-operative outcomes. Perioperative outcomes of interest included mechanical circulatory support, arrhythmias, infections, pneumothoraces and pericardial effusions. Post-operative outcomes of interest included symptoms of ischemia including aborted sudden cardiac death (aSCD), abnormal electrocardiogram (ECG), echocardiogram or exercise stress test, new aortic insufficiency (AI) or aortic stenosis (AS), arrhythmia and reoperation. The ECG, echocardiogram and exercise stress test results were obtained from the most recent available visit.
The majority of data were obtained via retrospective chart review. For patients operated on at CUMC, all patients were also contacted by phone. Verbal consent was obtained to use patients/parents’ subjective reporting of symptoms and vital status. For patients followed by a cardiologist at an outside institution, written consent was obtained to contact the primary cardiologist for follow-up records. Data on patients treated at WCMC were obtained via retrospective chart review with waiver of informed consent. Longitudinal follow-up data had recently been obtained and recorded in the medical records as part of an internal quality improvement effort. In those patients for whom no longitudinal follow-up was available, most recent vital status was obtained using the Social Security death index.
Statistical methods
Clinical and demographic variables were described using standard summary statistics. Change in pre- and post-operative symptoms and test data were described graphically. Standard univariable analyses were used to compare pre- and post-operative characteristics including Chi-square, Fisher’s exact, unpaired T-test and Kruskall Wallis test, as appropriate. Analyses were performed using Stata software, version 13.1(Statacorp, College Station, Texas). This study was approved by the respective Institutional Review Boards at CUMC and WCMC.
Results
Baseline patient characteristics
In total, 60 patients (38 male, 22 female) were identified who had surgery for AAOCA, 30 (50%) with ALCA and 30 (50%) with ARCA. All patients had an interarterial course and nearly all (n=56;93%) patients had an intramural course of the anomalous coronary, which was confirmed at the time of surgery. Median age at surgery was 15.4 years (IQR 11.9–17.9 yrs; range 4mos–68yrs). 12 patients were over 21 years at the time of surgery, 4 with ALCA and 8 with ARCA. Patient characteristics are summarized in Table 1.
Table 1.
Patient Characteristics and Presentation
| All Patients | ARCA | ALCA | P value | |
|---|---|---|---|---|
| All patients | 60 (100%) | 30 (50%) | 30(50%) | |
|
| ||||
| Male sex | 38 (63%) | 19 (63%) | 19 (63%) | 1.00 |
|
| ||||
| Age at surgery in years | 15.4 (IQR 11.9–17.9) | 15.5 (IQR 13.4–41.0) | 15.3 (IQR 10.8–17.5) | 0.225 |
|
| ||||
| Age at last followup in years | 16.8 (IQR 14.5–19.6) | 16.4 (IQR 14.7–19.6) | 17.1 (IQR 14.5–19.2) | 0.986 |
|
| ||||
| Weight at surgery in kilograms | 60.2 +/− 24.9 | 62.9 +/− 23.5 | 57.4 +/− 26.3 | 0.400 |
|
| ||||
| Symptoms at presentation | 53 (88%) | 28 (93%) | 25 (83%) | 0.424 |
| Chest pain | 38 (63%) | 22 (73%) | 16 (53%) | 0.108 |
| Shortness of breath | 17 (28%) | 10 (33%) | 7 (23%) | 0.390 |
| Palpitations | 12 (20%) | 8 (27%) | 4 (13%) | 0.197 |
| Syncope | 12 (20%) | 5 (17%) | 7 (23%) | 0.519 |
| Aborted sudden cardiac death | 4 (7%) | 0 (0%) | 4 (13%) | 0.112 |
|
| ||||
| No symptoms at presentation | 7 (12%) | 2 (7%) | 5 (17%) | 0.424 |
| Family history | 3 (5%) | 2 (7%) | 1 (3%) | 1.00 |
| Murmur | 2 (3%) | 0 (0%) | 2 (7%) | 0.492 |
| Abnormal ECG | 1 (2%) | 0 (0%) | 1 (3%) | 1.00 |
| History of PDA | 1 (2%) | 0 (0%) | 1 (3%) | 1.00 |
Numbers represent means/medians (SD/IQR) for continuous variables and numbers (%) for categorical variables.
P value for comparison between ARCA and ALCA.
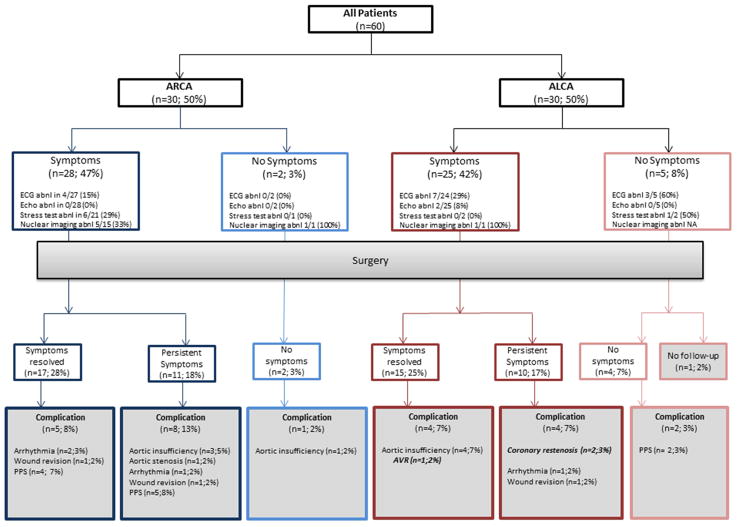
Symptoms were present in 53 (88%) patients at presentation (Table 1). Aborted sudden cardiac death (aSCD) was the presenting symptom in 4 (7%) patients with ALCA. There were no significant differences between presentation in patients with ARCA and ALCA (Figure 1).
Figure 1. Consort diagram for patient outcomes.
Describes the outcome for patients based on initial anatomy and symptoms. ARCA = anomalous right coronary artery off the left sinus; ALCA = anomalous left coronary off the right sinus; Abnl = abnormal; ECG = electrocardiogram; Echo = echocardiogram; AVR = aortic valve replacement; PPS = post-pericardiotomy syndrome. Abnormal echocardiograms include those with abnormal function; all echocardiograms demonstrated an anomalous coronary. All percentages represent the percent of total patients, n=60.
Preoperative testing
Of the 58 (97%) patients with a pre-operative ECG available for review, 13 (22%) were abnormal (Supplemental Figure 1A). All but 2 (3%) patients had normal ventricular function on pre-operative echocardiogram. The two patients with abnormal function both presented with cardiac arrest (Supplemental Table 1).
A total of 26 (43%) patients underwent exercise stress testing with stress ECG prior to surgical repair and 17 (28%) also had nuclear myocardial perfusion imaging (Table 2). More patients with ARCA compared to ALCA underwent exercise stress testing (p<0.001) and nuclear myocardial perfusion testing (p<0.001). Of the 26 patients with exercise testing, 7 (27%) had abnormal ST changes on ECG during the test that could represent ischemia and 6 (23%) patients complained of chest pain. On nuclear testing, 7 (41%) had abnormal perfusion imaging (Supplemental Figure 1B and Supplemental Table 1). Additional imaging including cardiac CT angiogram, MRI and cardiac catheterization was obtained in 46 (77%) patients (Table 2).
Table 2.
Pre- and post-operative testing
| All Patients | ARCA | ALCA | P value | |
|---|---|---|---|---|
| Pre-operative testing | ||||
| Electrocardiogram1 | 58 (97%) | 29 (97%) | 29 (97%) | 1.00 |
| Echocardiogram | 60 (100%) | 30 (100%) | 30 (100%) | 1.00 |
| Exercise stress test | 26 (43%) | 22 (73%) | 4 (13%) | *<0.001 |
| Nuclear stress test | 17 (28%) | 16 (53%) | 1 (3%) | *<0.001 |
| CT angiogram | 37 (62%) | 22 (73%) | 15 (50%) | 0.063 |
| MR angiogram | 8 (13%) | 3 (10%) | 5 (17%) | 0.706 |
| Cardiac catheterization | 12 (20%) | 7 (23%) | 5 (17%) | 0.519 |
| Post-operative testing2 | ||||
| Electrocardiogram | 50 (93%) | 28 (97%) | 22 (88%) | 0.326 |
| Echocardiogram | 48 (89%) | 27 (93%) | 21 (84%) | 0.399 |
| Exercise stress test | 34 (63%) | 19 (66%) | 15 (60%) | 0.675 |
| Nuclear stress test | 18 (33%) | 11 (38%) | 7 (28%) | 0.440 |
| CT angiogram | 3 (6%) | 0 (0%) | 3 (13%) | 0.086 |
| MR angiogram | 4 (7%) | 0 (0%) | 4 (16%) | *0.040 |
| Cardiac catheterization | 1 (2%) | 0 (0%) | 1 (4%) | 0.463 |
All patients had pre-operative electrocardiograms but several patients did not have records available for our review.
Percentages are of patients with follow-up available (n=54)
Indicates P value < 0.05. All P values are for comparison between ARCA to ALCA.
Surgical repair
Six surgeons operated during the study period. Coronary unroofing was performed in 56 (93%) patients and coronary translocation in 4 (7%) patients. Of the 4 patients who had coronary translocation, 3 did not have an intramural course. In the 4th patient, an unrooofing was initially attempted but the intramural portion was short and there was still an acute angle of takeoff on echocardiogram; the patient was put back on cardiopulmonary bypass and a translocation was performed. Operative details are reported in Table 3. There were no reported intraoperative complications and no intraoperative mortalities.
Table 3.
Intraoperative Findings
| All Patients | ARCA | ALCA | P value* | |
|---|---|---|---|---|
| Type of Surgical Repair | ||||
| Unroofing | 56 (93%) | 29 (97%) | 27 (90%) | 0.612 |
| Coronary translocation | 4 (7%) | 1 (3%) | 3 (10%) | 0.612 |
| Commissure involvement | 16 (27%) | 9 (30%) | 7 (23%) | 0.559 |
| Cardiopulmonary bypass time in minutes | 55 (IQR 45–66) | 55 (IQR 45–61) | 53 (IQR 43–74) | 0.827 |
| Aortic cross-clamp time in minutes | 35 (IQR 26–43) | 35 (IQR 27–43) | 32 (IQR 26–43) | 0.894 |
Numbers represent means/medians (SD/IQR) for continuous variables and numbers (%) for categorical variables.
P value for comparison between ARCA and ALCA.
Perioperative outcomes
Only one patient had a major acute postoperative complication. This patient experienced an out-of-hospital cardiac arrest and was cannulated onto extracorporeal membrane oxygenation (ECMO) pre-operatively and required ECMO post-operatively due to inability to wean from cardiopulmonary bypass. Minor acute post-operative complications occurred in 6 (10%) patients, including transient arrhythmia requiring treatment (n=4;7%), superficial wound infection (n=1;2%) and pneumothorax requiring chest tube placement (n=1;2%). After discharge, 11 (18%) patients developed post-pericardiotomy syndrome (PPS) at a median of 11 days (IQR 7–29 days; range 6–67 days), two of whom required percardiocentesis. PPS was more common in patients with ARCA compared to those with ALCA (p= 0.020) (Table 4).
Table 4.
Complications
| All Patients | ARCA | ALCA | P value | |
|---|---|---|---|---|
| Acute perioperative complications | ||||
| Peri-operative ECMO and hemorrhage | 1 (2%) | 0 (0%) | 1 (3%) | 1.00 |
| Arrhythmia requiring treatment | 4 (7%) | 4 (13%) | 0 (0%) | 0.112 |
| Pneumothorax requiring chest tube | 1 (2%) | 1 (3%) | 0 (0%) | 1.00 |
| Superficial wound infection | 1 (2%) | 0 (0%) | 1 (3%) | 1.00 |
| Post-pericardiotomy syndrome | 11 (18%) | 9 (30%) | 2 (7%) | *0.020 |
|
| ||||
| Follow-up complications | ||||
| Reoperation for coronary stenosis | 2 (3%) | 0 (0%) | 2 (7%) | 0.492 |
| Aborted sudden cardiac death | 1 (2%) | 0 (0%) | 1 (3%) | 1.00 |
| Aortic insufficiency1 | 7 (12%) | 3 (10%) | 4 (13%) | 1.00 |
| Aortic valve replacement for aortic insufficiency | 1 (2%) | 0 (0%) | 1(3%) | 1.00 |
| Arrhythmia | 4 (7%) | 3 (10%) | 1 (3%) | 0.612 |
| Wound revision | 3 (5%) | 2 (7%) | 1 (3%) | 1.00 |
| Supravalvar aortic stenosis | 1 (2%) | 1 (3%) | 0 (0%) | 1.00 |
Only mild or greater AI as assessed on echocardiogram was included
Indicates P value < 0.05. All P values are for comparison between ARCA to ALCA.
Median length of stay in the ICU was 2 days (IQR 2–3 days; range 1–23 days) and median total length of stay was 4 days (IQR 4–5; range 2–42 days).
Follow-up
Symptoms
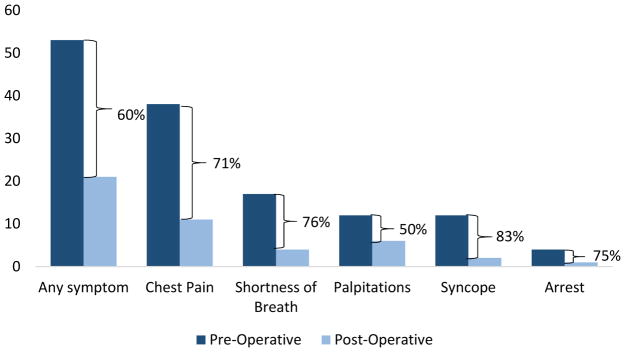
Longitudinal follow-up data were available from the primary cardiologist for 54 (90%) patients over a median of 1.6 years (IQR 0.6–2.5 yrs; range 1 month–9.4 years). An additional 5 (8%) patients responded to phone calls regarding their symptoms but did not complete consent to contact their cardiologist. Of 53 patients with symptoms at presentation, 32 (60%) had complete resolution post-operatively and 9 (17%) had a change in their symptoms (Figure 2). No asymptomatic patients developed symptoms post-operatively.
Figure 2. Change in symptoms of ischemia.
Bar chart depicts the number of patients with symptoms pre- and post-operatively. Parentheses represent percent change in symptoms. Includes patients in whom both pre- and post-operative data are available (n=59). All patients with post-operative symptoms experience pre-operative symptoms. No asymptomatic patients developed symptoms post-operatively.
Electrocardiogram
On initial post-operative ECG, 32 (53%) of patients had ST changes. Of 50 patients with post-discharge ECG records available at a median of 583 days (IQR 179–884 days, range 13–3360 days), 6 (12%) had abnormal findings, none of which were ischemic changes (Supplemental Figure 1A).
Echocardiogram
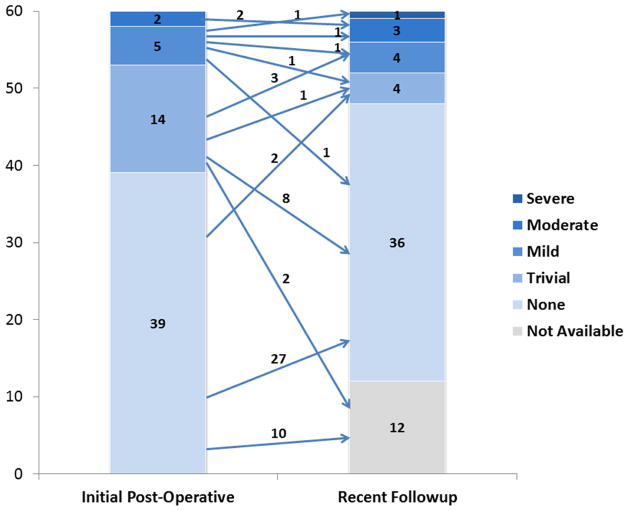
Post-discharge echocardiograms were available in 48 (80%) patients at a median of 583 days (IQR 222–874 days, range 12–3360 days) post-operatively. Of those, 12 (25%) had abnormal findings, including mild AI (n=4,8%), moderate AI (n=3,6%) and severe AI (n=1,2%), (Figure 3), moderate supravalvar AS (n=1;2%) presumed to be from the aortotomy closure, ventricular wall motion abnormality (n=2;4%) and pericardial effusion (n=1;2%). None of these abnormalities was present on pre-operative echocardiogram. There was no correlation between commissure takedown and AI (p=0.074).
Figure 3. Post-operative Aortic Insufficiency.
Bar graphs represent the number of patients with any degree of aortic insufficiency on initial post-operative and most recent follow-up echocardiogram. As noted, there were 12 patients without follow-up echocardiogram available, represented by the light gray box. The median time for follow-up echocardiogram was 583 days post-operatively.
Exercise testing
A total of 34 (57%) patients had an exercise stress test post-operatively which consisted of a stress electrocardiogram at a median 346 days post-operative (IQR 117–882 days, range 38–3360 days). Eighteen (30%) of those patients also had nuclear myocardial perfusion imaging. There was no significant difference between patients with and without post-operative stress testing based on type of anomalous coronary (p=0.30), age at surgery (p= 0.08), pre-operative symptoms (p=0.22), post-operative symptoms (p= 0.96) or length of follow-up (p=0.70). Of the 34 patient with exercise testing, 5 (15%) patients complained of chest pain during the study and 2 (6%) patients had ST changes indicative of ischemia. Both patients with ST changes had a similarly abnormal pre-operative stress test. An additional 2 (11%) patients had abnormal nuclear myocardial perfusion imaging. Only one patient had a nuclear perfusion scan pre-operatively; it was abnormal (Supplemental Figure 1B). None of the patients who complained of chest pain had ischemic changes.
Of the 4 patients with ST changes during exercise study or abnormal nuclear perfusion results, 1 had intermittent palpitations post-operatively and the others remained asymptomatic. The patient with palpitations is restricted from activity, but the remaining patients have been cleared by their cardiologists and are all active.
Reoperation
Reoperation was required in three (5%) patients with ALCA. One patient developed moderate AI and had worsening left ventricular dilation. This patient required aortic valve replacement 4.7 years after initial surgery. Two (3%) patients with ALCA required reoperation for restenosis of the anomalous coronaries. One patient who was 68 at the time of initial surgery and was repaired due to chest pain with minimal exertion, had recurrent chest pain three months post-surgery. ECG showed nonspecific ST and T wave abnormalities. An exercise stress test was concerning for ischemia in multiple regions. Significant stenosis was found at the origin of the anomalous left coronary orifice on cardiac catheterization, and coronary bypass grafting was performed 66 days after initial surgery without complication. Another patient was 10 years old at the time of initial surgery and had pre-operative symptoms including chest pain with exertion, palpitations and syncope with exertion. This patient experienced a cardiac arrest during tryouts for a high school sports team six years post-operatively. The patient had had a negative stress test and reportedly normal echocardiograms prior to being cleared for athletic participation. She did complain of occasional dizziness prior to the event but denied any prior episodes of chest pain. Post-arrest noninvasive imaging by cardiac CT angiogram revealed ostial narrowing with hypo-attenuated material at the area of unroofing, which appeared consistent with recurrent fibrosis (Supplemental Figure 2). Intra-operative examination revealed fibrous tissue around the left coronary orifice, which was resected. The patient continued to have premature ventricular contractions on routine post-operative monitoring; an implantable cardiac defibrillator was placed and the patient was restricted from competitive sports. Both patients with restenosis are asymptomatic at 3 and 1 years after the second operation, respectively.
Other complications
There were 3 (5%) patients who required scar revision either for discomfort (n=2) or abnormal healing (n=1). There were no reported deaths at the time of authorship. In those patients without follow-up data, this was confirmed using the Social Security death index.
Discussion
Surgical repair of AAOCA is generally safe and major acute post-operative complications are rare. In our study, 60% of patients had resolution of their symptoms post-operatively. However, 40% of patients still complained of some subjective symptoms although the vast majority had normal post-operative testing including exercise tests and ECGs. This may indicate that many of the pre-operative symptoms were not, in fact, attributable to the AAOCA and it is unclear whether symptoms are predictive of true ischemia. Recent studies have drawn attention to the possibility that patients with repaired AAOCA may continue to experience not only subjective symptoms, but also objective signs of ischemia.11,15,16 While the theoretical risk was known due to scarring of the coronary ostia, our study is the first to report restenosis of an unroofed anomalous coronary and aSCD after surgical repair of AAOCA. Our study also reports risks of supravalvar aortic stenosis after coronary unroofing and aortic insufficiency even in patients who do not undergo aortic commissural takedown.
Mortality after surgical repair of AAOCA is very rare. A survey of 113 participants from the Congenital Heart Surgeons Society (CHSS) reports only two deaths after surgical repair.17 However, restenosis of the anomalous coronary can occur. To our knowledge, there is one case in the literature of SCD during exercise after surgical repair of AAOCA in a patient who did not have an unroofing, but who underwent ostial plasty and pulmonary artery translocation.18 In both that patient and our own, stress testing was performed prior to clearing for sports participation and was reportedly normal. Our patient with aSCD presented 6 years after initial repair. Our patient did have some non-specific dizziness prior to her arrest, but no evidence of restenosis or ischemia on routine testing. These data highlight the limitations of functional testing in predicting ostial stenosis.
In our second patient with ostial restenosis, chest pain was the presenting symptom. The patient had an abnormal exercise stress test prior to her second repair, which lead to cardiac catheterization. This patient presented within weeks of initial surgery and was in her 60s, thus the mechanism of restenosis in this patient might have been different than in the first patient described—either due to post-operative changes or due to underlying coronary artery disease and abnormal healing of the surgical repair, rather than fibrous scarring over time.
Brothers et al. demonstrated that 38% of patients with AAOCA repair had evidence of ischemia on post-operative testing with patent coronary ostia on echocardiogram, while only one had evidence of ischemia pre-operatively, perhaps indicating that surgery itself may cause myocardial changes or that these changes may develop over time regardless of the operation.16 In one report by Mainwaring et al, of 115 patients who underwent surgical repair of AAOCA, two required reoperation for persistent ischemia, one for revision of the initial repair and one for a myocardial bridge. However, in these patients, anatomic issues were identified that were likely to contribute to the post-operative ischemia, including a narrow coronary orifice and myocardial bridging.15. In our study, no patients developed new ischemic changes on post-operative ECG and one patient had abnormal myocardial perfusion imaging with no baseline for comparison. Three patients showed persistent abnormalities consistent with ischemia on exercise stress testing or stress myocardial perfusion imaging. Although these patients all had patent coronary ostia on echocardiogram, perhaps these patients deserve further imaging to better define the coronary ostia and coronary perfusion post-operatively.
Based on the risk of postoperative complications, all patients who undergo surgery for AAOCA should have lifelong cardiovascular care, but post-operative follow-up is not yet standardized and it is unclear what testing is predictive of adverse events. Given available data, it is difficult to determine which patients are at highest risk. Larger studies, such as those currently being conducted by the Congenital Heart Surgeons Society, are needed to risk-stratify post-operative patients. Several institutions, including our own, have worked to develop standardized management protocols for evaluating and managing AAOCA longitudinally, in an effort to track and study outcomes systematically. Brothers and colleagues recently published guidelines recommending routine ECGs and echocardiograms and an exercise stress test with myocardial perfusion imaging three months post-operatively.19 They also recommend cardiac MRIs six months after surgery to assess for changes in the coronary anatomy or myocardial scarring.19
Current guidelines suggest that asymptomatic patients with normal stress tests can be cleared for exercise three months after surgery if there is no history of aSCD and twelve months after surgery if there is a history of aSCD.19,20 Our patient who arrested post-operatively, met criteria for exercise participation under these guidelines. An MRI was not performed as it was not part of that cardiologist’s practice. The post-arrest CT scan did demonstrate narrowing of the coronary ostium. It is not known at what point these changes developed. It stands to reason that if one is creating and manipulating a neo-ostium surgically, some scar will form, eventually leading, in some cases, to re-stenosis. It is possible that patients would benefit from MRI or CT screening at regular intervals and that advanced imaging should be incorporated into follow-up. Families should be counseled that even after patients are cleared for exercise, they remain at risk for ischemic complications and that it is critically important that first responders trained in CPR and equipped with an AED be present.
The incidence of more minor complications in our study was within the previously reported range—10 to 67%. 9,11,12 This wide range is likely due to different definitions and methods of data collection rather than from significant differences among centers. Our study confirms that the risk of post-operative AI, leading to a reoperation in one case, is not trivial (17% in our cohort vs. 21% in previously reported populations) and may be even higher as 20% of our patients did not have post-operative echocardiogram results available.11 Other studies have also reported low rates of aortic valve replacement secondary to AI.9,10 Post-operative AI is thought to be a result of commissural take-down. However, our study suggests that there may be more complex issues at play since three of the seven patients who developed post-operative AI did not have takedown of the aortic commissure during their operation and one of them underwent coronary translocation alone, without unroofing. This raises the possibility of a structural deficiency of the aortic wall at the level of the commissure/crossing intramural coronary. Indeed, the one patient who required aortic valve replacement was found to have extra laxity of the portion of the wall that carried the commissure. The commissure was well healed after the take-down and resuspension, but due to this laxity, was able to prolapse into the aortic lumen. Based on our data, one surgeon has modified his technique during coronary unroofing to minimize disruption of the aortic valve by fenestrating the intramural tunnel as opposed to splaying it open from beginning to end.
One of our patients developed post-operative supravalvar AS, thought to be due to the aortotomy closure. To our knowledge, this complication has not been previously reported. Other complications cited in the literature include ventricular dysfunction, heart block and other arrhythmias and post-pericardiotomy syndrome.9,17,21
There are some notable limitations of this study. As a retrospective review of surgical outcomes, it is subject to inherent bias, including selection bias based on the types of patients that are referred for surgery and underwent more extensive pre- and post-operative testing. We lacked a non-operative control cohort to compare long-term outcomes, which limits our ability to accurately assess the magnitude of risk reduction or to opine on the decision to operate. We included patients of all ages, including 12 patients over 21 years of age. These patients, on the one hand, may have subclinical acquired coronary artery disease in addition to their coronary anomaly that affects outcomes. On the other hand, patients with AAOCA who have already survived to older age might be at lower risk for SCD than those who have not already proven that they can survive.
The true incidence of restenosis and SCD after repair is difficult to determine from this sized cohort and it is also difficult to determine what types of post-operative testing are most useful for predicting adverse outcomes. Multicenter studies examining postoperative testing and outcomes, such as the CHSS AAOCA Registry, will be critical in determining the frequency and predictors of post-operative SCD with greater precision. It is notable that in our series all serious complications occurred in patients with ALCA (i.e., in patients who had an absolute indication for surgery). It will be important to see whether this remains true in a larger cohort. If so, this might suggest that more aggressive post-operative follow-up is warranted in patients with repaired ALCA.
Conclusion
Repair of AAOCA can be accomplished with relatively few complications and can lead to improvement in symptoms and signs of ischemia. Restenosis, and even sudden cardiac death, can occur and long-term surveillance is critical. Multi-institutional collaboration is vital to identify at risk sub-populations and refine current recommendations for long-term management and best practices for follow-up imaging.
Supplementary Material
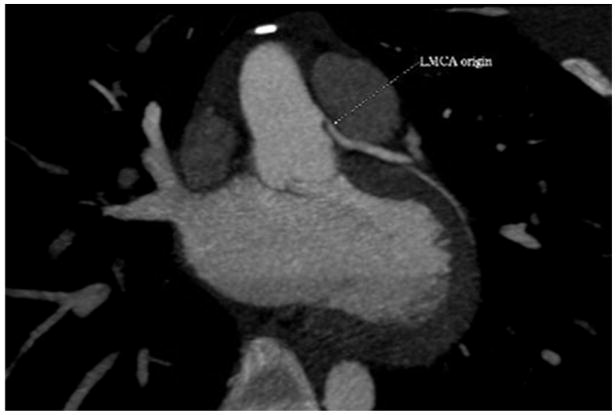
Central Picture.
Cardiac CT angiogram in patient with aborted sudden cardiac death 6 years after unroofing. Coronal view demonstrating the takeoff of the anomalous left coronary artery.
Video 1.
Axial multiplanar reconstruction images from cardiac CT performed after cardiac arrest in this patient who underwent ALCA unroofing demonstrates a ridge of hypoattenuation at the left coronary origin that extends rightward of the intercoronary commissure, with associated orifice narrowing (indicated by the yellow arrow).
Central Message.
Repair of anomalous aortic origin of the coronary artery can be performed safely; restenosis, and even sudden cardiac death, can occur and long-term surveillance is critical.
Perspective Statement.
Repair of anomalous aortic origin of the coronary artery can be accomplished safely and can lead to improvement in symptoms and signs of ischemia. Restenosis, and even sudden cardiac events, can occur and long-term surveillance is critical. Multi-institutional collaboration is vital to identify at risk sub-populations and refine current recommendations for long-term management.
Glossary of Abbreviations
- AAOCA
anomalous aortic origin of a coronary artery
- AI
aortic insufficiency
- ALCA
anomalous left coronary artery arising from the right sinus of Valsalva
- ARCA
anomalous right coronary artery arising from the left sinus of Valsalva
- AS
aortic stenosis
- aSCD
aborted sudden cardiac death
- CHSS
Congenital Heart Surgeons Society
- CUMC
Columbia University Medical Center
- ECG
electrocardiogram
- ECMO
extracorporeal membrane oxygenation
- SCD
sudden cardiac death
- WCMC
Weill Cornell Medical Center
Footnotes
There are more than 7 authors because this was a study at two institutions that required collaboration from investigators at both institutions for data collection and analysis.
This research was presented as an oral session at the AATS Centennial Meeting in Boston on May 2, 2017.
None of the authors have any conflicts of interest to disclose. Funding was provided through the Division of Cardiac, Thoracic and Vascular Surgery at Columbia University.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Eckart RE, Scoville SL, Campbell CL, et al. Sudden Death in Young Adults: A 25-Year Review of Autopsies in Military Recruits. Ann Intern Med. 2004;141(11):829. doi: 10.7326/0003-4819-141-11-200412070-00005. [DOI] [PubMed] [Google Scholar]
- 2.Maron BJ, Doerer JJ, Haas TS, Tierney DM, Mueller FO. Sudden deaths in young competitive athletes analysis of 1866 deaths in the united states, 1980–2006. Circulation. 2009;119(8):1085–1092. doi: 10.1161/CIRCULATIONAHA.108.804617. [DOI] [PubMed] [Google Scholar]
- 3.Cheitlin MD, De Castro CM, McAllister HA. Sudden Death as a Complication of Anomalous Left Coronary Origin From the Anterior Sinus of Valsalva: A Not-So-Minor Congenital Anomaly. Circulation. 1974;50(4):780–787. doi: 10.1161/01.cir.50.4.780. [DOI] [PubMed] [Google Scholar]
- 4.Taylor AJ, Byers JP, Cheitlin MD, Virmani R. Anomalous right or left coronary artery from the contralateral coronary sinus: “High-risk” abnormalities in the initial coronary artery course and heterogeneous clinical outcomes. Am Heart J. 1997;133(4):428–435. doi: 10.1016/S0002-8703(97)70184-4. [DOI] [PubMed] [Google Scholar]
- 5.Taylor AJ, Rogan KM, Virmani R. Sudden cardiac death associated with isolated congenital coronary artery anomalies. J Am Coll Cardiol. 1992;20(3):640–647. doi: 10.1016/0735-1097(92)90019-j. http://www.ncbi.nlm.nih.gov/pubmed/1512344. [DOI] [PubMed] [Google Scholar]
- 6.Tuo G, Marasini M, Brunelli C, Zannini L, Balbi M. Incidence and clinical relevance of primary congenital anomalies of the coronary arteries in children and adults. Cardiol Young. 2013;23(03):381–386. doi: 10.1017/S1047951112000959. [DOI] [PubMed] [Google Scholar]
- 7.Davis JA, Cecchin F, Jones TK, Portman MA. Major coronary artery anomalies in a pediatric population: incidence and clinical importance. J Am Coll Cardiol. 2001;37(2):593–597. doi: 10.1016/S0735-1097(00)01136-0. [DOI] [PubMed] [Google Scholar]
- 8.Angelini P, Shah NR, Uribe CE, et al. Novel MRI–Based Screening Protocol to Identify Adolescents at High risk of Sudden Cardiac Death. Journal of the American College of Cardiology. 2013;61 doi: 10.1016/S0735-1097(13)61621-6. [DOI] [Google Scholar]
- 9.Romp RL, Herlong JR, Landolfo CK, et al. Outcome of unroofing procedure for repair of anomalous aortic origin of left or right coronary artery. Ann Thorac Surg. 2003;76(2):589–596. doi: 10.1016/s0003-4975(03)00436-3. [DOI] [PubMed] [Google Scholar]
- 10.Fabozzo A, DiOrio M, Newburger JW, et al. Anomalous Aortic Origin of Coronary Arteries: A Single Center Experience. Semin Thorac Cardiovasc Surg. 2016;0(0):593–597. doi: 10.1053/j.semtcvs.2016.08.012. [DOI] [PubMed] [Google Scholar]
- 11.Wittlieb-Weber CA, Paridon SM, Gaynor JW, Spray TL, Weber DR, Brothers JA. Medium-term outcome after anomalous aortic origin of a coronary artery repair in a pediatric cohort. J Thorac Cardiovasc Surg. 2014;147(5):1580–1586. doi: 10.1016/j.jtcvs.2013.07.022. [DOI] [PubMed] [Google Scholar]
- 12.Frommelt PC, Sheridan DC, Berger S, Frommelt MA, Tweddell JS. Ten-year experience with surgical unroofing of anomalous aortic origin of a coronary artery from the opposite sinus with an interarterial course. J Thorac Cardiovasc Surg. 2011;142(5):1046–1051. doi: 10.1016/j.jtcvs.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 13.Davies JE, Burkhart HM, Dearani JA, et al. Surgical management of anomalous aortic origin of a coronary artery. Ann Thorac Surg. 2009;88(3):844–848. doi: 10.1016/j.athoracsur.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 14.Mumtaz MA, Lorber RE, Arruda J, Pettersson GB, Mavroudis C. Surgery for anomalous aortic origin of the coronary artery. Ann Thorac Surg. 2011;91(3):811–815. doi: 10.1016/j.athoracsur.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 15.Mainwaring RD, Murphy DJ, Rogers IS, et al. Surgical Repair of 115 Patients With Anomalous Aortic Origin of a Coronary Artery From a Single Institution. World J Pediatr Congenit Hear Surg. 2016;7(3):353–359. doi: 10.1177/2150135116641892. [DOI] [PubMed] [Google Scholar]
- 16.Brothers JA, McBride MG, Seliem MA, et al. Evaluation of myocardial ischemia after surgical repair of anomalous aortic origin of a coronary artery in a series of pediatric patients. J Am Coll Cardiol. 2007;50(21):2078–2082. doi: 10.1016/j.jacc.2007.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brothers J, Gaynor JW, Paridon S, Lorber R, Jacobs M. Anomalous aortic origin of a coronary artery with an interarterial course: Understanding current management strategies in children and young adults. Pediatr Cardiol. 2009 doi: 10.1007/s00246-009-9461-y. [DOI] [PubMed] [Google Scholar]
- 18.Nguyen AL, Haas F, Evens J, Breur JM. Sudden cardiac death after repair of anomalous origin of left coronary artery from right sinus of Valsalva with an interarterial course3: Case report and review of the literature. Neth Hear J. 2012;20(11):463–471. doi: 10.1007/s12471-012-0324-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brothers JA, Frommelt MA, Jaquiss RDB, Myerburg RJ, Fraser CD, Tweddell JS. Expert consensus guidelines: Anomalous aortic origin of a coronary artery. J Thorac Cardiovasc Surg. 2017;153(6):1440–1457. doi: 10.1016/j.jtcvs.2016.06.066. [DOI] [PubMed] [Google Scholar]
- 20.Van Hare GF, Ackerman MJ, Evangelista JK, et al. Eligibility and Disqualification Recommendations for Competitive Athletes With Cardiovascular Abnormalities: Task Force 4: Congenital Heart Disease. Circulation. 2015;132(22):e281–e291. doi: 10.1161/CIR.0000000000000240. [DOI] [PubMed] [Google Scholar]
- 21.Penalver JM, Mosca RS, Weitz D, Phoon CK. Anomalous aortic origin of coronary arteries from the opposite sinus: a critical appraisal of risk. BMC Cardiovasc Disord. 2012;12:83. doi: 10.1186/1471-2261-12-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.