Abstract
The striatum and globus pallidus are principal nuclei of the basal ganglia. Nissl- and acetylcholinesterase-stained sections of the tree shrew brain showed the neuroanatomical features of the caudate nucleus (Cd), internal capsule (ic), putamen (Pu), accumbens, internal globus pallidus, and external globus pallidus. The ic separated the dorsal striatum into the Cd and Pu in the tree shrew, but not in rats and mice. In addition, computer-based 3D images allowed a better understanding of the position and orientation of these structures. These data provided a large-scale atlas of the striatum and globus pallidus in the coronal, sagittal, and horizontal planes, the first detailed distribution of parvalbumin-immunoreactive cells in the tree shrew, and the differences in morphological characteristics and density of parvalbumin-immunoreactive neurons between tree shrew and rat. Our findings support the tree shrew as a potential model for human striatal disorders.
Electronic supplementary material
The online version of this article (10.1007/s12264-018-0212-z) contains supplementary material, which is available to authorized users.
Keywords: Striatum, Globus pallidus, Basal ganglia, Reconstruction, Rodent, Parvalbumin
Introduction
The striatum and globus pallidus are principal nuclei of the basal ganglia, the striatum being the largest. In primates, the striatum is composed of the caudate nucleus (Cd), internal capsule (ic), putamen (Pu), and accumbens (Acb) [1, 2]. The globus pallidus contains the external globus pallidus (EGP) and the internal globus pallidus (IGP; known as the entopeduncular nucleus (EP) in rodents) [3]. The striatum receives afferents from most regions of the cerebral cortex, thalamus, hippocampus, and amygdala [4–8]. The striatum is the principal input nucleus of the basal ganglia and provides efferent projections to other basal ganglia nuclei (globus pallidus, subthalamic nucleus, and substantia nigra), the thalamus, and the bed nucleus of the stria terminalis [9–13]. The dorsal striatum (Pu, Cd, and ic) integrates these inputs for the control of motor and cognitive behaviors [14–16]. The ventral striatum (Acb) integrates various afferent projections and plays a specific role in reward-based decision-making and cocaine abuse [17–20].
Recently, the tree shrew, a close living relative of primates, has been used in studies of viral infections [21], stress-related disorders [22], pharmacological tests [23], and Parkinson’s disease (PD) [24]. There are many antibodies (such as those for neuropeptide Y [25], NeuN [26], corticotropin-releasing factor [27], and tubulin [28]), pharmacological reagents (e.g., 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine [24], amyloidbetapeptide1-40 [29], and morphine [30]), surgical methods (stereotaxic surgery [11], monocular deprivation[31], and intravenous drug self-administration [30]), disease models (of depression [22], breast cancer [32], fatty liver disease [33], acute stress [34, 35], glioblastoma [36], and hepatitis B virus infection [37]) and behavioral tests (open field test [38], conditioned place preference/aversion [30], sucrose preference [22], and visuo-spatial trials [39]) which have been used in biomedical research using tree shrews. The tree shrew has been successfully used to study the structure and function of the brain [40–42], PD [24], Alzheimer’s disease [29], the visual system [43, 44], diffusion tensor imaging [45], and the limbic system [11, 46].
Previous studies have indicated that the morphological and anatomical characteristics of the striatum in the common tree shrew (Tupaia glis belangeri) resemble those in primates [47, 48]. The immunomarkers calcium-binding protein parvalbumin and acetylcholinesterase (AChE) have been used to define homologous nuclei among species [49–51]. The distribution of parvalbumin and AChE has been used to study the morphological characteristics of the caudate and putamen in tree shrews [25, 47]. A previous report indicated that the dorsal striatum in the tree shrew has many similarities to other mammals in the distribution of these markers [47]. The spiny output cells of the dorsal striatum receive afferents from the pulvinar nucleus in the tree shrew [6].
However, an anatomically comprehensive atlas of the striatum and globus pallidus in the tree shrew (Tupaia belangeri chinensis) has not been entirely explored. In the present study, we aimed to compare the anatomical features of the striatum and globus pallidus in the tree shrew with those in the rat and mouse. In addition, we provide detailed mapping and three-dimensional (3D) imaging of the striatum and globus pallidus in the tree shrew. Finally, we provide a comprehensive description of parvalbumin-immunoreactive (-ir) cells in these regions of the tree shrew and rat.
Materials and Methods
Animals
All adult male tree shrews (Tupaia belangeri chinensis), Sprague-Dawley rats, and C57/BL6J mice were housed in rooms under a 12-h dark/light cycle (lights off at 20:00). All animal procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals of the University of Science and Technology of China and were approved by the Animal Care and Use Committee at the University of Science and Technology of China.
Tissue Preparation
The tree shrews (n = 4, 10–19 months old), rats (n = 4, 6 months old), and mice (n = 3, 5 months old) were anesthetized with pentobarbital sodium (80 mg/kg, i.p.), then perfused with 0.9% saline and 4% paraformaldehyde (PFA). All brains were removed and segmented in the coronal plane according to brain atlases [52–55]. The forebrain was dehydrated in 30% sucrose and cut at 40 µm on a microtome (Leica CM1950, Wetzlar, Germany).
Nissl Staining
Three series of sections from tree shrews (24 sections at 320-µm intervals), rats (23 sections at 240-µm intervals), and mice (16 sections at 240-µm intervals) were rinsed in phosphate-buffered saline (PBS; 0.1 mol/L, pH 7.4), and then stained in 0.02% thionin acetate salt solution for 20 min [25]. The sections were dehydrated, coverslipped, and visualized under a light microscope (Olympus U-TV0.5XC-3, Tokyo, Japan).
Acetylcholinesterase Histochemistry
The sections were treated as previously described [25]. Briefly, another set of slides from tree shrews was incubated in 10 mL stock solution (50 mmol/L sodium acetate buffer, 4.0 mmol/L copper sulfate, 16 mmol/L glycine, pH 5.0, 11.6 mg acetylthiocholine iodide, and 0.3 mg ethopropazine hydrochloride) for 15 h. The slides were then rinsed and developed for 10 min in 1% sodium sulfide (pH 7.5). The sections were immersed for 8 h in 4% PFA, then dehydrated and coverslipped. The finished sections were visualized using the Olympus microscope (U-TV0.5XC-3).
Antibody Characterization and Immunohistochemistry
The specificity of the primary antibody, mouse monoclonal anti-parvalbumin (MAB1572, Millipore, Billerica, MA), was tested extensively in tree shrews [47] and rats [56]. Another two sets of adjacent sections were taken at the level of the striatum and globus pallidus of tree shrews (24 sections at 320-µm intervals) and rats (23 sections at 240-µm intervals). These sections were processed as previously described [57]. Briefly, floating sections were treated with 0.3% hydrogen peroxide and 0.5% Triton X-100 in PBS for 0.5 h. Then, following incubation with 5% normal horse serum (Solarbio, Beijing, China) in PBST (PBS containing 0.5% Triton X-100), the sections were incubated with mouse monoclonal anti-parvalbumin (MAB1572, Millipore; 1:2000) in 5% normal goat serum for 24 h at 4°C. The sections were then incubated with biotinylated horse anti-mouse IgG (1:200; Vector Laboratories, Burlingame, CA) and avidin-biotin peroxidase complex (1:200; Vector Laboratories). The sections were stained with 0.05% 3,3′-diaminobenzidine (Sigma-Aldrich) as chromogen, dehydrated, and coverslipped. Microphotographs were captured using an Imager.Z2 microscope with an automated acquisition system (TissueFAXS Plus, TissueGnostics GmbH, Vienna, Austria).
Some sections following parvalbumin immunoperoxidase labeling were stained with 0.02% thionin as described above. Then, sections were scanned using Imager.Z2 (Carl Zeiss Microscopy GmbH, Jena, Germany) with an automated acquisition system (TissueFAXS Plus, TissueGnostics GmbH).
3D Reconstruction
Every third section (133.98 µm between sections) was photographed and drawn using Adobe software. The TIF-format images (12.68 µm per pixel) from camera lucida drawings were used to reconstruct 3D models. These images were aligned with The Tree Shrew Brain in Stereotaxic Coordinates [52]. Based on the manually-defined representative color of each structure, brain regions were calculated using Imaris 7.2.3. (Bitplane AG, Zurich, Switzerland) and the image properties were set. Then the reconstructed images and movie were generated and exported [58].
Digital Photomicrographs and Drawings
Neuroanatomical localization and designation of the striatum and globus pallidus were based on previously-published atlases for the tree shrew [52], rat [54], and mouse [53]. Light microscopic images were captured using a series scanning microscope (Olympus U-TV0.5XC-3) and an Imager.Z2 microscope (Carl Zeiss) with an automated acquisition system (TissueFAXS Plus, TissueGnostics GmbH). All digital microphotographs of sections were adjusted for cropping and brightness/contrast using Adobe Photoshop CS5 (Adobe Systems, San Jose, CA). All drawings were depicted using Adobe Illustrator (Adobe Systems).
For quantitative analysis of the density of parvalbumin-ir cells, more than ten sections from each animal (4 tree shrews and 4 rats) were chosen for study. The density of positive cells was measured at 100× total magnification. Automatic cell counting methods were used to analyze these sections based on previous studies [59]. In ImageJ, a random-offset grid (200 µm × 200 µm) was applied to each image. At least three grids were counted for each section. The density of parvalbumin-ir cells was determined from the number of positive cells per grid. The data for all grids in each animal were pooled.
Statistics
All data are presented as the mean ± SEM and no data were excluded. The unpaired t-test and Mann-Whitney U test were used to make statistical comparisons between the tree shrew and rat using SPSS 19 software (SPSS Inc., Chicago, IL). In all cases, a probability value of P < 0.05 was considered statistically significant. The histogram for the density of parvalbumin-ir cells was plotted using GraphPad Prism (GraphPad Software, San Diego, CA).
Results
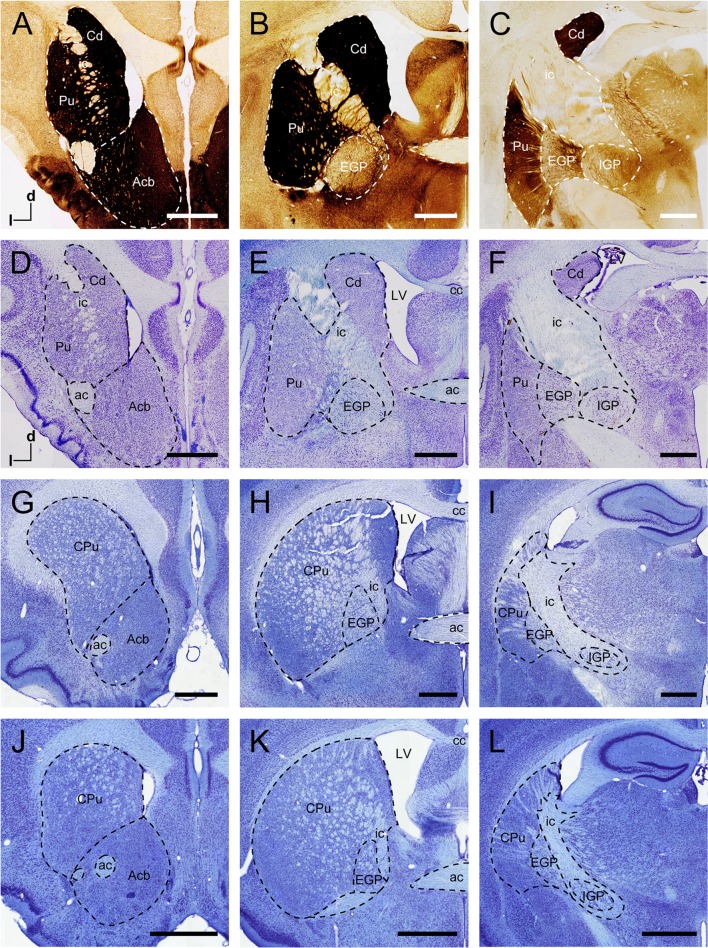
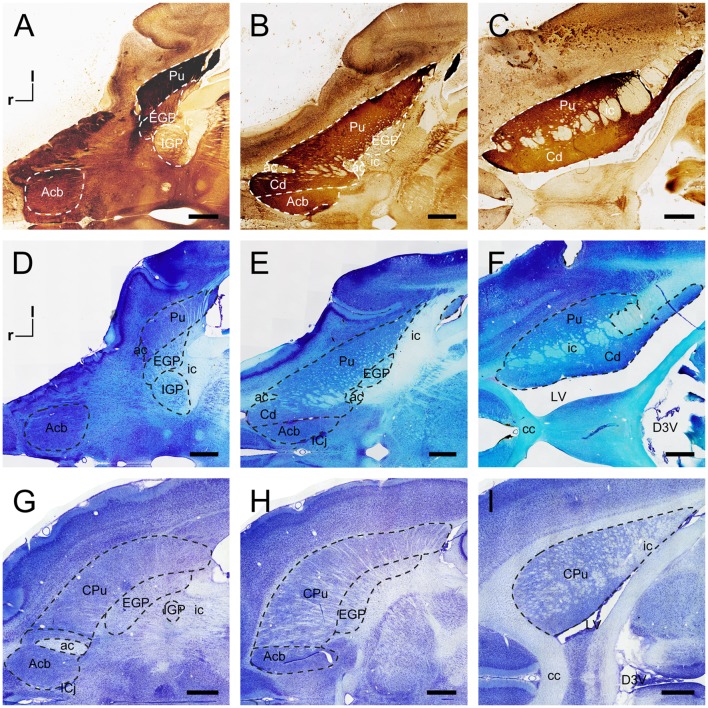
In the coronal sections from tree shrews, the dorsal striatum was composed of the ic, Cd, and Pu (Fig. 1A–F). The ic was mainly white matter that separated the dorsal striatum into the Cd and Pu. In the rostral and middle parts of the dorsal striatum, the Cd and Pu were not completely segmented by the ic (Fig. 1A, B). The Cd was located along the lateral ventricle and the dorsal and medial borders of the ic. The Acb was a region of the ventral striatum (Fig. 1A, D), located in the rostral part of the striatum. The AChE-stained sections showed darkly-staining neurons and fibers in the Cd, Pu, and Acb but not in the ic (Fig. 1A–C). In addition to the striatum, the EGP and IGP were the principal structures of the basal ganglia (Fig. 1B, C, E, F). The EGP was medial to the Pu and adjacent to the IGP (Fig. 1C, F). These two structures were approximately round in coronal sections. The EGP contained a moderate density of AChE-stained neurons and fibers, whereas only numerous fibers were observed in the IGP (Fig. 1F).
Fig. 1.
Coronal sections of the striatum and globus pallidus in the tree shrew, rat, and mouse. AChE-stained sections (A–C) and adjacent Nissl-stained sections (D–F) from rostral to caudal showing distinct subregions of the basal ganglia in the tree shrew. Coronal sections at similar levels show the organization of the basal ganglia in the rat (G–I) and mouse (J–L) in Nissl-stained sections. Dashed lines show the borders of these structures. ac, anterior commissure; Acb, nucleus accumbens; cc, corpus callosum; Cd, caudate nucleus; CPu, caudate putamen (dorsal striatum); d, dorsal; EGP, external globus pallidus; ic, internal capsule; IGP, internal globus pallidus; l, lateral; LV, lateral ventricle; Pu, putamen. Scale bars, 1 mm.
In the rodents, coronal sections at similar levels were Nissl stained to show the organization of the basal ganglia of the rat (Fig. 1G–I) and mouse (Fig. 1J–L). The neuroanatomical characteristics of the striatum and globus pallidus were similar in rat and mouse. The striatum was inconspicuously subdivided by the ic (Fig. 1H, K), which was fused into most regions of the striatum. The shape of the EGP and EP was approximately elliptical and separated by the ic in the rat and mouse (Fig. 1I, L).
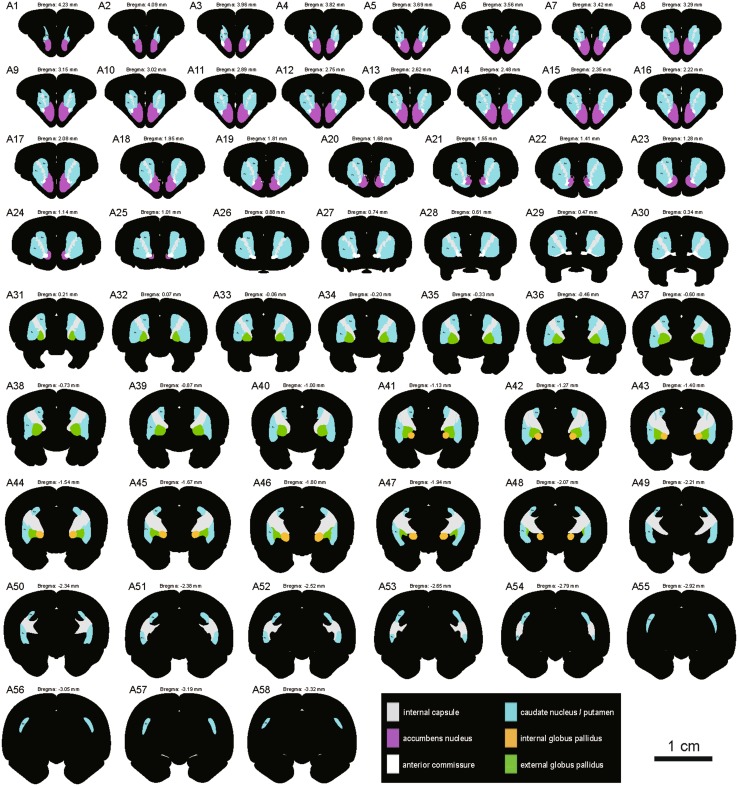
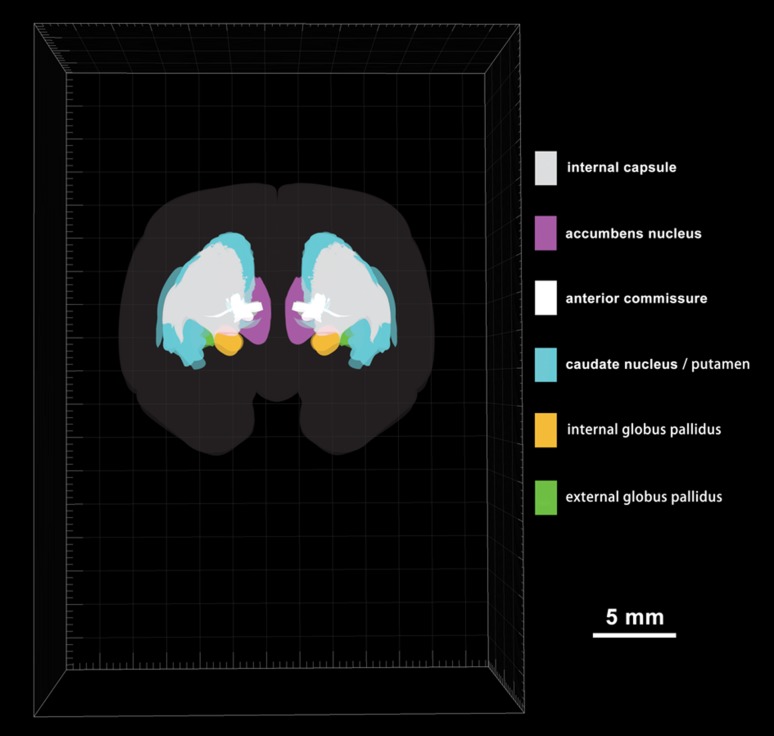
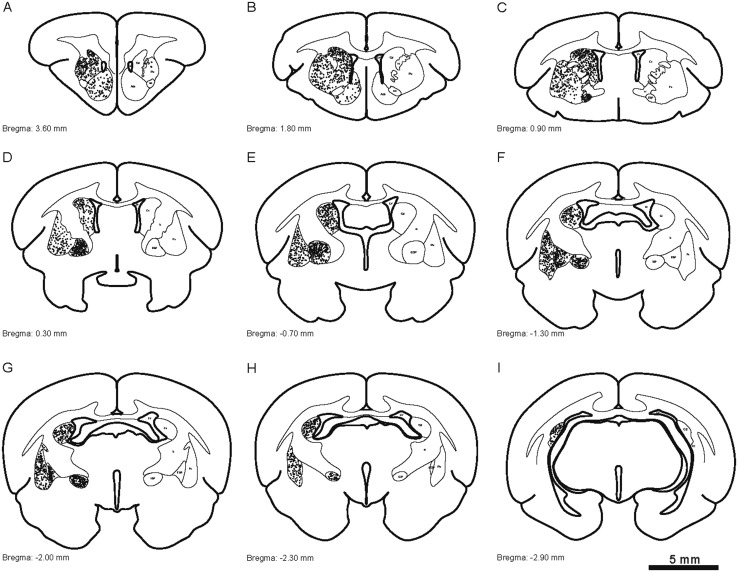
To display the striatum and globus pallidus of the tree shrew from rostral to caudal, a series of coronal sections was selected and referenced to the bregma (the reference zero point) (Fig. 2A1–A58). The ic, Cd, Pu, Acb, EGP, and IGP were clearly discernible in the coronal sections (Fig. 2). The outlines of the structures on the right side were derived from the left side of each section using mirror-image construction. In addition, deep brain structures were visualized by producing a translucent image of the 3D reconstruction using Imaris software (Fig. 3; Supplementary Material). The positional relationships among these brain regions were clearly represented in the 3D images.
Fig. 2.
Schematic drawings showing the general morphology of the striatum and globus pallidus in the coronal plane from rostral to caudal in the tree shrew (A1-A58, referring to The Tree Shrew (Tupaia belangeri chinensis) Brain in Stereotaxic Coordinates [52]). Colors represent distinct subregions of the basal ganglia. ICj, islands of Calleja. Scale bar, 1 cm.
Fig. 3.
Rostral view of a 3D reconstruction of the striatum and globus pallidus based on serial histological sections of tree shrew brain (58 sections shown in Fig. 2).
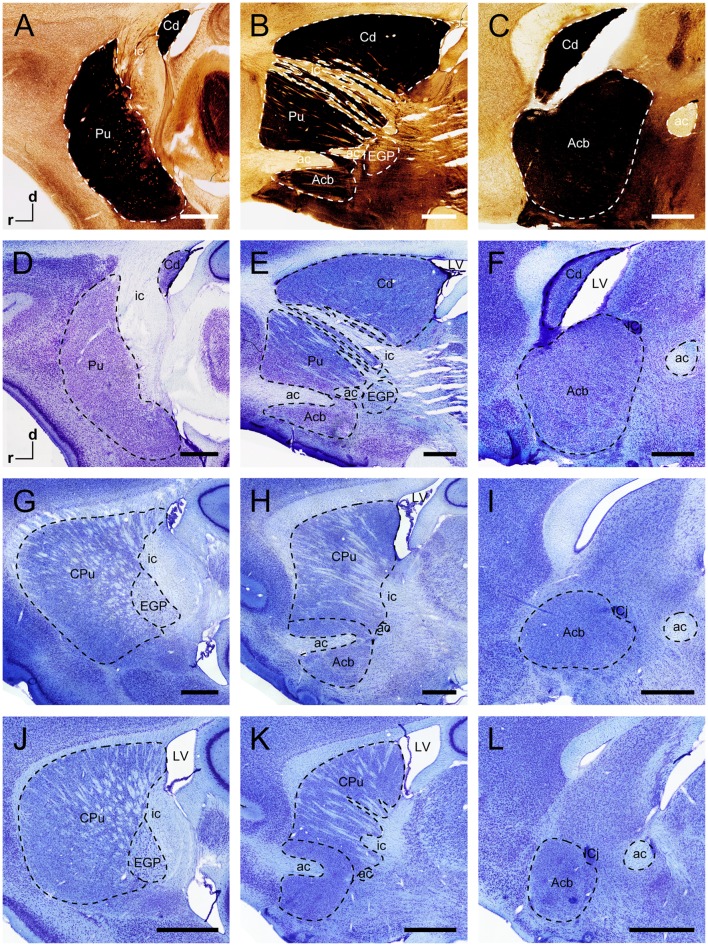
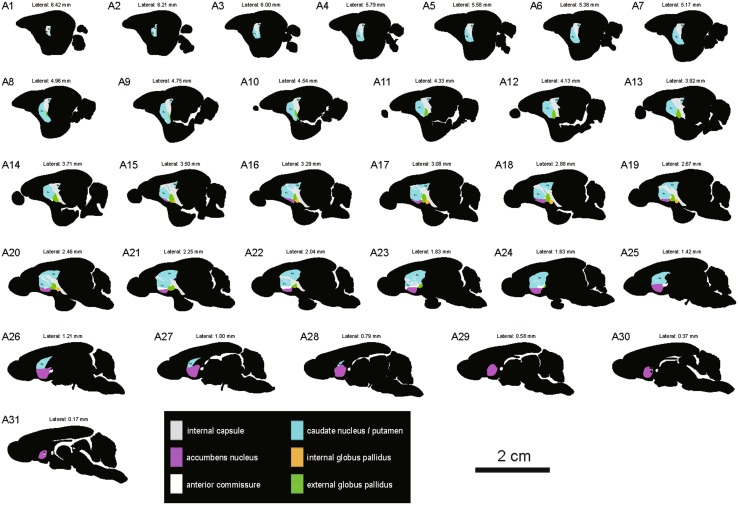
In sagittal sections from the tree shrew, the dorsal striatum was separated into the Cd and Pu by the ic (Fig. 4A–F). The middle part of the Cd was larger than the outer and inner parts (Fig. 4D–F). In the rodents, sagittal sections at similar levels showed the anatomical characteristics of the striatum and globus pallidus (Fig. 4G–L). The circular/oval-shaped ic was present in the outer part of the dorsal striatum in the rat and mouse (Fig. 4G, J). The strip-shaped ic was located in the inner part in the sagittal plane (Fig. 4E, H, K). The anterior commissure passed through the Acb in the sagittal sections (Fig. 4E, H, K). A series of sagittal sections was selected from lateral to medial in the tree shrew (Fig. 5). The outlines of the striatum and globus pallidus in the sagittal plane were depicted in the light of stained sections (Fig. 5).
Fig. 4.
Sagittal sections of the striatum and globus pallidus of the tree shrew, rat, and mouse. AChE-stained sections (A–C) and adjacent Nissl-stained sections (D–F) from lateral to medial show distinct subregions of the basal ganglia in the tree shrew. Sagittal sections at similar levels show the organization of the basal ganglia of the rat (G–I) and mouse (J–L) in Nissl-stained sections. Dashed lines show the borders of these structures. ac, anterior commissure; Acb, accumbens nucleus; Cd, caudate nucleus; CPu, caudate putamen (dorsal striatum); d, dorsal; EGP, external globus pallidus; ic, internal capsule; ICj, islands of Calleja; LV, lateral ventricle; Pu, putamen; r, rostral. Scale bars, 1 mm.
Fig. 5.
Schematic drawings showing the general morphology of the striatum and globus pallidus in the sagittal plane from lateral to medial in the tree shrew (A1–A31, referring to The Tree Shrew (Tupaia belangeri chinensis) Brain in Stereotaxic Coordinates [52]). Colors represent distinct subregions of the basal ganglia. ICj, islands of Calleja. Scale bar, 2 cm.
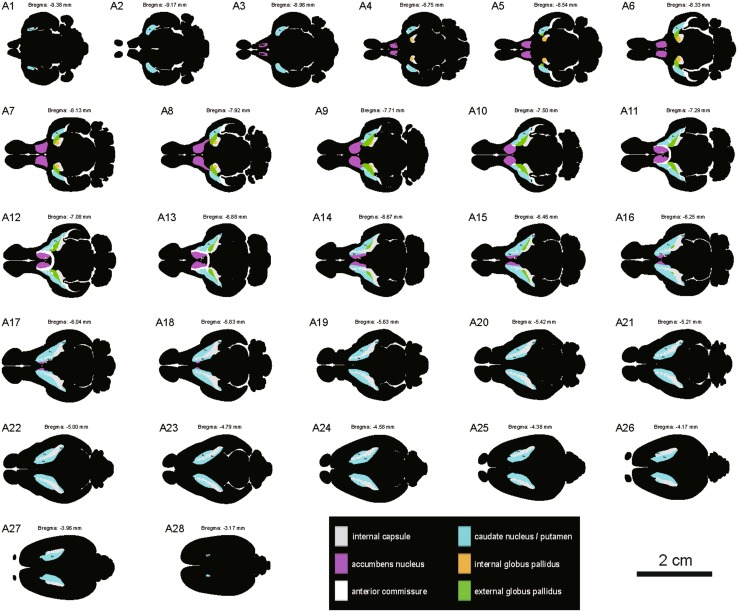
In horizontal sections, the striatum and globus pallidus were delineated in the tree shrew and rat (Fig. 6). The dorsal striatum of the tree shrew was not adjacent to the Acb in horizontal sections through the ventral Acb (Fig. 6A, D). The dorsal striatum in the tree shrew formed a strip which was divided by the ic (Fig. 6B, C, E, F). However, the dorsal striatum and EGP in the rat formed an arch or bridge (Fig. 6G, H). The Acb was medial to the dorsal striatum and adjacent to the midline in the tree shrew and rat (Fig. 6E, H). The ic in the rat was seldom visible in the ventral part of the striatum in horizontal sections (Fig. 6G). The strip-shaped ic was found in the middle part of the striatum and oriented perpendicular to the pial surface (Fig. 6H). The circular- and oval-shaped ic was scattered in the dorsal part of the striatum (Fig. 6I). A series of horizontal sections was selected from ventral to dorsal in the tree shrew (Fig. 7). Based on AChE- and Nissl-stained sections, the morphology and location of the striatum and globus pallidus in the horizontal plane were determined in the tree shrew (Fig. 7).
Fig. 6.
Horizontal sections of the striatum and globus pallidus in the tree shrew and rat. AChE-stained sections (A–C) and adjacent Nissl-stained sections (D–F) from ventral to dorsal show distinct subregions of the basal ganglia in the tree shrew. Horizontal sections at similar levels show the organization of the basal ganglia of the rat in Nissl-stained sections (G–I). Dashed lines show the borders of these structures. ac, anterior commissure; Acb, accumbens nucleus; cc, corpus callosum; Cd, caudate nucleus; CPu, caudate putamen (dorsal striatum); D3V, dorsal 3rd ventricle; EGP, external globus pallidus; ic, internal capsule; ICj, islands of Calleja; IGP, internal globus pallidus; l, lateral; LV, lateral ventricle; Pu, putamen; R, rostral. Scale bars, 1 mm.
Fig. 7.
Schematic drawings showing the general morphology of the striatum and globus pallidus in the horizontal plane from ventral to dorsal in the tree shrew (A1–A28, referring to The Tree Shrew (Tupaia belangeri chinensis) Brain in Stereotaxic Coordinates [52]). Colors represent distinct subregions of the basal ganglia. ICj, islands of Calleja. Scale bar, 2 cm.
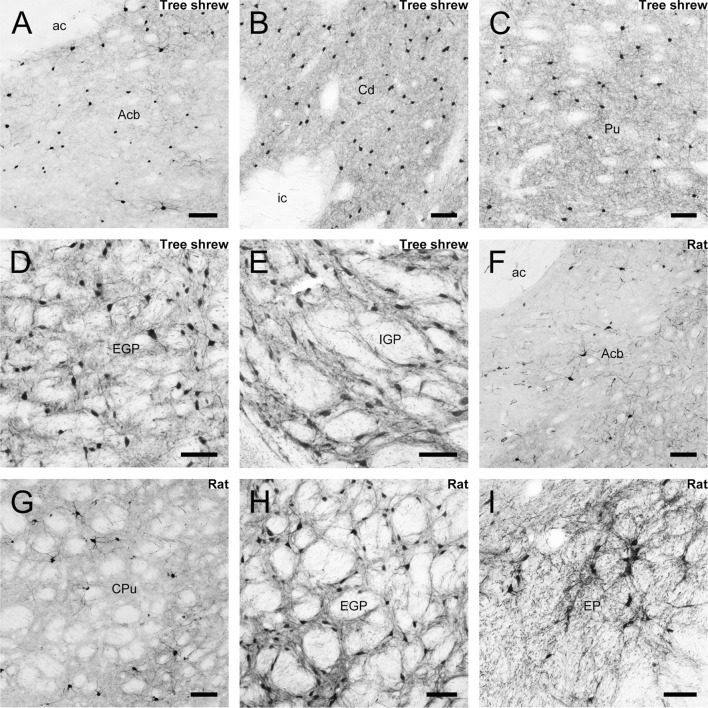
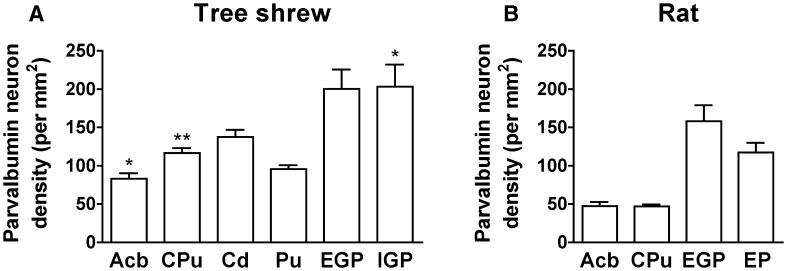
Parvalbumin immunoreactivity was assessed in the striatum and globus pallidus of the tree shrew. Camera lucida drawings were made to present the distribution of parvalbumin-ir cells throughout the striatum and globus pallidus from rostral to caudal (Fig. 8). The Acb contained a number of positive neurons and fibers (Fig. 9A). A moderate density of parvalbumin-ir cells and fibers was seen in the Cd and Pu (Fig. 9B, C). Immunoreactive neurons were absent from the ic (Fig. 9B). A high density of parvalbumin-ir neurons and fibers was found in the EGP and IGP of the tree shrew (Fig. 9D, E). However, a low density of parvalbumin-ir cells and fibers was scattered in the Acb and CPu of the rat (Fig. 9F, G). A high density of positive neurons and fibers was present in the rat EGP (Fig. 9H), while a moderate density was observed in the EP (Fig. 9I). The EGP and IGP (EP) had the highest density of parvalbumin-ir cells in the striatum and globus pallidus of the tree shrew and rat (Fig. 10). The density of parvalbumin-ir cells in the Cd of the tree shrew was higher than that in the Pu (Fig. 10A). A higher density of parvalbumin-ir cells was located in the CPu, Acb, and IGP of the tree shrew than the rat (Fig. 10A, B).
Fig. 8.
Camera lucida drawings showing the distribution of parvalbumin-ir cells (dots) in the striatum and globus pallidus of the tree shrew from rostral to caudal (A–I). Representative coronal sections were selected, with reference to bregma. The density of dots represents the relative density of cells in the areas. Each dot represents approximately one parvalbumin-labeled neuron. ac, anterior commissure; Acb, accumbens nucleus; Cd, caudate nucleus; EGP, external globus pallidus; ic, internal capsule; IGP, internal globus pallidus; LV, lateral ventricle; Pu, putamen.
Fig. 9.
Photomicrographs of parvalbumin-ir staining in the nucleus accumbens (Acb; A), caudate nucleus (Cd; B), putamen (Pu; C), external globus pallidus (EGP; D), and internal globus pallidus (IGP; E) in the tree shrew. Parvalbumin-ir neurons were also present in the Acb (F), caudate putamen (CPu; G), EGP (H), and entopeduncular nucleus (EP; I) of the rat. Scale bar, 100 μm.
Fig. 10.
Quantitative analysis of the density of parvalbumin-ir cells in distinct subregions of the striatum and globus pallidus in the tree shrew (A) and rat (B) (*P < 0.05; **P < 0.01 compared with rat; Mann–Whitney U test and unpaired t-test). Acb, accumbens nucleus; Cd, caudate nucleus; CPu, caudate putamen; EGP, external globus pallidus; EP, entopeduncular nucleus; IGP, internal globus pallidus; Pu, putamen.
Discussion
Our results present a detailed mapping of the striatum and globus pallidus in the Chinese tree shrew (Tupaia belangeri chinensis) in the coronal, sagittal, and horizontal planes. In addition, the morphological and anatomical features of the striatum and globus pallidus in all three planes in the tree shrew are clearly compared with those in rodents. The neurochemical features of the dorsal striatum in the common tree shrew (Tupaia glis belangeri) have been examined in the coronal plane [47]. In this paper, Rice et al. [47] analyzed the neurochemical composition and distribution of the striosome and matrix compartments using immunohistochemistry, but positional information of the dorsal striatum sections was not provided. The neurochemical characteristics of the ventral striatum in the common tree shrew, rat, and monkey were examined in the coronal plane using immunohistochemistry and electron microscopic analysis in another paper by McCollum and Roberts [48]. Their results indicated that the ultrastructure in the tree shrew closely resembles the characteristics of the primate Acb [48]. Our present study has described the comparative anatomical organization of the striatum and globus pallidus in the tree shrew, rat, and mouse. The dorsal striatum in the tree shrew contains the main anatomical feature of a well-developed ic that clearly separates the Cd and Pu, similar to previous reports in primates and humans [60, 61]. Actually, rats and mice present an under-developed ic in the striatum. The under-developed ic was scattered in most regions of the rostral and middle parts of the striatum in rat and mouse. The ic was absent in the dorsomedial part of the mid-striatum along the lateral ventricle in rat and mouse, resembling the well-developed Cd in the tree shrew. The morphological characteristics of the EGP and IGP in the tree shrew differed from those in rat and mouse. Although differences between the tree shrew and the rat have been reported in the organization within the Acb shell and the proportion of symmetrical and asymmetrical synapses formed by dopaminergic terminals [48], the morphological characteristics of the Acb were similar in these species in our study.
According to our data, the location of parvalbumin-ir neurons in the Acb, CPu, EGP, and IGP (EP) of the tree shrew is similar to that in rodents [62, 63]. However, the density of parvalbumin immunoreactivity in the globus pallidus and striatum differs between the tree shrew and rat. The density of parvalbumin neurons in the striatum of monkeys is higher than that in rats [64]. Similarly, a higher density of parvalbumin-ir neurons was present in the striatum of the tree shrew than in the rat. Previous work indicates that the density of parvalbumin-ir neurons in the striatum is related to behavioral performance in fear extinction and latent inhibition [65] and that the parvalbumin-expressing neurons in the basal ganglia play a role in regulating movement-related behaviors [66, 67]. Furthermore, the age of the animal affects the density of parvalbumin-ir neurons [68], especially in the main olfactory bulb and septum [69, 70]. However, no significant age-related changes have been found in the number of parvalbumin-ir cells in the rodent striatum [70, 71]. A previous report has identified two classes of neurons in the rodent EP on the basis of their electrophysiological characteristics [72] and that it contains somatostatin-ir neurons and parvalbumin-ir neurons [73]. Wallace et al. [74] used large-scale single-cell transcriptional profiling and molecular analyses to reveal that the rodent EP contains at least three classes of projection neurons: glutamate/GABA co-releasing somatostatin neurons, glutamatergic parvalbumin neurons, and GABAergic parvalbumin neurons. These classes in the EP have functionally and anatomically distinct outputs that differentially affect their targets, such as the epithalamus and thalamus [74].
It is possible that these differences in morphological and anatomical characteristics reflect species differences in their natural habitats. Wild tree shrews mainly live in an arboreal habitat [75], while rats and mice live mainly in burrow systems at ground level [76]. In addition, tree shrews in the laboratory prefer to jump, leap, and somersault in their cages [75]. Such a preference is not seen in rats and mice. The dorsal striatum receives numerous afferent projections from the motor cortex and somatosensory cortex [77, 78]. A recent report provides evidence that neuronal clusters in the dorsal striatum of mice encode locomotor information which is important for striatum-controlled behaviors [79]. The pulvinar in the tree shrew receives afferent inputs from the superior colliculus and projects to spiny output neurons in the Cd and Pu [6]. These subcortical circuits in diurnal tree shrews differ from those in rodents [80]. The rodents lack the apparent pulvinar nucleus observed in tree shrews [52–54].
Our data showed that the morphological and anatomical features of the striatum in the tree shrew are dissimilar to those in rodents. Rodent transgenic and neurotoxic models are widely used to study PD [81, 82], and although they have greatly improved our knowledge of its pathogenic and pathophysiological mechanisms [83], the rodent striatum differs significantly from that of primates, and this must be carefully taken into consideration when the findings in rodent models of PD are translated from laboratory species to human pathologies and clinical trials. The tree shrew has the potential for development as an animal model of PD because it is diurnal, small, easy to handle and feed, and has a short gestation period and duration of puberty [75]. Previous research indicates that tree shrews treated with the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine exhibit classic PD symptoms at a lower dose than in rodents [24].
There are several advantages of using the tree shrew as a model in which to study human diseases associated with the striatum. The tree shrew has a larger striatum than rodents [75], making it beneficial for magnetic resonance scanning and analysis. In addition, the tree shrew striatum displays clear-cut borders, providing a new animal model in which to study the subregional functions of the human striatum. The cytoarchitectonics of the striatum in tree shrews has similarities to that of primates [47]. These advantages may provide further insights into the human diseases associated with the striatum.
In summary, our results present a comprehensive comparison of the Cd, ic, Pu, Acb, EGP, and IGP (EP) in the tree shrew and rodents in the coronal, sagittal, and horizontal planes. Moreover, our data provide detailed coronal, sagittal, and horizontal atlases of the striatum and globus pallidus in the Chinese tree shrew. The computer-based 3D images allow a better understanding of the position and orientation of the striatum and globus pallidus in relation to each other. In addition, the present findings provide the first detailed distribution of parvalbumin-ir cells in the tree shrew striatum and globus pallidus and demonstrate species differences in the density of positive neurons between tree shrew and rat. Our results support the tree shrew as a potential animal model for human striatal disorders which may help to further elucidate and understand pathogenic and pathophysiological mechanisms.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (31500859 and 91432305) and the Strategic Priority Research Program of the Chinese Academy of Science (XDB02030001).
Authors’ Contributions
RJN was involved in tissue processing, histochemical analysis, collection and interpretation of data, and writing the manuscript; ZHH and YMS were involved in tissue processing and data interpretation; YW was involved in 3D brain reconstruction; TL was responsible for revising the manuscript; and JNZ was responsible for experimental design and manuscript preparation.
Compliance with Ethical Standards
Conflict of interest
All authors claim that there are no conflicts of interest.
Footnotes
Electronic supplementary material
The online version of this article (10.1007/s12264-018-0212-z) contains supplementary material, which is available to authorized users.
References
- 1.Fujiyama F. Anatomical connections of the basal ganglia. Brain Nerve. 2009;61:341–349. [PubMed] [Google Scholar]
- 2.Prensa L, Cossette M, Parent A. Dopaminergic innervation of human basal ganglia. J Chem Neuroanat. 2000;20:207–213. doi: 10.1016/S0891-0618(00)00099-5. [DOI] [PubMed] [Google Scholar]
- 3.Yelnik J, Damier P, Bejjani BP, Francois C, Gervais D, Dormont D, et al. Functional mapping of the human globus pallidus: contrasting effect of stimulation in the internal and external pallidum in Parkinson’s disease. Neuroscience. 2000;101:77–87. doi: 10.1016/S0306-4522(00)00364-X. [DOI] [PubMed] [Google Scholar]
- 4.Gimenez-Amaya JM. McFarland NR, de las Heras S, Haber SN. Organization of thalamic projections to the ventral striatum in the primate. J Comp Neurol. 1995;354:127–149. doi: 10.1002/cne.903540109. [DOI] [PubMed] [Google Scholar]
- 5.Khibnik LA, Tritsch NX, Sabatini BL. A direct projection from mouse primary visual cortex to dorsomedial striatum. PLoS One. 2014;9:e104501. doi: 10.1371/journal.pone.0104501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Day-Brown JD, Wei H, Chomsung RD, Petry HM, Bickford ME. Pulvinar projections to the striatum and amygdala in the tree shrew. Front Neuroanat. 2010;4:143. doi: 10.3389/fnana.2010.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.French SJ, Totterdell S. Quantification of morphological differences in boutons from different afferent populations to the nucleus accumbens. Brain Res. 2004;1007:167–177. doi: 10.1016/j.brainres.2004.02.018. [DOI] [PubMed] [Google Scholar]
- 8.Schmitt O, Eipert P, Kettlitz R, Lessmann F, Wree A. The connectome of the basal ganglia. Brain Struct Funct. 2016;221:753–814. doi: 10.1007/s00429-014-0936-0. [DOI] [PubMed] [Google Scholar]
- 9.Calabresi P, Picconi B, Tozzi A, Ghiglieri V, Di Filippo M. Direct and indirect pathways of basal ganglia: a critical reappraisal. Nat Neurosci. 2014;17:1022–1030. doi: 10.1038/nn.3743. [DOI] [PubMed] [Google Scholar]
- 10.Gerfen CR, Surmeier DJ. Modulation of striatal projection systems by dopamine. Annu Rev Neurosci. 2011;34:441–466. doi: 10.1146/annurev-neuro-061010-113641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ni RJ, Luo PH, Shu YM, Chen JT, Zhou JN. Whole-brain mapping of afferent projections to the bed nucleus of the stria terminalis in tree shrews. Neuroscience. 2016;333:162–180. doi: 10.1016/j.neuroscience.2016.07.017. [DOI] [PubMed] [Google Scholar]
- 12.Gao HR, Zhuang QX, Zhang YX, Chen ZP, Li B, Zhang XY, et al. Orexin directly enhances the excitability of globus pallidus internus neurons in rat by co-activating OX1 and OX2 receptors. Neurosci Bull. 2017;33:365–372. doi: 10.1007/s12264-017-0127-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eid L, Parent M. Chemical anatomy of pallidal afferents in primates. Brain Struct Funct. 2016;221:4291–4317. doi: 10.1007/s00429-016-1216-y. [DOI] [PubMed] [Google Scholar]
- 14.McFarland NR, Haber SN. Convergent inputs from thalamic motor nuclei and frontal cortical areas to the dorsal striatum in the primate. J Neurosci. 2000;20:3798–3813. doi: 10.1523/JNEUROSCI.20-10-03798.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Darvas M, Henschen CW, Palmiter RD. Contributions of signaling by dopamine neurons in dorsal striatum to cognitive behaviors corresponding to those observed in Parkinson’s disease. Neurobiol Dis. 2014;65:112–123. doi: 10.1016/j.nbd.2014.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou D, Pang F, Liu S, Shen Y, Liu L, Fang Z, et al. Altered motor-striatal plasticity and cortical functioning in patients with schizophrenia. Neurosci Bull. 2017;33:307–311. doi: 10.1007/s12264-016-0079-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heekeren HR, Wartenburger I, Marschner A, Mell T, Villringer A, Reischies FM. Role of ventral striatum in reward-based decision making. Neuroreport. 2007;18:951–955. doi: 10.1097/WNR.0b013e3281532bd7. [DOI] [PubMed] [Google Scholar]
- 18.Hurd YL, Svensson P, Ponten M. The role of dopamine, dynorphin, and CART systems in the ventral striatum and amygdala in cocaine abuse. Ann N Y Acad Sci. 1999;877:499–506. doi: 10.1111/j.1749-6632.1999.tb09285.x. [DOI] [PubMed] [Google Scholar]
- 19.Wang B, Yang X, Sun A, Xu L, Wang S, Lin W, et al. Extracellular signal-regulated kinase in nucleus accumbens mediates propofol self-administration in rats. Neurosci Bull. 2016;32:531–537. doi: 10.1007/s12264-016-0066-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li C, Yan Y, Cheng J, Xiao G, Gu J, Zhang L, et al. Toll-like receptor 4 deficiency causes reduced exploratory behavior in mice under approach-avoidance conflict. Neurosci Bull. 2016;32:127–136. doi: 10.1007/s12264-016-0015-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsukiyama-Kohara K, Kohara M. Tupaia belangeri as an experimental animal model for viral infection. Exp Anim. 2014;63:367–374. doi: 10.1538/expanim.14-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang J, Chai A, Zhou Q, Lv L, Wang L, Yang Y, et al. Chronic clomipramine treatment reverses core symptom of depression in subordinate tree shrews. PLoS One. 2013;8:e80980. doi: 10.1371/journal.pone.0080980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao F, Guo X, Wang Y, Liu J, Lee WH, Zhang Y. Drug target mining and analysis of the Chinese tree shrew for pharmacological testing. PLoS One. 2014;9:e104191. doi: 10.1371/journal.pone.0104191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma KL, Gao JH, Huang ZQ, Zhang Y, Kuang DX, Jiang QF, et al. Motor function in MPTP-treated tree shrews (Tupaia belangeri chinensis) Neurochem Res. 2013;38:1935–1940. doi: 10.1007/s11064-013-1099-8. [DOI] [PubMed] [Google Scholar]
- 25.Ni RJ, Shu YM, Luo PH, Fang H, Wang Y, Yao L, et al. Immunohistochemical mapping of neuropeptide Y in the tree shrew brain. J Comp Neurol. 2015;523:495–529. doi: 10.1002/cne.23696. [DOI] [PubMed] [Google Scholar]
- 26.Lu JS, Yue F, Liu X, Chen T, Zhuo M. Characterization of the anterior cingulate cortex in adult tree shrew. Mol Pain. 2016;12:1744806916684515. doi: 10.1177/1744806916684515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kozicz T, Bordewin LA, Czeh B, Fuchs E, Roubos EW. Chronic psychosocial stress affects corticotropin-releasing factor in the paraventricular nucleus and central extended amygdala as well as urocortin 1 in the non-preganglionic Edinger-Westphal nucleus of the tree shrew. Psychoneuroendocrinology. 2008;33:741–754. doi: 10.1016/j.psyneuen.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 28.Wolter R, Tauer U, Fuchs E, Volk B. Mapping of cytoskeletal components in the hippocampal formation of the tree shrew (Tupaia belangeri) J Chem Neuroanat. 1999;17:65–74. doi: 10.1016/S0891-0618(99)00026-5. [DOI] [PubMed] [Google Scholar]
- 29.Lin N, Xiong LL, Zhang RP, Zheng H, Wang L, Qian ZY, et al. Injection of Abeta1-40 into hippocampus induced cognitive lesion associated with neuronal apoptosis and multiple gene expressions in the tree shrew. Apoptosis. 2016;21:621–640. doi: 10.1007/s10495-016-1227-4. [DOI] [PubMed] [Google Scholar]
- 30.Shen F, Duan Y, Jin S, Sui N. Varied behavioral responses induced by morphine in the tree shrew: a possible model for human opiate addiction. Front Behav Neurosci. 2014;8:333. doi: 10.3389/fnbeh.2014.00333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guggenheim JA, McBrien NA. Form-deprivation myopia induces activation of scleral matrix metalloproteinase-2 in tree shrew. Invest Ophthalmol Vis Sci. 1996;37:1380–1395. [PubMed] [Google Scholar]
- 32.Ge GZ, Xia HJ, He BL, Zhang HL, Liu WJ, Shao M, et al. Generation and characterization of a breast carcinoma model by PyMT overexpression in mammary epithelial cells of tree shrew, an animal close to primates in evolution. Int J Cancer. 2016;138:642–651. doi: 10.1002/ijc.29814. [DOI] [PubMed] [Google Scholar]
- 33.Zhang L, Zhang Z, Li Y, Liao S, Wu X, Chang Q, et al. Cholesterol induces lipoprotein lipase expression in a tree shrew (Tupaia belangeri chinensis) model of non-alcoholic fatty liver disease. Sci Rep. 2015;5:15970. doi: 10.1038/srep15970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fang H, Sun YJ, Lv YH, Ni RJ, Shu YM, Feng XY, et al. High activity of the stress promoter contributes to susceptibility to stress in the tree shrew. Sci Rep. 2016;6:24905. doi: 10.1038/srep24905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yao YG. Creating animal models, why not use the Chinese tree shrew (Tupaia belangeri chinensis)? Zool Res. 2017;38:118–126. doi: 10.24272/j.issn.2095-8137.2017.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tong Y, Hao J, Tu Q, Yu H, Yan L, Li Y, et al. A tree shrew glioblastoma model recapitulates features of human glioblastoma. Oncotarget. 2017;8:17897–17907. doi: 10.18632/oncotarget.15225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang C, Ruan P, Ou C, Su J, Cao J, Luo C, et al. Chronic hepatitis B virus infection and occurrence of hepatocellular carcinoma in tree shrews (Tupaia belangeri chinensis) Virol J. 2015;12:26. doi: 10.1186/s12985-015-0256-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Van Kampen M, Schmitt U, Hiemke C, Fuchs E. Diazepam has no beneficial effects on stress-induced behavioural and endocrine changes in male tree shrews. Pharmacol Biochem Behav. 2000;65:539–546. doi: 10.1016/S0091-3057(99)00190-2. [DOI] [PubMed] [Google Scholar]
- 39.Ohl F, Oitzl MS, Fuchs E. Assessing cognitive functions in tree shrews: visuo-spatial and spatial learning in the home cage. J Neurosci Methods. 1998;81:35–40. doi: 10.1016/S0165-0270(98)00011-9. [DOI] [PubMed] [Google Scholar]
- 40.Casagrande VA, Harting JK, Hall WC, Diamond IT, Martin GF. Superior colliculus of the tree shrew: a structural and functional subdivision into superficial and deep layers. Science. 1972;177:444–447. doi: 10.1126/science.177.4047.444. [DOI] [PubMed] [Google Scholar]
- 41.Day-Brown JD, Slusarczyk AS, Zhou N, Quiggins R, Petry HM, Bickford ME. Synaptic organization of striate cortex projections in the tree shrew: A comparison of the claustrum and dorsal thalamus. J Comp Neurol. 2017;525:1403–1420. doi: 10.1002/cne.23998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Familtsev D, Quiggins R, Masterson S, Dang W, Slusarczyk AS, Petry HM, et al. Ultrastructure of geniculocortical synaptic connections in the tree shrew striate cortex. J Comp Neurol. 2016;524:1292–1306. doi: 10.1002/cne.23907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Takahata T, Kaas JH. c-FOS expression in the visual system of tree shrews after monocular inactivation. J Comp Neurol. 2017;525:151–165. doi: 10.1002/cne.24053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.De Luna P, Veit J, Rainer G. Basal forebrain activation enhances between-trial reliability of low-frequency local field potentials (LFP) and spiking activity in tree shrew primary visual cortex (V1) Brain Struct Funct. 2017;222:4239–4252. doi: 10.1007/s00429-017-1468-1. [DOI] [PubMed] [Google Scholar]
- 45.Dai JK, Wang SX, Shan D, Niu HC, Lei H. A diffusion tensor imaging atlas of white matter in tree shrew. Brain Struct Funct. 2017;222:1733–1751. doi: 10.1007/s00429-016-1304-z. [DOI] [PubMed] [Google Scholar]
- 46.Magarinos AM, McEwen BS, Flugge G, Fuchs E. Chronic psychosocial stress causes apical dendritic atrophy of hippocampal CA3 pyramidal neurons in subordinate tree shrews. J Neurosci. 1996;16:3534–3540. doi: 10.1523/JNEUROSCI.16-10-03534.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rice MW, Roberts RC, Melendez-Ferro M, Perez-Costas E. Neurochemical characterization of the tree shrew dorsal striatum. Front Neuroanat. 2011;5:53. doi: 10.3389/fnana.2011.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McCollum LA, Roberts RC. Ultrastructural localization of tyrosine hydroxylase in tree shrew nucleus accumbens core and shell. Neuroscience. 2014;271:23–34. doi: 10.1016/j.neuroscience.2014.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Watson C, Lind CR, Thomas MG. The anatomy of the caudal zona incerta in rodents and primates. J Anat. 2014;224:95–107. doi: 10.1111/joa.12132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hardman CD, Ashwell KWS. Stereotaxic and Chemoarchitectural Atlas of the Brain of the Common Marmoset (Callithrix jacchus) 1. Florida: CRC Press; 2012. [Google Scholar]
- 51.Paxinos G, Watson CR, Emson PC. AChE-stained horizontal sections of the rat brain in stereotaxic coordinates. J Neurosci Methods. 1980;3:129–149. doi: 10.1016/0165-0270(80)90021-7. [DOI] [PubMed] [Google Scholar]
- 52.Zhou JN, Ni RJ. The Tree Shrew (Tupaia belangeri chinensis) Brain in Stereotaxic Coordinates. 1. Beijing: Science Press and Springer; 2016. [Google Scholar]
- 53.Franklin KBJ, Paxinos G. The Mouse Brain in Stereotaxic Coordinates, 3rd Edition. 3. New York: Academic Press; 2007. [Google Scholar]
- 54.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 6. New York: Academic Press; 2007. [Google Scholar]
- 55.Burke MW, Zangenehpour S, Boire D, Ptito M. Dissecting the non-human primate brain in stereotaxic space. J Vis Exp 2009: 1–5. [DOI] [PMC free article] [PubMed]
- 56.Yang Z, You Y, Levison SW. Neonatal hypoxic/ischemic brain injury induces production of calretinin-expressing interneurons in the striatum. J Comp Neurol. 2008;511:19–33. doi: 10.1002/cne.21819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ni RJ, Shu YM, Wang J, Yin JC, Xu L, Zhou JN. Distribution of vasopressin, oxytocin and vasoactive intestinal polypeptide in the hypothalamus and extrahypothalamic regions of tree shrews. Neuroscience. 2014;265:124–136. doi: 10.1016/j.neuroscience.2014.01.034. [DOI] [PubMed] [Google Scholar]
- 58.Kamisaka Y, Ronnestad I. Reconstructed 3D models of digestive organs of developing Atlantic cod (Gadus morhua) larvae. Mar Biol. 2011;158:233–243. doi: 10.1007/s00227-010-1554-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Grishagin IV. Automatic cell counting with ImageJ. Anal Biochem. 2015;473:63–65. doi: 10.1016/j.ab.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 60.Lanciego JL, Vazquez A. The basal ganglia and thalamus of the long-tailed macaque in stereotaxic coordinates. A template atlas based on coronal, sagittal and horizontal brain sections. Brain Struct Funct. 2012;217:613–666. doi: 10.1007/s00429-011-0370-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cho ZH. 7.0 Tesla MRI brain atlas: in vivo atlas with cryomacrotome correlation. New York: Springer, 2015.
- 62.Todtenkopf MS, Stellar JR, Williams EA, Zahm DS. Differential distribution of parvalbumin immunoreactive neurons in the striatum of cocaine sensitized rats. Neuroscience. 2004;127:35–42. doi: 10.1016/j.neuroscience.2004.04.054. [DOI] [PubMed] [Google Scholar]
- 63.Saunders A, Huang KW, Sabatini BL. Globus pallidus externus neurons expressing parvalbumin interconnect the subthalamic nucleus and striatal interneurons. PLoS One. 2016;11:e0149798. doi: 10.1371/journal.pone.0149798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wu Y, Parent A. Striatal interneurons expressing calretinin, parvalbumin or NADPH-diaphorase: a comparative study in the rat, monkey and human. Brain Res. 2000;863:182–191. doi: 10.1016/S0006-8993(00)02135-1. [DOI] [PubMed] [Google Scholar]
- 65.Ammassari-Teule M, Sgobio C, Biamonte F, Marrone C, Mercuri NB, Keller F. Reelin haploinsufficiency reduces the density of PV+ neurons in circumscribed regions of the striatum and selectively alters striatal-based behaviors. Psychopharmacology (Berl) 2009;204:511–521. doi: 10.1007/s00213-009-1483-x. [DOI] [PubMed] [Google Scholar]
- 66.Xu M, Li L, Pittenger C. Ablation of fast-spiking interneurons in the dorsal striatum, recapitulating abnormalities seen post-mortem in Tourette syndrome, produces anxiety and elevated grooming. Neuroscience. 2016;324:321–329. doi: 10.1016/j.neuroscience.2016.02.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Marrone MC, Marinelli S, Biamonte F, Keller F, Sgobio CA, Ammassari-Teule M, et al. Altered cortico-striatal synaptic plasticity and related behavioural impairments in reeler mice. Eur J Neurosci. 2006;24:2061–2070. doi: 10.1111/j.1460-9568.2006.05083.x. [DOI] [PubMed] [Google Scholar]
- 68.Hamann M, Richter A, Meillasson FV, Nitsch C, Ebert U. Age-related changes in parvalbumin-positive interneurons in the striatum, but not in the sensorimotor cortex in dystonic brains of the dt(sz) mutant hamster. Brain Res. 2007;1150:190–199. doi: 10.1016/j.brainres.2007.02.074. [DOI] [PubMed] [Google Scholar]
- 69.Choi JH, Lee CH, Yoo KY, Hwang IK, Lee IS, Lee YL, et al. Age-related changes in calbindin-D28k, parvalbumin, and calretinin immunoreactivity in the dog main olfactory bulb. Cell Mol Neurobiol. 2010;30:1–12. doi: 10.1007/s10571-009-9425-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Krzywkowski P, De Bilbao F, Senut MC, Lamour Y. Age-related changes in parvalbumin- and GABA-immunoreactive cells in the rat septum. Neurobiol Aging. 1995;16:29–40. doi: 10.1016/0197-4580(95)80005-C. [DOI] [PubMed] [Google Scholar]
- 71.Bae EJ, Chen BH, Shin BN, Cho JH, Kim IH, Park JH, et al. Comparison of immunoreactivities of calbindin-D28k, calretinin and parvalbumin in the striatum between young, adult and aged mice, rats and gerbils. Neurochem Res. 2015;40:864–872. doi: 10.1007/s11064-015-1537-x. [DOI] [PubMed] [Google Scholar]
- 72.Benhamou L, Cohen D. Electrophysiological characterization of entopeduncular nucleus neurons in anesthetized and freely moving rats. Front Syst Neurosci. 2014;8:7. doi: 10.3389/fnsys.2014.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Miyamoto Y, Fukuda T. Immunohistochemical study on the neuronal diversity and three-dimensional organization of the mouse entopeduncular nucleus. Neurosci Res. 2015;94:37–49. doi: 10.1016/j.neures.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 74.Wallace ML, Saunders A, Huang KW, Philson AC, Goldman M, Macosko EZ, et al. Genetically distinct parallel pathways in the entopeduncular nucleus for limbic and sensorimotor output of the basal ganglia. Neuron. 2017;94(138–152):e135. doi: 10.1016/j.neuron.2017.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zheng YT, Yao YG, Xu L. Basic Biology and Disease Models of Tree Shrews. Yunnan, China: Yunnan Science and Technology Press; 2014. [Google Scholar]
- 76.Hubrecht R, Kirkwood J. The UFAW Handbook on the Care and Management of Laboratory and Other Research Animals (Eighth Edition) London: Universities Federation for Animal Welfare; 2010. pp. 262–275. [Google Scholar]
- 77.Flaherty AW, Graybiel AM. Motor and somatosensory corticostriatal projection magnifications in the squirrel monkey. J Neurophysiol. 1995;74:2638–2648. doi: 10.1152/jn.1995.74.6.2638. [DOI] [PubMed] [Google Scholar]
- 78.Hintiryan H, Foster NN, Bowman I, Bay M, Song MY, Gou L, et al. The mouse cortico-striatal projectome. Nat Neurosci. 2016;19:1100–1114. doi: 10.1038/nn.4332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Barbera G, Liang B, Zhang L, Gerfen CR, Culurciello E, Chen R, et al. Spatially compact neural clusters in the dorsal striatum encode locomotion relevant information. Neuron. 2016;92:202–213. doi: 10.1016/j.neuron.2016.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Masterson SP, Li J, Bickford ME. Synaptic organization of the tectorecipient zone of the rat lateral posterior nucleus. J Comp Neurol. 2009;515:647–663. doi: 10.1002/cne.22077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lee Y, Dawson VL, Dawson TM. Animal models of Parkinson’s disease: vertebrate genetics. Cold Spring Harb Perspect Med 2012, 2. [DOI] [PMC free article] [PubMed]
- 82.Tieu K. A guide to neurotoxic animal models of Parkinson’s disease. Cold Spring Harb Perspect Med. 2011;1:a009316. doi: 10.1101/cshperspect.a009316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Blandini F, Armentero MT. Animal models of Parkinson’s disease. FEBS J. 2012;279:1156–1166. doi: 10.1111/j.1742-4658.2012.08491.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.