Abstract
Background
Antimicrobial drug resistance is a global threat for treatment of infectious diseases and costs life and money and threatens health delivery system's effectiveness. The resistance of E. coli to frequently utilized antimicrobial drugs is becoming a major challenge in Ethiopia. However, there is no inclusive countrywide study. Therefore, this study intended to assess the prevalence of E. coli resistance and antimicrobial-specific resistance pattern among E. coli clinical isolates in Ethiopia.
Methods
Articles were retrieved from PubMed, Embase, and grey literature from 2007 to 2017. The main outcome measures were overall E. coli and drug-specific resistance patterns. A random-effects model was used to determine pooled prevalence with 95% confidence interval (CI), using DerSimonian and Laird method. In addition, subgroup analysis was conducted to improve the outcome. The study bias was assessed by Begg's funnel plot. This study was registered in PROSPERO as follows: PROSPERO 2017: CRD42017070106.
Results
Of 164 articles retrieved, 35 articles were included. A total of 19,235 study samples participated in the studies and 2,635 E. coli strains were isolated. Overall, E. coli antibacterial resistance was 45.38% (95% confidence interval (CI): 33.50 to 57.27). The resistance pattern ranges from 62.55% in Addis Ababa to 27.51% in Tigray region. The highest resistance of E. coli reported was to ampicillin (83.81%) and amoxicillin (75.79%), whereas only 13.55% of E. coli isolates showed resistance to nitrofurantoin.
Conclusion
E. coli antimicrobial resistance remains high with disparities observed among regions. The bacterium was found to be highly resistant to aminopenicillins. The finding implies the need for effective prevention strategies for the E. coli drug resistance and calls for multifaceted approaches with full involvement of all stakeholders.
1. Background
Escherichia coli (E. coli) is one of the most widespread bacteria throughout the world. Some strains of E. coli can cause serious illness for humankind [1] including urinary tract infections [2–4], bloodstream infections [5], skin infection, otitis media [6, 7], and diarrhea [8].
E. coli resistance to antimicrobials is creating trouble to the healthcare system worldwide [9, 10]. This complicates treatment outcomes, increases the cost of treatment, and limits the therapeutic options that contribute to the global spectra of a postantimicrobial age in which some of the most effective drugs lose their efficiency [11]. The bacterium is becoming highly resistant to conventionally used antibiotics (to both the newer and older medicines) as evidenced by many previous studies [12–16]. Adaptive resistance was supposed to be the main mechanism for the development of resistance including that to lethal doses of the antimicrobials [17].
Antimicrobial resistance of E. coli in developing countries including Ethiopia is reported to be one major reason for failure of treatment of infectious diseases [18]. A number of studies conducted in Ethiopia from various clinical settings show increments in the prevalence of antimicrobial resistance patterns of E. coli [6, 19, 20]. However, there is no comprehensive and aggregated nationwide study to show the pattern of antimicrobial resistance in E. coli. Hence, the purpose of this meta-analysis was to sum up the available data and to establish the pooled prevalence and antimicrobial resistance of E. coli in Ethiopia.
2. Methods
This study was conducted in a similar approach to Eshetie et al. (2016) [21] and according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) Checklist [22] (additional S1 file).
2.1. Study Selection
A systematic literature search was conducted in PubMed and Embase, and also manual search for articles potentially relevant to our study was identified. We built our search strategy by combining the three main arms (Table 1): E. coli, drugs-related terms, and Ethiopia.
Table 1.
Searching strategies including search arm and terms used in the study.
| Search arm | Search terms | |
|---|---|---|
| 1 | E. coli | “Escherichia coli” OR “E. coli” |
| 2 | Drug and/or resistance | “antibiotic resistance” OR “drug resistance” OR “drug resistance, microbial” OR “drug resistance, microbial” OR “antibacterial resistance” OR “antibiotic resistance” OR “antimicrobial resistance” |
| 3 | Ethiopia | “Ethiopia” |
The word “OR” was used to combine search terms within each arm and the word “AND” was used to connect the three search arms (#1 AND #2 AND #3).
Among the citations extracted, abstracts were reviewed to retrieve the clinical studies on E. coli colonization. Articles that were relevant, by title and abstract, were accessed in full text to determine those that provided sufficient information to be included in our meta-analysis. Finally, the references cited by each eligible study were screened to identify additional articles.
2.2. Inclusion and Exclusion Criteria
Studies included in this meta-analysis were those that had extractable data on the prevalence of drug resistance of E. coli on a human in Ethiopian hospitals or research centers and were only published from 2007 to 2017 and were only in English language.
2.3. Outcome of Interest
The main outcome of interest was the prevalence of drug resistance or antimicrobial susceptibility of E. coli among the total E. coli clinical isolates. The prevalence was calculated by dividing the numbers of resistant E. coli isolates by the total number of clinically isolated E. coli. As a secondary outcome of interest, we had also calculated the pooled resistance pattern of E. coli isolates to specific antibiotics.
2.4. Data Extraction and Assessment of Quality of Study
Screening by title, abstract, and full text and data extraction were done independently by two authors (Kald Beshir Tuem and Abadi Kahsu Gebre) at each step and Derbew Fikadu Berhe was involved in consensus for discrepancies (if any) between the two authors (Kald Beshir Tuem and Abadi Kahsu Gebre). In cases of insufficient data, the authors reviewed the full text of the article for further information and clarification. The extracted data from each article were summarized into a spreadsheet. References and data for each study were carefully cross-checked to ensure that no overlapping data were present and to maintain the integrity of the meta-analysis. Information extracted from each paper was region, study area, study design, study population, culture specimens, number of E. coli isolated, the average percentage of resistant E. coli, antimicrobial resistance rate of E. coli, and references. With all the articles used in this study being cross-sectional, the score for the quality of the study was assessed using the modified Newcastle-Ottawa scale for the representativeness of sample, appropriateness of sample size, response rate, validity of method, strategy to control confounding factors, reliability of outcome determination, and appropriate statistical analyses. The quality score (Table 2) disagreements were resolved by consensus and a final agreed-upon rating was assigned to each study (S2 file) [58].
Table 2.
Summary of 35 studies reporting the prevalence and resistance pattern of E. coli in different parts of Ethiopia.
| References | Region | Study area | Study period | Study design | Study population | Culture specimens | Study samples | Number of E. coli isolated | Average %resistant E. coli | NOS quality score out of 7 |
|---|---|---|---|---|---|---|---|---|---|---|
| Abejew et al., 2014 [23] | Amhara | Dessie | January to March 2012 | RCS | Test results of patients diagnosed with UTI | Urine | 2486 | 410 | 51.8 | 6 |
| Kibret and Abera, 2014 [24] | Dessie | — | R | Patients | Mid-stream morning urine | 1404 | 203 | 53.2 | 6 | |
| Kibret and Abera, 2011 [25] | Dessie | — | R | Patients | Urine, ear discharge, pus swab from wounds, and eye discharge | 3,149 | 446 | 44.2 | 6 | |
| Azene and Beyene, 2011 [26] | Dessie | — | R | Patents with wound infections | Wound swab | 599 | 82 | 49.8 | 6 | |
| Alebachew et al., 2016 [27] | Gondar | March 1 to May 2, 2013 | CS | HIV patients | Blood | 100 | 01 | 44.4 | 7 | |
| Alemu et al., 2012 [28] | Gondar | March 22 to April 30, 2011 | CS | Pregnant women attending ANC | Urine | 385 | 19 | 29.2 | 7 | |
| Wondimeneh et al., 2014 [29] | Gondar | January to May 2013 | CS | Fistula patients | “Clean-catch” mid-stream urine specimen | 53 | 6 | 59.3 | 6 | |
| Wondimu et al., 2013 [30] | Gondar | December 2011 to June 2012 | CS | Blood donated from donors at blood bank | blood | 137 | 2 | 0.7 | 7 | |
| Eshetie et al., 2015 [31] | Gondar | February to May 2014 | CS | UTI-suspected patients | Urine | 442 | 112 | 48.0 | 4 | |
| Tiruneh et al., 2014 [32] | Gondar | September 1, 2011, to June 30, 2012 | CS | UTI-suspected patients | Urine | 284 | 120 | 68.0 | 4 | |
| Eshetie et al., 2016 [33] | Gondar | February to June 2014 | CS | Patients with UTI | Urine | 446 | 112 | 38.8 | 6 | |
| Mulu et al., 2017 [34] | Debre Markos | January 2015 | R | Patients | Pus/swab from wound, urine, ear discharge, blood, stool, urethral or cervical discharge, nasal or throat swab, and CSF | 575 | 39 | 31.5 | 6 | |
| Abera and Kibret, 2014 [35] | West Gojjam | November 2009 to February 2010 | CS | Adult patients who underwent trachomatous trichiasis surgery | Conjunctival swabs | 1413 | 20 | 26.5 | 5 | |
| Adugna et al., 2015 [36] | Bahir Dar | December 2011 to February 2012 | CS | Children below five years of age with acute diarrhea | Stool sample | 422 | 204 | 42.8 | 7 | |
| Demilie et al., 2012 [37] | Bahir Dar | October 2010 to January 2011 | CS | Pregnant women | Clean-catch urine | 367 | 16 | 41.7 | 4 | |
| Derbie et al., 2017 [38] | Bahir Dar | January 2015 | R | Patients | Urine | 446 | 72 | 55.3 | 5 | |
| Melaku et al., 2012 [39] | Bahir Dar | April to August 2010 | CS | Patients | Urine | 1254 | 33 | 87.8 | 6 | |
| Mulu et al., 2012 [40] | Bahir Dar | October 2010 to January 2011 | CS | Patients | Wound swab and venous blood samples | 294 | 8 | 53.1 | 6 | |
| Wondemagegn et al., 2015 [41] | Bahir Dar | May to November 2013 | CS | Women of reproductive age | Vaginal swab | 409 | 105 | 50.5 | 7 | |
|
| ||||||||||
| Dereje et al., 2017 [42] | Central Ethiopia | Addis Ababa | February to May 2015 | CS | Fistula patients | Clean-catch mid-stream urine | 210 | 65 | 38.2 | 7 |
| Dessie et al., 2016 [43] | Addis Ababa | October 2013 and March 2014 | CS | Surgical site infected patients | Wound swab | 107 | 24 | 68.4 | 6 | |
| Desta et al., 2016 [44] | Addis Ababa | December 2012 | CS | All age groups patients | Fecal samples/swabs | 267 | 235 | 83.0 | 7 | |
| Mamuye, 2016 [45] | Addis Ababa | August 2013 to January 2014 | CS | Outpatient and inpatient and pregnant women | Mid-urine samples | 424 | 53 | 52.2 | 5 | |
| Legese et al., 2017 [46] | Addis Ababa | January to March 2014 | CS | Septicemia and UTI-suspected patients | Blood and urine | 322 | 6 | 72.9 | 6 | |
|
| ||||||||||
| Ramos et al., 2014 [47] | Oromia | West Arsi | July to December 2013 | CS | Leprosy patients | Pus produced by ulcer | 68 | 17 | 35.8 | 5 |
| Derese et al., 2016 [48] | Dire Dawa | February 18, 2015, to March 25, 2015 | CS | Pregnant women | Urine specimens | 186 | 9 | 34.4 | 6 | |
| Beyene and Tsegaye, 2011 [49] | Jimma | April to June 2010 | CS | UTI cases patients | Urine | 228 | 7 | 27.1 | 5 | |
| Debalke et al., 2014 [50] | Jimma | September to December 2012 | CS | HIV/AIDS patients | Urine | 481 | 31 | 36.6 | 7 | |
| Mama et al., 2014 [51] | Jimma | May to September 2013 | CS | Patients with wound infection | Wound swab | 150 | 29 | 61.6 | 6 | |
| Mulualem, 2012 [52] | Jimma | February to March 2007 | CS | Inpatients and outpatients | Urine, sputum, stool, and wound | 359 | 67 | 45.7 | 6 | |
| Zenebe et al., 2011 [53] | Jimma | October 27, 2009, to March 26, 2010 | CS | Febrile patients | Venous blood | 260 | 4 | 25.0 | 6 | |
|
| ||||||||||
| Nigussie and Amsalu, 2017 [54] | SNNP | Hawassa | June to October 2014 | CS | Diabetic patients | Mid-stream urine | 240 | 11 | 47.7 | 7 |
| Amsalu et al., 2016 [55] | Hawassa | January 2012 to December 2014 | RCS | Patients registered at microbiology lab book | Pus, ear discharge, nasal swab, urine, genital swab, CSF, and stool | 510 | 35 | 64.0 | 7 | |
| Tadesse et al., 2014 [56] | Hawassa | March to September 2012 | CS | Pregnant women attending ANC | Mid-stream urine | 244 | 16 | 56.3 | 6 | |
|
| ||||||||||
| Wasihun et al., 2015 [57] | Tigray | Mekelle | March to October 2014 | PCS | Febrile patients | Venous blood | 514 | 16 | 27.5 | 6 |
|
| ||||||||||
| Total | 19,235 | 2,635 | ||||||||
ANC: antenatal clinic, CSF: cerebrospinal fluid, CS: cross-sectional, PCS: prospective cross-sectional, R: retrospective review of culture, RCS: retrospective cross-sectional, SNNP: Southern Nations, Nationalities, and Peoples, UTI: urinary tract infection, —: no data.
2.5. Quality Control
The quality of eligible studies was checked independently by two authors (Kald Beshir Tuem and Abadi Kahsu Gebre) using a set of predetermined criteria such as research design quality of paper, completeness of extractable information, and employed methods for E. coli isolation. The study bias was measured by Begg's funnel plot [59]. This study was registered in PROSPERO as follows: PROSPERO 2017: CRD42017070106.
2.6. Data Analysis
A random-effects model was used to determine pooled prevalence, subgroup analysis, and 95% confidence interval (CI) by employing the approach of DerSimonian and Laird [60]. Variances and CIs were stabilized using Freeman-Tukey arc-sine methodology [61]; the reason is that using the standard approach of inverse variance method to calculate pooled prevalence does not work well in meta-analysis of single-arm study because, for studies with small or large prevalence, the inverse variance method causes the variance to become small and the calculated CI may be outside of the range [62]. Heterogeneity of study results was assessed using I2 test and significant heterogeneity was considered at p < 0.10 and I2 > 50% [60, 63]. Statistical analyses were performed using Open Meta-Analyst (version 3.13) and Comprehensive Meta-Analysis (version 3.1). In addition, we performed subgroup analyses according to the region of the country and the mechanism of action of the tested drugs to improve the specificity of the assessment.
3. Results
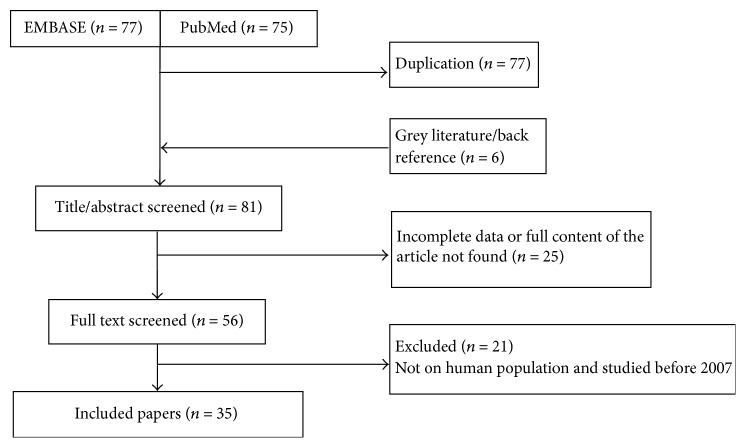
From Embase, PubMed, and manual searching, we found 164 potentially relevant studies, of which 35 were included for analysis (Figure 1).
Figure 1.
Flowchart shows selected articles for meta-analysis.
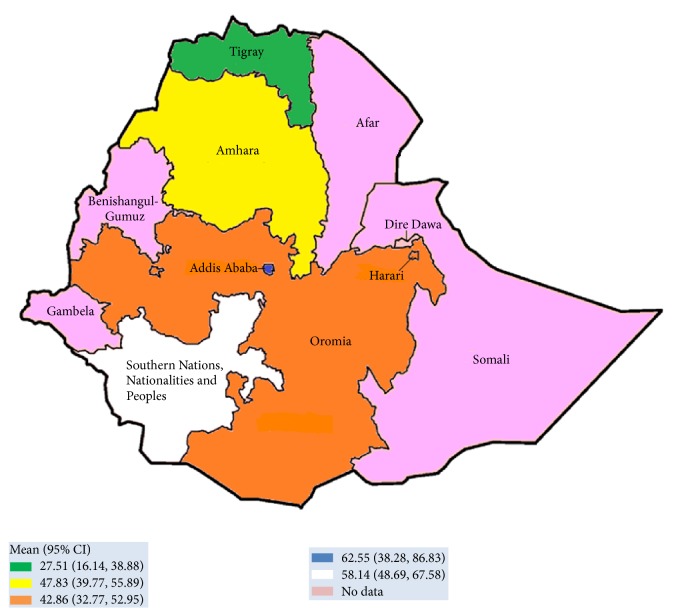
The study design of all included articles (35) was cross-sectional study. In these studies, a total of 19,235 study samples participated, from which 2,635 E. coli strains were isolated. The published studies were from four regions in Ethiopia (Table 2) which include the federal capital city of Ethiopia, Addis Ababa. No report was obtained from other regions in the country (Afar, Benishangul-Gumuz, Gambella, and Somali). Most of the studies indicated that various specimens had been utilized for screening of E. coli; particularly multisite swabbing was performed from different parts of the body, including skin, nasal, eye, ear, urethra, throat, vagina, or genital area (Table 2), and other biological fluids like blood, urine, pus, stool, and cerebrospinal fluid (CSF) were taken for test. A total of 2,635 E. coli strains were isolated from these various sites. The lowest and highest proportions of E. coli resistance were reported, respectively, from Bahir Dar (55.20%) and Mekelle (27.50%) cities. The average prevalence of E. coli resistance was also noted in different regions of Ethiopia; Addis Ababa region was ranked first (62.55%, 95% CI: 38.28–6.83%), followed by Southern Nations, Nationalities, and Peoples of Ethiopia (58.14%, 95% CI: 48.69–67.58%), Amhara (47.83%, 95% CI: 39.77–55.89%), and Oromia (42.86%, 95% CI: 32.77–52.95%), whereas relatively low magnitude of E. coli resistance was reported from Tigray region (27.51%, 95% CI: 16.14–38.88%) (Figures 2 and 3).
Figure 2.
Proportion of E. coli resistance in different regions of Ethiopia, 2007–2017. Values in parenthesis indicated 95% CI of E. coli resistance in different regions of Ethiopia.
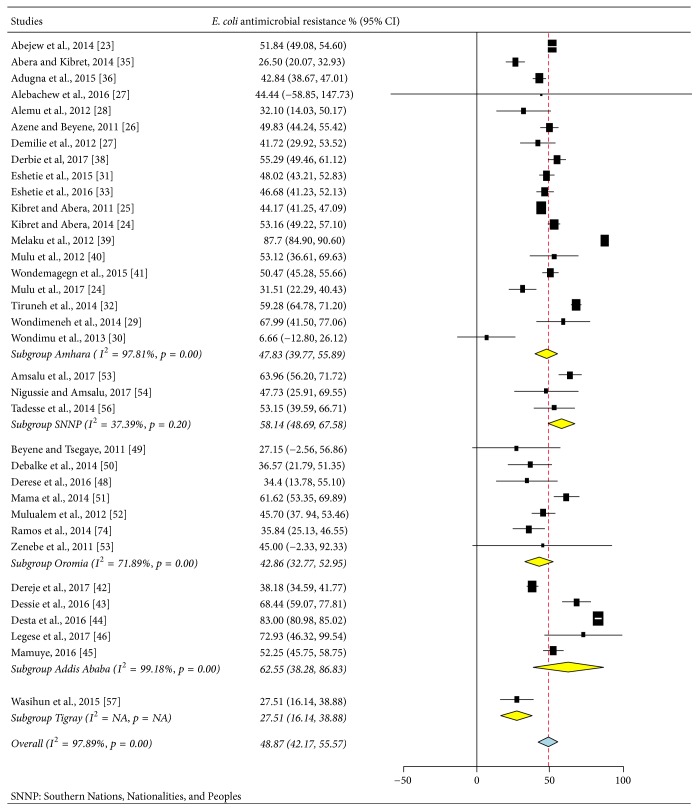
Figure 3.
Subgroup analysis of E. coli antibacterial resistance according to regions of Ethiopia.
Subgroup analyses (Figure 3) were carried out based on the region (Addis Ababa, Amhara, Oromia, SNNP, and Tigray) and the mechanism of action of the drugs (cell wall synthesis inhibitors, protein synthesis inhibitors, DNA synthesis inhibitors, and antimetabolites). A paper-based analysis in our study showed that the overall E. coli resistance in Ethiopia was 48.87% (95% CI: 42.17–55.57%) with highest prevalence in the capital city, Addis Ababa, 62.55% (95% CI: 38.28–86.83%) (Figure 3).
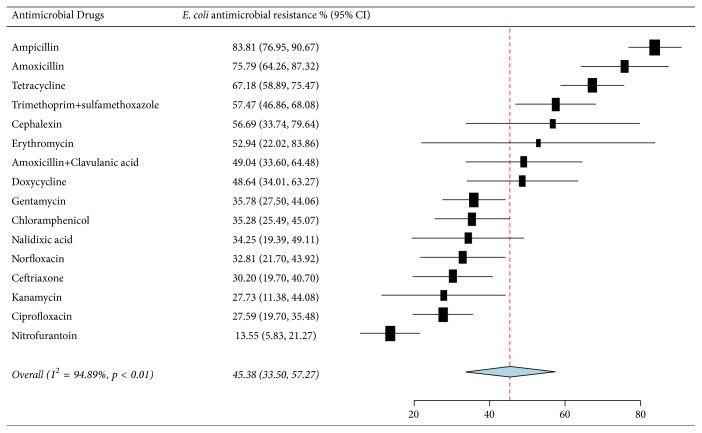
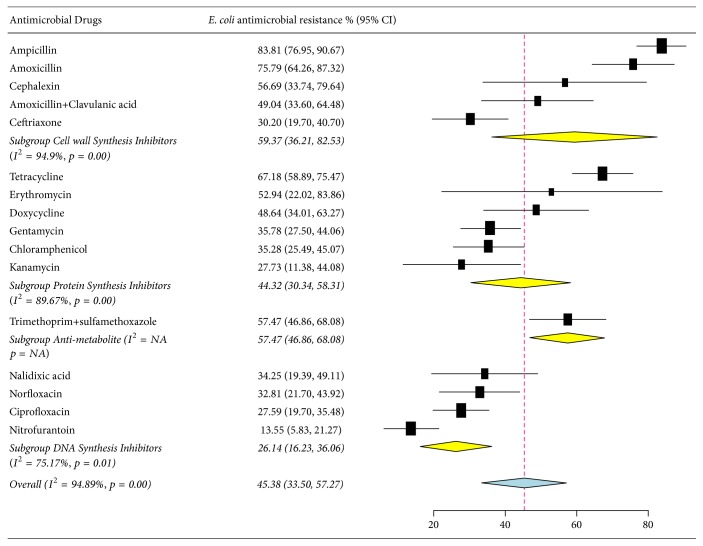
As presented in Figure 4 and Table 3, the pooled prevalence of E. coli resistance was 45.38% (95% CI: 33.50–57.27%), and high resistance rates were observed to ampicillin, 83.81% (95% CI: 76.95–90.67%), amoxicillin, 75.79% (95% CI: 64.26–87.32%), tetracycline, 67.18% (95% CI: 58.89–75.47%), trimethoprim-sulfamethoxazole, 57.47% (95% CI: 58.89–75.47%), and cephalothin, 56.69% (95% CI: 33.74–79.64%). A relatively low level of nitrofurantoin resistance was observed, 13.55% (95% CI: 5.83–21.27%).
Figure 4.
Forest plot of the pooled percentage and confidence interval of E. coli resistance to antibacterial drugs in Ethiopia from 2007 to 2017.
Table 3.
Percentage of pooled antibacterial resistance rates of E. coli in Ethiopia, 2007–2017.
| Studies | Antibacterial | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AML | AMP | AMC | KF | CRO | TE | DO | C | E | CN | K | CIP | NA | F | NOR | SXT | |
| Abejew et al., 2014 [23] | 84.6 | 80.0 | --- | 42.3 | 46.9 | 82.2 | 64.5 | 49.0 | 94.4 | 34.0 | 20.0 | 28.3 | 13.3 | 10.4 | --- | 75.8 |
| Abera and Kibret, 2014 [35] | --- | --- | --- | --- | 40 | 25 | --- | 35 | --- | 40 | --- | 4 | --- | --- | --- | 15 |
| Adugna et al., 2015 [36] | --- | 86.8 | 47.5 | --- | --- | 76 | --- | 36.2 | --- | 37.2 | --- | 6.9 | --- | --- | 9.3 | --- |
| Alebachew et al., 2016 [27] | --- | 100 | 100 | --- | 0 | 100 | --- | 0 | --- | 0 | --- | 0 | --- | 0 | --- | 100 |
| Alemu et al., 2012 [28] | 100 | 100 | 36.8 | --- | 0 | 52.6 | --- | 0 | --- | 5.3 | --- | 0 | --- | --- | 0 | 26.3 |
| Beyene and Tsegaye, 2011 [49] | 100 | 100 | --- | --- | 0 | 28.6 | --- | 0 | --- | 0 | --- | 14.3 | 0 | 0 | --- | 28.6 |
| Debalke et al., 2014 [50] | --- | 88 | --- | --- | 4 | --- | --- | 0 | --- | --- | --- | --- | 52 | 0 | 16 | 96 |
| Demilie et al., 2012 [37] | 75 | 81.7 | 37.5 | --- | --- | 68.8 | --- | 37.5 | --- | 31.2 | 18.8 | 18.8 | 43.8 | 6.3 | 25 | 56.2 |
| Derbie et al., 2017 [38] | --- | 89.1 | 78.6 | --- | --- | 66.1 | --- | --- | --- | 27.6 | --- | 64.4 | --- | 25 | 27 | 64.5 |
| Dereje et al., 2017 [42] | --- | --- | 21.6 | --- | 24.6 | --- | --- | 32.2 | --- | 53.8 | --- | 56.9 | --- | 40 | --- | --- |
| Derese et al., 2016 [48] | 77.8 | 77.8 | --- | --- | 0 | 66.7 | --- | 11.1 | --- | 0 | --- | 11.1 | 44.4 | 44.4 | --- | 11.1 |
| Dessie et al., 2016 [43] | --- | 95.8 | 70.8 | --- | 83.3 | 83.3 | --- | 25 | --- | 54.2 | --- | 66.7 | --- | --- | --- | --- |
| Desta et al., 2016 [44] | --- | --- | 93 | 98 | --- | --- | --- | --- | --- | 63 | --- | 78 | --- | --- | --- | --- |
| Eshetie et al., 2015 [31] | --- | 92.9 | 60.7 | --- | 17.9 | 53.6 | --- | 64.3 | --- | 57.1 | --- | 10.7 | 25 | --- | --- | 50 |
| Kibret and Abera, 2014 [24] | 87.3 | --- | --- | 56.9 | 33.9 | 80.1 | 61 | 37.4 | 93.7 | 24.5 | --- | 34.1 | --- | 3.8 | --- | 72.1 |
| Kibret and Abera, 2011 [25] | 86 | --- | --- | 59.5 | 37.4 | 72.4 | --- | 35.3 | 89.4 | 13 | --- | 19.9 | --- | 3.6 | 6.5 | 62.9 |
| Azene and Beyene, 2011 [26] | 85.0 | --- | --- | 64.7 | 66.7 | 71.4 | 33.3 | 36.1 | 51.9 | 14.4 | --- | 7.7 | --- | --- | --- | 67.1 |
| Mama et al., 2014 [51] | --- | 100 | --- | 100 | 62 | 79 | 44.8 | 65.5 | --- | 51.7 | --- | 34 | 41 | --- | 44.8 | 55 |
| Mamuye, 2016 [45] | 75.5 | 79.2 | --- | 32.1 | 45.3 | 83.0 | 71.7 | 30.2 | --- | 22.6 | --- | 54.7 | 73.6 | 20.8 | 67.9 | 22.6 |
| Melaku et al., 2012 [39] | 85.7 | 100 | --- | --- | --- | 81.6 | --- | 83.7 | --- | --- | --- | --- | --- | --- | --- | --- |
| Mulu et al., 2017 [34] | 25 | 33.3 | --- | --- | 3.3 | 75 | 75 | 13 | 0 | 69.6 | --- | 18.2 | --- | --- | 23.1 | 11.1 |
| Mulualem et al., 2012 [52] | 86 | 86 | 70.1 | --- | 9 | 73.1 | --- | 35.8 | --- | 3 | --- | 20.9 | --- | --- | 16.4 | 56.7 |
| Mulu et al., 2012 [40] | 90 | 78 | --- | --- | 55.6 | 66.7 | 66.7 | 0 | --- | 44.4 | 44.4 | 44.4 | 66.7 | 22.2 | 44.4 | 67 |
| Nigussie and Amsalu, 2017 [54] | --- | 100 | 36.4 | --- | 63.6 | --- | --- | --- | --- | 72.7 | --- | 18.2 | --- | 0 | 9.1 | 81.8 |
| Ramos et al., 2014 [47] | 64.7 | 70.6 | 5.9 | --- | 5.9 | 41.2 | 29.4 | 35.3 | 41.2 | 11.8 | --- | 29.4 | --- | --- | --- | 58.8 |
| Wasihun et al.2015 [57] | --- | --- | 6.7 | --- | 60 | --- | 40 | --- | --- | 13.3 | --- | 6.7 | --- | 26.7 | 60 | 6.7 |
| Wondimeneh et al., 2014 [29] | 50 | 66.7 | --- | --- | 50 | 100 | --- | 16.7 | --- | 66.7 | --- | 50 | --- | --- | 66.7 | 66.7 |
| Wondimu et al., 2013 [30] | --- | 33.3 | 0 | --- | 0 | 0 | 0 | 0 | 0 | 0 | --- | 33.3 | --- | --- | --- | 0 |
| Zenebe et al., 2011 [53] | 0 | 100 | --- | 0 | 0 | 75 | --- | 100 | --- | 75 | --- | 0 | 0 | --- | --- | 100 |
| Amsalu1 et al., 2016 [54] | --- | 100 | 26.1 | --- | 45.7 | --- | --- | 64.3 | --- | 60.6 | --- | 56.2 | --- | --- | 68.8 | 90 |
| Tadesse et al., 2014 [56] | --- | 68.8 | --- | --- | --- | --- | --- | --- | --- | 43.8 | --- | --- | --- | --- | 18.8 | 81.2 |
| Tiruneh et al., 2014 [32] | 87.5 | 83.3 | --- | --- | --- | 79.2 | --- | 58.3 | --- | 44.2 | --- | 46.7 | --- | --- | --- | 76.7 |
| Legese et al., 2017 [46] | 100 | --- | 100 | --- | --- | 83.3 | --- | 66.7 | --- | 66.7 | --- | --- | --- | 0 | 66.7 | 100 |
| Eshetie et al., 2016 [33] | --- | 98.2 | 42 | --- | --- | 43.8 | --- | 54.5 | --- | 52.7 | --- | 0.9 | 17 | --- | --- | 64.3 |
| Wondemagegn et al., 2015 [41] | 80 | 73.3 | --- | --- | --- | 73.3 | --- | --- | --- | 26.7 | --- | 20 | --- | --- | 20 | 60 |
AMC: amoxicillin-clavulanic acid, AML: amoxicillin, AMP: ampicillin, C: chloramphenicol, CIP: ciprofloxacin, CN: gentamicin, CRO: ceftriaxone, DO: doxycycline, E: erythromycin, F: nitrofurantoin, K: kanamycin, KF: cephalothin, NA: nalidixic acid, NOR: norfloxacin, SXT: trimethoprim/sulfamethoxazole, TE: tetracycline, ---: not done.
Comparing the prevalence of E. coli resistance among the antibacterial drugs, subgroup analysis (Figure 5) revealed that the cell wall synthesis inhibitors account for the greatest resistance percentage, 59.37% (95% CI: 36.21–82.53%), and DNA synthesis inhibitors account for the lowest resistance percentage, 26.14% (95% CI: 33.50–57.27%).
Figure 5.
Subgroup analysis of pooled percentage and confidence interval of E. coli resistance to antibacterial drugs according to drug mechanism of action.
There was a high level of heterogeneity by random model methods (I2 = 97.89%; p < 0.01). Hence, the included studies have been conducted in different study settings, study periods, and study populations, which could have an effect on the heterogeneity of the included studies. The symmetry of funnel plot showed small study bias, which yielded insignificant effect.
4. Discussion
Antibiotic resistance continues to be a major global challenge in the management of bacterial infection. The trouble behind antibiotic resistance is highly marked in undeveloped or developing countries, including Ethiopia, where infectious diseases are highly prevalent [64]. Factors responsible for an increase in rates of antimicrobial resistance include misuse/overuse of antibiotics by healthcare professionals and general public and inadequate surveillance systems due to lack of reliable microbiological techniques leading to the inappropriate prescription of antibiotics [33]. Antimicrobial resistance in E. coli has increased worldwide and its susceptibility patterns show substantial variation in different geographical locations [5]. To date, the overall epidemiology and burden of multidrug resistance (MDR) bacteria have not been fully understood, especially in resource-limited countries including Ethiopia [64, 65]. To the best of our knowledge, this is the first meta-analysis study conducted to determine the pooled prevalence of E. coli prevalence and resistance in Ethiopia. Our result revealed that E. coli strains displayed diverse resistance patterns, with percentages varying slightly based on sample type and geographical distribution. Based on the tested antimicrobials, the overall E. coli resistance in Ethiopia was nearly 50% (45.38% (95% CI: 33.50–57.27%)).
Developing countries have comparatively higher risk factors associated with MDR strains than the developed ones [64, 66]. Resistance to antibacterial agents is a normal evolutionary process for microorganisms, but it is highly aggravated by continuous deployment of antimicrobial drugs in treating infections [67, 68]. It is claimed that more than half of drugs are prescribed, sold, or dispensed without following standard protocols, and the situation is more pronounced in developing countries including Ethiopia [69]. Sosa et al. (2010) reported that antibiotic usage in most of the low-income countries is generally unregulated, which is a prime factor for the occurrence of resistant bacterial strains [64]. This implies that antibiotics are being used widely and inappropriately in resource-limited countries including Ethiopia. This may lead to an increase in the occurrence of drug-resistive bacterial strains such as E. coli.
In our study, the regional prevalence of E. coli resistance was estimated, and the subgroup analysis showed that the highest prevalence of E. coli resistance (62.55%) was noted in Addis Ababa city, which was almost two times higher than Tigray region (27.51%). The observed variation might be due to differences in study location, hospital setup, and antimicrobial utilization.
Subgroup analysis also showed that E. coli strains exhibited higher resistance with cell wall inhibitors, specifically aminopenicillins (ampicillin and amoxicillin), followed by protein synthesis inhibitors, mainly to tetracycline, and lesser resistance prevalence to nitrofurantoin. In line with our data, globally, E. coli strains were reported to be highly resistant to the above-mentioned antibiotics, mainly to aminopenicillins [70, 71]. Săndulescu (2016) reported that E. coli showed low resistance to nitrofurantoin, which is in line with the present finding.
Moreover, our finding indicated the higher magnitude of E. coli resistance. This may imply the need for intervention in prescribing and using antibacterial against E. coli infections. Interventional strategies may include creating public awareness, maintaining hand hygiene, applying infection prevention protocols, and maintaining environmental sanitation, which are encouraged for preventing infection. In addition to these, promoting health education, maintaining continuous professional educations, and advocating rational prescribing habits are evidently effective in the minimization of the unwanted use of antibiotics, which in turn decrease selective pressure of resistant strains.
5. Conclusion
In this meta-analysis, the pooled E. coli resistance is considerably high. E. coli strains were highly resistant to ampicillin but showed lesser resistance to nitrofurantoin. Adopting safety protocols and implementing proper antibiotic prescription policies could be potential interventional strategies to address the emerging resistance of E. coli.
Abbreviation
- ANC:
Antenatal clinic
- AMC:
Amoxicillin-clavulanic acid
- AML:
Amoxicillin
- AMP:
Ampicillin
- C:
Chloramphenicol
- CI:
Confidence interval
- CIP:
Ciprofloxacin
- CN:
Gentamicin
- CRO:
Ceftriaxone
- CS:
Cross-sectional
- CSF:
Cerebrospinal fluid
- DO:
Doxycycline
- E:
Erythromycin
- E. coli:
Escherichia coli
- F:
Nitrofurantoin
- K:
Kanamycin
- KF:
Cephalothin
- MDR:
Multidrug resistance
- NA:
Nalidixic acid
- NOR:
Norfloxacin
- PCS:
Prospective cross-sectional
- R:
Retrospective review of culture
- RCS:
Retrospective cross-sectional
- SNNP:
Southern Nations, Nationalities, and Peoples
- SXT:
Trimethoprim/sulfamethoxazole
- TE:
Tetracycline
- UTI:
Urinary tract infection.
Ethical Approval
Ethical clearance was not required and was not necessary for this study.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
Kald Beshir Tuem contributed to conception of the research protocol and study design. Kald Beshir Tuem, Abadi Kahsu Gebre, Tesfay Mehari Atey, and Derbew Fikadu Berhe contributed to literature review, data collection, data extraction, data analysis and interpretation, and drafting the manuscript. Kald Beshir Tuem, Abadi Kahsu Gebre, Tesfay Mehari Atey, Helen Bitew, Ebrahim M. Yimer, and Derbew Fikadu Berhe contributed to drafting and reviewing the manuscript. All authors have read and approved the manuscript.
Supplementary Materials
S1 file: PRISMA 2009 Checklist. We have conducted the meta-analysis according to Moher et al. (2009), “Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement.” We included all the criteria on the checklist, except in the discussion part; we did not include the limitations of the study related to incomplete retrieval of articles, as we did not face such a problem.
S2 file: Modified Newcastle-Ottawa Scale. This scale had been adapted from Wells et al. (2009), “The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomized Studies in Meta-Analyses.” We considered the comparability to be controlled if the standard laboratory procedure is described in the articles. Since the outcome of the bacterial sensitivity test is obtained after full growth of the bacterial strains, we assigned one star for the assessment outcomes of all studies.
References
- 1.Levine M. M. Escherichia coli that cause diarrhea: enterotoxigenic, enteropathogenic, enteroinvasive, enterohemorrhagic, and enteroadherent. 1987;155(3):377–389. doi: 10.1093/infdis/155.3.377. [DOI] [PubMed] [Google Scholar]
- 2.Wagenlehner F. M., Naber K. G., Weidner W. Rational antibiotic therapy of urinary tract infections. 2008;31(10):385–90. [PubMed] [Google Scholar]
- 3.De Francesco M. A., Giuseppe R., Laura P., Riccardo N., Nin M. Urinary tract infections inBrescia, Italy: Etiology of uropathogens and antimicrobial resistance of common Uropathogens. 2007;13(6):136–144. [PubMed] [Google Scholar]
- 4.Kashef N., Djavid G. E., Shahbazi S. Antimicrobial susceptibility patterns of community-acquired uropathogens in Tehran, Iran. 2010;4(4):202–206. doi: 10.3855/jidc.540. [DOI] [PubMed] [Google Scholar]
- 5.Biedenbach D. J., Moet G. J., Jones R. N. Occurrence and antimicrobial resistance pattern comparisons among bloodstream infection isolates from the SENTRY Antimicrobial Surveillance Program (1997-2002) 2004;50(1):59–69. doi: 10.1016/j.diagmicrobio.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 6.Gebre-Sellassie S. Antimicrobial resistance patterns of clinical bacterial isolates in southern Ethiopia. 2007;45(4):363–370. [PubMed] [Google Scholar]
- 7.Khan N. A., Saba N., Abdus A. S., Ali A. A. Incidence and Antibiogram Patterns of Escherichia coli Isolated from Various Clinical Samples from Patients at NIH Islamabad. 2002;5(1):111–113. doi: 10.3923/pjbs.2002.111.113. [DOI] [Google Scholar]
- 8.Turner S. M., Scott-Tucker A., Cooper L. M., Henderson I. R. Weapons of mass destruction: Virulence factors of the global killer enterotoxigenic Escherichia coli. 2006;263(1):10–20. doi: 10.1111/j.1574-6968.2006.00401.x. [DOI] [PubMed] [Google Scholar]
- 9.Bell J. M., Turnidge J. D., Gales A. C., Pfaller M. A., Jones R. N. Prevalence of extended spectrum β-lactamase (ESBL)-producing clinical isolates in the Asia-Pacific region and South Africa: regional results from SENTRY Antimicrobial Surveillance Program (1998–99) 2002;42(3):193–198. doi: 10.1016/s0732-8893(01)00353-4. [DOI] [PubMed] [Google Scholar]
- 10.El Kholy A., Baseem H., Hall G. S., Procop G. W., Longworth D. L. Antimicrobial resistance in Cairo, Egypt 1999–2000: a survey of five hospitals. 2003;51(3):625–630. doi: 10.1093/jac/dkg101. [DOI] [PubMed] [Google Scholar]
- 11.Dromigny J. A., Nabeth P., Juergens-Behr A., Perrier-Gros-Claude J. D. Risk factors for antibiotic-resistant Escherichia coli isolated from community-acquired urinary tract infections in Dakar, Senegal. 2005;56(1):236–239. doi: 10.1093/jac/dki158. [DOI] [PubMed] [Google Scholar]
- 12.Tadesse D. A., Zhao S., Tong E. Antimicrobial drug resistance in Escherichia coli from humans and food animals, United States, 1950–2002. 2012;18(5):741–749. doi: 10.3201/eid1805.111153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.US Food and Drug Administration. National antimicrobial resistance monitoring system –enteric bacteria (NARMS): 2008 executive report. Rockville (MD); 2010. http://www.fda.gov/AnimalVeterinary/SafetyHealth/AntimicrobialResistance/NationalAntimicrobialResistanceMonitoringSystem/default.htm.
- 14.Atkinson B. A., Lorian V. Antimicrobial agent susceptibility patterns of bacteria in hospitals from 1971 to 1982. 1984;20:791–796. doi: 10.1128/jcm.20.4.791-796.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blaettler L., Mertz D., Frei R., et al. Secular trend and risk factors for antimicrobial resistance in escherichia coli isolates in Switzerland 1997-2007. 2009;37(6):534–539. doi: 10.1007/s15010-009-8457-0. [DOI] [PubMed] [Google Scholar]
- 16.Kronvall G. Antimicrobial resistance 1979-2009 at Karolinska hospital, Sweden: Normalized resistance interpretation during a 30-year follow-up on Staphylococcus aureus and Escherichia coli resistance development. 2010;118(9):621–639. doi: 10.1111/j.1600-0463.2010.02660.x. [DOI] [PubMed] [Google Scholar]
- 17.Patel T., Levitin A. Escherichia Coli Adaptive Resistance to Clinical Antibiotics. 2014;2(1) [Google Scholar]
- 18.Erb A., Stürmer T., Marre R., Brenner H. Prevalence of antibiotic resistance in Escherichia coli: Overview of geographical, temporal, and methodological variations. 2007;26(2):83–90. doi: 10.1007/s10096-006-0248-2. [DOI] [PubMed] [Google Scholar]
- 19.Endalafer N., Gebre-Selassie S., Kotiso B. Nosocomial bacterial infections in a tertiary hospital in Ethiopia. 2011;12(1):38–43. doi: 10.1177/1757177410376680. [DOI] [Google Scholar]
- 20.Yismaw G., Abay S., Asrat D., Yifru S., Kassu A. Bacteriological profile and resistant pattern of clinical isolates from pediatric patients, Gondar University Teaching Hospital, Gondar, Northwest Ethiopia. 2010;48(4):293–300. [PubMed] [Google Scholar]
- 21.Eshetie S., Tarekegn F., Moges F., Amsalu A., Birhan W., Huruy K. Methicillin resistant Staphylococcus aureus in Ethiopia: A meta-analysis. 2016;16(1, article no. 689) doi: 10.1186/s12879-016-2014-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moher D., Liberati A., Tetzlaff J., Altman D. G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. 2009;339, article b2535 doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abejew A. A., Denboba A. A., Mekonnen A. G. Prevalence and antibiotic resistance pattern of urinary tract bacterial infections in Dessie area, Northeast Ethiopia. 2014;7(1, article 687) doi: 10.1186/1756-0500-7-687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kibret M., Abera B. Prevalence and antibiogram of bacterial isolates from urinary tract infections at Dessie Health Research Laboratory, Ethiopia. 2014;4(2):164–168. doi: 10.1016/S2221-1691(14)60226-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kibret M., Abera B. Antimicrobial susceptibility patterns of E. coli from clinical sources in northeast Ethiopia. 2011;11:S40–S45. doi: 10.4314/ahs.v11i3.70069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Azene M. K., Beyene B. A. Bacteriology and antibiogram of pathogens from wound infections at Dessie Laboratory, North-east Ethiopia. 2011;13(4):68–74. doi: 10.4314/thrb.v13i4.64901. [DOI] [PubMed] [Google Scholar]
- 27.Alebachew G., Teka B., Endris M., Shiferaw Y., Tessema B. Etiologic Agents of Bacterial Sepsis and Their Antibiotic Susceptibility Patterns among Patients Living with Human Immunodeficiency Virus at Gondar University Teaching Hospital, Northwest Ethiopia. 2016;2016 doi: 10.1155/2016/5371875.5371875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alemu A., Moges F., Shiferaw Y., et al. Bacterial profile and drug susceptibility pattern of urinary tract infection in pregnant women at University of Gondar Teaching Hospital, Northwest Ethiopia. 2012;5, article no. 197 doi: 10.1186/1756-0500-5-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wondimeneh Y., Muluye D., Alemu A., et al. Urinary tract infection among obstetric fistula patients at Gondar University Hospital, Northwest Ethiopia. 2014;14(1, article no. 12) doi: 10.1186/1472-6874-14-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wondimu H., Addis Z., Moges F., Shiferaw Y. Bacteriological Safety of Blood Collected for Transfusion at University of Gondar Hospital Blood Bank, Northwest Ethiopia. 2013;2013:1–7. doi: 10.1155/2013/308204.308204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eshetie S., Unakal C., Gelaw A., Ayelign B., Endris M., Moges F. Multidrug resistant and carbapenemase producing Enterobacteriaceae among patients with urinary tract infection at referral Hospital, Northwest Ethiopia. 2015;4(1, article no. 12) doi: 10.1186/s13756-015-0054-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tiruneh M., Yifru S., Gizachew M., et al. Changing Trends in Prevalence and Antibiotics Resistance of Uropathogens in Patients Attending the Gondar University Hospital, Northwest Ethiopia. 2014;2014:1–7. doi: 10.1155/2014/629424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eshetie S., Tarekegn F., Kumera G., Mekonnen F. Multidrug resistant Escherichia coli strains isolated from urine sample, University of Gondar Hospital, Northwest Ethiopia. 2016;4(2):140–142. doi: 10.12980/jclm.4.2016j5-247. [DOI] [Google Scholar]
- 34.Mulu W., Abera B., Yimer M., Hailu T., Ayele H., Abate D. Bacterial agents and antibiotic resistance profiles of infections from different sites that occurred among patients at Debre Markos Referral Hospital, Ethiopia: A cross-sectional study. 2017;10(1, article no. 254) doi: 10.1186/s13104-017-2584-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abera B., Kibret M. Azithromycin, fluoroquinolone and chloramphenicol resistance of non-chlamydia conjunctival bacteria in rural community of Ethiopia. 2014;62(2):236–239. doi: 10.4103/0301-4738.99974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Adugna A., Kibret M., Abera B., Nibret E., Adal M. Antibiogram of e. Coli serotypes isolated from children aged under five with acute diarrhea in bahir dar town. 2015;15(2):656–664. doi: 10.4314/ahs.v15i2.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Demilie T., Beyene G., Melaku S., Tsegaye W. Urinary bacterial profile and antibiotic susceptibility pattern among pregnant women in north west Ethiopia. 2012;22(2):121–128. [PMC free article] [PubMed] [Google Scholar]
- 38.Derbie A., Hailu D., Mekonnen D., Abera B., Yitayew G. Antibiogram profile of uropathogens isolated at bahir dar regional health research laboratory centre, northwest ethiopia. 2017;26, article no. 134 doi: 10.11604/pamj.2017.26.134.7827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Melaku S., Kibret M., Abera B., Gebre-Sellassie S. Antibiogram of nosocomial urinary tract infections in Felege Hiwot referral hospital, Ethiopia. 2012;12(2):134–139. doi: 10.4314/ahs.v12i2.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mulu W., Kibru G., Beyene G., Damtie M. Postoperative nosocomial infections and antimicrobial resistance pattern of bacteria isolates among patients admitted at Felege Hiwot Referral Hospital, Bahirdar, Ethiopia. 2012;22(1):7–18. [PMC free article] [PubMed] [Google Scholar]
- 41.Wondemagegn M., Yimer M., Zenebe Y., Abera B. Common causes of vaginal infections and antibiotic susceptibility of aerobic bacterial isolates in women of reproductive age attending at Felegehiwot referral Hospital, Ethiopia: a cross sectional study. 2015;15(42):1–9. doi: 10.1186/s12905-015-0197-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dereje M., Woldeamanuel Y., Asrat D., Ayenachew F. Urinary tract infection among fistula patients admitted at Hamlin fistula hospital, Addis Ababa, Ethiopia. 2017;17(1, article no. 150) doi: 10.1186/s12879-017-2265-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dessie W., Mulugeta G., Fentaw S., Mihret A., Hassen M., Abebe E. Pattern of bacterial pathogens and their susceptibility isolated from surgical site infections at selected referral hospitals, Addis Ababa, Ethiopia. 2016;2016:8. doi: 10.1155/2016/2418902.2418902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Desta K., Woldeamanuel Y., Azazh A., et al. High gastrointestinal colonization rate with extended-spectrum β-lactamase-producing Enterobacteriaceae in hospitalized patients: Emergence of carbapenemase-producing K. Pneumoniae in Ethiopia. 2016;11(8) doi: 10.1371/journal.pone.0161685.e0161685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mamuye Y. Antibiotic Resistance Patterns of Common Gram-negative Uropathogens in St. Paul's Hospital Millennium Medical College. 2016;26(2):93–100. doi: 10.4314/ejhs.v26i2.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Legese M. H., Weldearegay G. M., Asrat D. Extended-spectrum beta-lactamase- and carbapenemase-producing Enterobacteriaceae among ethiopian children. 2017;10:27–34. doi: 10.2147/IDR.S127177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ramos J. M., Pérez-Tanoira R., García-García C., et al. Leprosy ulcers in a rural hospital of Ethiopia: Pattern of aerobic bacterial isolates and drug sensitivities. 2014;13(1, article no. 47) doi: 10.1186/s12941-014-0047-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Derese B., Kedir H., Teklemariam Z., Weldegebreal F., Balakrishnan S. Bacterial profile of urinary tract infection and antimicrobial susceptibility pattern among pregnant women attending at Antenatal Clinic in Dil Chora Referral Hospital, Dire Dawa, Eastern Ethiopia. 2016;12:251–260. doi: 10.2147/TCRM.S99831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Beyene G., Tsegaye W. Bacterial Uropathogens in Urinary Tract Infection and Antibiotic Susceptibility Pattern in Jimma University Specialized Hospital, Southwest Ethiopia. 2011;21(2) doi: 10.4314/ejhs.v21i2.69055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Debalke S., Cheneke W., Tassew H., Awol M. Urinary tract infection among antiretroviral therapy users and nonusers in jimma university specialized hospital, Jimma, Ethiopia. 2014;2014 doi: 10.1155/2014/968716.968716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mama M., Abdissa A., Sewunet T. Antimicrobial susceptibility pattern of bacterial isolates from wound infection and their sensitivity to alternative topical agents at Jimma University Specialized Hospital, South-West Ethiopia. 2014;13, article 14 doi: 10.1186/1476-0711-13-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mulualem Y., Kasa T., Mekonnen Z., Suleman S. Occurrence of extended spectrum beta (b)-lactamases in multi-drug resistant Escherichia coli isolated from a clinical setting in Jimma University Specialized Hospital, Jimma, southwest Ethiopia. 2012;9(2):58–61. [PubMed] [Google Scholar]
- 53.Zenebe T., Kannan S., Yilma D., Beyene G. Invasive Bacterial Pathogens and their Antibiotic Susceptibility Patterns in Jimma University Specialized Hospital, Jimma, Southwest Ethiopia. 2011;21(1) doi: 10.4314/ejhs.v21i1.69038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nigussie D., Amsalu A. Prevalence of uropathogen and their antibiotic resistance pattern among diabetic patients. 2017;43(1):85–92. doi: 10.5152/tud.2016.86155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Amsalu A., Geto Z., Asegu D., Eshetie S. Antimicrobial resistance pattern of bacterial isolates from different clinical specimens in Southern Ethiopia: A three-year retrospective study. 2017;9(1):1–8. [Google Scholar]
- 56.Tadesse E., Teshome M., Merid Y., Kibret B., Shimelis T. Asymptomatic urinary tract infection among pregnant women attending the antenatal clinic of Hawassa Referral Hospital, Southern Ethiopia. 2014;7(1, article no. 155) doi: 10.1186/1756-0500-7-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wasihun A. G., Wlekidan L. N., Gebremariam S. A., et al. Bacteriological profile and antimicrobial susceptibility patterns of blood culture isolates among febrile patients in mekelle hospital, Northern Ethiopia. 2015;4(1) doi: 10.1186/s40064-015-1056-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wells G., Shea B., O'Connell D. Ottawa, Canada: Ottawa Hospital Research Institute; 2009. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. [Google Scholar]
- 59.Ioannidis J. P. A. Interpretation of tests of heterogeneity and bias in meta-analysis. 2008;14(5):951–957. doi: 10.1111/j.1365-2753.2008.00986.x. [DOI] [PubMed] [Google Scholar]
- 60.DerSimonian R., Laird N. Meta-analysis in clinical trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 61.Fazel S., Khosla V., Doll H., Geddes J. The prevalence of mental disorders among the homeless in Western countries: Systematic review and meta-regression analysis. 2008;5(12):1670–1681. doi: 10.1371/journal.pmed.0050225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Barendregt J. J., Doi S. A., Lee Y. Y., Norman R. E., Vos T. Meta-analysis of prevalence. 2013;67(11):974–978. doi: 10.1136/jech-2013-203104. [DOI] [PubMed] [Google Scholar]
- 63.Rücker G., Schwarzer G., Carpenter J. R., Schumacher M. Undue reliance on I2 in assessing heterogeneity may mislead. 2008;8, article no. 79 doi: 10.1186/1471-2288-8-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sosa A. D. J., Amábile-Cuevas C. F., Byarugaba D. K., Hsueh -R., Kariuki S., Okeke I. N. New York, Dordrecht Heidelberg, London: Springer; 2010. [DOI] [Google Scholar]
- 65.World Health Organization. Antimicrobial resistance: global report on surveillance. World Health Organization; 2014. http://www.who.int/drugresistance/documents/surveillancereport/en/
- 66.Nelson N., Joshi M., Kirika R. Antimicrobial Resistance: The Need for Action in the East, Central and Southern Africa Region. Submitted to the US Agency for International Development by the Strengthening Pharmaceutical Systems (SPS) Program. Arlington, VA: Management Sciences for Health. 2009. http://apps.who.int/medicinedocs/documents/s18415en/s18415en.pdf.
- 67.Laxminarayan R., Duse A., Wattal C. Antibiotic resistance—the need for global solutions. 2013;13(12):1057–1098. doi: 10.1016/S1473-3099(13)70318-9. [DOI] [PubMed] [Google Scholar]
- 68.O'Brien T. F. Emergence, spread, and environmental effect of antimicrobial resistance: how use of an antimicrobial anywhere can increase resistance to any antimicrobial anywhere else. 2002;34(supplement 3):S78–S84. doi: 10.1086/340244. [DOI] [PubMed] [Google Scholar]
- 69.World Health Organization. Promoting rational use of medicines: core components. Geneva: WHO Press, 2002. http://apps.who.int/medicinedocs/pdf/h3011e/h3011e.pdf.
- 70.Săndulescu O. Global distribution of antimicrobial resistance in E. coli. 2012;2(2):69–74. doi: 10.18683/jccp.2016.1015. [DOI] [Google Scholar]
- 71.Bryce A., Costelloe C., Hawcroft C., Wootton M., Hay A. D. Faecal carriage of antibiotic resistant Escherichia coli in asymptomatic children and associations with primary care antibiotic prescribing: A systematic review and meta-analysis. 2016;16(1, article no. 359) doi: 10.1186/s12879-016-1697-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
S1 file: PRISMA 2009 Checklist. We have conducted the meta-analysis according to Moher et al. (2009), “Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement.” We included all the criteria on the checklist, except in the discussion part; we did not include the limitations of the study related to incomplete retrieval of articles, as we did not face such a problem.
S2 file: Modified Newcastle-Ottawa Scale. This scale had been adapted from Wells et al. (2009), “The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomized Studies in Meta-Analyses.” We considered the comparability to be controlled if the standard laboratory procedure is described in the articles. Since the outcome of the bacterial sensitivity test is obtained after full growth of the bacterial strains, we assigned one star for the assessment outcomes of all studies.