Abstract
Attention deficit hyperactivity disorder (ADHD) is a common childhood neuropsychiatric disorder that has been linked to the dopaminergic system. This study aimed to investigate the effects of regulation of the dopamine D4 receptor (DRD4) on functional brain activity during the resting state in ADHD children using the methods of regional homogeneity (ReHo) and functional connectivity (FC). Resting-state functional magnetic resonance imaging data were analyzed in 49 children with ADHD. All participants were classified as either carriers of the DRD4 4-repeat/4-repeat (4R/4R) allele (n = 30) or the DRD4 2-repeat (2R) allele (n = 19). The results showed that participants with the DRD4 2R allele had decreased ReHo bilaterally in the posterior lobes of the cerebellum, while ReHo was increased in the left angular gyrus. Compared with participants carrying the DRD4 4R/4R allele, those with the DRD4 2R allele showed decreased FC to the left angular gyrus in the left striatum, right inferior frontal gyrus, and bilateral lobes of the cerebellum. The increased FC regions included the left superior frontal gyrus, medial frontal gyrus, and rectus gyrus. These data suggest that the DRD4 polymorphisms are associated with localized brain activity and specific functional connections, including abnormality in the frontal-striatal-cerebellar loop. Our study not only enhances the understanding of the correlation between the cerebellar lobes and ADHD, but also provides an imaging basis for explaining the neural mechanisms underlying ADHD in children.
Keywords: Attention deficit hyperactivity disorder, Dopamine D4 receptor, Frontal-striatal-cerebellar loop, Resting-state functional magnetic resonance imaging, Regional homogeneity, Functional connectivity
Introduction
Attention deficit hyperactivity disorder (ADHD) is one of the most common childhood mental and behavioral disorders, affecting emotions, communication, and cognitive functions in children. The main clinical symptoms include inattention, hyperactivity, and impulsiveness. With a global prevalence of 5.3% [1], ADHD significantly affects the academic performance, and the families and communities of these children.
With the development of molecular genetics for diagnosis and treatment [2], ADHD is now considered to be a polygenic hereditary disease, and is correlated with abnormal dopamine transmission systems. Currently, conventional drugs for ADHD, such as methylphenidate hydrochloride (MPH), directly or indirectly increase the dopamine concentration in central synapses and improve ADHD symptoms. In this system, the dopamine D4 receptor (DRD4) gene is a leading candidate gene associated with ADHD, located near the telomere of chromosome 11p15.5 [3]. Most of the diversity is in a variable number of 48-bp (base pair) tandem repeats (VNTRs) on exon III [4]. The variant alleles range from 2-repeats to 11-repeats [5]. The 4- (4R), 7- (7R), and 2-repeat (2R) alleles are common, with global mean frequencies of approximately 67%, 12%, and 10%, respectively [6]. Meta-analyses have revealed that individuals with the 7R allele have an increased risk for ADHD (OR = 1.1–1.4) [7, 8]. The 7R carriers are considered to be at risk for problematic social behaviors, such as alcoholism, financial risk-taking, ADHD, and infidelity [9]. However, the majority of correlation studies have been carried out in non-Asian populations. Compared to American populations, the mean allele frequency of the 7R allele is generally lower in Asian populations [10], while the 2R allele frequency is higher [11]. Research on a small sample of Chinese participants indicates that those carrying the 2R allele have an increased risk of developing ADHD [12, 13]. Moreover, 2R allele carriers perform worse than 7R allele carriers in attention tests [14]. Based on the evolutionary and biochemical similarities between the 2R and 7R alleles [15], it has been recommended that carriers of these two alleles be combined in studies of Asian populations [16].
Previously, research was designed to investigate the effect of DRD4 gene polymorphism on brain structure and function using imaging genetics. In a large longitudinal study, Shaw et al. found differences in cortical thickness in the orbital frontal, medial prefrontal, and parietal cortex in 7R carriers and non-7R carriers among children with ADHD[17]. DRD4 polymorphism also results in different images in task-state functional magnetic resonance imaging (fMRI) studies [18]. Compared to individuals with the 4R/4R allele, those carrying the 2R or 7R allele perform worse in the GO/NO-GO task [19]. Furthermore, the 7R allele influences regional brain activation and the connectivity patterns between neural networks for incompatibility and temporal processing [18].
Regional homogeneity (ReHo) and seed-based functional connectivity (FC) analysis are common analytical methods in resting-state fMRI [20]. The basis of ReHo is the similarity of the time series of different voxels in the functional mass. The Kendall’s coefficient concordance (KCC) value, also known as the ReHo value, is used to measure the similarity or synchronization between the time series of a given voxel and its nearest neighbors [21]. Based on the fact that there are temporal similarities in neurophysiological measurement indexes in different remote brain areas, FC in brain function studies is often used as a measure for the synchronization of functional activity in non-adjacent brain regions [22, 23]. In this study, seed-based FC analysis was used to evaluate the functional connection pattern.
Currently, studies on resting-state fMRI related to DRD4 polymorphisms are rare. The aim of this study was to explore the effects of DRD4 polymorphisms on brain function in Chinese Han children with ADHD during the resting-state by means of local brain activity and specific FC, which might provide an imaging basis for further research on the pathogenesis of ADHD.
Materials and Methods
Participants
Participants were recruited and studied between December 2012 and September 2014 at the Psychiatry Outpatient Unit for ADHD, First Affiliated Hospital of Wenzhou Medical University. A diagnosis of ADHD was determined by three experienced clinical psychiatrists using the criteria in the Diagnostic and Statistical Manual of Mental Disorders, 4th Revision. The Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children – Present and Lifetime Versions was used to exclude conduct disorder and oppositional defiant disorder, or any other axis I comorbid psychiatric disorders [24]. Children who had prior treatment with MPH or atomoxetine were excluded. Other exclusion criteria included substance abuse history or a positive urine screen, participation in a treatment study in the past 30 days, a history of head injury with loss of consciousness, a past or present diagnosis of a psychotic disorder, neurological or cardiovascular disease, full-scale IQ below 75 as measured by the Wechsler Abbreviated Scale of Intelligence, or any other condition that could affect brain function. Then, 56 Chinese Han children with ADHD, aged 7 to 15 years, were recruited. The study was approved by the Institutional Review Board of the First Affiliated Hospital of Wenzhou Medical University. Written informed consent was given by parents and children who were asked to complete the Conners’ Parent Rating Scale and the Stroop Color Word Test.
Genotyping
All participants were genotyped for DRD4 48-bp VNTR polymorphisms.
DNA was extracted from 10 mL of peripheral venous blood followed by cell lysis and heme/protein precipitation. Genotyping was performed in the following manner. The polymerase chain reaction (PCR) was carried out in a 25 μL volume containing 1 μL of template DNA, 1 μL of each primer 5′-GCG ACT ACG TGG TCT ACT CG-3′ and 5′-AGG ACC CTC ATG GCC TTG-3′, 9.5 μL of ddH2O, 12.5 μL of PCR reagents (KAPA2G Fast Multiplex Mix, KAPA Biosystems, Cape Town, South Africa, Code No: KM5802). Samples were amplified on a ABI 2720 thermal cycler (ABI Applied Biosystems, CA) with an initial denaturation step at 95°C for 10 min. Products were injected onto an ABI 3730XL multi-capillary array genetic analyzer (ABI Applied Biosystems, CA). Alleles were called with ABI PRISM Genemapper v 3.5, blind to the phenotypic data.
Image Acquisition
Participants were scanned on a GE Signa HDx 3.0 Tesla MR scanner (GE Healthcare, USA), using a standard 8-channel head coil at the Department of Radiology of the First Affiliated Hospital of Wenzhou Medical University. Participants were asked to fit sponge earplugs in their ears to reduce scanner noise and were given foam pads to minimize head motion. Participants were instructed to relax, remain motionless, keep their eyes closed, stay awake, and not think of anything in particular. Scan sessions began with shimming and localization. First, axial T2-weighted images were acquired; these were used to exclude structural lesions. Resting-state functional images depicting the blood oxygenation level-dependent (BOLD) signal were acquired using an echo-planar imaging sequence (EPI) (repetition time = 2000 ms, echo time = 30 ms, flip angle = 90°, field of view = 192 mm, matrix = 64 × 64, 31 slices, slice thickness = 4 mm, gap = 0.2 mm, and 240 volumes). Children were accompanied by a guardian to ensure their safety during the scanning process.
ReHo Data Analysis
The EPI data were preprocessed using Data Processing Assistant for resting-state fMRI on MATLAB 7.12.0 (R2011a)(https://cn.mathworks.com/products/matlab.html). The first 10 volumes of each scanning session were removed to allow for scanner calibration and participant adaptation to the scanning environment, and the remaining 230 volumes were analyzed. For each participant, EPI images were slice-time corrected and realigned. Acceptable scans had no more than 3 mm of displacement in any direction and no more than 3° of angular motion during the entire fMRI scan. Seven participants were excluded from further analysis because of excessive head movement. After realignment, all data were normalized to Montreal Neurological Institute (MNI) space and resampled to 3 mm × 3 mm × 3 mm voxels. The resulting fMRI data were temporally band-pass filtered (0.01 Hz–0.08 Hz) to reduce low-frequency drift and physiological high-frequency respiratory and cardiac noise for further ReHo and functional connectivity analyses.
The KCC value (also called the ReHo value) was calculated to measure the similarity of the ranked time series of a given voxel to its nearest 26 neighbor voxels in a voxel-wise manner. By calculating the KCC value of every voxel in the whole brain, an individual ReHo map was obtained for each participant. The intracranial voxels were extracted to make a mask [25]. For standardization purposes, each individual ReHo map was divided by its own mean ReHo within the mask. Then, the data were smoothed with a Gaussian kernel of 4-mm full-width at half-maximum (FWHM).
FC Data Analysis
The preprocessing of format conversion, removal of the first 10 time points, slice timing, realignment, and normalization was the same as for ReHo. The difference was that spatial smoothing with a Gaussian kernel of 4-mm FWHM occurred directly after normalization. All voxel time-series were detrended to correct for linear drift over time. Temporal filtering (0.01 Hz–0.08 Hz) was used to reduce low-frequency drift and respiratory and cardiac noise. Then, nuisance covariates, including six head motion parameters, global mean signal, white matter signal, and cerebrospinal fluid signal of the data were regressed out. The left angular gyrus was selected as the region of interest (ROI) (Fig. 1). Finally, to perform seed-based correlation analysis, the BOLD time series of the voxels within the left angular gyrus were averaged to generate the reference time series. The resulting time course was used to perform Pearson linear correlation analysis with all voxels of the brain data. Mask images were acquired for the spatial masks of two groups. A Fisher z-transform was applied to normalize the correlation coefficients.
Fig. 1.
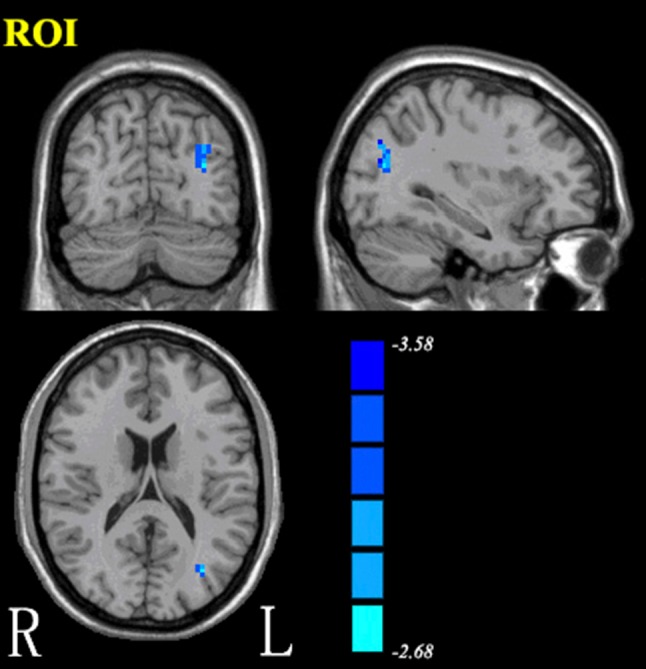
The left angular gyrus was selected as the region of interest for functional connectivity.
Statistics Analysis
Statistical analysis was performed using the Resting-State fMRI Data Analysis Toolkit (REST V1.8) (http://restfmri.net/forum/REST_V1.8). To investigate the ReHo differences and differences in connectivity to the left angular gyrus between children who were homozygous for the DRD4 4R allele and those for the 2R allele, two-sample t-tests were executed in REST. The covariates were age, sex, IQ, and head motions. The results were corrected for multiple comparisons with the Alphasim program included in the REST V1.8, threshold at voxel-level uncorrected P < 0.01 and cluster-level corrected P < 0.01 (cluster size > 378 mm3). Regions with statistically significant differences were masked on the MNI brain template.
Results
Clinical Characteristics
The participants were classified as either carriers of the DRD4 4R/4R allele (n = 30) or carriers of a 2R allele (n = 19; 16 with a 2R/4R genotype, and one each with a 2R/2R, a 2R/5R, and a 2R/3R genotype). The genotypes were in Hardy-Weinberg equilibrium. The two groups did not differ in age, sex, IQ, ADHD subtype, or handedness (P > 0.05). However, the two groups also did not differ in the behavioral assessment scales (Table 1).
Table 1.
Demographic and clinical characteristics of ADHD children with different DRD4 48-bp VNTR polymorphism genotypes.
| Characteristic | DRD4-4R/4R (n = 30) | DRD4-2R (n = 19) | Statistics |
|---|---|---|---|
| Age (years) | 10.50 ± 1.87 | 9.50 ± 1.76 | t = 1.846, P = 0.071 |
| Male | 27 (90%) | 16 (84%) | χ2 = 0.024, P = 0.877 |
| Female | 3 (10%) | 3 (16%) | χ2 = 0.024, P = 0.877 |
| Right-handed | 30 (100%) | 19 (100%) | |
| IQ | 116.50 ± 18.89 | 115.60 ± 13.74 | t = 0.173, P = 0.863 |
| ADHD subtype | |||
| Inattentive | 14 (47%) | 6 (31%) | χ2 = 1.097, P = 0.578 |
| Hyperactive-Impulsive | 5 (16%) | 4 (21%) | χ2 = 1.097, P = 0.578 |
| Combined | 11 (37%) | 9 (47%) | χ2 = 1.097, P = 0.578 |
| Conners’ Parent Rating Scale | |||
| Mean Impulsivity-Hyperactivity | 1.59 ± 0.67 | 1.70 ± 0.70 | t = − 0.531, P = 0.598 |
| Mean Conners Index of Hyperactivity | 1.56 ± 0.56 | 1.61 ± 0.62 | t = − 0.282, P = 0.779 |
| Stroop Color Word Test | |||
| Stroop reaction time (s) | 2.77 ± 1.12 | 2.83 ± 1.09 | t = − 0.185, P = 0.854 |
ReHo Imaging
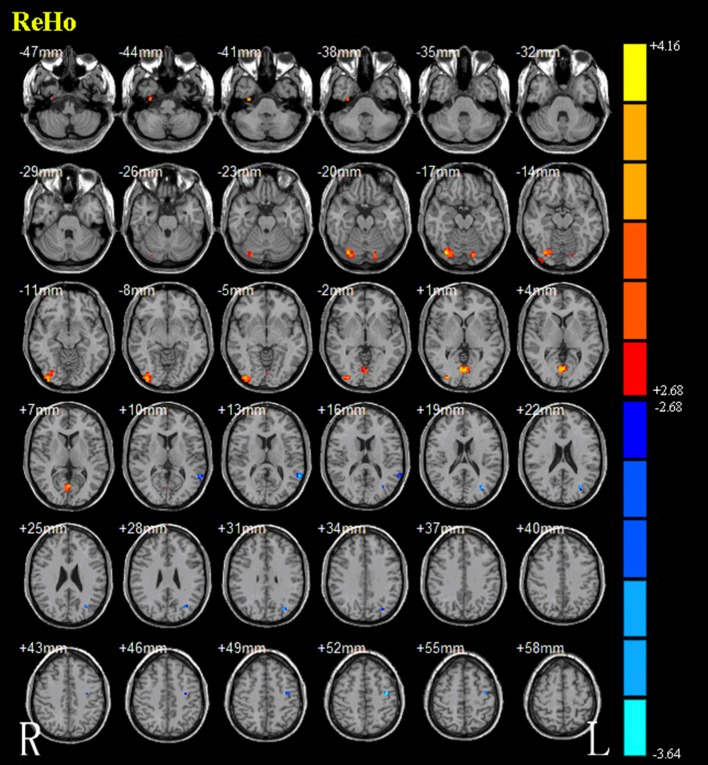
The results from the two-sample t-test clearly showed significant ReHo differences between the two groups (Alphasim correction, P < 0.01). Those with the DRD4 2R allele showed lower ReHo bilaterally in the posterior lobes of the cerebellum and lingual gyrus, right occipital lobes, medial occipital temporal gyrus, and lateral occipital temporal gyrus. ReHo was higher in the left supramarginal gyrus, angular gyrus, and precentral gyrus (Fig. 2 and Table 2).
Fig. 2.
Brain areas with ReHo differences between carriers of the DRD4 4R/4R allele and carriers of the DRD4 2R allele. Hot and cold colors indicate increased and decreased ReHo values in carriers of the DRD4 4R/4R allele. Color bar represents t-values ranging from − 3.64 to 4.16.
Table 2.
Regions showing ReHo differences between carriers of the DRD4 4R/4R allele and carriers of the DRD4 2R allele.
| Brain regions | Voxels | Peak MNIa coordinate | T valueb | ||
|---|---|---|---|---|---|
| X | Y | Z | |||
| Increased (DRD4-4R/4R > DRD4-2R) | |||||
| Medial occipital temporal gyrus (R) | 18 | 33 | − 9 | − 42 | 3.8352 |
| Cerebellar posterior lobe, occipital lobe, medial occipital temporal gyrus, lateral occipital temporal gyrus (R) | 168 | 33 | − 75 | − 18 | 4.1076 |
| Cerebellar posterior lobe (L) | 21 | − 15 | − 78 | − 18 | 3.4663 |
| Lingual gyrus (L/R) | 73 | − 3 | − 72 | 0 | 4.1582 |
| Decreased (DRD4-4R/4R < DRD4-2R) | |||||
| Supramarginal gyrus (L) | 28 | − 60 | − 48 | 12 | − 3.6265 |
| Angular gyrus (L) | 31 | − 33 | − 72 | 18 | − 3.5846 |
| Precentral gyrus (L) | 18 | − 39 | − 12 | 51 | − 3.6397 |
aCoordinates of the MNI three-dimensional positioning system.
bPeak intensity.
FC Imaging
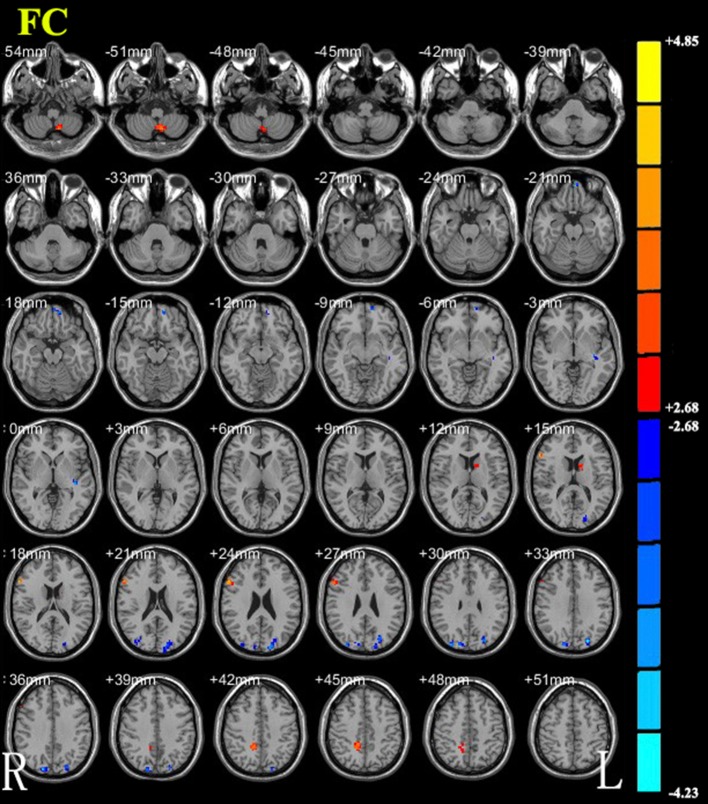
Compared with the DRD4 4R/4R allele, the DRD4 2R allele showed decreased FC to the left angular gyrus in the bilateral anterior lobes of the cerebellum, right inferior frontal gyrus, precuneus, and left striatum. Some regions showed increased FC to the left angular gyrus. These regions included the bilateral occipital lobes, left superior frontal gyrus, medial frontal gyrus, rectus gyrus, superior parietal lobule, and temporal lobe (Fig. 3 and Table 3).
Fig. 3.
Brain areas with functional connectivity differences between carriers of the DRD4 4R/4R allele and carriers of the DRD4 2R allele. Hot and cold colors indicate increased and decreased functional connectivity carriers of the DRD4 4R/4R allele, respectively. Color bar represents t-values ranging from − 4.23 to 4.85.
Table 3.
Regions showing differences in FC to the left angular gyrus between carriers of the DRD4 4R/4R allele and carriers of the DRD4 2R allele.
| Brain regions | Voxels | Peak MNIa coordinate | T valueb | ||
|---|---|---|---|---|---|
| X | Y | Z | |||
| Increased (DRD4-4R/4R > DRD4-2R) | |||||
| Cerebellar anterior lobe (L/R) | 44 | − 9 | − 60 | − 51 | 3.9776 |
| Striatum/Caudate (L) | 15 | − 15 | 6 | 15 | 3.3814 |
| Inferior frontal gyrus (R) | 36 | 60 | 24 | 18 | 4.8513 |
| Precuneus (R), white matter in R parietal lobe | 42 | 15 | − 48 | 45 | 3.5883 |
| Decreased (DRD4-4R/4R < DRD4-2R) | |||||
| Superior frontal gyrus, medial frontal gyrus, rectus gyrus (L) | 26 | − 3 | 60 | − 21 | − 4.1043 |
| White matter in temporal lobe (L) | 15 | − 39 | − 24 | 0 | − 3.4049 |
| Occipital lobe, superior parietal lobule (L) | 87 | − 24 | − 81 | 33 | − 3.9882 |
| Occipital lobe (R) | 22 | 30 | − 90 | 30 | − 3.4283 |
| Occipital lobe, cuneus (R) | 37 | 18 | − 87 | 36 | − 3.6372 |
aCoordinates of the MNI three-dimensional positioning system.
bPeak intensity.
Discussion
With the development of molecular genetics, disorder of the dopamine system is considered to be a pathological mechanism underlying many mental illnesses, and is also one of the important factors in the pathogenesis of ADHD. DRD4 is a gene encoding a G-protein-coupled receptor. The distribution of DRD4 in the human brain is still controversial [26]. A study of human brain tissue using RT-PCR revealed that DRD4 has a relatively high expression level in regions of the prefrontal lobe and temporolimbic structures [27]. Our study also showed a significant change in the ReHo value and FC abnormality during the resting state in the brain regions with DRD4 overexpression. Compared with the DRD4 4R/4R allele, the DRD4 2R allele showed decreased ReHo mainly on the right side, temporal occipital lobe, and cerebellum; ReHo was higher in the left angular gyrus; and FC decreased to the left angular gyrus in the frontal-striatal-cerebellar loop.
Our results showed that, compared with carriers of the DRD4 4R/4R allele, the DRD4 2R allele carriers showed a lower ReHo in the right lateral occipital temporal gyrus, the right occipital lobe, the bilateral lingual gyrus, and the cerebellum. An fMRI study has shown that the intensity and extent of the BOLD signal in the lingual gyrus regions are closely related to neuronal myelination in those regions during brain development in children aged 7 years–13 years [28]. Since then, Pang et al. have used resting-state fMRI to study school-aged children. With increasing age, neuronal myelin in the lingual gyrus gradually matures, resulting in increasing ReHo values in those regions, and is associated with a gradual enhancement of cognitive function [29]. It can be speculated that children with DRD4 2R have delayed neuronal development relative to those with DRD4 4R/4R, consistent with the etiological hypothesis of delayed neurodevelopment of ADHD [30, 31]. The lingual gyrus is involved in visual space processing, and the lingual gyrus, superior parietal lobule, and lateral occipital temporal gyrus are associated with advanced visual function [32]. These three brain regions are closely linked to each other, forming a complex visual loop, i.e., the dorsal and ventral pathways.
Our results showed that the increased ReHo values were mainly concentrated in the left angular gyrus. Imaging studies have reflected that the angular gyrus is associated with ADHD [33, 34]. The angular gyrus is also part of the frontal-parietal lobe network and is one of the nodes of the default mode network [35]; it serves as an intermediate station for the integration of internal and external information and highlights the convergence of different multimodal inputs into the angular gyrus and interactions with different subsystems that include memory and attention (superior parietal area) [36, 37]. Moreover, the left angular gyrus integrates spatial information from both cerebral hemispheres [38]. Our study showed that the ReHo of the left angular gyrus in carriers of the DRD4 2R allele was significantly increased, indicating that the DRD4 gene polymorphism might be involved in regulating the activity of the angular gyrus and influencing ADHD symptoms. So, we selected the left angular gyrus as the ROI for FC analysis.
We found that the DRD4 gene not only regulated local brain activity, but also played an important role in functional connections. The ReHo value of the bilateral cerebellum was lower in children with the DRD4 2R genotype than in those with the DRD4 4R/4R genotype, and the decreased FC to the left angular gyrus also included the frontal-striatal-cerebellar loop, consistent with previous studies [39, 40]. Other studies have found significant functional changes in the striatum area in ADHD patients [41–43], and abnormalities in the frontal-striatal pathway play an important role in the pathogenesis of ADHD [44, 45]. Caudate abnormalities may remain relatively unchanged throughout development and recovery [46].
However, conventional wisdom holds that the cerebellum is involved in the regulation of balance and muscle tone as well as the coordination of voluntary movement. Recently, studies have shown that the cerebellum also receives information from association cortex related to cognitive and emotional functions [47]. A structural fMRI study found a decrease in cerebellar volume in children with ADHD and a reduction in white matter fibers in the frontal-cerebellar loop [48, 49]. It was suggested that the cerebellum is associated with a genetic risk of ADHD in a sample of boys with ADHD and the unaffected siblings of probands with ADHD [50]. Furthermore, Gilsbach et al. found that non-7R allele carriers (mainly the 4R genotype) in the implementation of executive control tasks showed a strong hemodynamic response between the cerebellum, anterior cingulate gyrus, and inferior frontal gyrus [18]. Like the cerebrum, the cerebellum has complex neural connections [51]. According to these structural and functional findings in the cerebellum, it can be speculated that the cerebellum plays a key role in the pathogenesis of ADHD.
In our study, DRD4 2R allele carriers showed significantly higher FC in the left superior frontal gyrus, medial frontal gyrus, and rectus gyrus than DRD4 4R/4R allele carriers. Similar results were obtained in a study of changes in cortical volume in adolescents [52]. Mouse experiments have revealed that synchronized network activity in the prefrontal cortex is regulated by DRD4 polymorphisms [53], and this result has been validated in a task-state fMRI study of healthy adolescents [18]. In addition, “cold” executive function is mainly regulated by the dorsal prefrontal lobe and anterior cingulate cortex, and is associated with attention deficit symptoms [54]. DRD4 gene polymorphisms can alter the action of dopamine in regions with inhibitory control functions, such as the prefrontal cortex [55, 56]. Therefore, it has been suggested that DRD4 2R has an effect on executive control [57]. Our study further validates the association between DRD4 gene polymorphism and left frontal lobe activity. Meanwhile, increased FC in the left frontal lobe may be a compensatory action for a reduction in the frontal-striatal-cerebellar loop. Some authors have suggested that the carriers of different DRD4 genotypes display significant differences in hyperactivity symptoms [17]. However, the results of our study failed to reveal statistical differences between the two groups in terms of functional tasks and hyperactivity-impulsive symptoms (Table 1); this may have been due to the relatively small sample size.
We found that, compared with carriers of the DRD4 4R/4R allele, DRD4 2R carriers showed increased FC to the left angular gyrus in the bilateral occipital lobes. The DRD4 gene has been shown to be able to regulate the mean BOLD signal change in a task-state fMRI study, with significant hemodynamic responses in the right inferior frontal gyrus, and the occipital and cerebellar regions [55], consistent with the results of our study. The currently effective ADHD drug MPH improves the clinical symptoms and significantly reduces local cerebral blood flow in the visual cortex of children with ADHD [58–60]. The increased activity of the visual cortex region is also thought to be associated with attention lapses in ADHD patients [44, 61]. These results suggest that DRD4 2R allele may be associated with more obvious symptoms of inattention than the DRD4 4R/4R allele. Although there were no reliable clinical indicators in the present study, it provides an imaging basis for the hypothesis that the DRD4 gene regulates occipital lobe activity in children with ADHD.
In our study, two methods (ReHo and FC) combining local brain activity with specific brain connections were used to obtain a comprehensive analysis of the pathological and physiological basis of ADHD in the resting state. This experimental sample was based on ADHD children who had not received MPH treatment, to reduce the effects of confounding factors [40]. Because of the uniqueness of Chinese populations, we studied the effects of DRD4 2R in children with ADHD in China. However, this study has limitations that need to be addressed. First, there were no differences in the clinical scale between the two DRD4 genotype groups in this study, including the implementation of control functions and hyperactivity-impulsive symptoms. So it is necessary to expand the research sample to find an association between the clinical indexes of ADHD and imaging genetics. Second, we did not preselect patients according to ADHD subtype (inattentive, hyperactive/impulsive, or combined subtypes), which might have confounded the results. Future studies can usefully focus on these. Finally, only ADHD patients were recruited in this study, which limited further interpretation of our results; however, this feasibility study provides a practical foundation for future longitudinal research paradigms.
Conclusion
In summary, this resting-state fMRI study suggests that DRD4 gene polymorphisms are associated with localized brain activity in children with ADHD, but also participate in the regulation of specific functional connections involved in inhibitory control and executive control, including abnormality in the frontal-striatal-cerebellar loop. These findings further suggest that there is a correlation between the cerebellum and ADHD and provide an imaging basis for explaining the neural mechanisms in children with ADHD. In future, we hope to study the imaging of different DRD4 phenotypes and redefine the classification of ADHD from the perspective of imaging genetics.
Acknowledgements
This work was supported by the Natural Science Foundation of Zhejiang Province, China (No. LY14H180006, LQ18H090009) and the Natural Science Foundation of Jiangsu Province (BK20160142). We thank all the volunteers for their participations.
Conflict of interest
All authors claim that there are no conflicts of interest.
Footnotes
Andan Qian and Xin Wang have contributed equally to this work
Contributor Information
Ke Zhao, Email: cocozk1986@163.com.
Meihao Wang, Email: wzwangmh@163.com.
References
- 1.Polanczyk G, de Lima MS, Horta BL, Biederman J, Rohde LA. The worldwide prevalence of ADHD: a systematic review and metaregression analysis. Am J Psychiatry. 2007;164:942–948. doi: 10.1176/ajp.2007.164.6.942. [DOI] [PubMed] [Google Scholar]
- 2.Ma G, Fan H, Shen C, Wang W. Genetic and neuroimaging features of personality disorders: state of the art. Neurosci Bull. 2016;32:286–306. doi: 10.1007/s12264-016-0027-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petronis A, Van Tol HH, Lichter JB, Livak KJ, Kennedy JL. The D4 dopamine receptor gene maps on 11p proximal to HRAS. Genomics. 1993;18:161–163. doi: 10.1006/geno.1993.1445. [DOI] [PubMed] [Google Scholar]
- 4.Kotler M, Manor I, Sever Y, Eisenberg J, Cohen H, Ebstein RP, et al. Failure to replicate an excess of the long dopamine D4 exon III repeat polymorphism in ADHD in a family-based study. Am J Med Genet. 2000;96:278–281. doi: 10.1002/1096-8628(20000612)96:3<278::AID-AJMG8>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 5.Ding YC, Chi HC, Grady DL, Morishima A, Kidd JR, Kidd KK, et al. Evidence of positive selection acting at the human dopamine receptor D4 gene locus. Proc Natl Acad Sci U S A. 2002;99:309–314. doi: 10.1073/pnas.012464099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kebir O, Tabbane K, Sengupta S, Joober R. Candidate genes and neuropsychological phenotypes in children with ADHD: review of association studies. J Psychiatry Neurosci. 2009;34:88–101. [PMC free article] [PubMed] [Google Scholar]
- 7.Gizer IR, Ficks C, Waldman ID. Candidate gene studies of ADHD: a meta-analytic review. Hum Genet. 2009;126:51–90. doi: 10.1007/s00439-009-0694-x. [DOI] [PubMed] [Google Scholar]
- 8.Li D, Sham PC, Owen MJ, He L. Meta-analysis shows significant association between dopamine system genes and attention deficit hyperactivity disorder (ADHD) Hum Mol Genet. 2006;15:2276–2284. doi: 10.1093/hmg/ddl152. [DOI] [PubMed] [Google Scholar]
- 9.Laucht M, Becker K, Blomeyer D, Schmidt MH. Novelty seeking involved in mediating the association between the dopamine D4 receptor gene exon III polymorphism and heavy drinking in male adolescents: results from a high-risk community sample. Biol Psychiatry. 2007;61:87–92. doi: 10.1016/j.biopsych.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 10.Chang FM, Kidd JR, Livak KJ, Pakstis AJ, Kidd KK. The world-wide distribution of allele frequencies at the human dopamine D4 receptor locus. Hum Genet. 1996;98:91–101. doi: 10.1007/s004390050166. [DOI] [PubMed] [Google Scholar]
- 11.Park S, Kim BN, Cho SC, Kim Y, Kim JW, Lee JY, et al. Association between urine phthalate levels and poor attentional performance in children with attention-deficit hyperactivity disorder with evidence of dopamine gene-phthalate interaction. Int J Environ Res Public Health. 2014;11:6743–6756. doi: 10.3390/ijerph110706743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leung PW, Lee CC, Hung SF, Ho TP, Tang CP, Kwong SL, et al. Dopamine receptor D4 (DRD4) gene in Han Chinese children with attention-deficit/hyperactivity disorder (ADHD): increased prevalence of the 2-repeat allele. Am J Med Genet B Neuropsychiatr Genet. 2005;133B:54–56. doi: 10.1002/ajmg.b.30129. [DOI] [PubMed] [Google Scholar]
- 13.Qian Q, Wang Y, Zhou R, Yang L, Faraone SV. Family-based and case-control association studies of DRD4 and DAT1 polymorphisms in Chinese attention deficit hyperactivity disorder patients suggest long repeats contribute to genetic risk for the disorder. Am J Med Genet B Neuropsychiatr Genet. 2004;128B:84–89. doi: 10.1002/ajmg.b.30079. [DOI] [PubMed] [Google Scholar]
- 14.Manor I, Tyano S, Eisenberg J, Bachner-Melman R, Kotler M, Ebstein RP. The short DRD4 repeats confer risk to attention deficit hyperactivity disorder in a family-based design and impair performance on a continuous performance test (TOVA) Mol Psychiatry. 2002;7:790–794. doi: 10.1038/sj.mp.4001078. [DOI] [PubMed] [Google Scholar]
- 15.Asghari V, Sanyal S, Buchwaldt S, Paterson A, Jovanovic V, Van Tol HH. Modulation of intracellular cyclic AMP levels by different human dopamine D4 receptor variants. J Neurochem. 1995;65:1157–1165. doi: 10.1046/j.1471-4159.1995.65031157.x. [DOI] [PubMed] [Google Scholar]
- 16.Reist C, Ozdemir V, Wang E, Hashemzadeh M, Mee S, Moyzis R. Novelty seeking and the dopamine D4 receptor gene (DRD4) revisited in Asians: haplotype characterization and relevance of the 2-repeat allele. Am J Med Genet B Neuropsychiatr Genet. 2007;144B:453–457. doi: 10.1002/ajmg.b.30473. [DOI] [PubMed] [Google Scholar]
- 17.Shaw P, Gornick M, Lerch J, Addington A, Seal J, Greenstein D, et al. Polymorphisms of the dopamine D4 receptor, clinical outcome, and cortical structure in attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2007;64:921–931. doi: 10.1001/archpsyc.64.8.921. [DOI] [PubMed] [Google Scholar]
- 18.Gilsbach S, Neufang S, Scherag S, Vloet TD, Fink GR, Herpertz-Dahlmann B, et al. Effects of the DRD4 genotype on neural networks associated with executive functions in children and adolescents. Dev Cogn Neurosci. 2012;2:417–427. doi: 10.1016/j.dcn.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Henriquez-Henriquez M, Villarroel L, Henriquez H, Zamorano F, Rothhammer F, Aboitiz F. Intratask variability as a correlate for DRD4 and SLC6A3 variants: A pilot study in ADHD. J Atten Disord. 2015;19:987–996. doi: 10.1177/1087054712455844. [DOI] [PubMed] [Google Scholar]
- 20.Zhang K, Zhu Y, Zhu Y, Wu S, Liu H, Zhang W, et al. Molecular, functional, and structural imaging of major depressive disorder. Neurosci Bull. 2016;32:273–285. doi: 10.1007/s12264-016-0030-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zang Y, Jiang T, Lu Y, He Y, Tian L. Regional homogeneity approach to fMRI data analysis. Neuroimage. 2004;22:394–400. doi: 10.1016/j.neuroimage.2003.12.030. [DOI] [PubMed] [Google Scholar]
- 22.Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 23.Friston KJ, Frith CD, Liddle PF, Frackowiak RS. Functional connectivity: the principal-component analysis of large (PET) data sets. J Cereb Blood Flow Metab. 1993;13:5–14. doi: 10.1038/jcbfm.1993.4. [DOI] [PubMed] [Google Scholar]
- 24.Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 25.Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McGeary J. The DRD4 exon 3 VNTR polymorphism and addiction-related phenotypes: a review. Pharmacol Biochem Behav. 2009;93:222–229. doi: 10.1016/j.pbb.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mulcrone J, Kerwin RW. The regional pattern of D4 gene expression in human brain. Neurosci Lett. 1997;234:147–150. doi: 10.1016/S0304-3940(97)00702-7. [DOI] [PubMed] [Google Scholar]
- 28.Fornari E, Knyazeva MG, Meuli R, Maeder P. Myelination shapes functional activity in the developing brain. Neuroimage. 2007;38:511–518. doi: 10.1016/j.neuroimage.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 29.Pang GF, Wang SH, Ren YL, Ma L, Chen J, Xing W, et al. Cognitive development of normal school age children: a resting-state fMRI study. Zhonghua Yi Xue Za Zhi. 2009;89:1313–1317. [PubMed] [Google Scholar]
- 30.Rubia K. Neuro-anatomic evidence for the maturational delay hypothesis of ADHD. Proc Natl Acad Sci U S A. 2007;104:19663–19664. doi: 10.1073/pnas.0710329105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rubia K, Overmeyer S, Taylor E, Brammer M, Williams SC, Simmons A, et al. Functional frontalisation with age: mapping neurodevelopmental trajectories with fMRI. Neurosci Biobehav Rev. 2000;24:13–19. doi: 10.1016/S0149-7634(99)00055-X. [DOI] [PubMed] [Google Scholar]
- 32.Xia S, Foxe JJ, Sroubek AE, Branch C, Li X. Topological organization of the “small-world” visual attention network in children with attention deficit/hyperactivity disorder (ADHD) Front Hum Neurosci. 2014;8:162. doi: 10.3389/fnhum.2014.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dickstein SG, Bannon K, Castellanos FX, Milham MP. The neural correlates of attention deficit hyperactivity disorder: an ALE meta-analysis. J Child Psychol Psychiatry. 2006;47:1051–1062. doi: 10.1111/j.1469-7610.2006.01671.x. [DOI] [PubMed] [Google Scholar]
- 34.Tamm L, Menon V, Reiss AL. Parietal attentional system aberrations during target detection in adolescents with attention deficit hyperactivity disorder: event-related fMRI evidence. Am J Psychiatry. 2006;163:1033–1043. doi: 10.1176/ajp.2006.163.6.1033. [DOI] [PubMed] [Google Scholar]
- 35.Andrews-Hanna JR, Smallwood J, Spreng RN. The default network and self-generated thought: component processes, dynamic control, and clinical relevance. Ann N Y Acad Sci. 2014;1316:29–52. doi: 10.1111/nyas.12360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen Q, Weidner R, Vossel S, Weiss PH, Fink GR. Neural mechanisms of attentional reorienting in three-dimensional space. J Neurosci. 2012;32:13352–13362. doi: 10.1523/JNEUROSCI.1772-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seghier ML. The angular gyrus: multiple functions and multiple subdivisions. Neuroscientist. 2013;19:43–61. doi: 10.1177/1073858412440596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hirnstein M, Bayer U, Ellison A, Hausmann M. TMS over the left angular gyrus impairs the ability to discriminate left from right. Neuropsychologia. 2011;49:29–33. doi: 10.1016/j.neuropsychologia.2010.10.028. [DOI] [PubMed] [Google Scholar]
- 39.Li F, He N, Li Y, Chen L, Huang X, Lui S, et al. Intrinsic brain abnormalities in attention deficit hyperactivity disorder: a resting-state functional MR imaging study. Radiology. 2014;272:514–523. doi: 10.1148/radiol.14131622. [DOI] [PubMed] [Google Scholar]
- 40.Zang YF, He Y, Zhu CZ, Cao QJ, Sui MQ, Liang M, et al. Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain Dev. 2007;29:83–91. doi: 10.1016/j.braindev.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 41.Durston S, Tottenham NT, Thomas KM, Davidson MC, Eigsti IM, Yang Y, et al. Differential patterns of striatal activation in young children with and without ADHD. Biol Psychiatry. 2003;53:871–878. doi: 10.1016/S0006-3223(02)01904-2. [DOI] [PubMed] [Google Scholar]
- 42.Booth JR, Burman DD, Meyer JR, Lei Z, Trommer BL, Davenport ND, et al. Larger deficits in brain networks for response inhibition than for visual selective attention in attention deficit hyperactivity disorder (ADHD) J Child Psychol Psychiatry. 2005;46:94–111. doi: 10.1111/j.1469-7610.2004.00337.x. [DOI] [PubMed] [Google Scholar]
- 43.Shafritz KM, Marchione KE, Gore JC, Shaywitz SE, Shaywitz BA. The effects of methylphenidate on neural systems of attention in attention deficit hyperactivity disorder. Am J Psychiatry. 2004;161:1990–1997. doi: 10.1176/appi.ajp.161.11.1990. [DOI] [PubMed] [Google Scholar]
- 44.Castellanos FX, Proal E. Large-scale brain systems in ADHD: beyond the prefrontal-striatal model. Trends Cogn Sci. 2012;16:17–26. doi: 10.1016/j.tics.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liston C, Malter Cohen M, Teslovich T, Levenson D, Casey BJ. Atypical prefrontal connectivity in attention-deficit/hyperactivity disorder: pathway to disease or pathological end point? Biol Psychiatry. 2011;69:1168–1177. doi: 10.1016/j.biopsych.2011.03.022. [DOI] [PubMed] [Google Scholar]
- 46.Halperin JM, Schulz KP. Revisiting the role of the prefrontal cortex in the pathophysiology of attention-deficit/hyperactivity disorder. Psychol Bull. 2006;132:560–581. doi: 10.1037/0033-2909.132.4.560. [DOI] [PubMed] [Google Scholar]
- 47.Middleton FA, Strick PL. Basal ganglia and cerebellar loops: motor and cognitive circuits. Brain Res Brain Res Rev. 2000;31:236–250. doi: 10.1016/S0165-0173(99)00040-5. [DOI] [PubMed] [Google Scholar]
- 48.Konrad K, Eickhoff SB. Is the ADHD brain wired differently? A review on structural and functional connectivity in attention deficit hyperactivity disorder. Hum Brain Mapp. 2010;31:904–916. doi: 10.1002/hbm.21058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Valera EM, Faraone SV, Murray KE, Seidman LJ. Meta-analysis of structural imaging findings in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2007;61:1361–1369. doi: 10.1016/j.biopsych.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 50.Mulder MJ, Baeyens D, Davidson MC, Casey BJ, van den Ban E, van Engeland H, et al. Familial vulnerability to ADHD affects activity in the cerebellum in addition to the prefrontal systems. J Am Acad Child Adolesc Psychiatry. 2008;47:68–75. doi: 10.1097/chi.0b013e31815a56dc. [DOI] [PubMed] [Google Scholar]
- 51.Williams RW, Herrup K. The control of neuron number. Annu Rev Neurosci. 1988;11:423–453. doi: 10.1146/annurev.ne.11.030188.002231. [DOI] [PubMed] [Google Scholar]
- 52.Durston S, Fossella JA, Casey BJ, Hulshoff Pol HE, Galvan A, Schnack HG, et al. Differential effects of DRD4 and DAT1 genotype on fronto-striatal gray matter volumes in a sample of subjects with attention deficit hyperactivity disorder, their unaffected siblings, and controls. Mol Psychiatry. 2005;10:678–685. doi: 10.1038/sj.mp.4001649. [DOI] [PubMed] [Google Scholar]
- 53.Zhong P, Liu W, Yan Z. Aberrant regulation of synchronous network activity by the attention deficit/hyperactivity disorder-associated human dopamine D4 receptor variant D4.7 in the prefrontal cortex. J Physiol. 2016;594(1):135–47. doi: 10.1113/JP271317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Castellanos FX, Sonuga-Barke EJ, Milham MP, Tannock R. Characterizing cognition in ADHD: beyond executive dysfunction. Trends Cogn Sci. 2006;10:117–123. doi: 10.1016/j.tics.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 55.Mulligan RC, Kristjansson SD, Reiersen AM, Parra AS, Anokhin AP. Neural correlates of inhibitory control and functional genetic variation in the dopamine D4 receptor gene. Neuropsychologia. 2014;62:306–318. doi: 10.1016/j.neuropsychologia.2014.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Szekely E, Sudre GP, Sharp W, Leibenluft E, Shaw P. Defining the neural substrate of the adult outcome of childhood ADHD: A multimodal neuroimaging study of response inhibition. Am J Psychiatry. 2017;174:867–876. doi: 10.1176/appi.ajp.2017.16111313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ha RY, Namkoong K, Kang JI, Kim YT, Kim SJ. Interaction between serotonin transporter promoter and dopamine receptor D4 polymorphisms on decision making. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:1217–1222. doi: 10.1016/j.pnpbp.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 58.Lee JS, Kim BN, Kang E, Lee DS, Kim YK, Chung JK, et al. Regional cerebral blood flow in children with attention deficit hyperactivity disorder: comparison before and after methylphenidate treatment. Hum Brain Mapp. 2005;24:157–164. doi: 10.1002/hbm.20067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schweitzer JB, Lee DO, Hanford RB, Tagamets MA, Hoffman JM, Grafton ST, et al. A positron emission tomography study of methylphenidate in adults with ADHD: alterations in resting blood flow and predicting treatment response. Neuropsychopharmacology. 2003;28:967–973. doi: 10.1038/sj.npp.1300110. [DOI] [PubMed] [Google Scholar]
- 60.O’Gorman RL, Mehta MA, Asherson P, Zelaya FO, Brookes KJ, Toone BK, et al. Increased cerebral perfusion in adult attention deficit hyperactivity disorder is normalised by stimulant treatment: a non-invasive MRI pilot study. Neuroimage. 2008;42:36–41. doi: 10.1016/j.neuroimage.2008.04.169. [DOI] [PubMed] [Google Scholar]
- 61.Lee JS, Kim BN, Kang EJ, Lee DS, Kim YK, Chung JK, et al. Regional cerebral blood flow in children with attention deficit hyperactivity disorder: Comparison before and after methylphenidate treatment. Hum Brain Mapp. 2005;24:157–164. doi: 10.1002/hbm.20067. [DOI] [PMC free article] [PubMed] [Google Scholar]