Abstract
The neurocircuitries that constitute the cortico-striato-thalamo-cortical (CSTC) circuit provide a framework for bridging gaps between neuroscience and executive function in attention deficit hyperactivity disorder (ADHD), but it has been difficult to identify the mechanisms for regulating emotional problems from the understanding of ADHD comorbidity with disruptive behavior disorders (DBD). Research based on “cool” and “hot” executive functional theory and the dual pathway models, which are thought of as applied response inhibition and delay aversion, respectively, within the neuropsychological view of ADHD, has shed light on emotional responding before and after decontextualized stimuli, while CSTC circuit-related domains have been suggested to explain the different emotional symptoms of ADHD with or without comorbid DBD. This review discusses the role of abnormal connections in each CSTC circuit, especially in the emotion circuit, which may be responsible for targeted executive dysfunction at the neuroscience level. Thus, the two major domains – abstract thinking (cool) and emotional trait (hot) – trigger the mechanism of onset of ADHD.
Keywords: Attention deficit hyperactivity disorder, Disruptive behavior disorder, Emotion, Executive function
Introduction
Attention deficit hyperactivity disorder (ADHD) is one of the most common neurodevelopmental disorders in childhood with an overall prevalence of 6.26% in China [1]. It occurs more than twice as often in boys than in girls aged 4–17 [2]. The major clinical symptoms of ADHD are developmentally inappropriate and impaired inattention, motor hyperactivity, and impulsivity, with difficulties often continuing into adulthood [3]. The continuously increasing trend of ADHD in recent years plays an important role in the mental health of children and adolescents. Because of the pervasiveness of ADHD, comorbidity with childhood-onset neurodevelopmental disorders and psychiatric disorders is substantial. Neither the high rate of morbidity nor the comorbidity has received enough attention. Therefore, the phenomena of low treatment rate and high prevalence are regarded as serious public health problems worldwide.
The pathogenesis of ADHD is still unknown, and current diagnosis is based mainly on the medical history of children provided by their parent’s subjective expressions, rather than sufficient objective biomarkers. However, not until ADHD was catalogued as a neurodevelopmental disorder in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-V) [4] published in 2013, did the neuroscience research accelerate with a clear direction.
Focus on the Progress of Neuroscience in ADHD
The focus of neuropsychiatric research is not merely on the discussion of semiology that is emphasized in psychiatry, but also on the neuroscience perspective and disease phenotype. It is thought that the symptoms originate from improper connections in neuronal networks [5], which was a milestone in adding a new dimension of disease classification. Compelling evidence for neuroplasticity is the fact that children’s brains before maturity can establish bypass connections and compensate for impaired function through the differentiation and emergent responses of neuronal networks. For example, children with dyslexia can build extremely strong compensatory functions by constructing bypass connections, such that a new neurobiological compensatory pathway takes over language processing and motor activity instead of the primary language center [6]. Such processes hardly occur in adulthood, suggesting that less automatic processing of these structures is established in learning of a second language later in life [7].
According to the general pattern of development of the nervous system, the perception of young people is more sensitive than that of adults, as perception declines with age [8]. Cortico-striatal perception is easily damaged by separating cortico-cortical from cortico-striatal circuits within the fronto-striatal circuits [9]. Furthermore, myelination of the primary nerve occurs ahead of the senior nerve center. White matter maturation, including myelination, starts prenatally and progresses in an orderly manner during infancy and childhood from posterior-to-anterior, inferior-to-superior, and central-to-peripheral regions [10]. This raises the question of whether organic brain disease is responsible for the dysfunction of systems that require compensation by bypass connections, or mental retardation (MR) leads to permanently incomplete neuronal connections. The former may establish an abnormal channel connected to the cause of a variety of symptoms in ADHD, but the latter tends to be a state of disinhibition so ADHD symptoms in patients with MR may be less responsive to medical treatment than in patients without MR [11].
Cortico-Striato-Thalamo-Cortical (CSTC) Circuit-Related Symptoms with ADHD Traits
With the development of neuroscience, it is generally accepted that ADHD results from neurofibrillary dysplasia [12] or atypical neuronal development [13]. Complicated cognitive and emotional adjustments are integrated by the executive control system. It is no longer believed that a change in a specific brain functional region causes a corresponding symptom, but each problem in the integration process can cause a chain-reaction in the corresponding neuronal circuit [14].
By way of prefrontal hypofunction and an abnormal cortico-striatal pathway, the CSTC circuit has been associated with ADHD [15]. More focus on the neurocircuitry of ADHD is needed for rapid progress in biomedicine. Neuronal signals are projected from pyramidal neurons of the prefrontal cortex in the CSTC circuit into the striatum, and then to the thalamus. The signals in the CSTC circuit through the striatum are connected to a specific group of striatal neurons, then reach the thalamus, and finally return to the original area of prefrontal cortex as feedback loop, a shorter one from the cortico-striatal pathway and a longer one from the CSTC circuit [16]. Five CSTC circuits are described in Stahl’s Essential Psychopharmacology [17]: a sustained attention circuit originating in the dorsolateral prefrontal cortex (DLPFC) projecting to the superior caudate nucleus, called the dorsolateral prefrontal cortico-striato-thalamic-cortical circuit [18]; an emotion circuit originating in the ventrolateral prefrontal cortex (VLPFC) projecting to the nucleus accumbens, called the ventrolateral prefrontal cortico-striato-thalamic-cortical circuit [19]; a selective attention circuit originating in the dorsal anterior cingulate cortex (DACC) projecting to the inferior striatum, called the dorsal anterior cingulate cortico-striato-thalamic-cortical circuit [20]; a hyperactivity circuit originating in the motor cortex projecting to the putamen or lateral lenticular nucleus of the striatum, called the motor cortico-striato-thalamic-cortical circuit [21]; and an impulsivity/compulsivity circuit originating in the orbitofrontal cortex (OFC) projecting to the inferior caudate nucleus, called the orbitofrontal cortico-striato-thalamic-cortical circuit [22].
Neuropsychological Mechanism of the Emotion Circuit in ADHD and Disruptive Behavior Disorder (DBD)
The above four circuits, except for the emotion circuit, are related to almost every major clinical diagnosis of ADHD. Not only have their functions been found to be correlated with each cerebral region involved in the CSTC circuit of ADHD, but also subcortical structures, such as the striatum, play an important role in the transmitting process of pyramidal neuron by over-suppression of excitatory network bursts and under-suppression of inhibitory network bursts in the circuit [23]. Meanwhile, it was declined that thalamus, as the subcortical of the CSTC circuit of ADHD, is involved in motor planning and attentional processes [24], and cognitive functions [25]. Huge neural networks will be linked by many times of synaptic transmissions of each pyramidal neuron to subcortical structures. The result affects cognitive processing significantly in individuals to supervise and control their own cognition and behavior, which is called executive function (EF), providing compelling evidence that the transition to adult-level response inhibition depends on the refinement and strengthening of integration among specialized networks throughout adolescence [26].
Although the emotional dysregulation from the emotion circuit seems to be unrelated to the diagnostic standard of ADHD, it has been found that pathogenesis of the emotion circuit is also closely related to ADHD [27]. Because of the consequences of its impairment (25%–45% in childhood and 30%–70% in adulthood), emotional dysregulation is regarded as an important clinical feature of ADHD and an associated feature supporting its diagnosis in DSM-V [28]. ADHD patients with emotional dysregulation are generally unable to control and convert their emotions to a proper intensity with proper context-awareness, which makes them more likely to be frightened and frustrated by sudden emotional changes. The increased intensity of emotional network activation is associated with the deficit of emotional control in ADHD, and also indicates that patients with ADHD are more sensitive to emotional stimuli [29]. As a result, both the “cool” and “hot” EFs are affected, especially the “hot” EF, discussed below.
It has been found that the emotion circuit is responsible for serious emotional disorders such as DBD (conduct disorder and/or oppositional defiant disorder) with callous-unemotional traits [30]. The emotion circuit may be responsible for high comorbidity between ADHD and DBD. ADHD and DBD are also evaluated as a homogeneous variable in the design of the Mini International Neuropsychiatric Interview for Children and Adolescents [31]. Decreased activity of the VLPFC is associated with greater severity of symptoms of EF in youths with ADHD [32] and DBD [33], suggesting that analyses of neuronal networks might provide objective diagnostic neuroimaging biomarkers for ADHD with comorbid DBD.
The EF Model of ADHD with Comorbid DBD
EF is the cognitive control required to complete a series of cognitive tasks and behavior; it is a process of problem-solving supervised and controlled by self-consciousness and behavior. Attention, memory, planning, self-regulation, and other core cognitive and behavioral operations comprise what we know as EF [34]. Inhibition and metacognition are two broad domains of EF. Inhibition includes the ability to inhibit cognitive, verbal, motor, and emotional activities. Deficits in inhibition contribute to the impaired development of four aspects of EF in the domain of metacognition: planning and problem-solving, verbal working memory, nonverbal working memory, and emotional self-regulation [35].
In neuropsychological studies, deficits in response inhibition and emotion self-regulation contribute to dominant damage as the core symptom of ADHD [36, 37]. Many dysfunctions of cognition and behavior are due to deficits in self-control and sustained attention. The EF model is divided into two theoretical parts, “cool” EF and “hot” EF [38]. It has been suggested that the current dual pathway model of ADHD involving both the attention and emotion circuits, which correspond to “cool” and “hot” EF, respectively, might not be independent of the process of neurocognition. However, Posner et al. [39] have demonstrated that patients with ADHD have reduced connectivity in the two neuronal circuits underlying executive attention of “cool” EF and emotional regulation of “hot” EF. Decontextualization between these two EFs and the behavioral correlates of ADHD demonstrate that reduced connectivity of executive attention in the attention circuit is associated with executive inattention but not with emotional lability, while reduced connectivity of emotional regulation in the emotion circuit is associated with emotional lability but not with executive inattention. In the real world, however, “cool” EF cannot be separated from “hot” EF except that researcher adds a delay of gratification and affective decision-making tasks to distinguish hot-affection from cool-cognition in psychological experiments to clarify these two circuits [40].
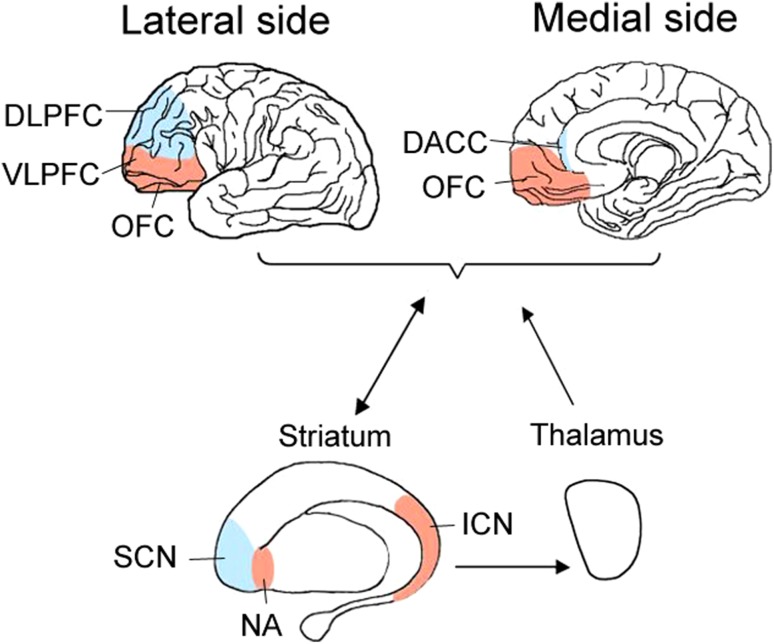
“Cool” EF is both a top-down executive control and an absolute process of cognitive control; it is required to solve abstract and contextualized problems including response inhibition, adaption, task-switching, or change of strategy [34]. The advanced cortical neurons originating from the DLPFC and DACC to the superior caudate nucleus (SCN) and thalamus downstream are shown in Fig. 1 (blue color). Top-down dysfunction constitutes a gradient extending from mainly non-emotional top-down processes to mainly emotional regulatory processes in ADHD [41].
Fig. 1.
The locations of EF models in the CSTC circuits in ADHD. Differential development of neural circuitry subserves “cool” EF (blue color) including the DLPFC and the DACC, and “hot” EF (red color) including OFC and the VLPFC, leading to abnormal downlink to the substructure of striatum (SCN or NA/ICN) and thalamus.
“Hot” EF is both top-down executive control and bottom-top feedback of cognitive control including the motivation, reward, and cognitive processes involved in emotional responses and emotional decision-making. Children with ADHD who are probably less sensitive to reinforcement tend to prefer immediate over delayed rewards. Their response to a reward might attenuate more rapidly than that of their unaffected peers [28]. The above process is called delay aversion in “hot” EF of ADHD. It has been debated whether brain processes associated with emotion can be separated from those associated with cognition. Since cognition is composed of emotional and non-emotional information, the processes interact [42]. Any emotional regulatory task contains non-emotional processes, in agreement with previous theoretical constructs of attention in distinguishing “cool” EF regulating non-emotional processes from “hot” EF regulating emotional processes [41]. The cortical neurons originating from the VLPFC and the OFC to the nucleus accumbens (NA) or the inferior caudate nucleus (ICN) and thalamus downstream are shown in Fig. 1 (red color). Bottom-up feedback is more important than upstream control in “hot” EF [43]. Altered reward-processing in aggressive behaviors can be explained by fronto-accumbal white matter connectivity and cortical thickness in the OFC of “hot” EF [44].
Response inhibition is classic “cool” EF that requires individuals to distinguish a target stimulus from a non-target stimulus at the same time. This reaction depends on the sustained attention circuit and the selective attention circuit. From Barkley’s theory of the primacy of inhibitory control in ADHD [45], the Stroop test mainly involves response inhibition. Transcranial direct-current stimulation significantly increases the activity of the DLPFC to provide better control of response inhibition [46]. The Stroop test is the classical paradigm of response inhibition. The Stroop effect was originally used to measure the level of response inhibition, but modified follow-up versions tend to make more guidance, such as standardized Stroop, Golden Stroop, and emotional Stroop. The emotional Stroop test was designed on the basis of advantages over the original version. The interference terms of emotional words not only assess the function of response inhibition, but emotional responses can also be recognized simultaneously as a tool for measuring “hot” EF, meanwhile, there are interaction effects on the inhibitory control and emotional regulation in youths [47].
A meta-analysis of neuroimaging studies has shown that consistent brain activation patterns of healthy participants that span the DLPFC and the DACC are more strongly interrelated to emotionally-salient stimuli during cognitive conflict trials of the emotional Stroop test than emotionally-neutral stimuli. These regions are consistently activated on intense but not on mild emotional interference trials [48]; this is the so-called slow effect of the emotional Stroop effect. The symptom of emotional stimulation is sustained after the first stimulus is presented, and affects the identification of subsequent stimuli [49]. Elevated behavioral and emotional dysregulation shows inverse activity in the VLPFC and DACC, and the subcortical amygdala [50]. Positive emotional experiences activate the ventromedial prefrontal cortex (VMPFC), and the neurocircuitry of the VMPFC and amygdala have been associated with response inhibition in emotional disturbance using fMRI studies during the emotional Stroop test. The decreased activity of the emotion circuit aggravates the severity of ADHD with an overlap between emotional responding and response inhibition, resulting in callous-unemotional traits and destructive behavior in youths with ADHD [30]. The involvement of reward-related emotional responding regions (including the OFC, VLPFC, VMPFC, ventral striatum, and thalamus) adds an impulsivity/compulsivity profile to the “hot” EF of ADHD [51], while decision-making of the “hot” EF in youths with DBD reveals reduced reward response regions (including the VMPFC, OFC, caudate, and thalamus) [52]. This is supposed to be the theoretical mechanism of ADHD with comorbid DBD in the EF.
Thus, the EF model of ADHD and its brain structure are related to the dual pathway model of ADHD [53]. Response inhibition and delay-related deficits represent independent neuropsychological components [54]. The response inhibition deficit results from the sustained attention circuit and the delay-related deficit from the emotion circuit, which is respectively familiar from the “cool” EF and “hot” EF in another theoretical system noted above. In basic studies of the dual pathway model, evidence has shown that cognitive and behavioral dysregulation comes from the response inhibition deficit, while the shortened delay gradient and inadequate delay gratification come from the delay-related deficit [54]. If disruptive behavior is chosen as the only factor to be discussed, the high comorbidity of ADHD and DBD may be explainable. More than half of youths with ADHD characterized by hyperactivity/impulsivity and oppositional defiant disorder are strongly associated with a general disruptive behavior factor, rather than their specific inattention, specific hyperactivity/impulsivity, and specific oppositional defiant disorder factors [55]. The above reveals the specific reason for the decrease in processing ability after decision-making conflict [56].
The congruence of different theories is based on pathology in the regions DLPFC, DACC, VLPFC, OFC, striatum, and thalamus. The connections between these regions are regarded as the foundation of the pathogenic mechanism [57]. Therefore, the phenomena of response inhibition deficit and delay-related deficit play important roles in the independent EF component in ADHD [58]. “Hot” EF-related CSTC circuits are likely to be associated with the emotional behavior problems of ADHD, and there is reason to infer that executive dysfunction in ADHD may be associated with the emotion circuit and caused by abnormal activity.
Perspectives
Many fMRI studies have attempted to determine whether the CSTC circuits differ statistically between an ADHD group and a healthy control group and have reached conclusions that provide information about disease and health. However, such studies ignored comparisons among neuropsychiatric diseases, and, consequently, the final conclusions do not help to distinguish between diseases. Therefore, the diversity and specificity of diseases cannot be explained, given the subjectivity and uncertainty of the differential diagnoses. Moreover, single-subject studies, the most common neuroscience method, provide limited results. It is difficult to find neuroscience research from the perspective of psychiatric traits rather than the classification of diseases. We believe that increasing numbers of evidence-based findings will be available from multiple interdisciplinary studies in neuroscience with the intensive study of ADHD. Interdisciplinary studies are not commonly seen in China, such as neuroimaging, neuropsychology, neurobiology, and computational psychiatry [59]. Based on the existing theory, it will be a breakthrough if the integration of present resources can optimize the allocation of resources within their scope. The findings may provide biological markers of ADHD, which are expected to provide strategies for future therapeutic targets of the neurobiological mechanisms.
Acknowledgements
The authors thank Liqiong Huang for proofreading the final edition. This review was supported by a Project of Shanghai Municipal Health and Family Planning Commission ( 201540114), a Key Specialty Project of Shanghai Municipal Health and Family Planning Commission grant for Child Psychiatry (ZK2015B01) and a Research Project of the Shanghai Changning Health and Family Planning Commission grant (20164Y013).
Compliance with Ethical Standards
Conflict of interest
All authors claim that there are no conflicts of interest.
References
- 1.Wang T, Liu K, Li Z, Xu Y, Liu Y, Shi W, et al. Prevalence of attention deficit/hyperactivity disorder among children and adolescents in China: a systematic review and meta-analysis. BMC Psychiatry. 2017;17:32. doi: 10.1186/s12888-016-1187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albert M, Rui P, Ashman JJ. Physician office visits for attention-deficit/hyperactivity disorder in children and adolescents aged 4-17 years: United States, 2012-2013. Nchs Data Brief. 2017;269:1–8. [PubMed] [Google Scholar]
- 3.Thapar A, Cooper M. Attention deficit hyperactivity disorder. Lancet. 2016;387:1240–1250. doi: 10.1016/S0140-6736(15)00238-X. [DOI] [PubMed] [Google Scholar]
- 4.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5®). American Psychiatric Pub, 2013.
- 5.Li Y, Fang H, Zheng W, Qian L, Xiao Y, Wu Q, et al. A fiber tractography study of social-emotional related fiber tracts in children and adolescents with autism spectrum disorder. Neurosci Bull. 2017;33:722–730. doi: 10.1007/s12264-017-0155-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mohamad NB, Lee KY, Mansor W, Mahmoodin Z, Fadzal CW, Amirin S. EEG-based time and spatial interpretation of activation areas for relaxation and words writing between poor and capable dyslexic children. Conf Proc IEEE Eng Med Biol Soc. 2015;2015:4757–4760. doi: 10.1109/EMBC.2015.7319457. [DOI] [PubMed] [Google Scholar]
- 7.Weber K, Luther L, Indefrey P, Hagoort P. Overlap and differences in brain networks underlying the processing of complex sentence structures in second language users compared with native speakers. Brain Connect. 2016;6:345–355. doi: 10.1089/brain.2015.0383. [DOI] [PubMed] [Google Scholar]
- 8.Lester AW, Moffat SD, Wiener JM, Barnes CA, Wolbers T. The aging navigational system. Neuron. 2017;95:1019–1035. doi: 10.1016/j.neuron.2017.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Szalisznyo K, Silverstein D, Teichmann M, Duffau H, Smits A. Cortico-striatal language pathways dynamically adjust for syntactic complexity: A computational study. Brain Lang. 2017;164:53–62. doi: 10.1016/j.bandl.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 10.Krogsrud SK, Fjell AM, Tamnes CK, Grydeland H, Mork L, Due-Tønnessen P, et al. Changes in white matter microstructure in the developing brain—A longitudinal diffusion tensor imaging study of children from 4 to 11 years of age. Neuroimage. 2016;124:473–486. doi: 10.1016/j.neuroimage.2015.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hassler F, Thome J. Mental retardation and ADHD. Z Kinder Jugendpsychiatr Psychother. 2012;40:83–93. doi: 10.1024/1422-4917/a000155. [DOI] [PubMed] [Google Scholar]
- 12.Poot M. Connecting the CNTNAP2 networks with neurodevelopmental disorders. Mol Syndromol. 2015;6:7–22. doi: 10.1159/000371594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pitzianti M, Grelloni C, Casarelli L, D’Agati E, Spiridigliozzi S, Curatolo P, et al. Neurological soft signs, but not theory of mind and emotion recognition deficit distinguished children with ADHD from healthy control. Psychiatry Res. 2017;256:96–101. doi: 10.1016/j.psychres.2017.06.029. [DOI] [PubMed] [Google Scholar]
- 14.Zhu Y, Yang D, Ji W, Huang T, Xue L, Jiang X, et al. The Relationship between neurocircuitry dysfunctions and attention deficit hyperactivity disorder: a review. Biomed Res Int. 2016;2016:3821579. doi: 10.1155/2016/3821579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Posner J, Park C, Wang Z. Connecting the dots: a review of resting connectivity MRI studies in attention-deficit/hyperactivity disorder. Neuropsychol Rev. 2014;24:3–15. doi: 10.1007/s11065-014-9251-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hauser TU, Fiore VG, Moutoussis M, Dolan RJ. Computational psychiatry of ADHD: neural gain impairments across marrian levels of analysis. Trends Neurosci. 2016;39:63–73. doi: 10.1016/j.tins.2015.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stahl SM. Stahl’s Essential Psychopharmacology: Neuroscientific Basis and Practical Applications. Cambridge University Press, 2008.
- 18.Norman LJ, Carlisi CO, Christakou A, Cubillo A, Murphy CM, Chantiluke K, et al. Shared and disorder-specific task-positive and default mode network dysfunctions during sustained attention in paediatric Attention-Deficit/Hyperactivity Disorder and obsessive/compulsive disorder. Neuroimage Clin. 2017;15:181–193. doi: 10.1016/j.nicl.2017.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qu Y, Telzer EH. Cultural differences and similarities in beliefs, practices, and neural mechanisms of emotion regulation. Cultur Divers Ethnic Minor Psychol. 2017;23:36–44. doi: 10.1037/cdp0000112. [DOI] [PubMed] [Google Scholar]
- 20.Bonath B, Tegelbeckers J, Wilke M, Flechtner HH, Krauel K. Regional gray matter volume differences between adolescents With ADHD and typically developing controls: further evidence for anterior cingulate involvement. J Atten Disord. 2016;8:1087054715619682. doi: 10.1177/1087054715619682. [DOI] [PubMed] [Google Scholar]
- 21.Baghdadi G, Towhidkhah F, Rostami R. A mathematical and biological plausible model of decision-execution regulation in “Go/No-Go” tasks: Focusing on the fronto-striatal-thalamic pathway. Comput Biol Med. 2017;86:113–128. doi: 10.1016/j.compbiomed.2017.05.014. [DOI] [PubMed] [Google Scholar]
- 22.Ho NF, Chong JS, Koh HL, Koukouna E, Lee TS, Fung D, et al. Intrinsic affective network is impaired in children with attention-deficit/hyperactivity disorder. PLoS One. 2015;10:e0139018–30306. doi: 10.1371/journal.pone.0139018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhong P, Liu W, Yan Z. Aberrant regulation of synchronous network activity by the attention-deficit/hyperactivity disorder-associated human dopamine D4 receptor variant D4.7 in the prefrontal cortex. J Physiol. 2016;594:135–147. doi: 10.1113/JP271317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reynolds JE, Billington J, Kerrigan S, Williams J, Elliott C, Winsor AM, et al. Mirror neuron system activation in children with developmental coordination disorder: A replication functional MRI study. Res Dev Disabil. 2017;20:30302–30306. doi: 10.1016/j.ridd.2017.11.012. [DOI] [PubMed] [Google Scholar]
- 25.Wu W, Cui L, Fu Y, Tian Q, Liu L, Zhang X, et al. Sleep and cognitive abnormalities in acute minor thalamic infarction. Neurosci Bull. 2016;32:341–348. doi: 10.1007/s12264-016-0036-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marek S, Hwang K, Foran W, Hallquist MN, Luna B, Zhang X, et al. The contribution of network organization and integration to the development of cognitive control. PLoS Biol. 2015;13:e1002328. doi: 10.1371/journal.pbio.1002328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Musser ED, Nigg JT. Emotion dysregulation across emotion systems in attention deficit/hyperactivity disorder. J Clin Child Adolesc Psychol. 2017;19:1–13. doi: 10.1080/15374416.2016.1270828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gallo EF, Posner J. Moving towards causality in attention-deficit hyperactivity disorder: overview of neural and genetic mechanisms. Lancet Psychiatry. 2016;3:555–567. doi: 10.1016/S2215-0366(16)00096-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Richard-Lepouriel H, Etain B, Hasler R, Bellivier F, Gard S, Kahn JP, et al. Similarities between emotional dysregulation in adults suffering from ADHD and bipolar patients. J Affect Disord. 2016;198:230–236. doi: 10.1016/j.jad.2016.03.047. [DOI] [PubMed] [Google Scholar]
- 30.Hwang S, Nolan Z, White S, Williams W, Sinclair S, Blair R. Dual neurocircuitry dysfunctions in disruptive behavior disorders: emotional responding and response inhibition. Psychol Med 2016: 1–12. [DOI] [PMC free article] [PubMed]
- 31.Sheehan DV, Sheehan KH, Shytle RD, Janavs J, Bannon Y, Rogers JE, et al. Reliability and validity of the Mini International Neuropsychiatric Interview for Children and Adolescents (MINI-KID) J Clin Psychiatry. 2010;71:313–326. doi: 10.4088/JCP.09m05305whi. [DOI] [PubMed] [Google Scholar]
- 32.Hart H, Chantiluke K, Cubillo AI, Smith AB, Simmons A, Brammer MJ, et al. Pattern classification of response inhibition in ADHD: Toward the development of neurobiological markers for ADHD. Human Brain Mapping. 2014;35:3083–3094. doi: 10.1002/hbm.22386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leibenluft E. Categories and dimensions, brain and behavior: the yins and yangs of psychopathology. JAMA Psychiatry. 2014;71:15–17. doi: 10.1001/jamapsychiatry.2013.2810. [DOI] [PubMed] [Google Scholar]
- 34.Goldstein S, Naglieri JA. Handbook of Executive Functioning. Ann Rehabil Med. 2014;56:618. [Google Scholar]
- 35.Barkley RA. Differential diagnosis of adults with ADHD: the role of executive function and self-regulation. J Clin Psychiatry. 2010;71:e17. doi: 10.4088/JCP.9066tx1c. [DOI] [PubMed] [Google Scholar]
- 36.Dalley JW, Robbins TW. Fractionating impulsivity: neuropsychiatric implications. Nat Rev Neurosci. 2017;18:158–171. doi: 10.1038/nrn.2017.8. [DOI] [PubMed] [Google Scholar]
- 37.Pauli-Pott U, Roller A, Heinzel-Gutenbrunner M, Mingebach T, Dalir S, Becker K. Inhibitory control and delay aversion in unaffected preschoolers with a positive family history of attention deficit hyperactivity disorder. J Child Psychol Psychiatry. 2014;55:1117–1124. doi: 10.1111/jcpp.12230. [DOI] [PubMed] [Google Scholar]
- 38.Antonini TN, Becker SP, Tamm L, Epstein JN. Hot and cool executive functions in children with attention-deficit/hyperactivity disorder and comorbid oppositional defiant disorder. J Int Neuropsychol Soc. 2015;21:584–595. doi: 10.1017/S1355617715000752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Posner J, Rauh V, Gruber A, Gat I, Wang Z, Peterson BS. Dissociable attentional and affective circuits in medication-naive children with attention-deficit/hyperactivity disorder. Psychiatry Res. 2013;213:24–30. doi: 10.1016/j.pscychresns.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O’Toole S, Monks CP, Tsermentseli S. Associations between and development of cool and hot executive functions across early childhood. Br J Dev Psychol. 2018;36:142–148. doi: 10.1111/bjdp.12226. [DOI] [PubMed] [Google Scholar]
- 41.Petrovic P, Castellanos FX. Top-down dysregulation-from ADHD to emotional instability. Front Behav Neurosci. 2016;10:70. doi: 10.3389/fnbeh.2016.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Okon-Singer H, Hendler T, Pessoa L, Shackman AJ. The neurobiology of emotion-cognition interactions: fundamental questions and strategies for future research. Front Hum Neurosci. 2015;9:58. doi: 10.3389/fnhum.2015.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krain AL, Castellanos FX. Brain development and ADHD. Clin Psychol Rev. 2006;26:433–444. doi: 10.1016/j.cpr.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 44.Cha J, Fekete T, Siciliano F, Biezonski D, Greenhill L, Pliszka SR, et al. Neural correlates of aggression in medication-naive children with ADHD: Multivariate analysis of morphometry and tractography. Neuropsychopharmacology. 2015;40:1717–1725. doi: 10.1038/npp.2015.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barkley RA. Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychol Bull. 1997;121:65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- 46.Soltaninejad Z, Nejati V, Ekhtiari H. Effect of anodal and cathodal transcranial direct current stimulation on DLPFC on modulation of inhibitory control in ADHD. J Attent Disord. 2015;101:291–302. doi: 10.1177/1087054715618792. [DOI] [PubMed] [Google Scholar]
- 47.Nakamichi K. Differences in young children’s peer preference by inhibitory control and emotion regulation. Psychol Rep. 2017;1:0033294117709260. doi: 10.1177/0033294117709260. [DOI] [PubMed] [Google Scholar]
- 48.Song S, Zilverstand A, Song H, d’Oleire Uquillas F, Wang Y, Xie C, et al. The influence of emotional interference on cognitive control: A meta-analysis of neuroimaging studies using the emotional Stroop task. Sci Rep. 2017;7:2088. doi: 10.1038/s41598-017-02266-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McKenna FP, Sharma D. Reversing the emotional Stroop effect reveals that it is not what it seems: the role of fast and slow components. J Exp Psychol Learn Mem Cogn. 2004;30:382–392. doi: 10.1037/0278-7393.30.2.382. [DOI] [PubMed] [Google Scholar]
- 50.Hafeman D, Bebko G, Bertocci MA, Fournier JC, Chase HW, Bonar L, et al. Amygdala-prefrontal cortical functional connectivity during implicit emotion processing differentiates youth with bipolar spectrum from youth with externalizing disorders. J Affect Disord. 2017;208:94–100. doi: 10.1016/j.jad.2016.09.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Castellanos FX, Aoki Y. Intrinsic functional connectivity in attention-deficit/hyperactivity disorder: A Science in development. Biol Psychiatry Cogn Neurosci Neuroimaging. 2016;1:253–261. doi: 10.1016/j.bpsc.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.White SF, Fowler KA, Sinclair S, Schechter JC, Majestic CM, Pine DS, et al. Disrupted expected value signaling in youth with disruptive behavior disorders to environmental reinforcers. J Am Acad Child Adolesc Psychiatry. 2014;53:579–588. doi: 10.1016/j.jaac.2013.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sonuga-Barke EJ. Psychological heterogeneity in AD/HD—a dual pathway model of behaviour and cognition. Behav Brain Res. 2002;130:29–36. doi: 10.1016/S0166-4328(01)00432-6. [DOI] [PubMed] [Google Scholar]
- 54.Sonuga-Barke E, Bitsakou P, Thompson M. Beyond the dual pathway model: evidence for the dissociation of timing, inhibitory, and delay-related impairments in attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2010;49:345–355. doi: 10.1016/j.jaac.2009.12.018. [DOI] [PubMed] [Google Scholar]
- 55.Burns GL, de Moura MA, Beauchaine TP, McBurnett K. Bifactor latent structure of ADHD/ODD symptoms: predictions of dual-pathway/trait-impulsivity etiological models of ADHD. J Child Psychol Psychiatry. 2014;55:393–401. doi: 10.1111/jcpp.12165. [DOI] [PubMed] [Google Scholar]
- 56.Beauchaine TP, Ben-David I, Sela A. Attention-deficit/hyperactivity disorder, delay discounting, and risky financial behaviors: A preliminary analysis of self-report data. PLoS One. 2017;12:e0176933. doi: 10.1371/journal.pone.0176933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xia S, Li X, Kimball AE, Kelly MS, Lesser I, Branch C. Thalamic shape and connectivity abnormalities in children with attention-deficit/hyperactivity disorder. Psychiatry Res. 2012;204:161–167. doi: 10.1016/j.pscychresns.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang BR, Chan RC, Gracia N, Cao XY, Zou XB, Jing J, et al. Cool and hot executive functions in medication-naive attention deficit hyperactivity disorder children. Psychol Med. 2011;41:2593–2602. doi: 10.1017/S0033291711000869. [DOI] [PubMed] [Google Scholar]
- 59.Wang XJ, Krystal JH. Computational psychiatry. Neuron. 2014;84:638–654. doi: 10.1016/j.neuron.2014.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]