Abstract
The ZNF804A variant rs1344706 has consistently been associated with schizophrenia and plays a role in hippocampal-prefrontal functional connectivity during working memory. Whether the effect exists in the resting state and in patients with schizophrenia remains unclear. In this study, we investigated the ZNF804A polymorphism at rs1344706 in 92 schizophrenic patients and 99 healthy controls of Han Chinese descent, and used resting-state functional magnetic resonance imaging to explore the functional connectivity in the participants. We found a significant main effect of genotype on the resting-state functional connectivity (RSFC) between the hippocampus and the dorsolateral prefrontal cortex (DLPFC) in both schizophrenic patients and healthy controls. The homozygous ZNF804A rs1344706 genotype (AA) conferred a high risk of schizophrenia, and also exhibited significantly decreased resting functional coupling between the left hippocampus and right DLPFC (F(2,165) = 13.43, P < 0.001). The RSFC strength was also correlated with cognitive performance and the severity of psychosis in schizophrenia. The current findings identified the neural impact of the ZNF804A rs1344706 on hippocampal-prefrontal RSFC associated with schizophrenia.
Electronic supplementary material
The online version of this article (10.1007/s12264-018-0221-y) contains supplementary material, which is available to authorized users.
Keywords: Schizophrenia, ZNF804A, Imaging genetics, Hippocampus, Dorsolateral prefrontal cortex
Introduction
Schizophrenia is a severe neuropsychiatric disorder with a complex etiology, which exhibits a considerable level of heritability [1, 2]. Previous studies have shown that abnormal brain structure and function are important intermediate phenotypes of schizophrenia [3, 4]. Imaging genetics and genomics research has further linked genetic variations to brain structure and function, indicating that genetic risk factors impact cognition, emotion, and behavior in both healthy persons and patients with diseases [5, 6].
The altered hippocampal–prefrontal connectivity along with related cognitive impairments in patients with schizophrenia might be an important aspect of the pathophysiology [7, 8]. Meyer-Lindenberg and colleagues found that, during a working memory task with a low load, both controls and patients with schizophrenia showed a negative correlation between activity in the hippocampal formation and that in the contralateral dorsolateral prefrontal cortex (DLPFC). In contrast, when switched to a high working memory load, the correlation remained in patients but diminished in controls, suggesting a region-specific alteration of hippocampal-DLPFC functional connectivity in schizophrenia [9]. The hippocampal–DLPFC coupling during working memory activation has also been reported in healthy relatives of patients [10] and healthy carriers of risk genotypes for schizophrenia [11, 12].
Zinc finger protein 804A (ZNF804A) is one of the candidate genes for schizophrenia recognized by genome-wide association studies of European samples (rs1344706, P = 1.61 × 10−7) [13]. In Asian populations, a few association studies have been conducted for ZNF804A rs1344706 and the results were inconsistent [14–17]. Overexpression of Znf804a mRNA in rat neural progenitor cells has been reported to significantly change the expression of schizophrenia-associated genes [18]. A landmark study of neuronal function with suppressed expression of ZNF804A in both human and rat cells has revealed its function in neurite formation, the maintenance of dendritic spine morphology, and responses to activity-dependent stimuli [19]. All these results suggest that ZNF804A is one of the most intriguing and promising risk genes for schizophrenia and a psychotic phenotype [20].
Rs1344706, a single nucleotide polymorphism (SNP) located at intron 2 of ZNF804A, has been reported to confer a risk of schizophrenia across several populations, A being the risk allele [21]. Neuroimaging studies have indicated its potential effect on brain structure [22, 23]. Efforts have also been made to understand its effect on multiple brain functions. Reduced inter-hemispheric DLPFC connectivity with a higher rs1344706 risk status has been reported during working memory, and this persists in both resting and cognitive states. However, the increased connectivity between the left hippocampus and right DLPFC has been exclusively reported in the n-back working memory task [11, 24]. Paulus and colleagues also reported positive functional connectivity between the left hippocampus and right DLPFC for the AA genotype and negative functional connectivity for the CC and CA genotypes [25].
Previous functional magnetic resonance imaging (fMRI) studies mainly concentrated on the association between the ZNF804A polymorphism and functional coupling of the left hippocampus and right DLPFC during working memory tasks. However, the effect of the ZNF804A polymorphism on the resting-state functional connectivity (RSFC) in patients with schizophrenia remains elusive. In the current study, we investigated the effects of ZNF804A rs1344706 on the RSFC of the hippocampus voxel-wise in the whole brain in patients with schizophrenia and healthy controls. Then, together with cognitive performance and assessment of psychosis severity, we tested whether their effects on RSFC are associated with behavior.
Materials and Methods
Participants
A total of 92 patients with schizophrenia and 99 healthy controls, who are all Han Chinese from northern China, were recruited in the Peking University Sixth Hospital. In the patients, two experienced psychiatrists made a diagnosis according to the Structured Clinical Interview for Diagnostic and the Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR) Axis I Disorders (SCID, patient edition). Patients with any other neurological disorder, a history of severe medical illness, substance dependence, pregnancy, or treatment with electroconvulsive therapy within the past 6 months, and those with a diagnosis of any other Axis I disorder, were excluded. The healthy controls were screened using SCID (non-patient edition) and recruited if they had no history of mental and/or neurological disorder, drug or alcohol abuse, traumatic brain injury, or visible brain lesions on conventional MRI. Written informed consent was given by all patients and their legal guardians (i.e., parents) and by all healthy controls. This study was approved by the Medical Research Ethics Committees of Mental Health of Peking University Sixth Hospital.
Assessment of Symptomatology and Cognitive Performance
The severity of symptoms in the schizophrenic patients was evaluated by trained and experienced psychiatrists within one week of MRI scanning, using the Positive and Negative Syndrome Scale (PANSS). Category Fluency Test-animal naming (CFT) [26], the Digit Symbol Substitution Task (DSST) from the Wechsler Adult Intelligence Scale-III (WAIS-III) [27], and the Wechsler Memory Scale-Revised (WMS-R) [28] were also assessed to measure cognitive performance in the speed of processing and memory domains that are commonly impaired in schizophrenia [29–31].
Genotyping
Peripheral blood samples were collected from all participants and genomic DNA was extracted using a Qiagen QIAamp DNA Mini Kit (Germany). The SNP ZNF804A rs1344706 was selected from dbSNP (http://www.ncbi.nlm.nih.gov/SNP/) and genotyped using the TaqMan SNP genotyping assay on an ABI PRISM 7900 Sequence Detection System (Applied Biosystems, Foster City, CA), as described previously [32]. Investigators were blind to the case or control status during the genotyping process. We repeated the genotyping assay in 1% of the samples and the results were 100% concordant. The DNA extraction and genotyping were centrally processed at the Key Laboratory of Mental Health in Beijing, China.
Imaging Data Acquisition
MRI scans were performed on a Siemens 3.0 Tesla Trio magnetic resonance scanner (Siemens Medical Systems, Erlangen, Germany) in Peking University Third Hospital. The resting-state functional imaging data were acquired with the following parameters: repetition time = 2000 ms, echo time = 30 ms, field of view = 220 × 220 mm2, matrix = 64 × 64, flip angle = 90°, voxel size = 3.4 × 3.4 × 4.0 mm3, 33 slices and 240 volumes. Before scanning, all participants were instructed to move as little as possible, keep their eyes closed, think of nothing in particular, and avoid falling asleep. After scanning, they were asked whether they fell asleep to reconfirm.
Resting-State fMRI Pre-processing
Data preprocessing of resting-state fMRI was completed using DPARSF (Data Processing Assistant for Resting State fMRI Advanced Edition, http://rfmri.org/DPARSF). The following steps were performed: (1) discarding the first 10 volumes from each participant; (2) slice timing correction; (3) realigning the volumes to the middle volume; (4) regressing out nuisance covariate signals including white matter, cerebrospinal fluid, and global signals; (5) spatial normalization by DARTEL (Diffeomorphic Anatomical Registration Through Exponentiated Lie Algebra); (6) smoothing with a 4-mm Gaussian kernel after resampling to 3-mm isotropic voxels; (7) linear regression to remove the effects of linear trends; and (8) temporal bandpass filtering (0.01–0.1 Hz). Specifically, healthy controls and schizophrenic patients exhibiting a maximum displacement of >3 mm in any of the cardinal directions (x, y, z) or a maximum rotation (x, y, z) of >3° were excluded. Finally, a total of 94 healthy controls and 79 schizophrenic patients were included in the functional connectivity analyses.
Statistical Analyses
The effect of gender was analyzed using Pearson’s χ2 test, and differences in continuous demographic variables, cognitive performance, and PANSS scores were analyzed by one-way ANOVA. Hardy-Weinberg equilibrium between expected and observed genotype distributions was tested using the χ2 test. Correlations between cognitive performance, symptom severity, and RSFC strength were computed using two-tailed Pearson correlations. These analyses were performed using the Statistical Package for the Social Sciences for Windows, version 13.0 (SPSS, Chicago, IL). P < 0.05 was considered statistically significant.
The functional imaging analysis was performed using SPM8 (http://www.fil.ion.ucl.ac.uk/spm). We calculated individual voxel-wise hippocampal RSFC maps with a mask of the whole brain by taking the left and right hippocampus as regions-of-interest (ROIs). Hippocampal masks were constructed using the automated anatomical labeling atlas implemented in Wake Forest University Pickatlas [33]. Individual RSFC maps were generated by calculating the Pearson correlation coefficient between the average blood oxygen level-dependent time series in the ROIs and those of each voxel in the whole brain. Then, the correlation coefficients were converted to z values by Fisher’s z transformation to improve normality. The individual z maps were then entered into a two-way ANOVA with diagnosis and genotype as between-subject factors and age and gender as covariates. A voxel-level threshold of P < 0.001 with a cluster extent k > 10 was considered significant. For each diagnostic group, a partial correlation analysis with age and gender as covariates in order to exclude their potential effect [34, 35] was further performed by genotype subgroup to explore the associations between the RSFC strength and cognitive performance as well as those between the RSFC strength and PANSS scores in patients.
Results
Cognitive Performance
There were no significant differences in gender distribution, age, education, and genotype between schizophrenic patients and healthy controls. Both case and control samples in each diagnostic group were in Hardy-Weinberg equilibrium (P > 0.05). Patients had significantly poorer cognitive performance in WMS-R (P = 1 × 10−6), DSST (P < 1×10−6), and CFT (P = 0.001) (Table 1). A significant difference of gender distribution was only found among the genotype subgroups of ZNF804A rs1344706 in schizophrenia due to fewer females in the AA subgroup (P = 0.011). No significant differences in WMS-R, DSST, and CFT were found between the genotype subgroups of rs1344706 in either diagnostic group (Table 2).
Table 1.
Demographic and clinical characteristics of healthy controls and schizophrenic patients.
| Variables | Schizophrenic patients | Healthy controls | F or χ2 | P value |
|---|---|---|---|---|
| Gender (male/female) | 49/30 | 49/45 | 1.71 | 0.19 |
| Age (years) | 27.2 ± 6.8 | 25.8 ± 5.4 | 2.30 | 0.25 |
| Education (years) | 13.9 ± 2.7 | 13.7 ± 3.5 | 0.19 | 0.64 |
| Genotype of rs1344706 (CC/CA/AA) | 18/41/20 | 24/45/25 | 0.30 | 0.86 |
| WMS-R scorea | 93.75 ± 21.07 | 109.31 ± 17.00 | 25.19 | <0.001 |
| DSST scoreb | 52.26 ± 13.33 | 67.67 ± 13.79 | 52.99 | <0.001 |
| CFT scoreb | 18.68 ± 5.35 | 21.73 ± 6.48 | 10.66 | 0.001 |
| PANSS total score | 76.85 ± 12.94 | n.a. | n.a. | n.a. |
| PANSS positive score | 23.63 ± 4.33 | n.a. | n.a. | n.a. |
| PANSS negative score | 18.51 ± 5.70 | n.a. | n.a. | n.a. |
| PANSS general score | 35.68 ± 5.43 | n.a. | n.a. | n.a. |
Data are given as mean ± SD; P-values refer to one-way ANOVA (parametric data) and the χ2 test (categorical data). WMS-R, Wechsler Memory Scale-Revised; DSST, Digit Symbol Substitution Test; CFT, Category Fluency Test-animal naming; PANSS, Positive and Negative Syndrome Scale; n.a., not applicable. aData were from 59 patients and 94 controls; bData were from 74 patients and 93 controls.
Table 2.
Demographic and clinical characteristics of healthy controls and schizophrenic patients with different genotypes of rs1344706.
| rs1344706 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SZ | HC | |||||||||
| CC | CA | AA | F or χ2 | P value | CC | CA | AA | F or χ2 | P value | |
| Gender (male/female) | 10/8 | 21/20 | 18/2 | 9.00 | 0.01 | 12/12 | 23/22 | 14/11 | 0.21 | 0.90 |
| Age (years) | 27.8 ± 6.7 | 27.5 ± 6.9 | 26.2 ± 7.0 | 0.36 | 0.70 | 24.5 ± 5.2 | 26.0 ± 5.4 | 26.8 ± 5.7 | 1.24 | 0.30 |
| Education (years) | 14.1 ± 2.3 | 14.3 ± 2.5 | 12.8 ± 3.3 | 2.08 | 0.13 | 14.4 ± 2.8 | 13.8 ± 3.0 | 12.9 ± 4.3 | 1.25 | 0.29 |
| WMS-R scorea | 91.47 ± 18.30 | 99.57 ± 21.12 | 86.39 ± 20.39 | 2.36 | 0.10 | 115.46 ± 12.76 | 108.07 ± 15.26 | 105.64 ± 21.96 | 2.34 | 0.10 |
| DSST scoreb | 56.18 ± 9.79 | 53.46 ± 14.27 | 46.70 ± 12.92 | 2.75 | 0.07 | 68.48 ± 13.39 | 68.07 ± 14.50 | 66.20 ± 13.29 | 0.20 | 0.82 |
| CFT scoreb | 19.59 ± 4.95 | 19.24 ± 5.70 | 16.85 ± 4.78 | 1.65 | 0.20 | 20.83 ± 5.18 | 22.05 ± 7.13 | 22.04 ± 6.57 | 0.31 | 0.74 |
| PANSS total score | 82.67 ± 10.19 | 73.15 ± 14.89 | 79.20 ± 7.79 | 4.13 | 0.02 | n.a. | n.a. | n.a. | n.a. | n.a. |
| PANSS positive score | 24.50 ± 3.81 | 23.32 ± 4.85 | 23.50 ± 3.68 | 0.47 | 0.62 | n.a. | n.a. | n.a. | n.a. | n.a. |
| PANSS negative score | 19.78 ± 5.96 | 17.03 ± 5.63 | 20.35 ± 5.03 | 2.99 | 0.06 | n.a. | n.a. | n.a. | n.a. | n.a. |
| PANSS general score | 38.39 ± 6.56 | 34.66 ± 5.09 | 35.35 ± 4.32 | 3.17 | 0.05 | n.a. | n.a. | n.a. | n.a. | n.a. |
| Chlorpromazine equivalent dose (mg/day) | 436.11 ± 219.50 | 438.75 ± 205.53 | 482.50 ± 163.25 | 0.86 | 0.65 | n.a. | n.a. | n.a. | n.a. | n.a. |
Data are given as mean ± SD; P-values refer to one-way ANOVA (parametric data) and the χ2 test (categorical data). SZ, schizophrenic patients; HC, healthy controls; WMS-R, Wechsler Memory Scale-Revised; DSST, Digit Symbol Substitution Test; CFT, Category Fluency Test-animal naming; PANSS, Positive and Negative Syndrome Scale; n.a., not applicable. aData were from 59 patients and 94 controls; bData were from 74 patients and 93 controls.
Effect of Diagnosis on Hippocampal-Thalamic RSFC
Two-way ANOVA showed a significant main effect of diagnosis on RSFC. We found increased RSFCs between the bilateral hippocampus and the right thalamus in schizophrenic patients, compared with the healthy controls (Table S1, Fig. S1). We maintained a cluster-level false discovery rate (FDR) at P < 0.05 using a voxel-level threshold of P < 0.001 with a cluster extent k > 10.
Effect of ZNF804A rs1344706 on Hippocampal-Frontal RSFC
Two-way ANOVA showed a significant main effect of genotype on the RSFC between the bilateral hippocampus and the right DLPFC. No significant interactive effect between genotype and diagnosis was found. As the hippocampus on each side had similar results, we only present results for the left hippocampus here. Data for the right hippocampus are available on request.
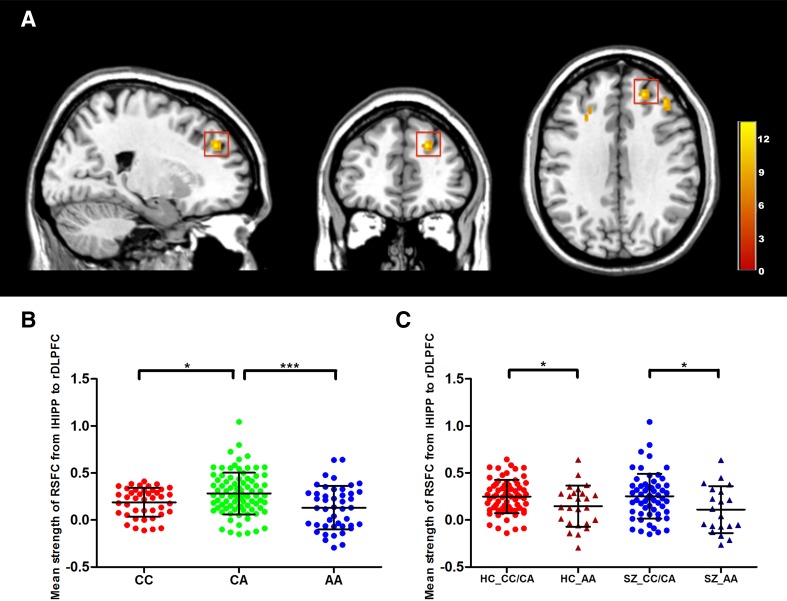
When the left hippocampus was treated as the seed region, genotype had a significant effect on the RSFC between the left hippocampus and the right dorsolateral superior and middle frontal gyri in the right DLPFC (Table S2). Results in the right dorsolateral superior frontal gyrus are shown in Fig. 1A (details of the right dorsolateral middle frontal gyrus are shown in Table S3 and Fig. S2) and those in the left middle frontal gyrus are shown in Table S4 and Fig. S3.
Fig. 1.
Effects of ZNF804A rs1344704 on left hippocampal-frontal functional connectivity. A Frontal region (red box) with a significant genotype main effect for functional connectivity from the left hippocampus to the right dorsolateral prefrontal cortex (DLPFC) (peak MNI coordinates: x = 21, y = 42, z = 33, peak F-score = 13.43, P < 0.001, cluster size = 19). The color bar indicates the F-score. B Scatterplot of the mean strength of resting-state functional connectivity (RSFC) (mean ± SD) between the left hippocampus and the frontal region in the genotype groups. The Y-axis indicates the Z-score. C Scatterplot of the mean strength of RSFC (mean ± SD) from the left hippocampus to the right DLPFC in the genotype groups (CC/CA and AA genotype groups) in different diagnostic groups. The Y-axis indicates the Z-score. SZ, schizophrenic patients; HC, healthy controls; lHIPP, left hippocampus; rDLPFC, right dorsolateral prefrontal cortex; *P < 0.05, **P < 0.01, ***P < 0.001.
Furthermore, we extracted the mean functional connectivity strength of the right DLPFC with the peak MNI coordinates (x = 21, y = 42, z = 33) as the center and 6 mm as the radius (the sphere regions were similar to the frontal regions as shown in Fig. S4 and the results were almost the same). The results also showed a significant genotype effect on the mean RSFC strength of the right DLPFC (P = 4.70 × 10−4). The post hoc analysis showed that the RSFC strength was higher in CA heterozygotes than in CC (P = 0.019) and AA (P < 0.001) homozygotes (Fig. 1B). This significant difference still existed for each diagnosis. In the control group, CA heterozygotes showed higher RSFC strength than CC (P = 0.037) and AA (P = 0.004) homozygotes. In the schizophrenic group, CA heterozygotes only showed higher RSFC strength than AA (P = 0.012) homozygotes, with no significant difference from CC homozygotes (P = 0.202). When CC and CA were grouped as C carriers, as has been done in many previous studies exploring the effects of ZNF804A variation [23, 36–38], the mean RSFC strength in the CC/CA group was higher than that in AA homozygotes in both the control (P = 0.023) and schizophrenia (P = 0.025) groups (Fig. 1C).
Correlations Between RSFC and Behavioral Measures
With age and gender as confounding factors, partial correlation analysis showed that, in CA heterozygotes, the strength of RSFC was significantly negatively correlated with the DSST score, and positively correlated with scores of total and general psychopathological syndrome in the schizophrenic group. For AA (risk) homozygotes, the RSFC strength was significantly negatively correlated with the WMS-R score in the control group while positively correlated with the WMS-R score, and negatively correlated with the PANSS total score in the schizophrenic group (Table 3).
Table 3.
Significant correlations of the left hippocampus-right DLPFC resting-state functional connectivity with behavior.
| Cognitive Performance | PANSS score | |||
|---|---|---|---|---|
| SZ | HC | SZ | HC | |
| CA genotype | DSST (r = −0.341, P = 0.045) | DSST (r = −0.305, P = 0.047) | Total (r = 0.363, P = 0.025) | – |
| – | – | General (r = 0.389, P = 0.014) | – | |
| AA genotype | WMS-R (r = 0.610, P = 0.012) | WMS-R (r = −0.551, P = 0.006) | Total (r = −0.486, P = 0.041) | – |
The mean strength of resting-state functional connectivity (RSFC) between the left hippocampus and the right dorsolateral frontal region was significantly correlated with different measures of cognitive performance and symptom severity in patients. SZ, schizophrenic patients; HC, healthy controls; DSST, Digit Symbol Substitution Test; WMS-R, Wechsler Memory Scale-Revised; PANSS, Positive and Negative Syndrome Scale; Total, total PANSS score; General, score for general psychopathological syndrome in PANSS.s
Taken together, for CA heterozygotes, higher hippocampal-prefrontal functional connectivity may predict a lower speed of information processing in healthy controls and schizophrenic patients, and higher PANSS scores for total and general symptoms indicating the severity of psychosis in schizophrenia. For AA (risk) homozygotes, schizophrenic patients showed a lower hippocampal-prefrontal functional connectivity, which may predict worse memory functions, a slower speed of word processing, and more severe symptoms.
Discussion
In this study, we used an imaging genetic approach to investigate the effects of ZNF804A genetic variation on the resting-state fMRI in a sample of schizophrenic patients and healthy individuals. We found that schizophrenic patients who were homozygous for the rs1344706 risk allele (AA) exhibited a lower strength of RSFC between the bilateral hippocampus and the right DLPFC. The mean RSFC strength between these two regions in patients was positively correlated with the WMS-R and CFT scores, and negatively correlated with the PANSS score. This means that a lower RSFC strength indicates a worse cognitive performance and a more severe psychiatric syndrome in patients. The current findings confirmed the likelihood that this SNP confers susceptibility to schizophrenia and identified potential neural mechanisms linking rs1344706 with schizophrenia.
Schizophrenia is regarded as a disorder of connectivity between components of large-scale brain networks [39–42]. The thalamus and hippocampus have been reported to exhibit greater fMRI activity during a sensory gating task, which is a common deficit in schizophrenia [43]. Both of these regions also show increased whole-brain functional connectivity strength with all other voxels in the brain compared with healthy controls [44]. Duan and colleagues found that the frequency of delta bursts in particular thalamic nuclei has a causal role in producing the working memory deficits in schizophrenia [45]. Furthermore, a higher functional connectivity with the hippocampal complex as the seed, between the hippocampal complex and the thalamus, has been reported in patients with auditory hallucinations alone, when compared with patients who had audio-visual hallucinations in the resting state [46], which suggested the specific functional involvement of the hippocampus and thalamus in schizophrenia. Our results revealed the functional coupling between the hippocampus and thalamus in schizophrenia; this is consistent with previous findings and might provide a potential pathogenesis of schizophrenia.
Previous studies reported a dysregulation of functional coupling between the left hippocampus and the right DLPFC during working memory in patients with schizophrenia and high-risk individuals [11, 24], and reduced hippocampal-prefrontal coupling during the resting state in chronic schizophrenia [47, 48]. Rasetti et al. reported the effect of the ZNF804A rs1344706 polymorphism on left hippocampus-right DLPFC coupling during the n-back working memory task in patients with schizophrenia, their healthy siblings, and healthy controls [10]. Recently, a study using both fMRI and diffusion tensor imaging data from healthy individuals has replicated the association between rs1344706 and the left hippocampus-right DLPFC coupling during the n-back working memory task, and also found impaired integrity of the white matter connection from the left hippocampus to the posterior cingulate cortex, one of the sub-connections between the left hippocampus and the right DLPFC, in healthy controls homozygous for the risk allele [38]. Therefore, our findings extend previous studies by reporting a consistent association between rs1344706 and left hippocampus-right DLPFC coupling during the resting state in schizophrenic patients.
The SNP rs1344706 has been associated with episodic and working memory in patients from different ethnic groups [49, 50] and with functional coupling of the right DLPFC and the anterior cingulate cortex during a cognitive control task [51]. The DLPFC is known to be a site for sustained attention and working memory [52, 53] and the hippocampus is believed to have close relationship with many areas of the cerebral cortex and constitutes a memory network to modulate and facilitate communication [54]. The correlation of hippocampus-DLPFC resting-state functional connectivity and cognitive performance demonstrated in our study provides further evidence that ZNF804A variation modulates the cortical network connectivity involved in memory and executive control. The rs1344706 allele has also been associated with the core symptoms of schizophrenia and the response to antipsychotic treatment. Larger white matter volumes and more severe symptoms have been reported in risk allele carriers with schizophrenia spectrum disorders [22]. When treated with antipsychotics, patients homozygous for the risk allele show poorer improvement of positive symptoms [55] and first-episode patients who are risk allele carriers exhibit significantly less improvement in the total PANSS score and positive sub-score [56]. Our results showing a correlation between RSFC strength and symptom severity in schizophrenia adds evidence for rs1344706 playing a role in the development of schizophrenia and being a potential target for future intervention.
Interestingly, in our study, effects of the ZNF804A polymorphism on the functional connectivity between the left hippocampus and the right DLPFC showed an “inverted U-shaped” form. We speculate that the highest RSFC strength in CA heterozygotes might result from cis-effects on ZNF804A expression in the brain. Ilaria Guella and colleagues found that total RNA of postmortem DLPFC samples from individuals with rs1344706 heterozygous for CA exhibited higher ZNF804A allelic expression than those homozygous for AA [57]. Another prior analysis revealed that the risk allele (A) was significantly associated with higher ZNF804A expression in postmortem prefrontal brain tissue from Irish control samples (n = 30, A: 32/60, C: 28/60) [58] whereas the opposite effect was found later in Caucasian patients with schizophrenia, using the same methods [59]. Various studies have demonstrated a cis-effect of rs1344706 on ZNF804A expression with controversial direction. Our results were partly consistent with the transcript expression model in which ZNF804A expression in CA heterozygotes is similar to CC homozygotes but significantly higher than in AA homozygotes [60]. Further study is needed to explore whether the distinguishing functional connectivity indeed has a relationship with the expression of ZNF804A.
Finally, several limitations of our study need to be clarified. First, we specifically focused on functional connectivity from the hippocampus based on previous findings; further studies focused on the functional connectivity of other brain regions and more functional measures are needed. Second, although we replicated significant differences of hippocampal-prefrontal connectivity among individuals of the three genotypes, the differences were not significant in multiple testing corrections. It would be necessary to expand the sample to verify the stability and reliability of the results. In general, the study sheds light on the effect of a risk variant on functional connectivity during resting-state that needs to be verified in an independent sample.
In conclusion, our findings add to the evidence supporting ZNF804A as a promising risk gene for schizophrenia, and suggest that ZNF804A variation may be involved in the development and progression of schizophrenia by affecting resting-state brain function.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by the National Key Research and Development Program of China (2016YFC1307000 and 2015BAI13B01), the National Natural Science Foundation of China (91432304, 81370032, 81571313 and 81221002), Capital Health Development Research (2016-2-4112), and Beijing Nova Program Interdisciplinary Studies Cooperative Project (Z161100004916038). We thank the Department of Radiology, The Third Hospital, Peking University, for providing the equipment for neuroimaging. We thank all the participants in this study.
Compliance with Ethical Standards
Conflict of interest
All authors claim that there are no conflicts of interest.
Footnotes
Electronic supplementary material
The online version of this article (10.1007/s12264-018-0221-y) contains supplementary material, which is available to authorized users.
Contributor Information
Qi Liu, Email: liu_qee@sohu.com.
Weihua Yue, Email: dryue@bjmu.edu.cn.
References
- 1.Sullivan PF, Kendler KS, Neale MC. Schizophrenia as a complex trait: evidence from a meta-analysis of twin studies. Arch Gen Psychiatry. 2003;60:1187–1192. doi: 10.1001/archpsyc.60.12.1187. [DOI] [PubMed] [Google Scholar]
- 2.Bienvenu OJ, Davydow DS, Kendler KS. Psychiatric ‘diseases’ versus behavioral disorders and degree of genetic influence. Psychol Med. 2011;41:33–40. doi: 10.1017/S003329171000084X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Erp TG, Hibar DP, Rasmussen JM, Glahn DC, Pearlson GD, Andreassen OA, et al. Subcortical brain volume abnormalities in 2028 individuals with schizophrenia and 2540 healthy controls via the ENIGMA consortium. Mol Psychiatry. 2016;21:585. doi: 10.1038/mp.2015.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hibar DP, Stein JL, Renteria ME, Arias-Vasquez A, Desrivieres S, Jahanshad N, et al. Common genetic variants influence human subcortical brain structures. Nature. 2015;520:224–229. doi: 10.1038/nature14101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meyer-Lindenberg A. From maps to mechanisms through neuroimaging of schizophrenia. Nature. 2010;468:194–202. doi: 10.1038/nature09569. [DOI] [PubMed] [Google Scholar]
- 6.Bogdan R, Salmeron BJ, Carey CE, Agrawal A, Calhoun VD, Garavan H, et al. Imaging genetics and genomics in psychiatry: A critical review of progress and potential. Biol Psychiatry. 2017;82:165–175. doi: 10.1016/j.biopsych.2016.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bahner F, Demanuele C, Schweiger J, Gerchen MF, Zamoscik V, Ueltzhoffer K, et al. Hippocampal-dorsolateral prefrontal coupling as a species-conserved cognitive mechanism: a human translational imaging study. Neuropsychopharmacology. 2015;40:1674–1681. doi: 10.1038/npp.2015.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bähner F, Meyer-Lindenberg A. Hippocampal–prefrontal connectivity as a translational phenotype for schizophrenia. Eur Neuropsychopharmacol. 2017;27:93–106. doi: 10.1016/j.euroneuro.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 9.Meyer-Lindenberg AS, Olsen RK, Kohn PD, Brown T, Egan MF, Weinberger DR, et al. Regionally specific disturbance of dorsolateral prefrontal-hippocampal functional connectivity in schizophrenia. Arch Gen Psychiatry. 2005;62:379–386. doi: 10.1001/archpsyc.62.4.379. [DOI] [PubMed] [Google Scholar]
- 10.Rasetti R, Sambataro F, Chen Q, Callicott JH, Mattay VS, Weinberger DR. Altered cortical network dynamics: a potential intermediate phenotype for schizophrenia and association with ZNF804A. Arch Gen Psychiatry. 2011;68:1207–1217. doi: 10.1001/archgenpsychiatry.2011.103. [DOI] [PubMed] [Google Scholar]
- 11.Esslinger C, Walter H, Kirsch P, Erk S, Schnell K, Arnold C, et al. Neural mechanisms of a genome-wide supported psychosis variant. Science. 2009;324:605. doi: 10.1126/science.1167768. [DOI] [PubMed] [Google Scholar]
- 12.Paulus FM, Bedenbender J, Krach S, Pyka M, Krug A, Sommer J, et al. Association of rs1006737 in CACNA1C with alterations in prefrontal activation and fronto-hippocampal connectivity. Hum Brain Mapp. 2014;35:1190–1200. doi: 10.1002/hbm.22244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Donovan MC, Craddock N, Norton N, Williams H, Peirce T, Moskvina V, et al. Identification of loci associated with schizophrenia by genome-wide association and follow-up. Nat Genet. 2008;40:1053–1055. doi: 10.1038/ng.201. [DOI] [PubMed] [Google Scholar]
- 14.Li M, Shi CJ, Shi YY, Luo XJ, Zheng XB, Li ZQ, et al. ZNF804A and schizophrenia susceptibility in Asian populations. Am J Med Genet B Neuropsychiatr Genet. 2012;159B:794–802. doi: 10.1002/ajmg.b.32084. [DOI] [PubMed] [Google Scholar]
- 15.Wang J, Zhao S, Shugart YY, Zhou Z, Jin C, Yuan J, et al. No association between ZNF804A rs1344706 and schizophrenia in a case-control study of Han Chinese. Neurosci Lett. 2016;618:14–18. doi: 10.1016/j.neulet.2016.02.048. [DOI] [PubMed] [Google Scholar]
- 16.Zhang C, Wang Z, Hong W, Wu Z, Peng D, Fang Y. ZNF804A genetic variation confers risk to bipolar disorder. Mol Neurobiol. 2016;53:2936–2943. doi: 10.1007/s12035-015-9193-3. [DOI] [PubMed] [Google Scholar]
- 17.Rao S, Yao Y, Ryan J, Jin C, Xu Y, Huang X, et al. Genetic association of rs1344706 in ZNF804A with bipolar disorder and schizophrenia susceptibility in Chinese populations. Sci Rep. 2017;7:41140. doi: 10.1038/srep41140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Girgenti MJ, LoTurco JJ, Maher BJ. ZNF804a regulates expression of the schizophrenia-associated genes PRSS16, COMT, PDE4B, and DRD2. PLoS One. 2012;7:e32404. doi: 10.1371/journal.pone.0032404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deans PJM, Raval P, Sellers KJ, Gatford NJF, Halai S, Duarte RRR, et al. Psychosis risk candidate ZNF804A localizes to synapses and regulates neurite formation and dendritic spine structure. Biol Psychiatry. 2017;82:49–61. doi: 10.1016/j.biopsych.2016.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang H, Xiao X, Li M. The schizophrenia risk gene ZNF804A: clinical associations, biological mechanisms and neuronal functions. Mol Psychiatry. 2017;22:944–953. doi: 10.1038/mp.2017.19. [DOI] [PubMed] [Google Scholar]
- 21.Schizophrenia Working Group of the Psychiatric Genomics Consortium Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511:421–427. doi: 10.1038/nature13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wassink TH, Epping EA, Rudd D, Axelsen M, Ziebell S, Fleming FW, et al. Influence of ZNF804a on brain structure volumes and symptom severity in individuals with schizophrenia. Arch Gen Psychiatry. 2012;69:885–892. doi: 10.1001/archgenpsychiatry.2011.2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Donohoe G, Rose E, Frodl T, Morris D, Spoletini I, Adriano F, et al. ZNF804A risk allele is associated with relatively intact gray matter volume in patients with schizophrenia. Neuroimage. 2011;54:2132–2137. doi: 10.1016/j.neuroimage.2010.09.089. [DOI] [PubMed] [Google Scholar]
- 24.Esslinger C, Kirsch P, Haddad L, Mier D, Sauer C, Erk S, et al. Cognitive state and connectivity effects of the genome-wide significant psychosis variant in ZNF804A. Neuroimage. 2011;54:2514–2523. doi: 10.1016/j.neuroimage.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 25.Paulus FM, Krach S, Bedenbender J, Pyka M, Sommer J, Krug A, et al. Partial support for ZNF804A genotype-dependent alterations in prefrontal connectivity. Hum Brain Mapp. 2013;34:304–313. doi: 10.1002/hbm.21434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Strauss E, Sherman EM, Spreen O. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. Oxford University Press, 2006.
- 27.Wechsler D. WAIS-III, Wechsler Adult Intelligence Scale: Administration and Scoring Manual. Psychological Corporation, 1997.
- 28.Wechsler D. WMS-R: Wechsler memory scale-revised: manual. Psychological Corporation, 1984.
- 29.Knowles EE, Weiser M, David AS, Glahn DC, Davidson M, Reichenberg A. The puzzle of processing speed, memory, and executive function impairments in schizophrenia: fitting the pieces together. Biol Psychiatry. 2015;78:786–793. doi: 10.1016/j.biopsych.2015.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reichenberg A, Harvey PD. Neuropsychological impairments in schizophrenia: Integration of performance-based and brain imaging findings. Psychol Bull. 2007;133:833–858. doi: 10.1037/0033-2909.133.5.833. [DOI] [PubMed] [Google Scholar]
- 31.Dickinson D, Ramsey ME, Gold JM. Overlooking the obvious: a meta-analytic comparison of digit symbol coding tasks and other cognitive measures in schizophrenia. Arch Gen Psychiatry. 2007;64:532–542. doi: 10.1001/archpsyc.64.5.532. [DOI] [PubMed] [Google Scholar]
- 32.Zheng F, Zhang Y, Xie W, Li W, Jin C, Mi W, et al. Further evidence for genetic association of CACNA1C and schizophrenia: new risk loci in a Han Chinese population and a meta-analysis. Schizophr Res. 2014;152:105–110. doi: 10.1016/j.schres.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 33.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 34.Zhang X, Yu JT, Li J, Wang C, Tan L, Liu B, et al. Bridging Integrator 1 (BIN1) genotype effects on working memory, hippocampal volume, and functional connectivity in young healthy individuals. Neuropsychopharmacology. 2015;40:1794–1803. doi: 10.1038/npp.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao X, Tian L, Yan J, Yue W, Yan H, Zhang D. Abnormal rich-club organization associated with compromised cognitive function in patients with schizophrenia and their unaffected parents. Neurosci Bull. 2017;33:445–454. doi: 10.1007/s12264-017-0151-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Voineskos AN, Lerch JP, Felsky D, Tiwari A, Rajji TK, Miranda D, et al. The ZNF804A gene: characterization of a novel neural risk mechanism for the major psychoses. Neuropsychopharmacology. 2011;36:1871–1878. doi: 10.1038/npp.2011.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O’Donoghue T, Morris DW, Fahey C, Da Costa A, Moore S, Cummings E, et al. Effects of ZNF804A on auditory P300 response in schizophrenia. Transl Psychiatry. 2014;4:e345. doi: 10.1038/tp.2013.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang Z, Chen X, Yu P, Zhang Q, Sun X, Gu H, et al. Effect of rs1344706 in the ZNF804A gene on the connectivity between the hippocampal formation and posterior cingulate cortex. Schizophr Res. 2016;170:48–54. doi: 10.1016/j.schres.2015.11.024. [DOI] [PubMed] [Google Scholar]
- 39.Dong D, Wang Y, Chang X, Luo C, Yao D. Dysfunction of large-scale brain networks in schizophrenia: a meta-analysis of resting-state functional connectivity. Schizophr Bull. 2018;44:168–181. doi: 10.1093/schbul/sbx034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lynall ME, Bassett DS, Kerwin R, McKenna PJ, Kitzbichler M, Muller U, et al. Functional connectivity and brain networks in schizophrenia. J Neurosci. 2010;30:9477–9487. doi: 10.1523/JNEUROSCI.0333-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Plaschke RN, Cieslik EC, Muller VI, Hoffstaedter F, Plachti A, Varikuti DP, et al. On the integrity of functional brain networks in schizophrenia, Parkinson’s disease, and advanced age: Evidence from connectivity-based single-subject classification. Hum Brain Mapp. 2017;38:5845–5858. doi: 10.1002/hbm.23763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hu ML, Zong XF, Mann JJ, Zheng JJ, Liao YH, Li ZC, et al. A review of the functional and anatomical default mode network in schizophrenia. Neurosci Bull. 2017;33:73–84. doi: 10.1007/s12264-016-0090-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tregellas JR, Davalos DB, Rojas DC, Waldo MC, Gibson L, Wylie K, et al. Increased hemodynamic response in the hippocampus, thalamus and prefrontal cortex during abnormal sensory gating in schizophrenia. Schizophr Res. 2007;92:262–272. doi: 10.1016/j.schres.2006.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhu J, Zhuo C, Xu L, Liu F, Qin W, Yu C. Altered coupling between resting-state cerebral blood flow and functional connectivity in schizophrenia. Schizophr Bull. 2017;43:1363–1374. doi: 10.1093/schbul/sbx051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Duan AR, Varela C, Zhang Y, Shen Y, Xiong L, Wilson MA, et al. Delta frequency optogenetic stimulation of the thalamic nucleus reuniens is sufficient to produce working memory deficits: relevance to schizophrenia. Biol Psychiatry. 2015;77:1098–1107. doi: 10.1016/j.biopsych.2015.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Amad A, Cachia A, Gorwood P, Pins D, Delmaire C, Rolland B, et al. The multimodal connectivity of the hippocampal complex in auditory and visual hallucinations. Mol Psychiatry. 2014;19:184–191. doi: 10.1038/mp.2012.181. [DOI] [PubMed] [Google Scholar]
- 47.Zhou Y, Shu N, Liu Y, Song M, Hao Y, Liu H, et al. Altered resting-state functional connectivity and anatomical connectivity of hippocampus in schizophrenia. Schizophr Res. 2008;100:120–132. doi: 10.1016/j.schres.2007.11.039. [DOI] [PubMed] [Google Scholar]
- 48.Rotarska-Jagiela A, van de Ven V, Oertel-Knochel V, Uhlhaas PJ, Vogeley K, Linden DE. Resting-state functional network correlates of psychotic symptoms in schizophrenia. Schizophr Res. 2010;117:21–30. doi: 10.1016/j.schres.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 49.Walters JT, Corvin A, Owen MJ, Williams H, Dragovic M, Quinn EM, et al. Psychosis susceptibility gene ZNF804A and cognitive performance in schizophrenia. Arch Gen Psychiatry. 2010;67:692–700. doi: 10.1001/archgenpsychiatry.2010.81. [DOI] [PubMed] [Google Scholar]
- 50.Chen M, Xu Z, Zhai J, Bao X, Zhang Q, Gu H, et al. Evidence of IQ-modulated association between ZNF804A gene polymorphism and cognitive function in schizophrenia patients. Neuropsychopharmacology. 2012;37:1572–1578. doi: 10.1038/npp.2012.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thurin K, Rasetti R, Sambataro F, Safrin M, Chen Q, Callicott JH, et al. Effects of ZNF804A on neurophysiologic measures of cognitive control. Mol Psychiatry. 2013;18:852–854. doi: 10.1038/mp.2012.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Manoach DS, Gollub RL, Benson ES, Searl MM, Goff DC, Halpern E, et al. Schizophrenic subjects show aberrant fMRI activation of dorsolateral prefrontal cortex and basal ganglia during working memory performance. Biol Psychiatry. 2000;48:99–109. doi: 10.1016/S0006-3223(00)00227-4. [DOI] [PubMed] [Google Scholar]
- 53.Callicott JH, Bertolino A, Mattay VS, Langheim FJ, Duyn J, Coppola R, et al. Physiological dysfunction of the dorsolateral prefrontal cortex in schizophrenia revisited. Cereb Cortex. 2000;10:1078–1092. doi: 10.1093/cercor/10.11.1078. [DOI] [PubMed] [Google Scholar]
- 54.Harrison PJ. The hippocampus in schizophrenia: a review of the neuropathological evidence and its pathophysiological implications. Psychopharmacology (Berl) 2004;174:151–162. doi: 10.1007/s00213-003-1761-y. [DOI] [PubMed] [Google Scholar]
- 55.Mossner R, Schuhmacher A, Wagner M, Lennertz L, Steinbrecher A, Quednow BB, et al. The schizophrenia risk gene ZNF804A influences the antipsychotic response of positive schizophrenia symptoms. Eur Arch Psychiatry Clin Neurosci. 2012;262:193–197. doi: 10.1007/s00406-011-0235-1. [DOI] [PubMed] [Google Scholar]
- 56.Zhang J, Wu X, Diao F, Gan Z, Zhong Z, Wei Q, et al. Association analysis of ZNF804A (zinc finger protein 804A) rs1344706 with therapeutic response to atypical antipsychotics in first-episode Chinese patients with schizophrenia. Compr Psychiatry. 2012;53:1044–1048. doi: 10.1016/j.comppsych.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 57.Guella I, Sequeira A, Rollins B, Morgan L, Myers RM, Watson SJ, et al. Evidence of allelic imbalance in the schizophrenia susceptibility gene ZNF804A in human dorsolateral prefrontal cortex. Schizophr Res. 2014;152:111–116. doi: 10.1016/j.schres.2013.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Riley B, Thiselton D, Maher BS, Bigdeli T, Wormley B, McMichael GO, et al. Replication of association between schizophrenia and ZNF804A in the Irish Case-Control Study of Schizophrenia sample. Mol Psychiatry. 2010;15:29–37. doi: 10.1038/mp.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schultz CC, Nenadic I, Riley B, Vladimirov VI, Wagner G, Koch K, et al. ZNF804A and cortical structure in schizophrenia: in vivo and postmortem studies. Schizophr Bull. 2014;40:532–541. doi: 10.1093/schbul/sbt123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tao R, Cousijn H, Jaffe AE, Burnet PW, Edwards F, Eastwood SL, et al. Expression of ZNF804A in human brain and alterations in schizophrenia, bipolar disorder, and major depressive disorder: a novel transcript fetally regulated by the psychosis risk variant rs1344706. JAMA Psychiatry. 2014;71:1112–1120. doi: 10.1001/jamapsychiatry.2014.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.