Abstract
Peanut (Arachis hypogaea) is one of the most common food allergens that can induce fatal anaphylaxis, and Ara h 2 is one of the major allergen components involved in peanut allergy. The aim of this study was to develop a quantitative method for detecting peanut allergen using monoclonal antibodies against Ara h 2. The splenocytes of immunized mice were fused with myeloma cells (SP2/0), and stable mAb-producing clones were obtained by limiting dilution. mAbs against Ara h 2 were isolated from mouse ascites, and specificity was confirmed by immunoblotting. Five mAbs with high purity and specific reactivity were obtained, which were referred to as 1-2E10, 2-1D5, 3-1C5, 4-1C2, and 5-1G4, respectively. After screening different mAb combinations for development of a sandwich ELISA, we selected 5-1G4 as the capture antibody and 1-2E10 as the detection antibody for the measurement of Ara h 2 from which an optimal correlation between the Ara h 2 concentration and the OD value was obtained. This sandwich ELISA could specifically detect Ara h 2 in peanut extract at concentrations as low as 5 ng/mL and up to 10 μg/mL. These mAbs can, therefore, serve as quantitative diagnostic reagents for peanut and peanut product risk assessment.
1. Introduction
The incidence of food allergy has been increasing in developing countries in recent decades and now affects up to 10% of the world population [1]. Food allergy-associated diseases have become a global health problem and a major food safety concern [2]. The gastrointestinal symptoms of food allergy include stomach pain, nausea, vomiting, anorexia, abdominal distension, abdominal pain, diarrhea, mucus production, and so on. Peanut allergy has been recognized as one of the most severe food allergies that can lead to life-threatening reactions after unintentional consumption [3]. Currently, the only effective treatment for peanut allergy is to avoid exposure to peanut allergens. However, avoidance is very difficult since peanut allergens are commonly found in many food products. Many foods are inadequately labeled and do not inform the consumer that peanut products might be present and, therefore, can lead to the accidental ingestion of peanut allergens. Moreover, in places where peanuts are cooked and consumed, peanut allergens can diffuse into the environment. Peanut-allergic patients often feel anxious because of the fear of accident consumption or exposure that commonly occurs and could send them into a medical emergency. Some parents with peanut-allergic children demand their children to avoid all food nuts, to the extreme of restricting the consumption of foods outside the home resulting in a deprivation of many childhood pleasures [4].
Peanut allergy is one of the prime causes of anaphylactic deaths, especially in Western countries [5]. Approximately 3~5% children suffer from peanut allergy beginning in infancy and up to adulthood [6, 7]. Peanut allergens can elicit a more severe reaction than allergens from other legumes [8]. At least 18 potentially important peanut allergens have been identified and listed in the World Health Organization/International Union of Immunological Societies (WHO/IUIS) allergen database, termed as Ara h 1 to Ara h 17 plus peanut oleosin [9, 10]. Based on the criteria suggested by the WHO/IUIS Allergen Nomenclature subcommittee in 1994 [11], Ara h 1, Ara h 2, and Ara h 3 were identified as the major peanut allergens. Ara h 2 belongs to the conglutinate family of seed storage proteins and is related to the 2S albumin family [12, 13]. Compared with Ara h 1 and h 3, Ara h 2 has a much higher potency in degranulation assays, suggesting that Ara h 2 is the most potent allergen in causing sensitization in the peanut-allergic patient [9, 14].
There are two Ara h 2 isoforms, Ara h 2.01 and Ara h 2.02, with Ara h 2.02 being the more sensitive diagnostic reagent due to its additional IgE binding epitopes compared to Ara h 2.01 [15]. In this study, we generated mAbs against rAra h 2.02, which has the same sequence with that of seed storage protein SSP1 of Arachis hypogaea (GenBank accession number AAT00598.1). The mAbs were used to develop a sandwich ELISA for quantification of Ara h 2 in peanut extract. This sandwich ELISA system can be used to precisely measure the amount of Ara h 2 in immunotherapy reagents. It may be also be used for standardizing clinical reagents, for monitoring of the allergen level in foods and the environment that allergic patients are exposed to daily, and for creating and ensuring a low-level allergen environment for patients; all of which could be beneficial to these peanut-allergic patients.
2. Material and Methods
2.1. Materials
All procedures performed in this study involving animals were in accordance with the ethical standards of the institution and have been approved by the research ethics committee of the Second Affiliated Hospital of Guangzhou Medical University and conform to the Guiding Principles in Use and Care of Animals published by the National Institutes of Health [16]. Balb/C mice were purchased from the Medical Laboratory Animal Center (Guangdong, China). Complete Freund's adjuvant (CFA) and Incomplete Freund's adjuvant (IFA) were purchased from Sigma-Aldrich Co. (St. Louis, USA). Sp 2/0 myeloma cell line was obtained from CAS Shanghai Life Science Cell Resource Center (Shanghai, China). HAT medium, HT medium, penicillin, streptomycin, fetal bovine serum (FBS), and RPMI-1640 medium plus L-glutamine were purchased from Life Technologies Inc. (New York, USA). Horse anti-mouse IgG-HRP conjugate was purchased from Cell Signaling Technology (Shanghai, China). Polyethylene glycol 4000(PEG 4000) was bought from Sigma-Aldrich Co. Strep-tag II column (5 × 1 mL Lot: 10183115) and HiTrap protein G (5 × 1 mL Lot: 17-0404-01) were bought from GE Healthcare (Freiburg, Germany). ISO-2 kit was purchased from Sigma-Aldrich Co. and the EZ-Link™ plus activated peroxidase kit from Thermo Scientific Co.
2.2. Generation of mAbs against Recombinant Ara h 2
Recombinant Ara h 2 (rAra h 2, referenced to GenBank accession number AAT00598.1) was expressed in E. coli and purified using a Strep-tag II column from inclusion bodies. Four 6–8-week-old Balb/C mice were immunized with purified rAra h 2 according to the procedure described previously [17, 18]. In brief, mouse mAbs against rAra h 2 were produced by the fusion of the myeloma cells sp2/0 and the spleen cells from the mice immunized with the purified rAra h 2 for three times at 2-week intervals. The hybridoma cells producing antibodies against rAra h 2 were screened by indirect ELISA and subcloned by limiting dilution to obtain stable mAb-producing cell lines. A commercial ISO-2 kit was used to identify the isotypes of the mAbs according to the manufacturer's instruction.
2.3. Purification and Characterization of mAbs
The stable mAb-producing cells were inoculated into the abdominal cavity of mice. The ascetic fluid was collected, and antibodies were purified using protein G affinity chromatography according to the manufacturer's instruction. The titers of the purified mAbs against Ara h 2 were measured by indirect ELISA, and the specificity was confirmed by immunoblotting (Supplementary Material 2.3).
2.4. Conjugation of Horseradish Peroxidase (HRP) to the mAbs
The Thermo Scientific™ EZ-Link plus activated peroxidase, an amine-reactive HRP, generated higher conjugate yields (>95%) than a method based on glutaraldehyde chemistry. The purified mAbs were dialyzed in carbonate-bicarbonate buffer (pH 9.4), and conjugation was carried out according to the manufacturer's recommendation.
2.5. Whole Peanut Extract
Peanut extract was made according to the protocol described by Caudrado et al. [19]. In brief, peanut flour from raw or roasted peanuts was suspended in a solution containing 100 mm Tris-HCl, 100 mm EDTA-Na2, and 100 mm NaCl at 1/10 w/v, rotated for 5 h at 4°C, and then centrifuged at 10,000g, 4°C for 20 min. The supernatant was collected and dialyzed in distilled water for 48 h at 4°C. The concentration of the protein was determined by BCA assay using bovine serum albumin (BSA) as the standard following the manufacturer's instruction.
2.6. Development of an ELISA for Ara h 2 Quantification
To build a sandwich ELISA, purified mAbs were used as the capture antibody to coat a polyvinyl microtiter plate (Costar, USA) with 100 μL of 1 μg/mL mAb solution. Purified rAra h 2 was used as the standard antigen, which was serially diluted from 10,000 ng/mL to 4.9 ng/mL, and the HRP-conjugated antibodies were used as the detection antibody. The optimal matching antibody pair was selected by comparing the correlation between the rAra h 2 concentration and the OD value of the different combinations of the 5 different antibodies.
2.7. Detection of the Cross-Reactivity of the mAbs
Since peanut allergens share significant amino acid similarity with antigens from other legumes and tree nuts, we further tested the specificity of the mAbs by assessing their cross-reactivity with other common food allergens by Western blotting. For this, we tested Ara h 8, Bet v 1, Gly m 4, and soybean extract as potential cross-reacting allergens with the Ara h 2 mAbs and used bovine serum albumin (BSA) as the negative control and peanut extract as the positive control. The HRP-conjugated mAbs were used as detection antibodies.
3. Results
3.1. Generation of mAbs against rAra h 2
After immunization, the sera from immunized and unimmunized mice were diluted at 1 : 500, 1 : 1000, 1 : 5000, 1 : 10,000, 1 : 20,000, 1 : 40,000, and 1 : 80,000 and the titers of rAra h 2-specific IgGs were measured by indirect ELISA. The results show that there were high titers of rAra h 2- specific antibodies in the sera of immunized mice (Figure 1). After fusion of splenocytes with the hybridoma cells, we obtained eight positive clones. After the first cycle of subcloning, five clones stably produced specific antibodies, while the other three clones were determined to be negative (Table 1). Four clones were IgG1 isotype while one clone was IgG2b isotype (Table 2).
Figure 1.
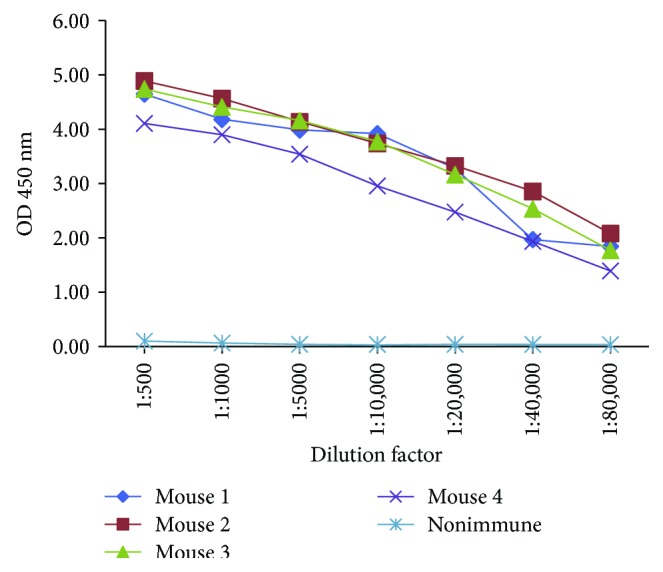
The serum titers of immunized mice.
Table 1.
Positive clones selected after fusion and the first subcloning.
| Clones selection after fusion | Clones selection after the first subcloning | ||
|---|---|---|---|
| Clones | OD at 450 nm | Clones | OD at 450 nm |
| Neg. ctrl | 0.051 | Neg. ctrl | 0.050 |
| Pos. ctrl | 3.46 | Pos. ctrl | 2.360 |
| 1-E4 | 3.042 | 1-1E4 | 2.26 |
| 4-B10 | 3.051 | 4-1B10 | 1.796 |
| 5-E2 | 3.867 | 5-1E2 | 2.233 |
| 5-E12 | 3.654 | 5-1E12 | 2.133 |
| 6-G9 | 3.676 | 6-1G9 | 2.4 |
| 8-D10 | 3.642 | 8-1D10 | 0.057 |
| 8-F8 | 3.846 | 8-1F8 | 0.055 |
| 8-H11 | 3.533 | 8-1H11 | 0.07 |
Neg. ctrl: negative control, serum from nonimmunized mouse. Pos. ctrl: positive control, serum from immunized mouse.
Table 2.
Ara h 2 mAb isotyping ELISA at OD 450 nm.
| Clone number | IgG1 | IgG2a | IgG2b | IgG3 | IgM | IgA |
|---|---|---|---|---|---|---|
| 1-2E10 | 2.447 | 0.335 | 0.337 | 0.344 | 0.358 | 0.353 |
| 2-1D5 | 0.246 | 1.279 | 0.243 | 0.242 | 0.236 | 0.242 |
| 3-1C5 | 2.3 | 0.414 | 0.404 | 0.377 | 0.364 | 0.369 |
| 4-1C2 | 1.947 | 0.342 | 0.343 | 0.343 | 0.34 | 0.357 |
| 5-1G4 | 1.951 | 0.379 | 0.381 | 0.36 | 0.336 | 0.375 |
| Medium | 0.109 | 0.101 | 0.113 | 0.122 | 0.133 | 0.116 |
The five mAb-producing clones were expanded and inoculated in Balb/C mice. The ascetic fluid was collected, and titration was performed by ELISA. The titers of the ascites were higher than 1 : 108 (Figure 2) and, thus, adequate for use in the subsequent experiments.
Figure 2.
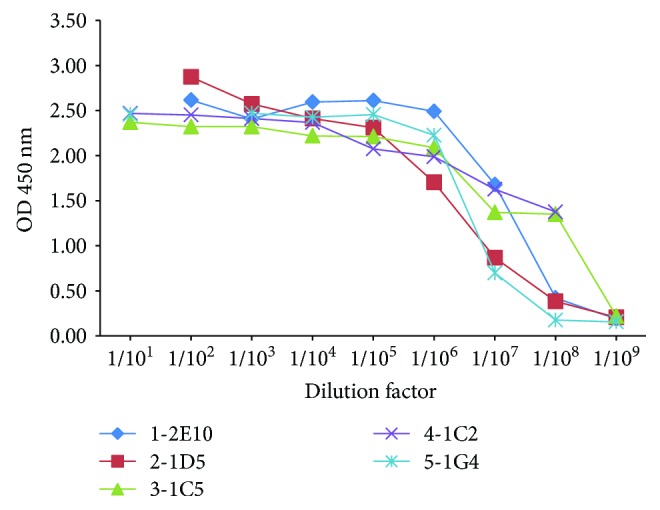
The mAb titers before purification (ascetic fluid).
3.2. Purification of the Ara h 2 mAbs
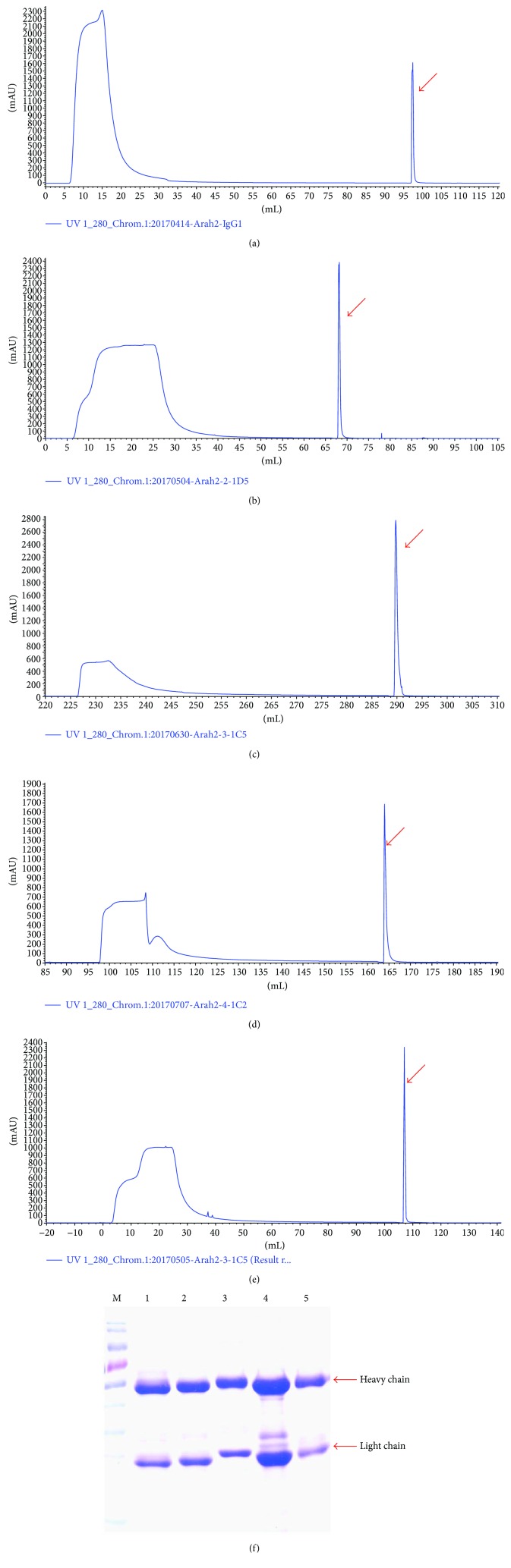
The Abs in the ascites were precipitated with 50% ammonium sulfate and purified by protein G affinity chromatography. As shown in Figures 3(a)–3(e), the first peak in the UV 280 graphs denoted the unbound protein and the second peak is the target protein (indicated with an arrow). The eluted proteins were subjected to SDS-PAGE. As shown in Figure 3(f), sharp individual bands of heavy chain and light chain antibody indicated that they are single clones.
Figure 3.
Purification of the mAbs against rAra h 2. (a) Clone 1-2E10 purification graph. (b) Clone 2-1D5 purification graph. (c) Clone 3-1C5 purification graph. (d) Clone 4-1C2 purification graph. (e) Clone 5-1G4 purification graph. (The arrows indicate the eluted peak of the target protein.) (f) The mAbs after purification. M: prestained protein marker. Lane 1: 1-2E10. Lane 2: 2-1D5. Lane 3: 3-1C5. Lane 4: 4-1C2. Lane 5: 5-1G4.
3.3. Characterization of the Ara h 2 mAbs
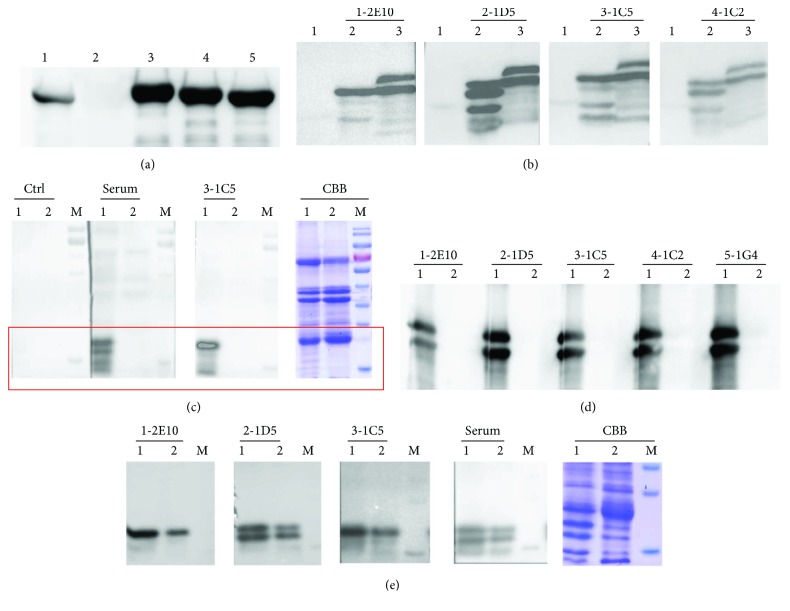
After purification, the mAb titers were measured by indirect ELISA. As shown in Figure 4, the titers were higher than 1 : 108, indicating highly efficient purification of mAbs. To confirm the isotypes and the specific reactivity of the mAbs against Ara h 2, Western blotting was performed. The isotype of the heavy chain was verified by goat anti-mouse IgG1-heavy chain (HRP) antibody. As shown in Figure 5(a), four mAbs were of the IgG1 isotype and only the 2-1D5 mAbs was of the IgG2a isotype. All mAbs recognized both recombinant Ara h 2 and native Ara h 2 in the peanut extract (Figure 5(b)). However, neither the mAbs nor the immune sera were able to recognize the peanut extract protein after desalting (Figures 5(c) and 5(d)), indicating that the desalting procedure may have affected the configuration and antigenic epitopes of the allergen. We then compared the mAb reactivity toward Ara h 2 in roasted and raw peanut extract. The result showed that the mAbs can recognize the allergen from both sources (Figure 5(e)).
Figure 4.
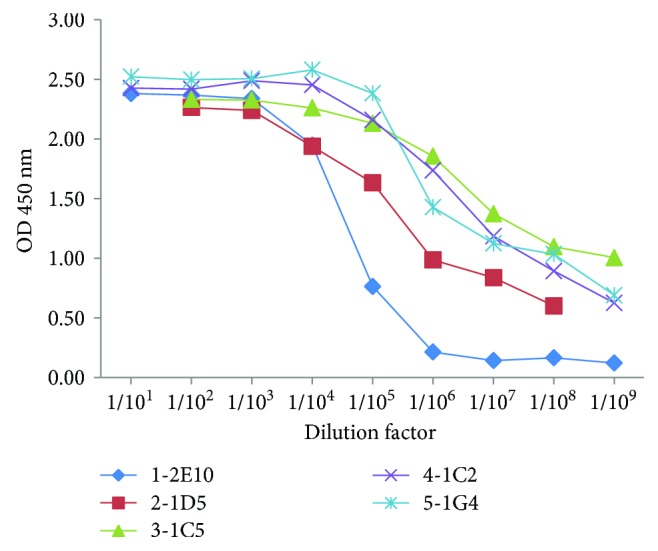
The mAb titers after purification.
Figure 5.
Characterization of the specificity of mAbs by Western blotting. (a) Isotyping of the IgG1 heavy chain of mAbs. Lane 1: 1-2E10-IgG1. Lane 2: 2-1D5-IgG2a. Lane 3: 3-1C5-IgG1. Lane 4: 4-1C2-IgG1. Lane 5: 5-1G4-IgG1. (b) Characterization of the specificity of the Ara h 2.02 mAbs. Lane 1: protein marker; lane 2: peanut extract before desalting; lane 3: rAra h 2; 1-2E10, 2-1D5, 3-1C5, and 4-1C2 were the mAbs applied individually. (c) Lane 1: peanut extract before desalting; lane 2: peanut extract after desalting; M: prestained protein marker; Ctrl: nonimmune serum; Serum: immunized serum; 3-1C5: the mAb was applied; CBB: Coomassie brilliant blue staining. (d) Lane 1: rAra h 2; lane 2: peanut extract after desalting; 1-2E10, 2-1D5, 3-1C5, 4-1C2, and 5-1G4 were the mAbs applied individually. (e) Lane 1: roasted peanut extract; lane 2: raw peanut extract; M: prestained protein marker; 1-2E10, 2-1D5, 3-1C5, and serum (immune serum) were applied individually. CBB: Coomassie brilliant blue staining.
3.4. Quantification of Ara h 2 by a Sandwich ELISA System
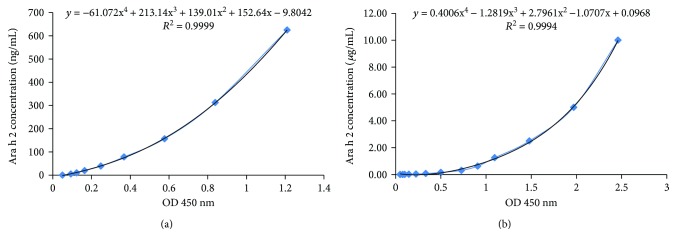
To develop a sandwich ELISA system, a purified mAb was used as the capture antibody and a different conjugated mAb was used as the detection antibody. After screening, 5-1G4 (1 μg/mL) was selected as the capture antibody and the HRP-conjugated 1-2E10 (at 1 : 2000 dilution) was chosen as the detection antibody. The correlation between the concentration of rAra h 2 and the OD value at 450 nm showed an excellent correlation (R 2 = 0.9999) (Figure 6(a)). The concentration range of Ara h 2 detectable in peanut extract was from 5 ng/mL to 625 ng/mL. Moreover, the correlation was still very high, up to 10 μg/mL (R 2 = 0.9994) (Figure 6(b), Supplementary Material Table 1), demonstrating the superb sensitivity and detection range of this ELISA system.
Figure 6.
The correlation between the rAra h 2 concentration and the OD value at 450 nm (R 2 = 0.9994). (a) Standard curve between 5 ng/mL and 625 ng/mL. (b) Standard curve between 5 ng/mL and 10 μg/mL.
3.5. Cross-Reactivity of the mAbs
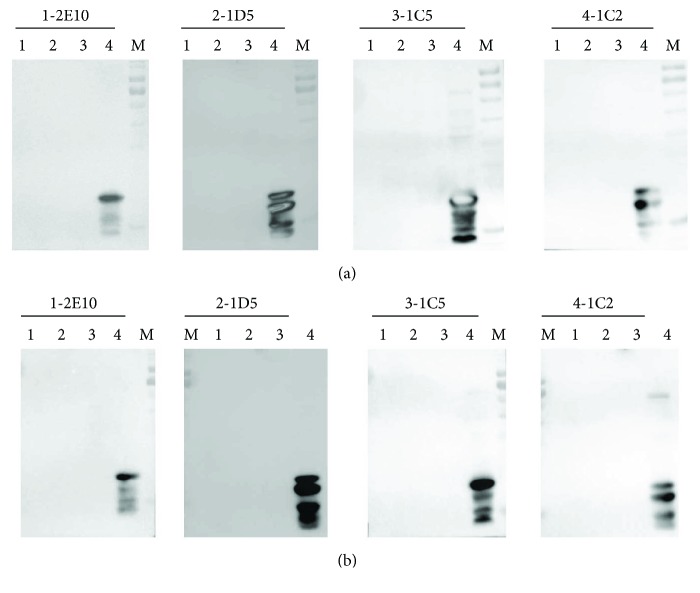
We selected Ara h 8, Bet v 1, Gly m 4, and soybean extract to examine potential antibody cross-reactivity, using BSA as the negative control and peanut extract (not desalted) as the positive control. The mAbs did not cross-react with any antigens except for Ara h 2 (Figure 7). Therefore, the mAbs can specifically distinguish Ara h 2 from the other food allergens tested.
Figure 7.
Evaluation of the cross-reactivity of the mAbs by Western blotting. (a) Lane 1: Ara h 8; lane 2: Bet v 1; lane 3: negative control (BSA); lane 4: positive control (peanut extract); M: prestained protein marker. (b) Lane 1: Gly m 4; lane 2: soybean extract; lane 3: negative control (BSA); lane 4: positive control (peanut extract); M: prestained protein marker.
4. Discussion
Quantification of the allergen level in foods and the environment is useful for preventing allergen exposure and standardizing immunotherapy reagents [20]. Here, we developed a sandwich ELISA to quantify the peanut allergen Ara h 2 by using mAbs raised against recombinant Ara h 2. All the mAbs created showed good reactivity to both rAra h 2 and peanut extract from both roasted and raw peanuts. However, they did not recognize peanut extract after the desalting process. This suggested that the desalting procedure might have changed the protein conformation, most likely the antigenic epitope-containing domains, or that Ara h 2 may have been lost during the desalting step due to its low molecular weight [21]. In any case, the mAbs recognized Ara h 2 from both raw and roasted peanuts, indicating that the antigen epitopes are quite stable.
Peanut-allergic patients can experience symptoms from urticaria to anaphylaxis and a significant decrease in their quality of life. Allergen immunotherapy (AIT) is an effective treatment for allergies since it can modify the immune system by shifting a Th2 response to a Th1 response, inducing anergy in epitope-specific T cells, and regulating differentiation of allergen-specific T cells [22–24]. Currently, whole peanut extract is the most commonly used reagent for skin prick test (SPT) and AIT study, which can potentially elicit adverse events including anaphylaxis [25]. Furthermore, the strategies of peanut immunotherapy have varied from subcutaneous, epicutaneous, and sublingual application to oral treatment but none of them are recommended for routine clinical usage because of the different dosing schedules and the varying duration of treatment [26, 27]. Here, we established a new method to quantify Ara h 2 using purified mAbs against the allergen, which may help to standardize other immunotherapeutic reagents. In addition, we propose a new treatment strategy that uses purified mAbs against rAra h 2 to block the systemic dispersion of the allergen, which may reduce or prevent the hypersensitivity reaction in subjects.
There are at least 18 allergen components in peanut; Ara h 1, Ara h 2, and Ara h 3 are the major allergens and Ara h 8 is defined as the minor allergen, and these share a significant amino acid similarity with the allergens Gly m 4 and Bet v 1 [28, 29]. Bet v 1 is the major allergen component of birch pollen, and a number of reports have been published on its cross-reactivity with peanut allergens [30, 31]. Peanuts and soybeans are frequently mixed consciously and unconsciously in foods made at home and in restaurants. Therefore, it is important and meaningful to specifically quantify the amount of potent allergens in foods apart from their cross-reacting allergens. The specificity of the mAbs against Ara h 2 is high enough to distinguish Ara h2 from the other common food allergens in mixtures. This could help the patient from ingesting peanut allergen, while reducing unnecessary avoidance.
The prevalence of peanut allergy has been steadily increasing over the past decade [32]. This is partially due to the peanut allergens hidden in various processed foods or prepared dishes. A study showed that the amount of peanut allergen in household dust is associated with peanut sensitization or peanut allergy in patients with atopic dermatitis, especially when exposure to the environmental allergen occurs within the first year of life through skin lesions or inhalation of dust particles [33]. Many sources of peanut allergens exist in our environment, such as peanut butter, household dust, and steam from food preparation in the kitchen, dining table surfaces, and even the sofa [34]. Data show that 50 to 100 peanut-allergic patients in the world will die annually because of accidental ingestion of peanut-contaminated food [35]. Therefore, for peanut allergy sufferers, it is important to know the presence of peanut allergen in foods and in the environment. Here, we have established a method to quantify the major peanut allergen Ara h 2, which would be helpful for peanut-allergic patients to avoid allergen exposure, as well as help improve their quality of life.
Food allergy is an IgE-mediated immune response disorder that involves many immune cells during the course of the disease [36, 37]. When constantly exposed to food allergens, chronic inflammation can develop in the intestinal tract. Mast cells, the main reactive immune cell in allergies, are also commonly found in tumor tissues [38]. Some preclinical studies have suggested that mast cells may contribute to tumor progression and that the inflammatory microenvironment could affect suppression of antitumor immunity [39]. We have detected and quantified the amount of Ara h 2 in intestinal samples by utilizing the mAbs produced in this study, and the preliminary results showed that there is a difference in Ara h 2 levels between precancerous and cancerous tissues (data not shown), thereby inviting further study.
5. Conclusion
We generated and purified five mAbs with highly specific reactivity toward Ara h 2 and established a quantitative ELISA by utilizing these mAbs to detect the level of Ara h 2 in the whole extract of both raw and roasted peanuts. This quantitative system can be utilized for standardization of allergen reagents, as well as for adequate food labeling and environmental allergen monitoring, which will help peanut-allergic patients avoid this dangerous allergen and increase the quality of their lives.
Acknowledgments
This work was supported by the National Science and Technology Major Project of China (2014ZX08011-005B and 2016ZX08011-005).
Disclosure
The sponsors had no role in the design of the study, the collection and analysis of the data, or in the preparation of the manuscript.
Conflicts of Interest
All authors declare that there is no conflict of interest regarding the publication of this article.
Supplementary Materials
Table 1: the correlation between the concentration of rAra h 2 and the OD value at 450 nm.
References
- 1.Nwaru B. I., Hickstein L., Panesar S. S., et al. The epidemiology of food allergy in Europe: a systematic review and meta-analysis. 2014;69(1):62–75. doi: 10.1111/all.12305. [DOI] [PubMed] [Google Scholar]
- 2.Bedolla-Barajas M., Valdez-López F., Alcalá-Padilla G., Bedolla-Pulido T. I., Rivera-Mejia V., Morales-Romero J. Prevalence and factors associated to peanut allergy in Mexican school children. 2017;45(1):69–76. doi: 10.1016/j.aller.2016.04.013. [DOI] [PubMed] [Google Scholar]
- 3.Anagnostou K., Clark A. The management of peanut allergy. 2015;100(1):68–72. doi: 10.1136/archdischild-2014-306152. [DOI] [PubMed] [Google Scholar]
- 4.Husain Z., Schwartz R. A. Food allergy update: more than a peanut of a problem. 2013;52(3):286–294. doi: 10.1111/j.1365-4632.2012.05603.x. [DOI] [PubMed] [Google Scholar]
- 5.Simms E., Foster G., Arias K., et al. Prediction of clinical peanut allergy status among children in Hamilton, Ontario using chart review data collected during 2012–2015. 2017;13(1):p. 10. doi: 10.1186/s13223-017-0179-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patel B. Y., Volcheck G. W. Food allergy: common causes, diagnosis, and treatment. 2015;90(10):1411–1419. doi: 10.1016/j.mayocp.2015.07.012. [DOI] [PubMed] [Google Scholar]
- 7.Dyer A. A., Rivkina V., Perumal D., Smeltzer B. M., Smith B. M., Gupta R. S. Epidemiology of childhood peanut allergy. 2015;36(1):58–64. doi: 10.2500/aap.2015.36.3819. [DOI] [PubMed] [Google Scholar]
- 8.Cabanillas B., Jappe U., Novak N. Allergy to peanut, soybean, and other legumes: recent advances in allergen characterization, stability to processing and IgE cross-reactivity. 2018;62(1) doi: 10.1002/mnfr.201700446. [DOI] [PubMed] [Google Scholar]
- 9.Schocker F., Baumert J., Kull S., Petersen A., Becker W. M., Jappe U. Prospective investigation on the transfer of Ara h 2, the most potent peanut allergen, in human breast milk. 2016;27(4):348–355. doi: 10.1111/pai.12533. [DOI] [PubMed] [Google Scholar]
- 10.Schwager C., Kull S., Behrends J., et al. Peanut oleosins associated with severe peanut allergy-importance of lipophilic allergens for comprehensive allergy diagnostics. 2017;140(5):1331–1338.e8. doi: 10.1016/j.jaci.2017.02.020. [DOI] [PubMed] [Google Scholar]
- 11.King T. P., Hoffman D., Lowenstein H., Marsh D. G., Platts-Mills T. A. E., Thomas W. Allergen nomenclature. 2004;105(3):224–233. doi: 10.1159/000236761. [DOI] [PubMed] [Google Scholar]
- 12.Valcour A., Jones J. E., Lidholm J., Borres M. P., Hamilton R. G. Sensitization profiles to peanut allergens across the United States. 2017;119(3):262–266.e1. doi: 10.1016/j.anai.2017.06.021. [DOI] [PubMed] [Google Scholar]
- 13.Becker W. M., Jappe U. Peanut allergens. 2014;100:256–267. doi: 10.1159/000359916. [DOI] [PubMed] [Google Scholar]
- 14.Ramos M. L., Huntley J. J., Maleki S. J., Ozias-Akins P. Identification and characterization of a hypoallergenic ortholog of Ara h 2.01. 2009;69(3):325–335. doi: 10.1007/s11103-008-9428-z. [DOI] [PubMed] [Google Scholar]
- 15.Lew M. H., Lim R. L. H. Expression of a codon-optimised recombinant Ara h 2.02 peanut allergen in Escherichia coli . 2016;100(2):661–671. doi: 10.1007/s00253-015-6953-y. [DOI] [PubMed] [Google Scholar]
- 16.The guide for the care and use of laboratory animals. 2016;57(1):NP.7–NPNP. doi: 10.1093/ilar/ilw013. [DOI] [Google Scholar]
- 17.Chen H., Zhang K., Wang S., Xu C., Zou Z., Tao A. Generation and purification of monoclonal antibodies against Der f 2, a major allergen from Dermatophagoides farinae . 2016;10(2):103–108. doi: 10.5582/ddt.2016.01029. [DOI] [PubMed] [Google Scholar]
- 18.Wei M., Zhao C., Zhang S., Wang L., Liu H., Ma X. Preparation and biological activity of the monoclonal antibody against the second extracellular loop of the angiotensin II type 1 receptor. 2016;2016:10. doi: 10.1155/2016/1858252.1858252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cuadrado C., Cabanillas B., Pedrosa M. M., et al. Influence of thermal processing on IgE reactivity to lentil and chickpea proteins. 2009;53(11):1462–1468. doi: 10.1002/mnfr.200800485. [DOI] [PubMed] [Google Scholar]
- 20.Tasaniyananda N., Tungtrongchitr A., Seesuay W., et al. Quantification of Fel d 1 in house dust samples of cat allergic patients by using monoclonal antibody specific to a novel IgE-binding epitope. 2017 doi: 10.12932/AP0876. [DOI] [PubMed] [Google Scholar]
- 21.Walczyk N. E., Smith P. M. C., Tovey E. R., Roberts T. H. Peanut protein extraction conditions strongly influence yield of allergens Ara h 1 and 2 and sensitivity of immunoassays. 2017;221:335–344. doi: 10.1016/j.foodchem.2016.10.063. [DOI] [PubMed] [Google Scholar]
- 22.Bonertz A., Roberts G. C., Hoefnagel M., et al. Challenges in the implementation of EAACI guidelines on allergen immunotherapy: a global perspective on the regulation of allergen products. 2018;73(1):64–76. doi: 10.1111/all.13266. [DOI] [PubMed] [Google Scholar]
- 23.Wambre E., Bajzik V., DeLong J. H., et al. A phenotypically and functionally distinct human TH2 cell subpopulation is associated with allergic disorders. 2017;9(401):p. eaam9171. doi: 10.1126/scitranslmed.aam9171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mayor S. Peanut immunotherapy provides long lasting suppression of allergy, finds study. 2017;358, article j3937 doi: 10.1136/bmj.j3937. [DOI] [Google Scholar]
- 25.Filep S., Block D. S., Smith B. R. E., et al. Specific allergen profiles of peanut foods and diagnostic or therapeutic allergenic products. 2018;141(2):626–631.e7. doi: 10.1016/j.jaci.2017.05.049. [DOI] [PubMed] [Google Scholar]
- 26.Hindley J. P., Filep S., Block D. S., King E. M., Chapman M. D. Dose of allergens in a peanut snack (Bamba) associated with prevention of peanut allergy. 2018;141(2):780–782. doi: 10.1016/j.jaci.2017.05.050. [DOI] [PubMed] [Google Scholar]
- 27.Gupta M., Sicherer S. H. Timing of food introduction and atopy prevention. 2017;35(4):398–405. doi: 10.1016/j.clindermatol.2017.03.013. [DOI] [PubMed] [Google Scholar]
- 28.Kim M. N., Lee K. E., Hong J. Y., et al. IgE cross-reactivity of peanut with walnut and soybean in children with food allergy. 2016;44(6):524–530. doi: 10.1016/j.aller.2016.04.012. [DOI] [PubMed] [Google Scholar]
- 29.Bublin M., Breiteneder H. Cross-reactivity of peanut allergens. 2014;14(4):p. 426. doi: 10.1007/s11882-014-0426-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmitz R., Ellert U., Kalcklosch M., Dahm S., Thamm M. Patterns of sensitization to inhalant and food allergens - findings from the German health interview and examination survey for children and adolescents. 2013;162(3):263–270. doi: 10.1159/000353344. [DOI] [PubMed] [Google Scholar]
- 31.Hurlburt B. K., Offermann L. R., McBride J. K., Majorek K. A., Maleki S. J., Chruszcz M. Structure and function of the peanut panallergen Ara h 8. 2013;288(52):36890–36901. doi: 10.1074/jbc.M113.517797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sicherer S. H. Epidemiology of food allergy. 2011;127(3):594–602. doi: 10.1016/j.jaci.2010.11.044. [DOI] [PubMed] [Google Scholar]
- 33.Brough H. A., Simpson A., Makinson K., et al. Peanut allergy: effect of environmental peanut exposure in children with filaggrin loss-of-function mutations. 2014;134(4):867–875.e1. doi: 10.1016/j.jaci.2014.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brough H. A., Makinson K., Penagos M., et al. Distribution of peanut protein in the home environment. 2013;132(3):623–629. doi: 10.1016/j.jaci.2013.02.035. [DOI] [PubMed] [Google Scholar]
- 35.Leung D. Y., Sampson H. A., Yunginger J. W., et al. Effect of anti-IgE therapy in patients with peanut allergy. 2003;348(11):986–993. doi: 10.1056/NEJMoa022613. [DOI] [PubMed] [Google Scholar]
- 36.Navines-Ferrer A., Serrano-Candelas E., Molina-Molina G. J., Martín M. IgE-related chronic diseases and anti-IgE-based treatments. 2016;2016:12. doi: 10.1155/2016/8163803.8163803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klemans R. J. B., van Os-Medendorp H., Blankestijn M., Bruijnzeel-Koomen C. A. F. M., Knol E. F., Knulst A. C. Diagnostic accuracy of specific IgE to components in diagnosing peanut allergy: a systematic review. 2015;45(4):720–730. doi: 10.1111/cea.12412. [DOI] [PubMed] [Google Scholar]
- 38.Liu J., Zhang Y., Zhao J., et al. Mast cell: insight into remodeling a tumor microenvironment. 2011;30(2):177–184. doi: 10.1007/s10555-011-9276-1. [DOI] [PubMed] [Google Scholar]
- 39.Wedemeyer J., Galli S. J. Decreased susceptibility of mast cell-deficient Kit W/Kit W-v mice to the development of 1, 2-dimethylhydrazine-induced intestinal tumors. 2005;85(3):388–396. doi: 10.1038/labinvest.3700232. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table 1: the correlation between the concentration of rAra h 2 and the OD value at 450 nm.