Abstract
Oligodendrocytes (OLs) are myelinating glial cells that form myelin sheaths around axons to ensure rapid and focal conduction of action potentials. Here, we found that an axonal outgrowth regulatory molecule, AATYK (apoptosis-associated tyrosine kinase), was up-regulated with OL differentiation and remyelination. We therefore studied its role in OL differentiation. The results showed that AATYK knockdown inhibited OL differentiation and the expression of myelin genes in vitro. Moreover, AATYK-deficiency maintained the proliferation status of OLs but did not affect their survival. Thus, AATYK is essential for the differentiation of OLs.
Keywords: AATYK, Differentiation, Oligodendrocytes
Introduction
Myelin sheaths elaborated by oligodendroglia (OLs) ensure rapid conduction and the integrity of axons in the central nervous system (CNS) [1, 2], which are crucial for the function of neural circuitry mediated by extensive neuron-glia interactions. Loss of OL function and myelin sheaths causes neurological diseases represented by multiple sclerosis [1, 3, 4]. In addition, emerging evidence has revealed OL and myelin deficits in psychiatric disorders such as schizophrenia [5]. It is now clear that OLs function in neuron survival, as OL impairment has been detected in neurodegenerative diseases including multiple sclerosis, Alzheimer’s disease, and Parkinson’s disease [6, 7]. To develop novel strategies to enhance OL differentiation and myelination in neurological disorders, it is essential to understand the mechanisms that underlie the control of OL differentiation and myelination.
OL progenitor cells (OPCs) are generated from pluripotent neural stem cells, and ultimately develop into mature and myelinating OLs after proliferation and differentiation. To identify candidate genes involved in OL myelinogenesis, we compared the mRNA array data from Nkx2.2-knockout mice with those from transgenic mice over-expressing Nkx2.2, two mouse models in which the differentiation of OLs is promoted or delayed, respectively, and many genes responded to altered OL differentiation (unpublished data). Interestingly, the axonal outgrowth regulatory molecule AATYK (apoptosis-associated tyrosine kinase) was up-regulated with OL differentiation. We therefore hypothesized that AATYK participates in OL differentiation and myelination.
Here, we further explored the role of AATYK in OL differentiation and myelinogenesis to test this hypothesis.
Materials and Methods
Animals
C57BL/6N mice were obtained from the Jackson Laboratory (Bar Harbor, ME). For statistics, cell number and relative expression levels of target proteins were calculated for each genotype (n = 3). The mice used in this study were handled according to protocols approved by Institutional Animal Care and Use Committee, University of Louisville (IACUC: 12034).
Immunohistochemistry and In Situ Hybridization (ISH)
Mice were deeply anesthetized with 2% tribromoethanol (Avertin) at the dose of 20 μL/g via intraperitoneal injection and perfused with 4% paraformaldehyde (PFA), and tissues were isolated and postfixed in 4% PFA overnight at 4°C, then kept in 20% sucrose in phosphate-buffered saline overnight at 4°C. The tissues were then embedded in OCT and cut into 16-μm sections. Immunohistochemistry and ISH were performed according to the description by Chen et al. [8]. The riboprobe sequence for AATYK was F: 5′-GCACAAAGGAATCACTCCCTAC-3′; R: 5′-GCTTACAGACACCCATAGACCC-3′. The dilution of anti-Olig2 antibody (Abcam, Cambridge, MA) was 1:1000.
Immunofluorescence Staining
Double-immunofluorescence procedures were as described by Chen et al. [8]. The dilutions of antibodies were as follows: anti-MBP (myelin basic protein) (Abcam, 1:500), anti-MAG (myelin-associated glycoprotein) (Millipore, Burlington, MA; 1:500), and anti-Ki67 (Thermo, Grand Island, NE, 1:500).
Cuprizone Treatment
Wild-type mice (8–10 weeks old) were fed a diet containing 0.2% cuprizone (w/w) (biscyclohexane oxaldihydrazone; Sigma-Aldrich) for 6 weeks to induce demyelination. Remyelination was assayed after returning them to normal chow for 2 weeks. Control mice were maintained on a normal diet.
Western Blotting
Cells were lysed in lysis buffer (Sigma) with a protease inhibitor cocktail (Sigma). Western blotting was performed as previously described [8]. SDS-PAGE gels were subsequently detected with anti-MBP (Abcam, 1:2 000), anti-caspase3 (Millipore, 1:1 000), and anti-GAPDH (Sigma, 1:5 000) antibodies.
Rat OPC Culture, Lentivirus Construction, and OPC Infection
Cerebral cortices from newborn Sprague-Dawley rats were used for OPC primary culture, according to protocols described by Chen et al. [8]. For RNAi-mediated depletion of AATYK, a lentivirus was used to express validated shRNA specifically targeting AATYK mRNA with the following sequence: 5′-GGC AAG AAG CAA GUG UCU TAC-3′ (GenePharma, Shanghai, China). A negative control shRNA lentivirus expressing a scrambled sequence without homology to any known mammalian mRNA was used in parallel. Rat OPCs were transfected with lentiviral AATYK-shRNA 72 h before experiments. The infection efficiency was analyzed on day 2 post-infection using GFP expression, and revealed to be > 90%. Six days after infection, OPCs were collected for experiments.
Statistical Analysis
Data are presented as mean ± SEM and were analyzed by Student’s t-test for two-group comparison. P < 0.05 was considered to be statistically significant.
Results and Discussion
AATYK is Selectively Expressed in Differentiating OLs
As our microarray results suggested that AATYK is involved in OL differentiation (unpublished data), we further tested this possibility by studying the AATYK expression pattern in the developing spinal cord using RNA ISH. The results showed that AATYK started to be expressed around embryonic day 18.5 (E18.5) in the spinal white matter, showing no specific expression at E16.5 (Fig. 1A, B). Its expression progressively increased thereafter (Fig. 1C, D), and at postnatal day 15 (P15), its expression was detected throughout the spinal cord (Fig. 1E). By P30, its expression was down-regulated in the white matter (Fig. 1F). These results suggested that AATYK is expressed in OLs. To test this possibility, we performed double-staining in the white matter of the brain. As expected, all AATYK+ cells in the P15 corpus callosum were co-labeled with the OL-specific marker Olig2 [9] (Fig. 1G). In addition, we examined AATYK expression in Olig1 mutant and Myrf conditional knock-out (CKO) mice, in which OL differentiation is significantly delayed and impaired [10–12]. We found that the AATYK+ cells in the white matter were completely lost in Olig1 mutant and Myrf-CKO mice (Fig. 1H, I). Together, these findings demonstrated that AATYK is expressed in differentiating OLs in the CNS. AATYK is known to be highly expressed in neurons, and is involved in axonal outgrowth [13]. Here we presented the first evidence that AATYK is also expressed in oligodendrocytes (Fig. 1A–G), although it was also detectable in neurons in the spinal gray matter (Fig. 1F). Moreover, AATYK was highly expressed by differentiating OLs, suggesting that AATYK helps to promote and stabilize OL differentiation.
Fig. 1.
AATYK is up-regulated in differentiating OLs. A–F Sections of spinal cord from E16.5, E18.5, P4, P7, P15, and P30 mice subjected to ISH with AATYK riboprobe. Expression of AATYK started at ~ E18.5 in the spinal white matter. Its expression progressively increased between E18.5 and P7, and then was scattered throughout the cord at P15. By P30, AATYK expression had gradually decreased in the white matter and was detectable in the gray matter. Arrows indicate white matter cells; arrowheads indicate gray matter cells. G AATYK-expressing cells immunoreactive to Olig2 in the corpus callosum at P15. H–I The numbers of AATYK+ cells were reduced drastically in Myrf CKO mice at P3. Arrows indicate white matter cells; arrowheads indicate AATYK+ Olig2+ double-positive cells. Scale bars, 50 μm.
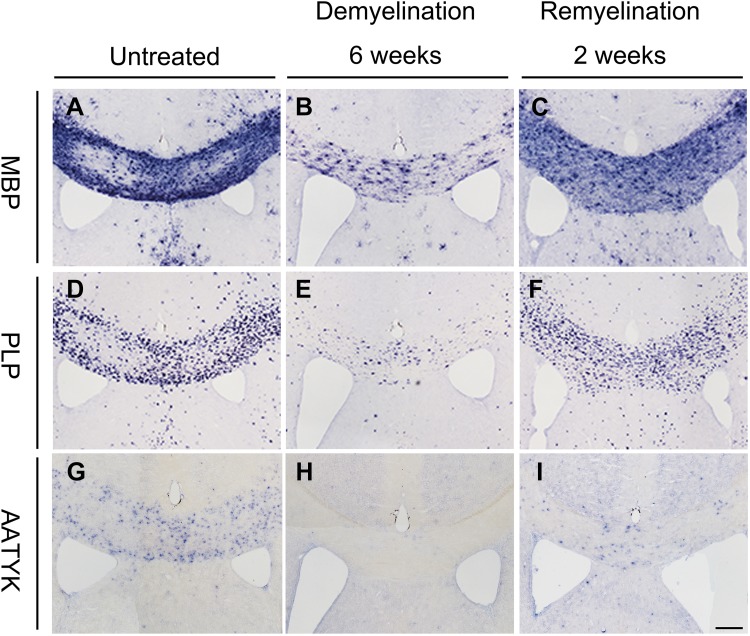
Up-regulation of AATYK During Remyelination in a Cuprizone-Induced Demyelination Model
The overlap of the spatiotemporal pattern of AATYK expression with the myelination process (Fig. 1) indicated that AATYK is involved in myelinogenesis in the CNS. Therefore we evaluated the expression of AATYK in the remyelination model induced by cuprizone [14]. Wild-type mice were fed cuprizone for 6 weeks, which induced massive demyelination in the corpus callosum, followed by spontaneous myelin repair within 2 weeks after cuprizone removal (Fig. 2A–I). We performed ISH with MBP and myelin proteolipid protein (PLP) probes to assess the remyelination status. The results showed that after 6 weeks of cuprizone treatment, severe demyelination of the corpus callosum occurred (Fig. 2B, E, H). Two weeks of recovery resulted in more MBP+ and PLP+ cells in the corpus callosum (Fig. 2C, F), similar to the AATYK+ cells (Fig. 2I), supporting the hypothesis that AATYK is involved in OL myelinogenesis.
Fig. 2.
AATYK is up-regulated during remyelination. In situ hybridization with MBP (A, B) or PLP probes (D, E) showed severe demyelination of the corpus callosum in wild-type mice after 6 weeks of cuprizone treatment as compared to untreated animals. Compared with the recovered expression of MBP and PLP (C, F), AATYK was observed in the corpus callosum of mice after recovery for 2 weeks (G, H, I). Scale bar, 100 μm.
Absence of AATYK Inhibits Oligodendrocyte Differentiation
Since AATYK is expressed in differentiating OLs and up-regulated during myelinogenesis, its deletion may affect the differentiation of OLs. To test this, primary cultures of OPCs were infected with lentivirus co-expressing green fluorescent protein (GFP) and short hairpin (sh) RNA of AATYK 3 days prior to differentiation assays. As expected, the qPCR results showed that AATYK expression in OLs was significantly inhibited by shRNA interference (Fig. 3Q). Meanwhile, the numbers of mature MAG+ and MBP+ OLs were remarkably decreased in the RNAi-AATYK cells compared to controls (Fig. 3C, G, K, O). Quantitative analysis also showed that the percentages of MBP+ and MAG+ OLs in GFP+ RNAi-AATYK-infected cells were significantly lower than in controls (Fig. 3R). Moreover, OLs from shRNA-AATYK cells displayed typical immature processes (Fig. 3F, N). Therefore, these results demonstrated that AATYK positively regulates the differentiation of OLs, and its absence inhibits OL differentiation. These findings raise the possibility that AATYK is essential for the differentiation of OLs and consequential OL myelination. This expression pattern (Fig. 1) and the function of AATYK in OL development are similar to myelin regulatory factor (MRYF), which is also expressed in differentiating OLs, and is required for CNS myelination and its maintenance [11, 15].
Fig. 3.
Ablation of AATYK inhibits OL differentiation. Primary cultured OPCs infected with GFP-tagged ShRNA-AATYK or control lentivirus (A, B, E, F, I, J, M, and N). AATYK was significantly down-regulated by shRNA in OPCs by qPCR (Q). Compared to control cells, the numbers of MAG+ and MBP+ cells were remarkably decreased in the RNAi-AATYK treated cells (C, G, D, H, K, O, L, and P). The percentages of co-labeled MAG+ or MBP+ with GFP+ OLs in GFP+ cells among RNAi-AATYK cells were significantly lower than that in the control (R). Arrows indicate GFP+ and MAG+/MBP+ double-positive cells; arrowheads indicate immature cells. Scale bars, 100 μm.
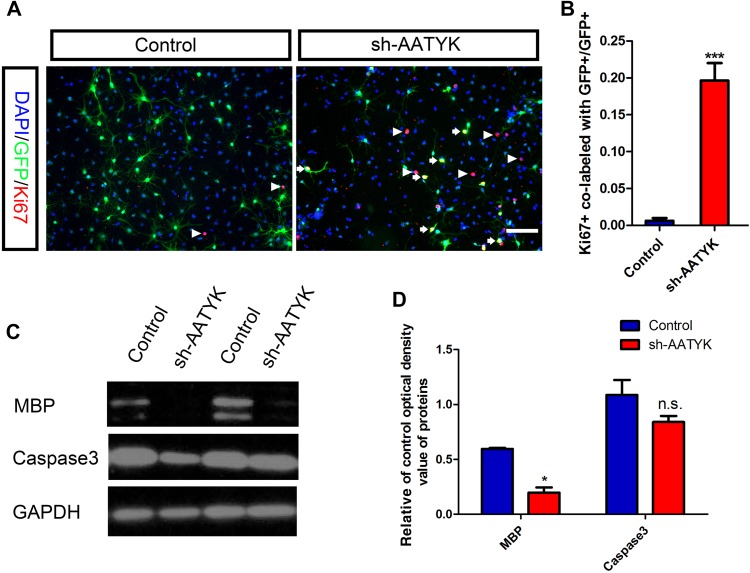
Ablation of AATYK Affects the Proliferation of OPCs but not Their Survival
To address the possibility that inhibition of OPC differentiation by AATYK ablation is due to the induction of cell death or proliferation, western blot with anti-MBP and anti-caspase3 antibodies and Ki67 immunostaining were performed. Consistent with the above results, the western immunoblotting revealed a similar decrease of MBP expression in AATYK-knockdown cells. However, there was no significant difference in caspase3 expression between control and RNAi-AATYK OPCs, indicating that cell death and apoptosis were not affected by AATYK-deficiency (Fig. 4C, D). Interestingly, we found that there were more Ki67+ and GFP+ co-labeled cells among the Sh-AATYK-treated OPCs than in controls (Fig. 4A), and statistical analysis showed significantly higher percentages of Ki67+ and GFP+ co-labeled cells among GFP+ cells treated with sh-AATYK than those with the control vector (Fig. 4B). These results suggest that AATYK knockdown does maintain the proliferation status of OLs, in other words, AATYK is essential for the differentiation of OLs.
Fig. 4.
AATYK knockdown does not affect the survival but maintains proliferation of OPCs. A Ki67 immunostaining showed there were more Ki67+ and GFP+ co-labeled cells in the sh-AATYK-treated OPCs than in the control. B Percentages of Ki67+ and GFP+ co-labelled OLs in GFP+ cells among RNAi-AATYK cells were significantly higher than in the control. C–D Compared to control cells, the expression of MBP protein was significantly decreased in shRNA-AATYK-treated cells and there was no difference in caspase3 between the two groups. Scale bar, 100 μm.
In the CNS, both extracellular signals and intracellular pathways control OPC differentiation and myelination during development [16]. Recently, a number of researchers have demonstrated that endocytosis plays a key role in regulating signal transduction during cell differentiation. For example, after activation by Wnt-ligands, Frizzled is internalized via a clathrin-mediated pathway and subsequently degraded, which can induce aberrant embryonic development [17–20]; GPR17, which plays a crucial role in OPC differentiation [21], is partially degraded or recycled to the plasma membrane through recycling endosomes after endocytosis [22]. Interestingly, AATYK regulates the trafficking of recycling endosomes in CHO-K1 cells and is involved in axonal outgrowth [23]. It is conceivable that AATYK-deficiency affects the formation of recycling endosomes, and thus upsets the balance between degradation and recycling for modulation of receptor levels on the cell surface such as GPR17, which is highly expressed in OPCs but down-regulated in mature OLs to ensure full myelination [24–26], and thereby, affect OL differentiation and myelination. However, the underlying mechanisms are currently unknown, and future studies with the conditional knockout approach are needed to elucidate the functions of AATYK and related signaling pathways in OL differentiation and myelination.
Acknowledgements
This work was supported by the National Natural Sciences Foundation of China (31471955) and the Natural Science Foundation of Zhejiang Province, China (LY17C090006; Q16C090017; LY18H090014).
Compliance with Ethical Standards
Conflict of interest
All authors claim that there are no conflicts of interest.
Contributor Information
Mengsheng Qiu, Email: m0qiu001@yahoo.com.
Xiaofeng Zhao, Email: xiaofengzhao@yahoo.com.
References
- 1.Miron VE, Kuhlmann T, Antel JP. Cells of the oligodendroglial lineage, myelination, and remyelination. Biochim Biophys Acta. 2010;1812:184–193. doi: 10.1016/j.bbadis.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 2.Nave KA. Myelination and support of axonal integrity by glia. Nature. 2011;468:244–252. doi: 10.1038/nature09614. [DOI] [PubMed] [Google Scholar]
- 3.Bruce CC, Zhao C, Franklin RJ. Remyelination - An effective means of neuroprotection. Horm Behav. 2009;57:56–62. doi: 10.1016/j.yhbeh.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 4.Steinman L. Multiple Sclerosis: a coordinated immunological attack against myelin in central nervous system. Cell. 1998;85:299–302. doi: 10.1016/S0092-8674(00)81107-1. [DOI] [PubMed] [Google Scholar]
- 5.Feng Y. Convergence and divergence in the etiology of myelin impairment in psychiatric disorders and drug addiction. Neurochem Res. 2008;33:1940–1949. doi: 10.1007/s11064-008-9693-x. [DOI] [PubMed] [Google Scholar]
- 6.Bartzokis G. Age-related myelin breakdown: a developmental model of cognitive decline and Alzheimer’s disease. Neurobiol Aging 2004, 25: 5–18; author reply 49–62. [DOI] [PubMed]
- 7.Sokolov BP. Oligodendroglial abnormalities in schizophrenia, mood disorders and substance abuse. Comorbidity, shared traits, or molecular phenocopies? Int J Neuropsychopharmacol 2007: 1–9. [DOI] [PubMed]
- 8.Chen YD, Mei RY, Teng P, Yang AF, Hu XM, Zhang ZY, et al. TAPP1 inhibits the differentiation of oligodendrocyte precursor cells via suppressing the Mek/Erk pathway. Neurosci Bull 2015: 1–10. [DOI] [PMC free article] [PubMed]
- 9.Redwine JM, Evans CF. Markers of central nervous system glia and neurons in vivo during normal and pathological conditions. Curr Top Microbiol Immunol. 2002;265:119–140. doi: 10.1007/978-3-662-09525-6_6. [DOI] [PubMed] [Google Scholar]
- 10.Zhu Q, Zhao XF, Zheng K, Li H, Huang H, Zhang Z, et al. Genetic evidence that Nkx2.2 and Pdgfra are major determinants of the timing of oligodendrocyte differentiation in the developing CNS. Development 2014, 141: 548–555. [DOI] [PMC free article] [PubMed]
- 11.Emery B, Agalliu D, Cahoy JD, Watkins TA, Dugas JC, Mulinyawe SB, et al. Myelin gene regulatory factor is a critical transcriptional regulator required for CNS myelination. Cell. 2009;138:172–185. doi: 10.1016/j.cell.2009.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koenning M, Jackson S, Hay CM, Faux C, Kilpatrick TJ, Willingham M, et al. Myelin gene regulatory factor is required for maintenance of myelin and mature oligodendrocyte identity in the adult CNS. J Neurosci. 2012;32:12528–12542. doi: 10.1523/JNEUROSCI.1069-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takano T, Tomomura T, Yoshioka N, Tsutsumi K, Terasawa Y, Saito T, et al. LMTK1/AATYK1 is a novel regulator of axonal outgrowth that acts via Rab11 in a Cdk5-dependent manner. J Neurosci. 2012;39:6587–6599. doi: 10.1523/JNEUROSCI.5317-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang A, Tourtellotte WW, Rudick R, Trapp BD. Premyelinating oligodendrocytes in chronic lesions of multiple sclerosis. N Engl J Med. 2002;346:165–173. doi: 10.1056/NEJMoa010994. [DOI] [PubMed] [Google Scholar]
- 15.Koenning M, Jackson S, Hay CM, Faux C, Kilpatrick TJ, Willingham M, et al. Myelin gene regulatory factor is required for maintenance of myelin and mature oligodendrocyte identity in the adult CNS. J Neurosci. 2012;32:12528–12542. doi: 10.1523/JNEUROSCI.1069-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fu P, Tang R, Yu Z, Li C, Chen X, Xie M, et al. Rho-associated kinase inhibitors promote microglial uptake via the ERK signaling pathway. Neurosci Bull. 2016;32:83–91. doi: 10.1007/s12264-016-0013-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Piddini E, Marshall F, Dubois L, Hirst E, Vincent JP. Arrow (LRP6) and Frizzled2 cooperate to degrade Wingless in Drosophila imaginal discs. Development. 2005;132:5479–5489. doi: 10.1242/dev.02145. [DOI] [PubMed] [Google Scholar]
- 18.Rives AF, Rochlin KM, Wehrli M, Schwartz SL, Di Nardo S. Endocytic trafficking of Wingless and its receptors, Arrow and DFrizzled-2, in the Drosophila wing. Dev Biol. 2006;293:268–683. doi: 10.1016/j.ydbio.2006.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seto ES, Bellen HJ. Internalization is required for proper Wingless signaling in Drosophila melanogaster. J Cell Biol. 2006;173:95–106. doi: 10.1083/jcb.200510123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu A, Rual JF, Tamai K, Harada Y, Vidal M, He X, et al. Association of Dishevelled with the clathrin AP-2 adaptor is required for Frizzled endocytosis and planar cell polarity signaling. Dev Cell. 2007;12:129–141. doi: 10.1016/j.devcel.2006.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen Y, Wu H, Wang H, Koito H, Li J, Ye F, et al. The oligodendrocyte-specific G protein-coupled receptor GPR17 is a cell-intrinsic timer of myelination. Nat Neurosci. 2009;12:1398–1406. doi: 10.1038/nn.2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fratangeli A, Parmigiani E, Fumagalli M, Lecca D, Benfante R, Passafaro M, et al. The regulated expression, intracellular trafficking and membrane recycling of the P2Y-like receptor GPR17 in Oli-neu oligodendroglial cells. J Biol Chem. 2013;288:5241–5256. doi: 10.1074/jbc.M112.404996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takano T, Tsutsumi K, Saito T, Asada A, Tomomura M, Fukuda M, et al. AATYK1A phosphorylation by Cdk5 regulates the recycling endosome pathway. Genes Cells. 2010;15:783–797. doi: 10.1111/j.1365-2443.2010.01419.x. [DOI] [PubMed] [Google Scholar]
- 24.Boda E, Viganò F, Rosa P, Fumagalli M, Labat-Gest V, Tempia F, et al. The GPR17 receptor in NG2 expressing cells: focus on in vivo cell maturation and participation in acute trauma and chronic damage. Glia. 2011;59:1958–1973. doi: 10.1002/glia.21237. [DOI] [PubMed] [Google Scholar]
- 25.Chen Y, Wu H, Wang S, Koito H, Li J, Ye F, et al. The oligodendrocyte-specific G protein-coupled receptor GPR17 is a cell-intrinsic timer of myelination. Nat Neurosci. 2009;12:1398–1406. doi: 10.1038/nn.2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fumagalli M, Daniele S, Lecca D, Lee PR, Parravicini C, Fields RD, et al. Phenotypic changes, signaling pathway, and functional correlates of GPR17-expressing neural precursor cells during oligodendrocyte differentiation. J Biol Chem. 2011;286:10593–10604. doi: 10.1074/jbc.M110.162867. [DOI] [PMC free article] [PubMed] [Google Scholar]