Abstract
Previous studies have shown that electroacupuncture (EA) promotes recovery of motor function in Parkinson’s disease (PD). However the mechanisms are not completely understood. Clinically, the subthalamic nucleus (STN) is a critical target for deep brain stimulation treatment of PD, and vesicular glutamate transporter 1 (VGluT1) plays an important role in the modulation of glutamate in the STN derived from the cortex. In this study, a 6-hydroxydopamine (6-OHDA)-lesioned rat model of PD was treated with 100 Hz EA for 4 weeks. Immunohistochemical analysis of tyrosine hydroxylase (TH) showed that EA treatment had no effect on TH expression in the ipsilateral striatum or substantia nigra pars compacta, though it alleviated several of the parkinsonian motor symptoms. Compared with the hemi-parkinsonian rats without EA treatment, the 100 Hz EA treatment significantly decreased apomorphine-induced rotation and increased the latency in the Rotarod test. Notably, the EA treatment reversed the 6-OHDA-induced down-regulation of VGluT1 in the STN. The results demonstrated that EA alleviated motor symptoms and up-regulated VGluT1 in the ipsilateral STN of hemi-parkinsonian rats, suggesting that up-regulation of VGluT1 in the STN may be related to the effects of EA on parkinsonian motor symptoms via restoration of function in the cortico-STN pathway.
Keywords: Parkinson’s disease, Electroacupuncture, Motor behavior, Vesicular glutamate transporter 1
Introduction
Parkinson’s disease (PD) is a neurodegenerative disease characterized by a progressive loss of dopaminergic neurons in the substantia nigra pars compacta (SNc). The loss of dopaminergic neurons projecting from the SNc to the striatum is believed to be responsible for many of the motor deficits that occur in PD, including resting tremor, rigidity, bradykinesia, and postural instability/gait difficulties [1]. Classical medical treatment with levodopa and surgical options, such as deep brain stimulation (DBS), play the most important roles in alleviating the motor symptoms; however both are associated with inevitable side-effects and a financial burden [2, 3]. Electroacupuncture (EA), also known as peripheral electrical stimulation, is a non-invasive complementary therapy, which has long been used to alleviate parkinsonian symptoms in patients [4]. Our previous research has shown that high-frequency EA stimulation at 100 Hz ameliorates motor function in various animal models of PD by normalizing neurotransmitters in the basal ganglia [5–7], inhibiting neuroinflammatory responses [8, 9], reducing oxidative stress [10, 11], and increasing the levels of neurotrophic factors [4, 12]. However, the exact mechanisms by which EA alleviates parkinsonian motor symptoms have not been fully elucidated.
The basal ganglia circuit has been shown to play important roles in normal motor modulation, as well as in the pathogenesis and treatment of PD. The glutamatergic projection from the motor cortex to the subthalamic nucleus (STN) is known as the hyper-direct pathway. This pathway plays a critical role in the control of movement [13] and is a crucial part of the basal ganglia circuit [14]. In PD, where depletion of dopamine occurs, both electrophysiological discharges and the functional connectivity of the hyper-direct pathway are impaired [15–19]. Indeed, electrophysiological studies have revealed synchronized discharges and β oscillations in the motor cortex and STN of parkinsonian patients and non-human primate models of PD [15–17]. Although an increase in functional connectivity between the motor cortex and the STN has been reported in parkinsonian patients via functional magnetic resonance imaging [18], the exact changes in motor cortex–STN connectivity have been contradictory. Vesicular glutamate transporter 1 (VGluT1) is a marker of primary motor cortex (M1)–STN glutamatergic terminals [20, 21] and plays a critical role in the modulation of glutamate in the STN. VGluT1 is decreased in animal models of PD [21–23]. In our previous work, using herpes simplex virus-green fluorescent protein (HSV-GFP) tracing, we showed that M1–STN connectivity is significantly reduced in hemi-parkinsonian rats, as indicated by a decrease in HSV-GFP-positive neurons in the STN [23]. Clinically, the STN is a key target of DBS for the treatment of PD [24]. DBS of the STN has been shown to alleviate parkinsonism by reducing excessive synchronization in M1, via the M1–STN hyper-direct pathway [25]. Our preliminary work showed that EA alleviates abnormal M1 electrophysiological discharges (unpublished). Unfortunately, whether M1–STN connectivity is involved in the alleviation of parkinsonian motor symptoms by EA has not been clarified.
In the present study, we set out to address this question in a rat model of PD with unilateral 6-hydroxydopamine (6-OHDA) lesions in the medial forebrain bundle (MFB).
Materials and Methods
Animals
Adult male Sprague-Dawley rats (Vital River Laboratory Animal Technology Co. Ltd., Beijing, China), weighing 200 g–220 g, were housed under a 12 h light/dark cycle with ad libitum access to food and water in the laboratory animal center of Capital Medical University. All experiments were approved by the Animal Ethics Committee of Capital Medical University and were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Animal welfare was monitored daily throughout the entire study.
Unilateral 6-OHDA Lesions of the MFB
Rats were treated with 6-OHDA unilaterally, according to a previously published regimen [26] and displayed comparable hemi-parkinsonian motor signs. Briefly, rats were anesthetized by intraperitoneal injection of chloral hydrate (350 mg/kg) and placed on a stereotaxic instrument (David Kopf Instruments, Tujunga, CA). The right MFB was injected with 8 μg of 6-OHDA (5 μg/μL dissolved in 0.1% ascorbic acid; Sigma-Aldrich, St. Louis, MO) at 1 μL/min, using the following stereotaxic coordinates: antero-posterior (AP) −4.3 mm, medio-lateral (ML) −1.5 mm, and dorso-ventral (DV) −7.8 mm relative to bregma [27]. The right MFB in sham rats was injected with normal saline containing 0.1% ascorbic acid.
Apomorphine (APO)-Induced Rotation Test
The APO-induced rotation test was performed in automatic Rotameter bowls (Panlab/Harvard Apparatus, Holliston, MA). Two weeks after 6-OHDA lesion, APO (0.05 mg/kg dissolved in normal saline, Cat#: A4393, Sigma-Aldrich) was administered by subcutaneous injection. The net number of contralateral rotations (i.e., contralateral rotations minus ipsilateral rotations) was recorded for a 30-min period, beginning 5 min after APO injection. PD was confirmed in rats with a net number of contralateral rotations > 60 turns/30 min [26]. Rotation behavior was then measured at 2 weeks, 3 weeks, 4 weeks, 5 weeks, and 6 weeks after generation of the 6-OHDA lesions (that is 0 week, 1 week, 2 weeks, 3 weeks, and 4 weeks after beginning EA treatment).
Rotarod Test
A Rotarod apparatus (Panlab/Harvard Apparatus), with a rod 21 cm in height and 60 cm in diameter, was used to measure the balance and crude motor coordination of rats. The rats were trained on the apparatus for three days (at 8 rpm on day 1, 10 rpm on day 2, and 12 rpm on day 3) until they reached stable performance. On the day of the test, the rats were placed on the rod and the rotation speed was started at 4 rpm and then accelerated to 40 rpm within 2 min. The latency to falling from the rod was automatically recorded. Each rat underwent three trials and the latency was averaged [26]. Rats were tested on the Rotarod at 2 weeks, 3 weeks, 4 weeks, 5 weeks, and 6 weeks after 6-OHDA lesion (that is, 0 week, 1 week, 2 weeks, 3 weeks, and 4 weeks after beginning EA treatment).
EA Stimulation
Rats were randomly divided into four groups: a sham group (sham), a 6-OHDA-lesioned group (PD), a 6-OHDA-lesioned group followed by 0 Hz stimulation (0 Hz), and a 6-OHDA-lesioned group followed by 100 Hz EA stimulation (100 Hz). EA stimulation was administered on day 15 following 6-OHDA lesion, that is, 2 days after the first APO-induced rotation and Rotarod test as described previously [26]. In brief, two stainless steel needles (0.25 mm in diameter) were inserted into the acupoints BAIHUI (GV 20, at the midpoint between the auricular apices) and DAZHUI (GV 14, directly below the spinous process of the vertebra prominens) to a depth of 5 mm. The rats were then treated with bidirectional square-wave electrical pulses (0.2 ms duration, 100 Hz) generated by a Han’s acupoint nerve stimulator (HANS, Neuroscience Research Institute, Peking University) for 30 min/day, 6 days/week for 4 weeks. The stimulation intensity was increased stepwise from 1 mA to 2 mA and then to 3 mA, each lasting for 10 min. During EA, the rats were kept in a cage in an awake, unrestrained condition. Those treated with EA at 0 Hz underwent the same procedures, but no electrical pulses were delivered.
Immunohistochemistry
Immunohistochemistry was performed as described previously [26]. Briefly, rats were anesthetized and perfused trans-cardially. Brains were post-fixed and cut into coronal sections (40 μm) containing the striatum, SNc, and STN. Every sixth section through the striatum and SNc was used for free-floating immunostaining for tyrosine hydroxylase (TH) and every sixth section through the STN was used for VGluT1 immunostaining. Sections were incubated with mouse antibody against TH (1:2000; Cat#: AMAB91112, Sigma-Aldrich) or rabbit antibody against VGluT1 (1:2000; Cat#: ab104898, Abcam, Cambridge, UK) overnight at 4°C. After rinsing with PBS, sections were incubated with biotinylated goat secondary antibody against mouse (1:200; Cat#: PK-4002, Vector Laboratories, Youngstown, OH) or rabbit (1:200; Cat#: PK-4001, Vector Laboratories). The antibody was visualized with DAB solution. Sections were fixed and cover-slipped. Immunohistochemistry and other molecular experiments shown below were all performed after 4 weeks of EA stimulation.
Quantification of TH-Immunoreactive Neurons and Fibers and VGluT1-Immunoreactive Fibers
The number of TH-immunoreactive neurons was counted under a 20× objective lens using Stereo Investigator 8.0 software (MBF Bioscience, Williston, VT). The relative number of TH neurons was calculated as the ratio of the number on the lesioned side to that on the unlesioned side. Images of TH-immunostained striatal sections and VGluT1-immunostained STN sections were analyzed by light microscopy (DP71, Olympus, Tokyo, Japan). Immunoreactive optical densities of TH-positive fibers in the striatum and VGluT1-positive fibers in the STN were calculated using ImageJ software (NIH, Bethesda, MD). The relative optical densities of TH- and VGluT1-immunoreactive fibers were calculated as the ratio of optical density on the lesioned side to that on the unlesioned side.
Western Blotting
After anesthesia, rats were euthanized and the brain removed. Coronal slices (200 µm) containing the STN were cut on a vibrating microtome (VT1000S, Leica Biosystems, Mannheim, Germany) in ice-cold phosphate-buffered saline (PBS). The STN was microdissected under a stereomicroscope (MZ6, Leica Microsystems, Wetzlar, Germany) and immediately frozen on dry ice, as described previously [28]. The STN tissue was lysed in RIPA (Cat#: P00138, Beyotime Institute of Biotechnology, Haimen, China) containing protease inhibitors. Protein concentrations were determined using a BCA protein assay kit (Thermo Fisher Scientific, Waltham, MA). A total of 30 µg protein was loaded on a 10% SDS-PAGE gel. The proteins were transferred to nitrocellulose membranes, and incubated with a primary antibody overnight at 4°C. The primary antibodies were rabbit antibody against VGluT1 (1:2000; Cat#: ab104898, Abcam) and mouse antibody against glyceraldehyde-phosphate dehydrogenase (GAPDH) (1:5000; Cat#: G8795, Sigma-Aldrich). The secondary antibodies were IRDye 680-conjugated goat anti-rabbit IgG (1:200; Cat#: P/N 925-68071, Li-Cor Biosciences, Lincoln, NE) and IRDye 680-conjugated goat anti-mouse antibody (1:200; Cat#: P/N 925-68070, Li-Cor Biosciences). Immunoreactive bands were visualized using an Odyssey imaging system (Li-Cor Biosciences). The optical densities of protein bands were normalized to the density of GAPDH bands visualized on the same membrane. The relative optical density of VGluT1 bands was calculated as a ratio of optical density on the lesioned side to that on the unlesioned side.
M1–STN Projection Assessment by Trans-Synaptic Anterograde Tracing
HSV-GFP was used in anterograde trans-synaptic tracing to analyze the structural connectivity of the hyper-direct pathway from M1 to the STN in hemi-parkinsonian rats. HSV-GFP was made by inserting binary tandemly-connected GFP cassettes into the HSV-1 strain H129 genome, a gift from Dr. Luo [29]. After EA treatment, HSV-GFP (5 × 109 pfu/mL) in a volume of 500 nL, was injected into the right M1 at the following coordinates: AP +3.0 mm, ML −2.6 mm, and DV −2.2 mm. The animals were trans-cardially perfused and the brains were post-fixed for 72 h after injection. Serial coronal sections (40 µm) containing M1 (AP +3.0 mm) and the STN (AP −3.36 mm) were cut and collected for quantification of HSV-GFP-positive neurons. Cell nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI) (1:5000; Cat#: D8417, Sigma-Aldrich). To quantify HSV-GFP-positive neurons, every sixth section of M1 and every section of the STN were collected, as described by Beier [30]. Each slide was then imaged using a slide scanner (VS120, Olympus). The boundaries of M1 and the STN were based on the rat brain atlas, as described previously [27]. The neurons infected by HSV-GFP in M1 and the STN were considered as starter cells and outputs, respectively. The stereo counting of starter cells and outputs was carried out using Imaris 7.6 software (Bitplane, Bern, Switzerland). The number of outputs in the STN was normalized by the number of starter cells in the M1, as described by Beier [30].
Statistics
Statistical analysis was performed using Prism version 5.0 (GraphPad Software, La Jolla, CA). One-way analysis of variance (ANOVA) followed by Tukey’s post hoc test was performed for comparisons among three or more groups. Two-way ANOVA analyses were used to show the effects of groups, weeks of treatment, and the interaction of groups × weeks. Data are reported as mean ± SEM. P < 0.05 was considered statistically significant.
Results
Effects of EA on Motor Deficits and Dopaminergic Neurodegeneration in 6-OHDA-Lesioned Rats
The APO-induced rotation test was performed 2, 3, 4, 5, and 6 weeks after the generation of 6-OHDA lesions. As shown in Fig. 1A, unilateral injection of 6-OHDA into the MFB produced intense contralateral APO-induced rotation in the hemi-parkinsonian rats compared to the sham rats (P < 0.001). The 100 Hz EA, but not the 0 Hz EA, reduced the intensity of APO-induced rotation after 3 and 4 weeks (P < 0.05, P < 0.01 respectively). Sham rats showed no rotation behavior. Motor coordination was assessed using the Rotarod test (Fig. 1B). The results showed that 6-OHDA-lesioned hemi-parkinsonian rats developed coordination deficits before EA (P < 0.05). However, the latency was reversed in hemi-parkinsonian rats after 3 and 4 weeks of 100 Hz EA (P < 0.05). Similar to our previous report, 0 Hz EA had no effect on the motor deficits [26]. These data suggest that 100 Hz EA stimulation alleviates motor dysfunction in 6-OHDA-lesioned hemi-parkinsonian rats.
Fig. 1.
EA at 100 Hz significantly reversed the motor deficits in hemi-parkinsonian rats. A 100 Hz but not 0 Hz EA significantly reversed APO-induced rotational behavior in hemi-parkinsonian rats. B 100 Hz but not 0 Hz EA significantly reversed the latency to falling in the Rotarod test of hemi-parkinsonian rats. Data represent mean ± SEM (n = 10–12 per group; *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001 versus sham rats; #P < 0.05 and ##P < 0.01 versus 100 Hz EA-treated rats).
Immunohistochemical staining for TH was performed 6 weeks after the generation of MFB lesions (after 4 weeks of EA treatment) (Fig. 2). The optical density of TH-immunoreactive profiles in the striatum (Fig. 2A, C) and the number of TH-immunoreactive neurons in the SNc (Fig. 2B, D) were quantified. The data showed that 6-OHDA lesions in the right MFB induced a loss of TH-positive profiles by 94.9 ± 2.1% in the striatum on the lesioned (right) side compared with the sham rats (P < 0.0001). Similarly, 6-OHDA lesions in the MFB induced a loss of TH-positive neurons by 91.3 ± 1.5% in the SNc on the lesioned side compared with the sham rats (P < 0.0001). The 100 Hz EA stimulation had no effect on the degeneration of TH-positive profiles in the lesioned striatum or TH-positive neurons in the lesioned SNc of hemi-parkinsonian rats compared with untreated hemi-parkinsonian rats (P > 0.05). There was also no effect of 0 Hz EA on TH expression in the striatum or SNc (P > 0.05). This was consistent with our previous results [26].
Fig. 2.
EA at 100 Hz had no significant effect on TH expression levels in the SNc and striatum of hemi-parkinsonian rats. A, C Neither 100 Hz nor 0 Hz EA stimulation had significant effects on TH-positive profiles in the lesioned (right) striatum of hemi-parkinsonian rats as shown by immunohistochemical staining. B, D Neither 100 Hz nor 0 Hz EA stimulation had significant effects on TH-positive neurons in the lesioned SNc of hemi-parkinsonian rats as shown by immunohistochemical staining. Scale bars, 1 mm in A and 500 μm in B. Data represent mean ± SEM (n = 4/group; ****P < 0.0001 versus sham rats).
These results collectively support the contention that 100 Hz EA treatment can alleviate the abnormal motor symptoms triggered by 6-OHDA lesions, but has no impact on the nigro-striatal dopaminergic pathway.
Effects of EA on HSV-GFP-Positive Neurons in the STN of Hemi-Parkinsonian Rats
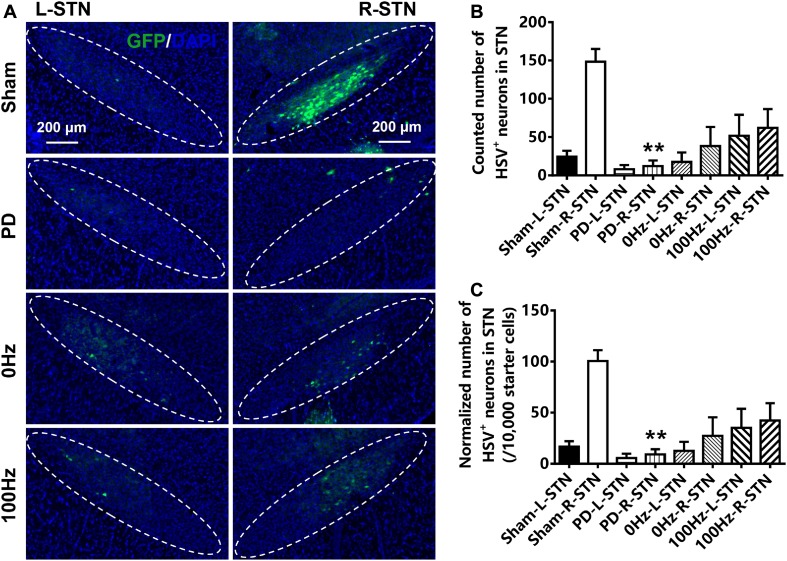
To investigate the effect of EA treatment on the connectivity of the M1–STN pathway, HSV-GFP tracing was performed and the HSV-GFP-positive neurons in M1 and the STN were counted. The number of HSV-GFP-positive neurons in the lesioned STN of hemi-parkinsonian rats (12.33 ± 7.13) was markedly lower than that in sham rats (148.4 ± 16.61) (P < 0.01, Fig. 3A, B). The normalized number of HSV-GFP neurons in the lesioned STN per number of M1 starter cells was also lower in the STN of hemi-parkinsonian rats (9.14 ± 5.10 per 10,000 starter cells) than in sham rats (100.5 ± 10.71 per 10,000 starter cells) (P < 0.01, Fig. 3A, C). There was no difference in the number of HSV-GFP-positive neurons in the lesioned STN of hemi-parkinsonian rats stimulated with 100 Hz EA for 4 weeks compared with untreated hemi-parkinsonian rats or hemi-parkinsonian rats treated with 0 Hz EA (P > 0.05).
Fig. 3.
EA at 100 Hz had no significant effect on the trans-synaptic infection of HSV-GFP neurons in the lesioned STN of hemi-parkinsonian rats. A Neither 100 Hz nor 0 Hz EA had significant effects on the trans-synaptic infection of HSV-GFP neurons in the lesioned STN of hemi-parkinsonian rats as shown by HSV-GFP tracing. B Neither 100 Hz nor 0 Hz EA stimulation had significant effects on the number of trans-synaptically infected HSV-GFP neurons in the lesioned STN of hemi-parkinsonian rats. C Neither 100 Hz nor 0 Hz EA stimulation had significant effects on the normalized number of trans-synaptically infected HSV-GFP neurons in the lesioned STN (the number of HSV-GFP neurons in the STN per 10,000 starter cells in the M1) of hemi-parkinsonian rats. Scale bars, 200 μm. Data represent mean ± SEM (n = 4/group; ****P < 0.0001 versus sham rats on the same ipsilateral (right) side).
Effect of EA on VGluT1 Expression in the Lesioned STN of Hemi-Parkinsonian Rats
To evaluate the linkage between possibly impaired glutamatergic terminals and the blunted M1–STN projection, intact glutamatergic terminals were investigated by detecting VGluT1 expression in the STN following 6-OHDA lesions and EA treatment. The VGluT1 levels in the lesioned STN were lower in hemi-parkinsonian rats than in sham rats as assessed by Western blotting (P < 0.01) (Fig. 4A, B) and immunohistochemistry (P < 0.05) (Fig. 4C, D). Treatment with 100 Hz EA significantly reversed the down-regulation of VGluT1 levels in the STN on the 6-OHDA-lesioned side, as revealed both by Western blotting (P < 0.01) and immunohistochemistry (P < 0.05), while stimulation with 0 Hz EA did not (Fig. 4). These data suggest that treatment with 100 Hz EA reverses the 6-OHDA-induced down-regulation of VGluT1 expression in the STN on the lesioned side.
Fig. 4.
EA at 100 Hz up-regulated VGluT1 expression levels in the lesioned STN of hemi-parkinsonian rats. A, B 100 Hz but not 0 Hz EA up-regulated the expression of VGluT1 protein in the lesioned STN of hemi-parkinsonian rats as shown by Western blotting. C, D 100 Hz but not 0 Hz EA up-regulated the expression of VGluT1 protein in the lesioned STN of hemi-parkinsonian rats as shown by immunohistochemical staining. Data represent mean ± SEM (n = 4/group; *P < 0.05, **P < 0.01 versus sham rats; #P < 0.05, ##P < 0.01 versus 100 Hz EA-treated rats).
Discussion
EA is one of the most widely-used complementary and alternative medicine treatment methods, like Chinese herbs [31, 32], and has long been used for the treatment of PD [4]. The behavioral and molecular findings from the present study demonstrated that high-frequency EA (100 Hz) at both GV 20 and GV 14 attenuated the motor deficiency in 6-OHDA-lesioned hemi-parkinsonian rats. More importantly, this treatment up-regulated the VGluT1 expression in the lesioned STN of hemi-parkinsonian rats. These results provide evidence that the effect of EA might be associated with increased expression of VGluT1 in the STN of 6-OHDA-lesioned hemi-parkinsonian rats.
Previous studies have confirmed that 6-OHDA lesions in the MFB induce a progressive loss of dopaminergic neurons in the SNc and progression of motor impairments in parkinsonian models [5]. Motor symptoms are manifested when striatal dopamine depletion exceeds 70% [1]. In the present study, ~91.3% of the dopaminergic neurons were lost in the SNc and the TH expression level was decreased by 94.9% in the ipsilateral striatum, 6 weeks after a 6-OHDA lesion was induced in the MFB. The 6-OHDA-lesioned hemi-parkinsonian rats showed a marked APO-induced rotation and a decreased latency to falling in the Rotarod test. Stimulation with 100 Hz EA, but not 0 Hz, ameliorated the APO-induced rotation and motor coordination as manifested by performance on the Rotarod test. These results are consistent with our previous studies [6, 12]. However, the mechanisms of 100 Hz EA treatment underlying this amelioration in motor function have not yet been fully clarified.
The restoration of dopamine levels in the striatum is thought to be a critical mechanism for the treatment of parkinsonian motor symptoms [2]. However, many studies have demonstrated motor ameliorations without restoration of striatal dopamine content. For example, DBS of the STN is a clinically effective therapy for parkinsonian motor symptoms, but does not affect dopamine levels in the striatum [33]. The STN is the primary projection target of motor cortical glutamatergic neurons [34, 35]. The glutamatergic projection to the STN from the motor cortex plays a critical role in the control of movement [14] and is known as the hyper-direct pathway. Within the glutamatergic terminals, VGluT1 is present on vesicle membranes [20] and is responsible for transporting glutamate into synaptic vesicles [36]. In PD, there are functional abnormalities in the M1–STN pathway [19]. Our previous study [23] and other studies [21, 22] have reported decreased cortical glutamatergic innervation of the STN in animal models of PD, as indicated by decreased VGluT1 expression levels in the STN of these models. In the present study, VGluT1 expression levels in the STN were also significantly down-regulated in parkinsonian rats, which may represent a decrease in the cortical glutamatergic terminals in the STN. We also used HSV-GFP tracing to assess the effect of EA on the connectivity of the M1–STN pathway in hemi-parkinsonian rats. HSV-GFP, a trans-synaptic anterograde viral tracer, was injected into M1, trans-synaptically transmitted to the STN, and infected STN neurons. The number of HSV-GFP-positive neurons in the STN represents the connectivity of the M1–STN pathway at the anatomical level. The M1–STN connectivity indicated by HSV-GFP-infected neurons in the STN has been shown to be significantly decreased in parkinsonian rats, which is also consistent with our previous study [23]. Results in the present study indicated that M1–STN connectivity is decreased in hemi-parkinsonian rats, and this may be related to the motor symptoms in these rats.
The STN and M1–STN pathway may be critical for the pathogenesis of parkinsonian motor symptoms and may be very important for the treatment of the symptoms. Research has shown that DBS of the STN can affect parkinsonian motor symptoms via the antidromic activation of M1 through the M1–STN pathway [37]. Other studies have shown that the effect of DBS of the STN might occur through the activation of afferent fibers of the STN from the motor cortex [38]. Our preliminary work also demonstrated that 100 Hz EA stimulation ameliorates the abnormal electrophysiological discharges in M1 (unpublished). Analysis of the VGluT1 content in the STN showed that the 100 Hz EA, but not the 0 Hz EA, markedly up-regulated the VGluT1 expression level in the lesioned STN of hemi-parkinsonian rats, although 100 Hz EA in the present study did not restore the M1–STN connectivity as indicated by HSV-GFP tracing. These results suggest that 100 Hz EA does not restore the neural connectivity of the M1–STN pathway, but up-regulates the VGluT1 expression levels in the STN of hemi-parkinsonian rats. This might be crucial for maintaining the balance of M1–STN pathway function in the basal ganglia circuit.
In conclusion, 100 Hz EA treatment alleviated the motor symptoms in 6-OHDA-lesioned hemi-parkinsonian rats, but did not elicit any significant changes in the M1–STN connectivity as shown by HSV-GFP tracing. Interestingly, the VGluT1 expression levels in the lesioned STN of hemi-parkinsonian rats were significantly up-regulated by 100 Hz EA treatment. The VGluT1 in the STN might play a role in the effectiveness of EA and might serve as a potential therapeutic target for PD.
Acknowledgements
This work was supported by the Beijing Municipal Science and Technology Commission (Z161100002616007), the National Key Research and Development Program (2016YFC1306300), the Major Program of the National Natural Science Foundation of China (81527901), and the Natural Science Foundation of Beijing Municipality (7082008).
Compliance with Ethical Standards
Conflict of interest
All authors claim that there are no conflicts of interest.
Contributor Information
Yong Wang, Email: kingwyong@sina.com.
Xiaomin Wang, Email: xmwang@ccmu.edu.cn.
References
- 1.Bernheimer H, Birkmayer W, Hornykiewicz O, Jellinger K, Seitelberger F. Brain dopamine and the syndromes of Parkinson and Huntington. Clinical, morphological and neurochemical correlations. J Neurol Sci. 1973;20:415–455. doi: 10.1016/0022-510X(73)90175-5. [DOI] [PubMed] [Google Scholar]
- 2.Dewey RB., Jr Management of motor complications in Parkinson’s disease. Neurology. 2004;62:S3–S7. doi: 10.1212/WNL.62.6_suppl_4.S3. [DOI] [PubMed] [Google Scholar]
- 3.Saint-Cyr JA, Taylor AE, Lang AE. Neuropsychological and psychiatric side effects in the treatment of Parkinson’s disease. Neurology. 1993;43:S47–S52. [PubMed] [Google Scholar]
- 4.Liang XB, Liu XY, Li FQ, Luo Y, Lu J, Zhang WM, et al. Long-term high-frequency electro-acupuncture stimulation prevents neuronal degeneration and up-regulates BDNF mRNA in the substantia nigra and ventral tegmental area following medial forebrain bundle axotomy. Brain Res Mol Brain Res. 2002;108:51–59. doi: 10.1016/S0169-328X(02)00513-2. [DOI] [PubMed] [Google Scholar]
- 5.Sun Z, Jia J, Gong X, Jia Y, Deng J, Wang X, et al. Inhibition of glutamate and acetylcholine release in behavioral improvement induced by electroacupuncture in parkinsonian rats. Neurosci Lett. 2012;520:32–37. doi: 10.1016/j.neulet.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 6.Jia J, Sun Z, Li B, Pan Y, Wang H, Wang X, et al. Electro-acupuncture stimulation improves motor disorders in Parkinsonian rats. Behav Brain Res. 2009;205:214–218. doi: 10.1016/j.bbr.2009.06.024. [DOI] [PubMed] [Google Scholar]
- 7.Jia J, Li B, Sun ZL, Yu F, Wang X, Wang XM. Electro-acupuncture stimulation acts on the basal ganglia output pathway to ameliorate motor impairment in Parkinsonian model rats. Behav Neurosci. 2010;124:305–310. doi: 10.1037/a0018931. [DOI] [PubMed] [Google Scholar]
- 8.Deng J, Lv E, Yang J, Gong X, Zhang W, Liang X, et al. Electroacupuncture remediates glial dysfunction and ameliorates neurodegeneration in the astrocytic alpha-synuclein mutant mouse model. J Neuroinflammation. 2015;12:103–116. doi: 10.1186/s12974-015-0302-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu XY, Zhou HF, Pan YL, Liang XB, Niu DB, Xue B, et al. Electro-acupuncture stimulation protects dopaminergic neurons from inflammation-mediated damage in medial forebrain bundle-transected rats. Exp Neurol. 2004;189:189–196. doi: 10.1016/j.expneurol.2004.05.028. [DOI] [PubMed] [Google Scholar]
- 10.Lv E, Deng J, Yu Y, Wang Y, Gong X, Jia J, et al. Nrf2-ARE signals mediated the anti-oxidative action of electroacupuncture in an MPTP mouse model of Parkinson’s disease. Free Radic Res. 2015;49:1296–1307. doi: 10.3109/10715762.2015.1067696. [DOI] [PubMed] [Google Scholar]
- 11.Wang HM, Pan YL, Xue B, Wang X, Zhao F, Jia J, et al. The antioxidative effect of electro-acupuncture in a mouse model of Parkinson’s disease. PLoS One. 2011;6:e19790. doi: 10.1371/journal.pone.0019790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liang XB, Luo Y, Liu XY, Lu J, Li FQ, Wang Q, et al. Electro-acupuncture improves behavior and upregulates GDNF mRNA in MFB transected rats. Neuroreport. 2003;14:1177–1181. doi: 10.1097/00001756-200306110-00015. [DOI] [PubMed] [Google Scholar]
- 13.Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–375. doi: 10.1016/0166-2236(89)90074-X. [DOI] [PubMed] [Google Scholar]
- 14.Nambu A, Tokuno H, Takada M. Functional significance of the cortico-subthalamo-pallidal ‘hyperdirect’ pathway. Neurosci Res. 2002;43:111–117. doi: 10.1016/S0168-0102(02)00027-5. [DOI] [PubMed] [Google Scholar]
- 15.Bergman H, Wichmann T, Karmon B, DeLong MR. The primate subthalamic nucleus. II. Neuronal activity in the MPTP model of parkinsonism. J Neurophysiol. 1994;72:507–520. doi: 10.1152/jn.1994.72.2.507. [DOI] [PubMed] [Google Scholar]
- 16.Moshel S, Shamir RR, Raz A, de Noriega FR, Eitan R, Bergman H, et al. Subthalamic nucleus long-range synchronization-an independent hallmark of human Parkinson’s disease. Front Syst Neurosci. 2013;7:79–92. doi: 10.3389/fnsys.2013.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shimamoto SA, Ryapolova-Webb ES, Ostrem JL, Galifianakis NB, Miller KJ, Starr PA. Subthalamic nucleus neurons are synchronized to primary motor cortex local field potentials in Parkinson’s disease. J Neurosci. 2013;33:7220–7233. doi: 10.1523/JNEUROSCI.4676-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baudrexel S, Witte T, Seifried C, von Wegner F, Beissner F, Klein JC, et al. Resting state fMRI reveals increased subthalamic nucleus-motor cortex connectivity in Parkinson’s disease. Neuroimage. 2011;55:1728–1738. doi: 10.1016/j.neuroimage.2011.01.017. [DOI] [PubMed] [Google Scholar]
- 19.Janssen ML, Temel Y, Delaville C, Zwartjes DG, Heida T, De Deurwaerdere P, et al. Cortico-subthalamic inputs from the motor, limbic, and associative areas in normal and dopamine-depleted rats are not fully segregated. Brain Struct Funct. 2016;221:1–13. doi: 10.1007/s00429-016-1351-5. [DOI] [PubMed] [Google Scholar]
- 20.Fremeau RT, Jr, Troyer MD, Pahner I, Nygaard GO, Tran CH, Reimer RJ, et al. The expression of vesicular glutamate transporters defines two classes of excitatory synapse. Neuron. 2001;31:247–260. doi: 10.1016/S0896-6273(01)00344-0. [DOI] [PubMed] [Google Scholar]
- 21.Mathai A, Ma Y, Pare JF, Villalba RM, Wichmann T, Smith Y. Reduced cortical innervation of the subthalamic nucleus in MPTP-treated parkinsonian monkeys. Brain. 2015;138:946–962. doi: 10.1093/brain/awv018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chu HY, McIver EL, Kovaleski RF, Atherton JF, Bevan MD. Loss of hyperdirect pathway cortico-subthalamic inputs following degeneration of midbrain dopamine neurons. Neuron. 2017;95:1306–1318. doi: 10.1016/j.neuron.2017.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang YY, Wang Y, Jiang HF, Liu JH, Jia J, Wang K, et al. Impaired glutamatergic projection from the motor cortex to the subthalamic nucleus in 6-hydroxydopamine-lesioned hemi-parkinsonian rats. Exp Neurol. 2017;300:135–148. doi: 10.1016/j.expneurol.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 24.Limousin P, Krack P, Pollak P, Benazzouz A, Ardouin C, Hoffmann D, et al. Electrical stimulation of the subthalamic nucleus in advanced Parkinson’s disease. N Engl J Med. 1998;339:1105–1111. doi: 10.1056/NEJM199810153391603. [DOI] [PubMed] [Google Scholar]
- 25.Malkki H. Parkinson disease: deep brain stimulation might alleviate parkinsonism by reducing excessive synchronization in primary motor cortex. Nat Rev Neurol. 2015;11:246. doi: 10.1038/nrneurol.2015.62. [DOI] [PubMed] [Google Scholar]
- 26.Sun M, Wang K, Yu Y, Su WT, Jiang XX, Yang J, et al. Electroacupuncture alleviates depressive-like symptoms and modulates BDNF signaling in 6-hydroxydopamine rats. Evid Based Complement Alternat Med. 2016;2016:7842362. doi: 10.1155/2016/7842362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Watson C, Paxinos G. The Rat Brain in Stereotaxic Coordinates 6th Edition. Academic Press, 2007: 1–463.
- 28.Swanger SA, Vance KM, Pare JF, Sotty F, Fog K, Smith Y, et al. NMDA receptors containing the GluN2D subunit control neuronal function in the subthalamic nucleus. J Neurosci. 2015;35:15971–15983. doi: 10.1523/JNEUROSCI.1702-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zeng WB, Jiang HF, Gang YD, Song YG, Shen ZZ, Yang H, et al. Anterograde monosynaptic transneuronal tracers derived from herpes simplex virus 1 strain H129. Mol Neurodegener. 2017;12:38–54. doi: 10.1186/s13024-017-0179-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beier KT, Steinberg EE, DeLoach KE, Xie S, Miyamichi K, Schwarz L, et al. Circuit architecture of VTA dopamine neurons revealed by systematic input-output mapping. Cell. 2015;162:622–634. doi: 10.1016/j.cell.2015.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tao K, Wang B, Feng D, Zhang W, Lu F, Lai J, et al. Salidroside protects against 6-hydroxydopamine-induced cytotoxicity by attenuating ER stress. Neurosci Bull. 2016;32:61–69. doi: 10.1007/s12264-015-0001-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao Q, Yang X, Cai D, Ye L, Hou Y, Zhang L, et al. Echinacoside protects against MPP(+)-induced neuronal apoptosis via ROS/ATF3/CHOP pathway regulation. Neurosci Bull. 2016;32:349–362. doi: 10.1007/s12264-016-0047-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Limousin P, Pollak P, Benazzouz A, Hoffmann D, Le Bas JF, Broussolle E, et al. Effect of parkinsonian signs and symptoms of bilateral subthalamic nucleus stimulation. Lancet. 1995;345:91–95. doi: 10.1016/S0140-6736(95)90062-4. [DOI] [PubMed] [Google Scholar]
- 34.DeLong MR, Wichmann T. Circuits and circuit disorders of the basal ganglia. Arch Neurol. 2007;64:20–24. doi: 10.1001/archneur.64.1.20. [DOI] [PubMed] [Google Scholar]
- 35.Mink JW, Thach WT. Basal ganglia intrinsic circuits and their role in behavior. Curr Opin Neurobiol. 1993;3:950–957. doi: 10.1016/0959-4388(93)90167-W. [DOI] [PubMed] [Google Scholar]
- 36.Gasnier B. The loading of neurotransmitters into synaptic vesicles. Biochimie. 2000;82:327–337. doi: 10.1016/S0300-9084(00)00221-2. [DOI] [PubMed] [Google Scholar]
- 37.Li Q, Ke Y, Chan DC, Qian ZM, Yung KK, Ko H, et al. Therapeutic deep brain stimulation in Parkinsonian rats directly influences motor cortex. Neuron. 2012;76:1030–1041. doi: 10.1016/j.neuron.2012.09.032. [DOI] [PubMed] [Google Scholar]
- 38.Gradinaru V, Mogri M, Thompson KR, Henderson JM, Deisseroth K. Optical deconstruction of parkinsonian neural circuitry. Science. 2009;324:354–359. doi: 10.1126/science.1167093. [DOI] [PMC free article] [PubMed] [Google Scholar]