Abstract
Palythoa caribaeorum is a zoanthid often dominant in shallow rocky environments along the west coast of the Atlantic Ocean, from the tropics to the subtropics. This species has high environmental tolerance and is a good space competitor in reef environments. Considering current and future scenarios in the global climate regime, this study aimed to model and analyze the distribution of P. caribaeorum, generating maps of potential distribution for the present and the year 2100. The distribution was modeled using maximum entropy (Maxent) based on 327 occurrence sites retrieved from the literature. Calcite concentration, maximum chlorophyll-a concentration, salinity, pH, and temperature range yielded a model with the smallest Akaike information criterion (2649.8), and were used in the present and future distribution model. Data from the HadGEM2-ES climate model were used to generate the projections for the year 2100. The present distribution of P. caribaeorum shows that parts of the Brazilian coast, Caribbean Sea, and Florida are suitable regions for the species, as they are characterized by high salinity and pH and small temperature variation. An expansion of the species’ distribution was forecast northward under mild climate scenarios, while a decrease of suitable areas was forecast in the south. In the climate scenario with the most intense changes, P. caribaeorum would lose one-half of its suitable habitats, including the northernmost and southernmost areas of its distribution. The Caribbean Sea and northeastern Brazil, as well as other places under the influence of coastal upwellings, may serve as potential havens for this species.
Keywords: Distribution forecast, Range shift, Maxent, IPCC, Global warming, Reef ecology, Niche modeling
Introduction
Palythoa caribaeorum (Duchassaing & Michelotti, 1860) is a sessile colonial anthozoan common along the west coast of the Atlantic Ocean (Kemp et al., 2006). This species can be found from Florida in the US (Kemp et al., 2006) to Arvoredo Island in Brazil (Bouzon, Brandini & Rocha, 2012), as well as around many oceanic islands in the Atlantic, such as Bermuda (Lesser et al., 1990) and the São Pedro e São Paulo archipelago (Edwards & Lubbock, 1983). Its distribution is not limited to the western side of the Atlantic Ocean, however, with records in the Cape Verde islands (Reimer, Hirose & Wirtz, 2010). This broad geographic distribution is possible because P. caribaeorum has a great tolerance to a wide range of environmental conditions (Sebens, 1982). Although studies on P. caribaeorum larvae are lacking, it is expected that its biology would be similar to its sister species, Palythoa turbeculosa (Esper 1791), which has symbiotic larvae with planktonic habits and a larval stage up to 170 days long (Polak et al., 2011; Ryland, 1997).
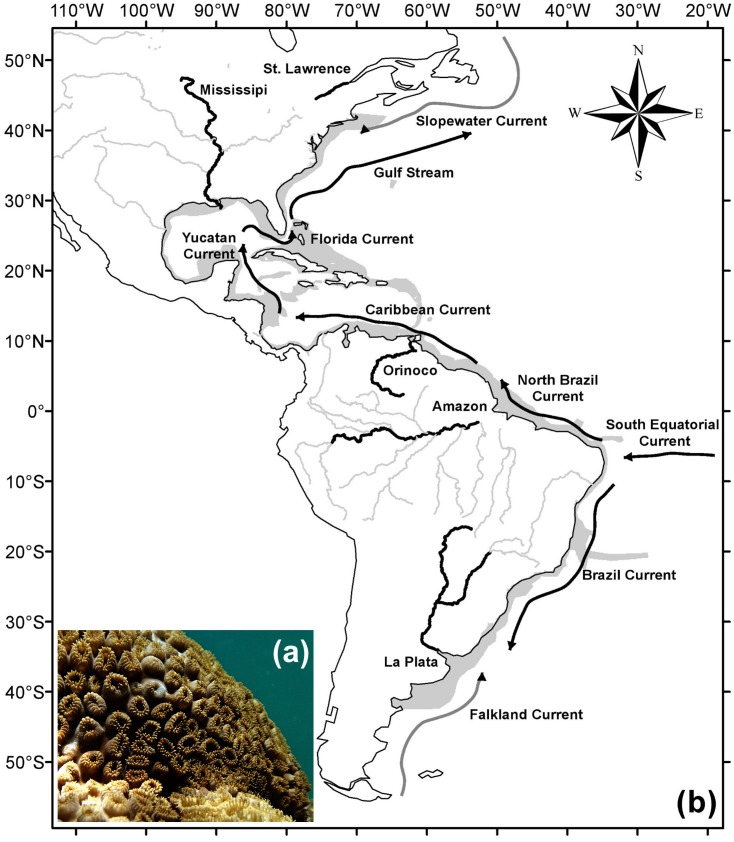
It is found on hard bottom environments, from the intertidal zone to depths up to 12 m (Sebens, 1982), where it can be dominant (Monteiro et al., 2008). Colonies have a mat format usually occupying large areas along the substratum (Sebens, 1982; Acosta, 2001; Silva et al., 2015). The animal’s color varies from yellow and brown to bronze, easily identifiable in the field (Fig. 1A).
Figure 1. Study area and major currents and rivers.
Underwater picture of (A) a Palythoa caribaeorum colony and (B) the study area (gray) with main rivers (black), adapted from the map “World Major Rivers” from ESRI; the schema of the main cold surface currents (gray) and warm surface currents (black) is adapted from Talley et al. (2011). Photo credit: Leonardo Durante.
P. caribaeorum is a mixotrophic organism, hosting symbiotic dinoflagellates (Symbiodinium) inside its tissues (Sebens, 1977; Suchanek & Green, 1981) and feeding on planktonic organisms, which contributes to the energy flux from plankton to benthic environments (Sorokin, 1991; Santana et al., 2015). Few studies have highlighted the importance of P. caribaeorum in association with other invertebrates (Pérez, Vila-Nova & Santos, 2005), reef dynamics (Silva et al., 2015), or the enrichment of the bottom fish community (Mendonça-Neto et al., 2008). However, a study conducted in the Todos os Santos Bay in Brazil (Cruz et al., 2015b) showed that the increased abundance of Palythoa sp. decreased habitat heterogeneity, and consequently the richness of fish and benthic organisms, changing the local trophic structure in that region.
P. caribaeorum is capable of overgrowing and thus adversely affecting the growth and recruitment of several organisms (Suchanek & Green, 1981; Castro et al., 2012) such as hard corals, hydrocorals, and other zoanthids, showing great efficiency competing for space. P. caribaeorum also has a fast growth rate estimated to vary between 0.04-0.15 mm per day (Bastidas & Bone, 1996; Silva et al., 2015). In general, zoanthids can dominate in areas where the environmental factors make it difficult for the settlement of scleractinian corals (Fautin, 1988; Cruz et al., 2015a), often becoming important as the main benthic component (Mendonça-Neto et al., 2008) and nursery habitat for other invertebrates (Pérez, Vila-Nova & Santos, 2005). Suchanek & Green (1981) and Sebens (1982) claimed that predation does not seem to be an ecological element controlling the distribution and abundance of P. caribaeorum, probably due to defense strategies related to nematocysts and palytoxin that are found in the animal’s tissue (Hines & Pawlik, 2011). However, Stampar, Silva & Luiz Jr (2007) reported a case of a Hawksbill turtle, Eretmochelys imbricata, preying colonies of P. caribaeorum in southeastern Brazil.
Regarding its ecological role, investigating the current and future distribution of P. caribaeorum will yield a prognosis of areas likely to experience changes in their benthic communities because of the presence or absence of P. caribaeorum. Assessing how species and biological systems are affected by climate change as well as the generation of forecast models are increasingly needed by decision makers and environmental managers (Gutt et al., 2012).
Species distribution models (SDM) are largely used in ecology, biogeography, and conservation biology to help solve practical problems in these fields (Guisan & Thuiller, 2005; Elith et al., 2011; Acosta et al., 2016). One of the main assumptions of SDM is that the environment is in equilibrium with its species, whose distribution is determined by environmental variability (Araújo & Pearson, 2005), excluding biological interactions, dispersion barriers, and fast or small-scale environmental disturbances (De Marco, Diniz-Filho & Bini, 2008). Temporal and climatic variations also interfere in the modeling, and environmental data must be gathered from the same period in which the species was recorded, making it difficult to acquire large amounts of data for mobile organisms. In this scenario, P. caribaeorum is a good candidate for the use of SDM.
The accelerating rates of environmental change pose a challenge to most organisms living in shallow waters throughout the world’s oceans. At a global scale, between 1971 and 2010, the warming rate of the oceans’ surface waters reached 0.13°C per decade (IPCC, 2014). Since 1950, coastal regions with high evaporation rates are becoming saltier; and for the same period, the pH of surface waters has decreased by 0.1 (IPCC, 2014). The forecast for the year 2050 shows a redistribution of marine organisms as a function of the increase in temperature. This redistribution is expected to be toward mid-latitude zones, increasing species richness in these areas while decreasing richness in the tropics due to extinction, migration, and reduction in genetic diversity (Fields et al., 1993). However, it is still unknown for sure how the effects of climate change, at the population level, will affect the environment at large ecological scales (Harley et al., 2006; Thomas et al., 2017).
This study aimed to investigate and model the distribution of P. caribaeorum along the western coast of the Atlantic Ocean, generating potential distribution maps for the present and the year 2100 based on forecast climate models. The maps were used to characterize the species’ habitat and identify areas where suitability was retained, lost, or gained for P. caribaeorum over time and under different climate scenarios.
Materials and Methods
Study area
The study area comprised the western Atlantic Ocean (from −42.5°to 42.5°latitude), between 2 m of altitude and 100 m of depth (Fig. 1B), hence extending beyond the current known distribution of P. caribaeorum. The oceanographic features of this region are influenced by the South Equatorial Current, from the east, which is divided into two components: northward (North Brazil Current) and southward along the coast (Brazil Current). The North Brazil Current flows toward the northwest, passing by the Caribbean Sea, Yucatan Channel, and Gulf of Mexico before merging with the Antilles Current in the Florida Strait to form the Gulf Stream, which flows toward Europe. Due to the strong tropical component, the region between 20°S and 30°N is strongly influenced by waters heated in equatorial regions (Ekman, 1953). In contrast, regions closer to the boundaries of this study are influenced by both tropical and colder currents, such as the Slopewater Current from the Labrador Current in the north (Churchill & Berger, 1998) and the Malvinas Current in the south (Talley et al., 2011). The study region is also influenced by large river discharges, mainly from the La Plata, Amazon, Orinoco, Mississippi, and Saint Lawrence rivers (Fig. 1B).
Biological data mining and filtering
An extensive literature review was performed to obtain the largest possible number of studies with occurrence data for P. caribaeorum (Supplemental Information 1). Occurrence data recorded by the authors of this study and from global databases, such as WORMS, OBIS, and GBIF, were also used, totaling 327 occurrence points for the species. The three identified synonyms (Reimer, 2015)—Palythoa caribaea Duerden, 1898, Palythoa caribbaea Goreau, 1959, and Palythoa caribdea Carballeira et al., 1998—were included.
Occurrence points found on land or at depths deeper than 100 m were automatically excluded, as were those localized in freshwater environments. Because sampled areas and methodologies varied among study sites, only one occurrence per pixel (5 arcminutes), based on the environmental variables dataset, was kept for analysis (Elith & Leathwick, 2009). This point was placed in the middle of each pixel, disregarding the exact occurrence point. After that, to avoid bias originating from densely sampled sites, the geographic filter “OccurrenceThinner” version 1.04 (Verbruggen, 2012), with thresholds of 0.5 and 1, was used, resulting in the final dataset.
Selecting environmental variables, the regulation multiplier value, and the running Maxent
Environmental and bathymetric data, with resolutions of 5 and 1 arcminute, were obtained from the Bio-Oracle (Tyberghein et al., 2012) dataset and the ETOPO1 Global Relief Model, respectively (Amante & Eakins, 2009). Twenty-seven layers of environmental data and their transformations available on the Bio-Oracle dataset were used in the analyses (Table 1). Each layer was cropped using ArcGIS 10.2 to only include areas within 2 m altitude and 100 m depth.
Table 1. Details about the variables retrieve from Bio-Oracle dataset and the layers kept in the final distribution model for P. caribaeorum.
| Variables | Unit | Measurement | Measurement transformations | Number of layers | Kept in the final model |
|---|---|---|---|---|---|
| Calcite concentration | mol/m3 | Remote sensor | Average | 1 | Average concentration |
| Chlorophyll-a concentration | mg/m3 | Remote sensor | Average, minimum, maximum and range | 4 | Maximum concentration |
| Cloud coverage | % | Remote sensor | Average, minimum and maximum | 3 | – |
| Difusive atenuation coeficient (490 nm) | 1/m | Remote sensor | Average, minimum and maximum | 3 | – |
| Photosynthetic available radiation | Einstein/m2/day | Remote sensor | Average and maximum | 2 | – |
| Air temperature above sea surface | °C | Remote sensor | Average, minimum, maximum and range | 4 | – |
| Sea surface temperature | °C | Remote sensor | Average, minimum, maximum and range | 4 | Range |
| Dissolved oxygen | ml/l | In situ | Diva interpolation | 1 | – |
| Nitrate | µmol/l | In situ | Diva interpolation | 1 | – |
| pH | – | In situ | Diva interpolation | 1 | Diva interpolation |
| Phosphate | µmol/l | In situ | Diva interpolation | 1 | – |
| Salinity | PSU | In situ | Diva interpolation | 1 | Diva interpolation |
| Silicate | µmol/l | In situ | Diva interpolation | 1 | – |
The habitat modeling was done using the Maxent 3.3.3 program (Phillips, Anderson & Schapire, 2006). Maxent has the advantage of using only occurrence points, instead of occurrence and absence/pseudo-absence, considering the lack of this type of data, especially for marine organisms. Maxent also demonstrates good performance identifying suitable habitats (Phillips, Anderson & Schapire, 2006; Reiss et al., 2011; Meißner et al., 2014) and predicting novel distributions for species (Costa et al., 2012; Acosta et al., 2016) when compared with other software.
All the features available in Maxent were used during the modeling (standard set up). To choose the environmental variables and the regulation multiplier (Rm) value, the “MaxentVariableSelection” package (Jueterbock, 2016) for R 3.3.1 (R Core Team, 2015) was used, with a contribution threshold of 4% and Pearson’s correlation coefficient of 0.9. A series of runs were conducted, varying the Rm from 0.5 to 10 with increments of 0.5, and were then assessed using the Akaike Information Criterion (AIC). The environmental variables and Rm of the model with a lower AIC were used to run the final Maxent model. The AIC tends to choose models that sort the most important variables, as well as models that better estimate environmental suitability (Warren et al., 2014), and therefore behave better when projected onto novel climate scenarios than models chosen using the Area Under the Receiver Operator Curve (AUC) value (Warren & Seifert, 2011). The AIC selects the optimal and most parsimonious model, taking into account its complexity (Bozdogan, 1987). Thirty percent of the data were randomly sampled to test the model along each of the 100 replicates, resulting in an average for all replicates. In all the analyses, 10,000 random points were used as the background along the whole study area.
Climate model data
Data from the HadGEM2-ES model (Collins et al., 2011) retrieved from the Coupled Model Intercomparison Project (CMIP5) were used on the projections for the year 2100 under three different Representative Concentration Pathway (RCP) climate scenarios: low, middle, and high radiative forcing of 2.6, 4.5, and 8.5 W/m2, respectively (Van Vuuren et al., 2011). The RCPs are related to retention of energy by the Earth’s surface due to accumulation of greenhouse gases from human activities, therefore higher numbers represent a greater anthropogenic impact on our biosphere. The HadGEM2-ES climate model was freely available and provided outputs of the main interest variables and high resolution in tropical areas under the RCP climate scenarios. When there were no available data for the projections, the layers were kept unchanged. NetCDF files from the climate model were interpolated using the bilinear interpolation “interp2” of Matlab 7.14, and were then cropped and converted to ASC format.
Projecting the results onto maps
Maps were generated using the cumulative likelihood of species occurrence obtained from Maxent, which can be interpreted as the sum of the relative occurrence rate of the species in one pixel and other pixels with the same or lower values of the relative occurrence rate (ROR). This type of output is best for drawing species distribution boundaries for different climate scenarios (Merow, Smith & Silander, 2013) and also has a better visual presentation. For the binary maps, the threshold equal to the maximum sensitivity plus specificity (max SSS) was chosen to detect suitable habitats for P. caribaeorum. This threshold improves the distinction between occurrence and absence sites, as well as between occurrence and background, also selecting the same threshold for both occurrence-only and occurrence-absence models (Liu, Newell & White, 2015).
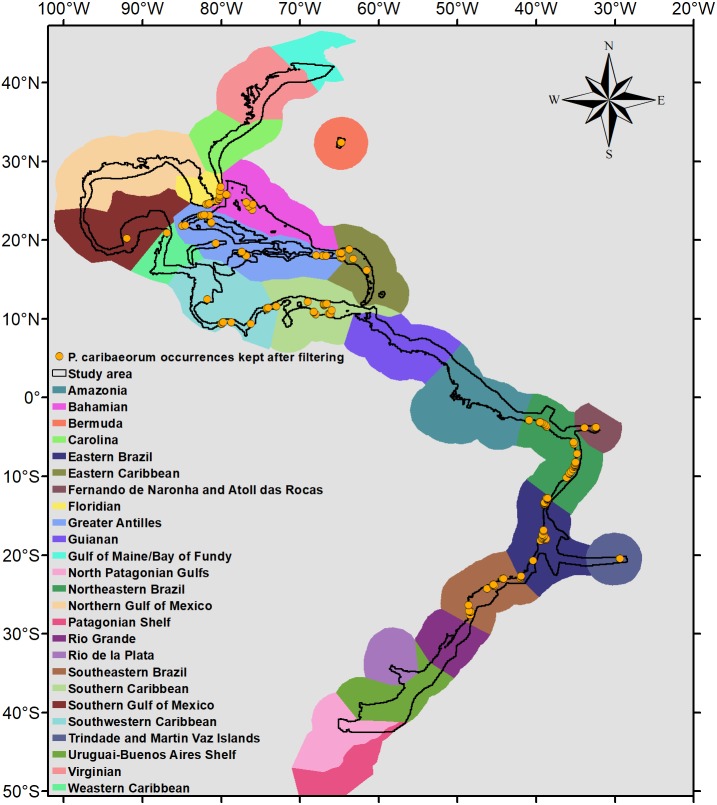
Because P. caribaeorum is distributed along coastal zones, the final results were cropped to depths up to 30 m, better defining its real habitat. The map of the Marine Ecoregions of the World (Spalding et al., 2007) was used as a reference for the results and discussion of specific areas (Fig. 2).
Figure 2. Occurrence points and Marine Ecoregions included in the study area.
Study area and occurrence points of P. caribaeorum after filtering, with the Marine Ecoregions of the World as a reference (Spalding et al., 2007).
Results
After filtering, 135 occurrence points of P. caribaeorum were kept for the analyses (Fig. 2). The model with a lower AIC (2649.8) presented an Rm of 3.5 and showed good performance defining occurrence and absence sites for P. caribaeorum (AUC = 0.860 ± 0.023) (Swets, 1988). The difference between the test AUC and the train AUC yielded a value of 0.015, showing that the model was not over-adjusted and is transferable to novel climate scenarios (Jueterbock, 2016; Warren & Seifert, 2011).
The environmental variables that contributed the most to the model were salinity (47.9%), concentration of calcite (21%), maximum concentration of chlorophyll-a (13.3%), pH (9.2%), and temperature range (8.5%), and were therefore kept in the model (Table 1). The habitat of P. caribaeorum is characterized by salinity values higher than 34, a maximum chlorophyll-a concentration from 0 to 28 mg/m3, a pH higher than 7.85, and temperature variation up to 10°C, although these intervals become much narrower when variable correlations are considered. For calcite, the model pointed to a modulating influence, as no minimum or maximum values were indicated.
The output of the HadGEM2-ES climate model for salinity, pH, and temperature (used to calculate temperature range) was used to project the SDM model to novel scenarios for the year 2100, while calcite and chlorophyll-a were kept constant. A max SSS equal to 25.97 was used as a threshold to infer suitable areas, thus identifying the potential distribution of the species.
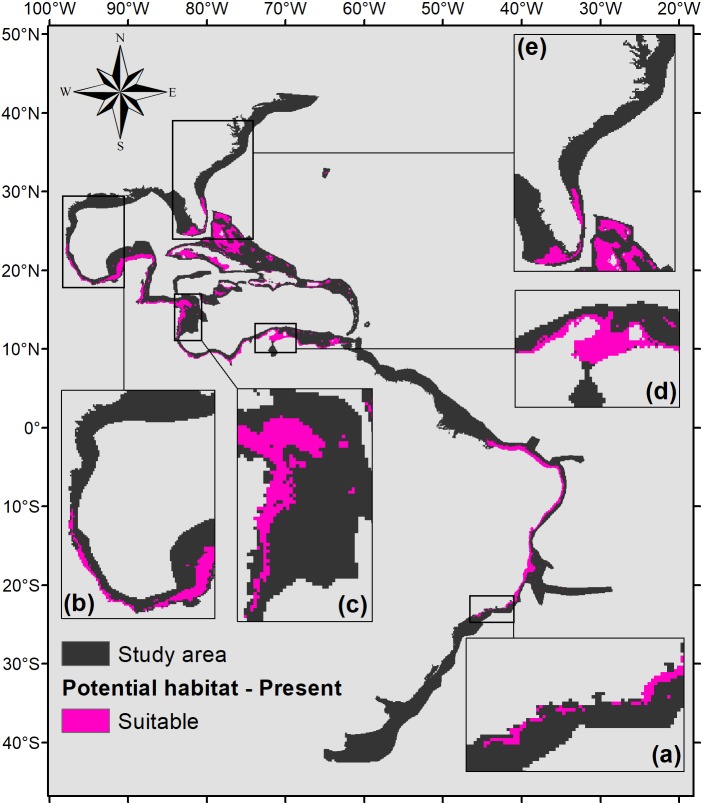
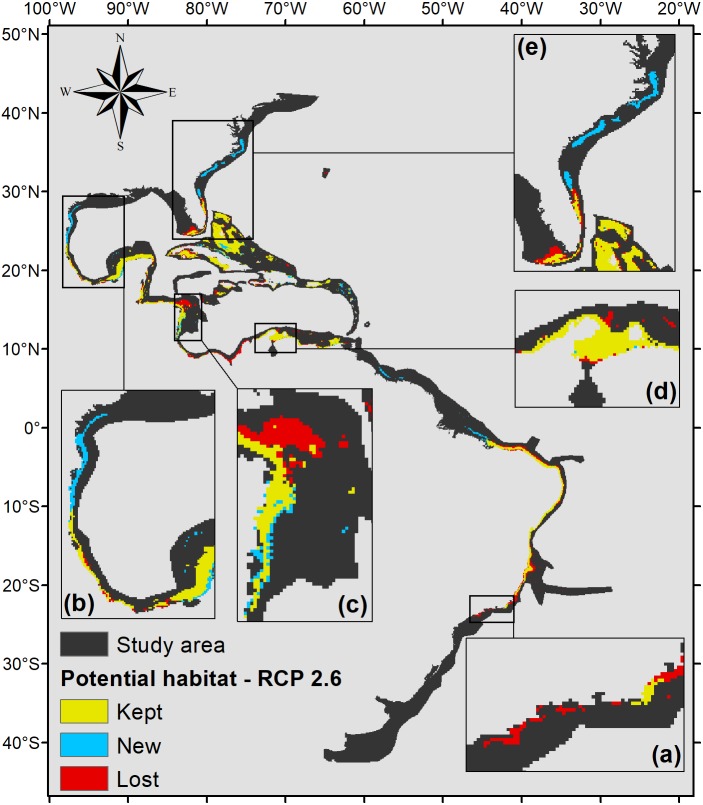
Five hundred forty eight square kilometers were characterized as currently suitable for P. caribaeorum (Fig. 3). Under the RCP 2.6 scenario (Fig. 4), the southern range of P. caribaeorum distribution would lose suitability, as would the Caribbean, Bermudan, and Floridian regions; however, the total suitable area is close to the one observed for the present: 492,000 km2. An increase in suitable areas in the Guianan, Amazonian, Bahamian, and Caribbean regions was also observed, but the biggest change occurred in the northern part of its distribution, in the northern Gulf of Mexico and along the Carolinian and Virginian regions. In these areas, the potential distribution of the species reached almost as far north as Chesapeake Bay. The southernmost area capable of retaining suitability for the species was near Cape Frio, in southeastern Brazil.
Figure 3. Suitable habitat for P. caribaeorum in the present.
Details for (A) southeastern Brazil, (B) southern Gulf of Mexico, (C) southwestern Caribbean, (D) southern Caribbean, and (E) Floridian regions.
Figure 4. Suitable habitat for P. caribaeorum for the year 2100 under the RCP 2.6 climate scenario, which includes regions with retained, new, and lost suitability compared with the present.
Details for (A) southeastern Brazil, (B) southern Gulf of Mexico, (C) southwestern Caribbean, (D) southern Caribbean, and (E) Floridian regions.
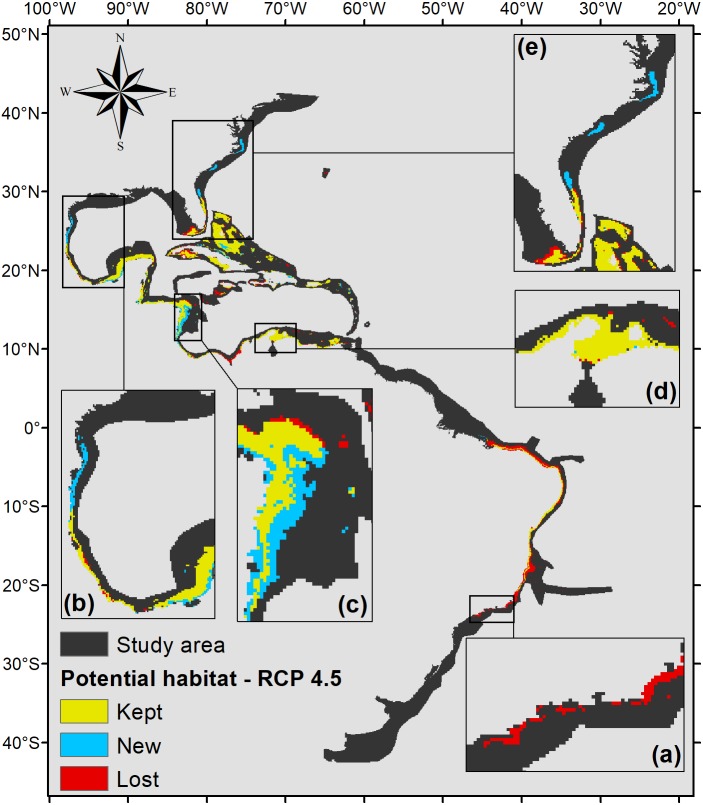
The projection under RCP 4.5 (Fig. 5) also shows that part of the Brazilian coast would lose habitat suitability, only remaining suitable around the Abrolhos Bank and in northeastern Brazil. In the north, the species’ potential distribution is similar to the 2.6 scenario, only presenting smaller suitable areas in those regions, 470,000 km2 in total.
Figure 5. Habitat suitability map for P. caribaeorum. in the year 2100 under the RCP 4.5 climate scenario.
The map includes regions with retained, new, and lost suitability compared with the present. Details for (A) southeastern Brazil, (B) southern Gulf of Mexico, (C) southwestern Caribbean, (D) southern Caribbean, and (E) Floridian regions.
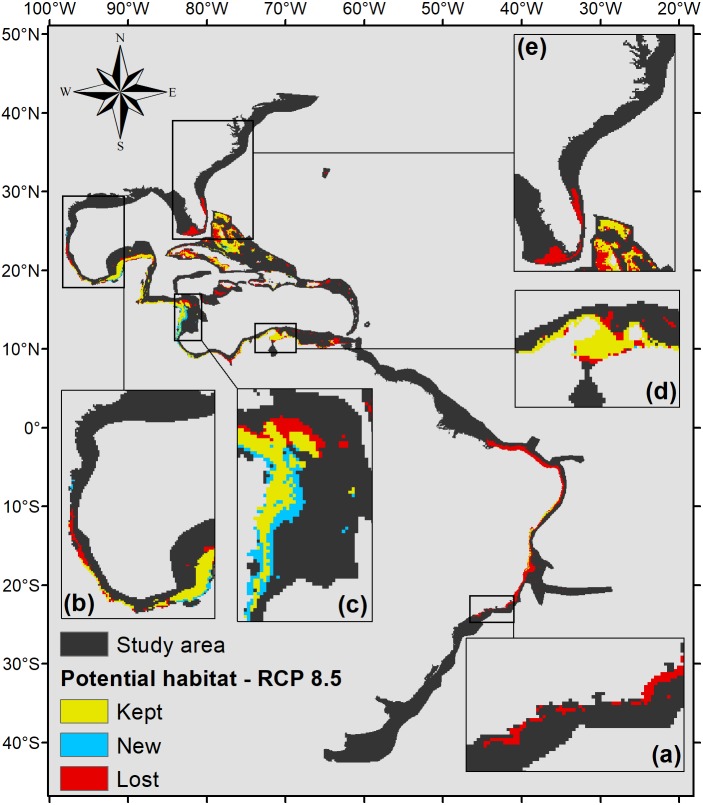
Under the scenario with the higher anthropogenic impact, RCP 8.5 (Fig. 6), almost the entire potential distribution for P. caribaeorum along the Brazilian coast would be lost, including the northeast region. Under RCP 8.5, the anthozoan would also lose suitable habitats in regions around Florida, the northern Gulf of Mexico, and the eastern and western Caribbean.
Figure 6. Habitat suitability map for P. caribaeorum. in the year 2100 under the RCP 8.5 climate scenario.
The map includes regions with retained, new, and lost suitability compared with the present. Details for (A) southeastern Brazil, (B) southern Gulf of Mexico, (C) southwestern Caribbean, (D) southern Caribbean, and (E) Floridian regions.
Overall, for this climate scenario, only one-half of the suitable areas seen in the present, around 275,000 km2, were characterized as a potential habitat for the species, comprising the southern, western, and southwestern Caribbean, the southern Gulf of Mexico, and the Bahamian, Greater Antilles, and eastern Brazil regions. Nevertheless, under the RCP 8.5 scenario, there would be an increase in suitable areas in the southwestern Caribbean, along Nicaragua’s coast, similar to the RCP 4.5 forecast (Fig. 5).
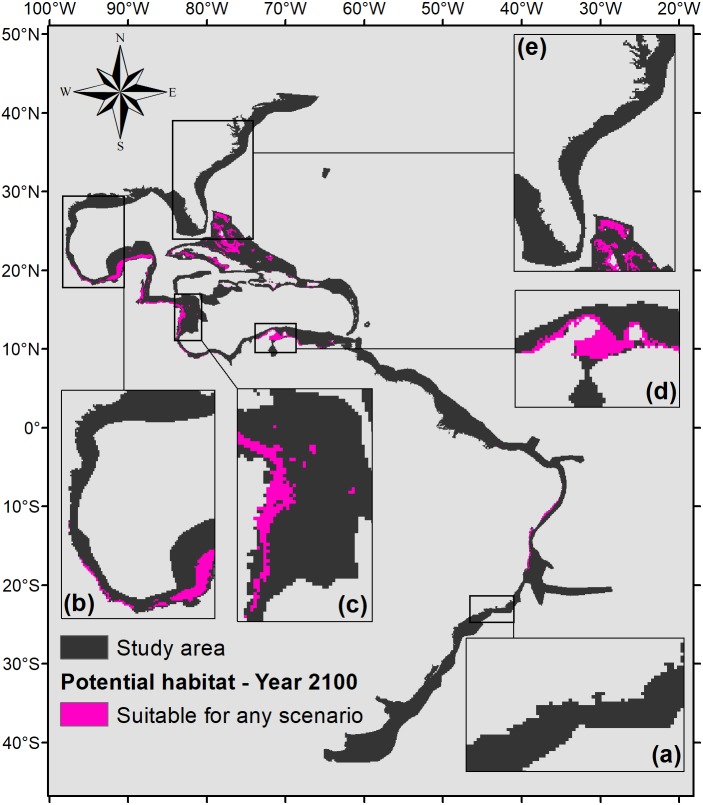
To demonstrate the most important regions for the species, a map of the intersection of all suitable areas under any climate scenarios for the year 2100 was made (Fig. 7). Figure 7 shows that the northern part of the map (from 0°northward) would have much more significance for the potential distribution of P. caribaeorum than the southern part. The suitable areas comprise six ecoregions in the north (southern, southwestern, and western Caribbean, southern Gulf of Mexico, and Bahamian and Greater Antilles regions) against only two in the south (northeastern and eastern Brazil).
Figure 7. Map of potential habitat for P. caribaeorum in the year 2100 under any climate scenario (pink).
Details for (A) southeastern Brazil, (B) southern Gulf of Mexico, (C) southwestern Caribbean, (D) southern Caribbean, and (E) Floridian regions.
Discussion
Environmental variables and P. caribaeorum’s habitat
The variables selected to model P. caribaeorum’s habitat suitability are in line with those used in studies about environmental controls for other anthozoans. For instance, temperature and salinity are two of the most important factors shaping the growth of modern reefs (Wood, 1993). Temperature is also related to coral (Hoegh-Guldberg, 1999; Hughes et al., 2003; Villamizar et al., 2008) and P. caribaeorum bleaching (Kemp et al., 2006). Variation in salinity may induce osmotic stress and can alter the zonation of zoanthids, including P. caribaeorum (Sebens, 1982). Changes in chlorophyll-a concentrations can also play a role in the distribution of P. caribaeorum due to its heterotrophic nutrition and dependency on the water column productivity, unlike most coral reef species, which depend solely on autotrophy and therefore, clear oligotrophic waters. This species also seems to be affected by lower pH (Kroeker et al., 2013), which is another consequence of global environmental changes (Okazaki et al., 2017).
When the projection of the habitat suitability of P. caribaeorum for the present (Fig. 3) is compared with the map of the biggest river discharges in the study area (Fig. 1), it becomes evident that salinity has a major influence on the distribution of this species. Salinity had a higher contribution to the model, sometimes reducing habitat suitability even when the values of other variables, like temperature and chlorophyll-a, were adequate for the species. Freshwater discharges from rivers are also an important source of calcite for oceans; however, calcite is 100 times more soluble in seawater than in freshwater (Libes, 2009), so high concentrations of this mineral are not limited to river-influenced regions. In fact, regarding only calcite, habitat suitability is positively correlated with its concentration, possibly because calcite concentration also indicates other important biogeochemical controls.
The maximum concentration of chlorophyll-a presented a distribution pattern similar to calcite and was also strongly affected by coastal processes, mainly big river discharges, which fertilize the sea and enhance primary production. From satellite data, chlorophyll-a cannot be distinguished from dissolved organic matter (DOM) without an algorithm specific to each region (Ali et al., 2016; Liew, Chia & Kwoh, 2001; Vazyulya et al., 2014). Away from river discharges, chlorophyll-a can be re-suspended back into the water column by wind, currents, and waves, and is therefore correlated with suspended sediment (Walsh, Dieterle & Esaias, 1987). The values of chlorophyll-a data used in this study are relative to its maximum concentration over several years. Hence, it is likely that these values are the sum of all processes listed above: river discharge, new production, and re-suspension. P. caribaeorum inhabits coastal regions with high chlorophyll-a concentrations, but when these concentrations are related to river discharge, the regions lose habitat suitability due to lower salinity and pH values. This can indicate that despite the low salinity, chlorophyll-a and turbidity can be important factors for the species’ niche. This information corroborates previous studies (Segal & Castro, 2011; Steiner et al., 2015), which suggested that low salinity was an exclusion factor for P. caribaeorum. However, it is difficult to decouple the influence of variables related to water quality from demands related more to depth and substrate in coastal areas, as both are intrinsically linked in continental shores. For instance, P. caribaeorum is quite abundant around remote oceanic islands where eutrophication, turbidity, and other processes typical of continental shores are not present. However, P. caribaeorum seems to be more common in regions influenced by coastal processes with high turbidity along the Brazilian coast (I Cruz, pers. obs., 2015).
Similar to calcite and chlorophyll-a, pH has a strong coastal component related to salinity, especially around the Amazonian river mouth, where pH drops below 7, and in the Virginian and Carolinian regions, where values exceed 8.25—both regions are devoid of the anthozoan. Regarding pH alone, a habitat with a pH over 7.85 is considered suitable, but due to correlations with the other variables, the habitat of P. caribaeorum is constrained to a pH between 8.05 and 8.25.
Lastly, temperature range seems to influence the species in a typical latitudinal fashion, presenting higher values in the northernmost and southernmost regions of the study area due to the intrusion of colder waters onto the continental shelf at higher latitudes. Although P. caribaeorum exhibits high tolerance to changes in temperature and water properties in tide pool environments (Bastidas & Bone, 1996) and in southeastern Brazil (Bouzon, Brandini & Rocha, 2012), extreme variations in temperature and decreases in salinity caused by rain can cause mortality (Sebens, 1977). For current environmental conditions, the latitudes 30°north and 30°south can be recognized as limits to the distribution of this organism, mainly due to the temperature range: between 9°C and 13°C in the south and 13°C and 24°C in the north. In these regions, salinity reaches a value of 34, with high seasonal variation (Piola et al., 2000; Rasmussen, Gawarkiewicz & Owens, 2005).
Present distribution of P. caribaeorum
There are regions characterized as potential habitats for P. caribaeorum that have no record of the species (Figs. 2 and 3). This indicates either a lack of adequate sampling effort in the Guianan, Greater Antilles, Bahamian, and western and southwestern Caribbean regions, the absence of proper substrata, or other conditions not considered in the present model.
In general, the region between Florida and the Yucatan Peninsula, with the exception of Flower Garden Banks off the coast of Texas (Tester et al., 2013), is characterized by the absence of coral reefs due to low temperatures during winter and high rainfall (Longhurst, 2007). In shallow areas in the northern Gulf of Mexico previously dominated by corals and sponges, changes in temperature and an increase in river discharge were followed by a community shift around 2005, and these areas are now dominated by algae (DeBose et al., 2013). This information corroborates our model predictions for P. caribaeorum in that region, strongly influenced by the Mississippi River Delta.
Using Briggs & Bowen’s (2012) classification, it is evident that all of P. caribaeorum’s current potential habitat is within warm marine provinces, characterizing the species’ distribution as tropical. However, the limits of its distribution are close to warm-temperate and cold provinces, which are characterized as marginal parts of the distribution. Studying reef communities located at higher latitudes can improve our knowledge of how this species will be affected during environmental and climatic changes (Thomas et al., 2017); thus, examining P. caribaeorum’s distribution in these regions is extremely important.
Palythoa caribaeorum’s response to climate change in the year 2100
According to the RCP 2.6 climate scenario for the year 2100, there would be an increase in salinity of 2.5 in the northern Gulf of Mexico, Virginian, and Carolinian regions, in turn increasing habitat suitability for P. caribaeorum (Fig. 4). Along the southeastern Brazilian coast, salinity would drop by 0.3, sufficient to become an unsuitable area for the species. Changes would also be seen in the plume of the Amazon river, influencing adjacent coastal regions to a lesser degree.
Values of pH in the year 2100 would drop for all regions, which is one of the factors that could exclude P. caribaeorum. Since the anthozoan response to changes in pH is related to salinity, its northernmost distribution should increase due to a weaker influence of rivers and a decline in the temperature range under the RCP 2.6 scenario. On the other hand, decreases in salinity and pH and more intense temperature fluctuations in the southeast and east of Brazil would result in a loss of suitable areas, except along the coast of Cape Frio in southeastern Brazil. This result may be associated with upwelling in this region (Castro et al., 2006), which modifies water characteristics and reduces the frequency of osmotic and thermal stress on the species (Hu & Guillemin, 2016), making that region more suitable for the animal. The same process seems to occur along the coast of Colombia, as seen for coral species in that region (Chollet, Mumby & Cortés, 2010). The Colombian coast is very important for P. caribaeorum since it would remain a suitable habitat under any climate scenario (Fig. 7).
The same trend observed under the RCP 2.6 scenario occurs in the RCP 4.5 scenario, with an increase in potential habitat in the northernmost range of its distribution (Fig. 5). However, for this scenario, P. caribaeorum would also lose habitat in the south, including around Cape Frio. Other areas, such as the continental shelf of the Yucatan Peninsula, would gain suitable zones in the RCP 4.5 scenario, due to higher salinity values and lower temperature variations. For regions experiencing a loss of suitable areas, such as the northeastern Brazil and Amazonian regions, the drop in pH values, salinity, and an increase in the temperature range would be responsible.
As expected, the climate scenario with the strongest anthropogenic impact, RCP 8.5, presented the smallest suitable area for the species (Fig. 6). Although it presented smaller temperature fluctuations compared to other scenarios, in the RCP 8.5 scenario P. caribaeorum would lose potential habitats in both the northernmost and southernmost ranges of its distribution, without any gain in suitable areas due to a drastic decrease in salinity and pH values. Freshwater intrusions from the La Plata and Amazon rivers would influence the species’ habitat along the Brazilian shelf, restricting its distribution to northeastern Brazil. Similarly, the Mississippi River plume would decrease the adequacy for the species in the north. In general, when looking at changes in salinity, pH, and temperature for any climate scenario, the variations are more intense in the north and south via the intrusion of polar and temperate currents into the continental shelf. These differences alter the habitat of P. caribaeorum and are more prominent in the Southern Hemisphere.
Regions such as the southern, southwestern, and western Caribbean, the southern Gulf of Mexico, the Bahamas, the Greater Antilles, and northeastern and eastern Brazil would retain suitable areas for the species under any climate scenario (Fig. 7), identifying them as refuges for the species under adverse environmental conditions (Haffer, 1969). Except for the Brazilian coast, all of these regions are within the Caribbean Province, which is considered the center of biodiversity in the Atlantic Ocean (Rocha et al., 2008; Briggs & Bowen, 2013). Under intense climate stress, it is likely that both P. caribaeorum and other invertebrate organisms will have their distributions restricted toward this diversity-gathering hotspot in the Atlantic Ocean.
The difference in suitable areas between the Northern and Southern Hemispheres suggests asymmetrical species distribution, with the Northern Hemisphere containing larger suitable habitats. This may result from differences in the oceanographic conditions of both basins. In contrast to other oceans, the Atlantic Ocean presents a net flux of superficial water transport to the north, with the Labrador Current being much weaker than the Malvinas Current; therefore, its principal component is toward the north (Onken, 1994). This occurs mainly because of thermohaline circulation and wind, both of which are likely to be altered by climate change (IPCC, 2014), in turn helping to remodel the distribution of marine organisms, as predicted in this study.
Likely changes in reef communities
Studies have shown that P. caribaeorum coexists with many other organisms, especially algae such as Caulerpa racemosa var. peltata, Bryopsis spp. (Magalhães et al., 2015), Sargassum, Halimeda, Laurencia, Dictyota, Dictyopteris, Ulva, and coralline algae (Steiner et al., 2015). Macroalgae are common in coral reefs, sometimes dominating the community and competing for space with corals (Tanner, 1995; Bruno et al., 2009). The disappearance of P. caribaeorum from many Brazilian reefs in the Abrolhos Bank would imply a change in the reef communities. It is possible that with the vanishing of P. caribaeorum, colonization of the available space would first be by algae, most likely cyanobacteria, due to their fast growth rate, especially in nutrient-poor environments (Villaça & Pitombo, 1997). However, the composition of the new community will depend on multiple factors, from changes in recruitment rates, growth, and mortality to changes in nutrient concentrations and herbivory (Littler, Littler & Taylor, 1983; Sandin & McNamara, 2012); community resilience is another factor (Roff & Mumby, 2012).
Regarding the colonization of new areas by P. caribaeorum, the temperate region of the northwestern Atlantic would see its suitable area increased under RCP 2.6 and 4.5, around Cape Hatteras and Chesapeake Bay. Zoanthids have the potential to disperse to great distances due to their long larval stage (Ryland et al., 2000; Polak et al., 2011) and asexual reproduction (Acosta, Sammarco & Duarte, 2005), alongside their potential to raft on floating objects and boats (Santos et al., 2016). Thus, it is possible that the colonization of P. caribaeorum in the northwestern Atlantic would occur under these climate scenarios for hard bottom environments.
Nicaragua’s coast, which is part of the southwestern Caribbean region, was particularly important for the species due to the increase in potential habitat area under RCP 4.5 and 8.5 (Figs. 5 and 6). Few studies were conducted about the benthic community in that region, which presents high coverage of algae of the genera Dictyota and Padina, corals such as Orbicella annularis, Agaricia spp., Porites spp., Acropora palmata, and Millepora alcicornis, and lower coverage of sponge and soft corals (Kramer et al., 2000). The same study discusses the likelihood of a phase shift in the coming years due to natural disturbances and climate change. Phase shifts from reef-building corals to zoanthids were previously reported in the western Atlantic (Cruz et al., 2015a), where they reduced fish diversity associated with the reefs (Cruz et al., 2015b), and have the potential to adversely affect regional fisheries and tourism, which are main income sources for the local population.
Although it was assumed in this work, it is not expected that all individuals will be affected in the same way by climate change. It is known that, at smaller scales, differences between symbiont algae (Kemp et al., 2006), as well as the anthozoan’s plasticity (Costa et al., 2011), can result in distinct outcomes for different populations under climate change. Average and maximum temperatures had small contributions to the model and were not considered here; however, it is known that surface waters are likely to experience an increase in temperature, especially in tropical areas (IPCC, 2014). The ability to endure such changes will also play a role in P. caribaeorum’s distribution. The authors highlight here the importance of future experimental work on temperature stress on key organisms, such as P. caribaeorum, to better understand the future of tropical reef communities.
It is possible that, due to the scale of the problem, some local variations were not accounted for, such as the discharge of small rivers around the Gulf of Mexico region. Smaller scales and regional studies should use higher resolution data for this matter. Sea level rise can modify wave stress atop coral reefs, favoring the colonization of P. caribaeorum over other organisms, including zoanthids (Rabelo et al., 2015). This effect was not considered in this study because the IPCC forecast for sea level rise did not surpass 0.8 m, which is smaller than the error of the bathymetric data used in this work.
Conclusions
The present habitat of P. caribaeorum can be characterized by high salinity values, marine pH, and a small to moderate range of temperature in coastal and shallow environments. The species’ current distribution in the western Atlantic Ocean is restricted to warm provinces, identifying it as a coastal and warm-water species, together with shallow water corals. According to this study, this organism’s potential distribution is likely to change by the year 2100. It is possible that, under low to mild anthropogenic impacts (RCP 2.6 and 4.5), the species’ distribution will be extended northward and will face a decrease of suitable habitat in the southernmost range of its distribution under any climate scenario. Under the scenario with the highest anthropogenic impacts (RCP 8.5), novel suitable areas poleward were not predicted, and only one-half of its potential habitat area would be retained when compared to the present. Under any climate scenario, the Caribbean Sea remained suitable for the species, characterizing this region as a possible refuge. Upwelling regions, such as Cape Frio and northern Colombia, could provide a refuge for this species as well, diminishing the local impacts of climate changes. Although community changes were discussed according to the available literature, biological and oceanographic processes not considered in this study may play an important role in the outcome of these changes.
Supplemental Information
Files contain all datasets in .csv format, along with the codes used for variable selection in R, and references list with original data.
Acknowledgments
We thank our colleagues Paulo Polito, André Acosta, Romina Barbosa, Bruno Ferrero, and Alexander Jueterbock, each of whom provided advice and expertise that contributed to this work. We would also like to thank Ana Flora Sarti for kindly providing part of the data used in this work, as well as Erica Donlon for the English review. This work is also a product of the SISBIOTA-Mar research network.
Funding Statement
This research was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP - Process No. 2016/05296-3) and CNPq, through the SISBIOTA network. Igor Cruz was supported by a postdoctoral scholarship (FAPESP - Process No. 2014/17815-0). Tito M.C. Lotufo is also a CNPq research fellow. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Leonardo M. Durante performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Igor C.S. Cruz analyzed the data, authored or reviewed drafts of the paper, approved the final draft, provided original data and enhanced discussion.
Tito M.C. Lotufo conceived and designed the experiments, analyzed the data, contributed reagents/materials/analysis tools, authored or reviewed drafts of the paper, approved the final draft.
Data Availability
The following information was supplied regarding data availability:
All the data used, including matrices, maxent codes, and full list of references used to assemble the matrices, are provided in the Supplemental File.
References
- Acosta (2001).Acosta A. Disease in Zoanthids: dynamics in space and time. Hydrobiologia. 2001;460:113–130. doi: 10.1023/A:1013135702430. [DOI] [Google Scholar]
- Acosta et al. (2016).Acosta AL, Giannini TC, Imperatriz-Fonseca VL, Saraiva AM. Worldwide alien invasion: a methodological approach to forecast the potential spread of a highly invasive pollinator. PLOS ONE. 2016;11:e0148295. doi: 10.1371/journal.pone.0148295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acosta, Sammarco & Duarte (2005).Acosta A, Sammarco PW, Duarte LF. New fission processes in the Zoanthid Palythoa caribaeorum: description and quantitative aspects. Bulletin of Marine Science. 2005;76:1–26. [Google Scholar]
- Ali et al. (2016).Ali KA, Ortiz J, Bonini N, Shuman M, Sydow C. Application of Aqua MODIS sensor data for estimating chlorophyll a in the turbid Case 2 waters of Lake Erie using bio-optical models. GIScience & Remote Sensing. 2016;53:483–505. doi: 10.1080/15481603.2016.1177248. [DOI] [Google Scholar]
- Amante & Eakins (2009).Amante C, Eakins BW. ETOPO1 1 arc-minute global relief model: procedures, data sources and analysis. NOAA Technical Memorandum NESDIS NGDC. Vol. 24. 2009:19. doi: 10.1594/PANGAEA.769615. [DOI]
- Araújo & Pearson (2005).Araújo MB, Pearson RG. Equilibrium of species’ distribution with climate. Ecography. 2005;28:693–695. doi: 10.1111/j.2005.0906-7590.04253.x. [DOI] [Google Scholar]
- Bastidas & Bone (1996).Bastidas C, Bone D. Competitive strategies between Palythoa Caribaeorum and Zoanthus Sociatus (Cnidaria: Anthozoa) at a reef flat environment in Venezuela. Bulletin of Marine Science. 1996;59:543–555. [Google Scholar]
- Bouzon, Brandini & Rocha (2012).Bouzon JL, Brandini FP, Rocha RM. Biodiversity of Sessile Fauna on rocky shores of coastal islands in Santa Catarina, Southern Brazil. Marine Science. 2012;2:39–47. doi: 10.5923/j.ms.20120205.01. [DOI] [Google Scholar]
- Bozdogan (1987).Bozdogan H. Model selection and Akaike’s information criterion (AIC): the general theory and its analytical extensions. Psychometrika. 1987;52:345–370. [Google Scholar]
- Briggs & Bowen (2012).Briggs JC, Bowen BW. A realignment of marine biogeographic provinces with particular reference to fish distributions. Journal of Biogeography. 2012;39:12–30. doi: 10.1111/j.1365-2699.2011.02613.x. [DOI] [Google Scholar]
- Briggs & Bowen (2013).Briggs JC, Bowen BW. Marine shelf habitat: biogeography and evolution. Journal of Biogeography. 2013;40:1023–1035. doi: 10.1111/jbi.12082. [DOI] [Google Scholar]
- Bruno et al. (2009).Bruno JF, Sweatman H, Precht WF, Selig ER, Schutte VGW. Assessing evidence of phase shifts from coral to macroalgal dominance on coral reefs. Ecology. 2009;90:1478–1484. doi: 10.1890/08-1781.1. [DOI] [PubMed] [Google Scholar]
- Castro et al. (2006).Castro BM, Brandini FP, Pires-Vanin AMS, Miranda LB. Multidisciplinary oceanography processes on the western atlantic continental shelf between 4°N and 34°S. In: Robinson AR, Brink KH, editors. The sea: the global coastal ocean. Cambridge: Harvard University Press; 2006. pp. 259–295. [Google Scholar]
- Castro et al. (2012).Castro CB, Segal B, Negrão F, Calderon EN. Four-year monthly sediment deposition on turbid southwestern Atlantic coral reefs, with a comparison of benthic assemblages. Brazilian Journal of Oceanography. 2012;60:49–63. doi: 10.1590/S1679-87592012000100006. [DOI] [Google Scholar]
- Chollet, Mumby & Cortés (2010).Chollet I, Mumby PJ, Cortés J. Upwelling areas do not guarantee refuge for coral reefs in a warming ocean. Marine Ecology Progress Series. 2010;416:47–56. doi: 10.3354/meps08775. [DOI] [Google Scholar]
- Churchill & Berger (1998).Churchill JH, Berger TJ. Transport of middle atlantic bight shelf water to the Gulf stream near Cape Hatteras. Journal of Geophysical Research. 1998;103:30605–30621. doi: 10.1029/98JC01628. [DOI] [Google Scholar]
- Collins et al. (2011).Collins WJ, Bellouin N, Doutriaux-Boucher M, Gedney N, Halloran P, Hinton T, Hughes J, Jones CD, Joshi M, Liddicoat S, Martin G, O’connor F, Rae J, Senior C, Sitch S, Totterdell I, Wiltshire A, Woodward S. Geoscientific model development development and evaluation of an earth-system model—HadGEM2. Geoscientific Model Development. 2011;4:1051–1075. doi: 10.5194/gmd-4-1051-2011. [DOI] [Google Scholar]
- Costa et al. (2012).Costa BM, Kendall MS, Parrish FA, Boland RC, Lecky J, Spalding H. Prediction of mesophotic coral distributions in the Au‘au Channel, Hawaii 2012.
- Costa et al. (2011).Costa DL, Gomes PB, Santos AM, Valenca NS, Vieira NA, Perez CD. Morphological plasticity in the reef zoanthid Palythoa caribaeorum as an adaptive strategy. Annales Zoologici Fennici. 2011;48:349–358. doi: 10.5735/086.048.0602. [DOI] [Google Scholar]
- Cruz et al. (2015a).Cruz ICS, De Kikuchi RKP, Longo LL, Creed JC. Evidence of a phase shift to Epizoanthus gabrieli Carlgreen, 1951 (Order Zoanthidea) and loss of coral cover on reefs in the Southwest Atlantic. Marine Ecology. 2015a;36:318–325. doi: 10.1111/maec.12141. [DOI] [Google Scholar]
- Cruz et al. (2015b).Cruz ICS, Loiola M, Albuquerque T, Reis R, De Anchieta CC, Nunes J, Reimer JD, Mizuyama M, Kikuchi RKP, Creed JC. Effect of phase shift from corals to Zoantharia on reef fish assemblages. PLOS ONE. 2015b;10:e0116944. doi: 10.1371/journal.pone.0116944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBose et al. (2013).DeBose JL, Nuttall MF, Hickerson EL, Schmahl GP. A high-latitude coral community with an uncertain future: Stetson Bank, northwestern Gulf of Mexico. Coral Reefs. 2013;32:255–267. doi: 10.1007/s00338-012-0971-3. [DOI] [Google Scholar]
- De Marco, Diniz-Filho & Bini (2008).De Marco P, Diniz-Filho JA, Bini LM. Spatial analysis improves species distribution modelling during range expansion. Biology Letters. 2008;4:577–580. doi: 10.1098/rsbl.2008.0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards & Lubbock (1983).Edwards A, Lubbock R. The ecology of Saint Paul’s rocks (Equatorial Atlantic) Journal of Zoology. 1983;200:51–69. doi: 10.1111/j.1469-7998.1983.tb06108.x. [DOI] [Google Scholar]
- Ekman (1953).Ekman S. Zoogeography of the sea. Sidgwick & Jackson; London: 1953. [Google Scholar]
- Elith & Leathwick (2009).Elith J, Leathwick JR. Species distribution models: ecological explanation and prediction across space and time. Annual Review of Ecology and Systematics. 2009;40:415–436. doi: 10.1146/annurev.ecolsys.l. [DOI] [Google Scholar]
- Elith et al. (2011).Elith J, Phillips SJ, Hastie T, Dudík M, Chee YE, Yates CJ. A statistical explanation of MaxEnt for ecologists. Diversity and Distributions. 2011;17:43–57. doi: 10.1111/j.1472-4642.2010.00725.x. [DOI] [Google Scholar]
- Fautin (1988).Fautin DG. Anthozoan dominated benthic environments. Proceedings of the 6th international coral reef symposium; Australia. 1988. pp. 1–6. [Google Scholar]
- Fields et al. (1993).Fields PA, Graham JB, Rosenblatt RH, Somero GN. Effects of expected global climate change on marine faunas. Trends in Ecology & Evolution. 1993;8:361–367. doi: 10.1016/0169-5347(93)90220-J. [DOI] [PubMed] [Google Scholar]
- Guisan & Thuiller (2005).Guisan A, Thuiller W. Predicting species distribution: offering more than simple habitat models. Ecology Letters. 2005;8:993–1009. doi: 10.1111/j.1461-0248.2005.00792.x. [DOI] [PubMed] [Google Scholar]
- Gutt et al. (2012).Gutt J, Zurell D, Bracegridle TJ, Cheung W, Clark MS, Convey P, Danis B, David B, De Broyer C, Di Prisco G, Griffiths H, Laffont R, Peck LS, Pierrat B, Riddle MJ, Saucède T, Turner J, Verde C, Wang Z, Grimm V. Correlative and dynamic species distribution modelling for ecological predictions in the Antarctic: a cross-disciplinary concept. Polar Research. 2012;31:1–23. doi: 10.3402/polar.v31i0.11091. [DOI] [Google Scholar]
- Haffer (1969).Haffer J. Speciation in amazonian forest birds. Science. 1969;165:131–137. doi: 10.1126/science.165.3889.131. [DOI] [PubMed] [Google Scholar]
- Harley et al. (2006).Harley CDG, Hughes AR, Kristin M, Miner BG, Sorte CJB, Carol S, Randall Hughes A, Hultgren KM, Thornber CS, Rodriguez LF, Tomanek L, Williams SL. The impacts of climate change in coastal marine systems. Ecology letters. 2006;9:228–241. doi: 10.1111/j.1461-0248.2005.00871.x. [DOI] [PubMed] [Google Scholar]
- Hines & Pawlik (2011).Hines DE, Pawlik JR. Assessing the antipredatory defensive strategies of Caribbean non-scleractinian zoantharians (Cnidaria): is the sting the only thing? Marine Biology. 2011;159:389–398. doi: 10.1007/s00227-011-1816-2. [DOI] [Google Scholar]
- Hoegh-Guldberg (1999).Hoegh-Guldberg O. Climate change, coral bleaching and the future of the world’s coral reefs. Marine Freshwater Research. 1999;50:839–866. doi: 10.1071/MF99078. [DOI] [Google Scholar]
- Hu & Guillemin (2016).Hu Z-M, Guillemin M-L. Coastal upwelling areas as safe havens during climate warming. Journal of Biogeography. 2016;43:1–2. doi: 10.1111/jbi.12887. [DOI] [Google Scholar]
- Hughes et al. (2003).Hughes TP, Baird AH, Bellwood DR, Card M, Connolly SR, Folke C, Grosberg R, Hoegh-Guldberg O, Jackson JBC, Kleypas J, Lough JM, Marshall P, Nystrom M, Palumbi SR, Pandolfi JM, Rosen B, Roughgarden J. Climate change, human impacts, and the resilience of coral reefs. Science. 2003;301:929–934. doi: 10.1126/science.1085046. [DOI] [PubMed] [Google Scholar]
- IPCC (2014).IPCC 2014. Climate change 2014: synthesis report. Contribution of working groups I, II and III to the fifth assessment report of the intergovernmental panel on climate change.
- Jueterbock (2016).Jueterbock A. 2016. Package “MaxentVariableSelection”: selecting the best set of relevant environmental variables along with the optimal regularization multiplier for maxent niche modeling.
- Kemp et al. (2006).Kemp DW, Cook CB, LaJeunesse TC, Brooks WR. A comparison of the thermal bleaching responses of the zoanthid Palythoa caribaeorum from three geographically different regions in south Florida. Journal of Experimental Marine Biology and Ecology. 2006;335:266–276. doi: 10.1016/j.jembe.2006.03.017. [DOI] [Google Scholar]
- Kramer et al. (2000).Kramer P, Kramer PR, Arias-Gonzalez E, McField M. Status of coral reefs of Northern Central America: Mexico, Belize, Guatemala, Honduras, Nicaragua and El Salvador 2000.
- Kroeker et al. (2013).Kroeker KJ, Kordas RL, Crim R, Hendriks IE, Ramajo L, Singh GS, Duarte CM, Gattuso JP. Impacts of ocean acidification on marine organisms: quantifying sensitivities and interaction with warming. Global Change Biology. 2013;19:1884–1896. doi: 10.1111/gcb.12179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesser et al. (1990).Lesser MP, Stochaj WR, Tapley DW, Shick JM. Bleaching in coral reef anthozoans: effects of irradiance, ultraviolet radiation, and temperature on the activities of protective enzymes against active oxygen. Coral Reefs. 1990;8:225–232. doi: 10.1007/BF00265015. [DOI] [Google Scholar]
- Libes (2009).Libes SM. Introduction to marine biogeochemistry. Amsterdam: Academic Press; 2009. Calcite, Alkalinity, and the pH of Seawater; p. 909. [Google Scholar]
- Liew, Chia & Kwoh (2001).Liew SC, Chia AS, Kwoh LK. Evaluating the validity of SeaWiFS chlorophyll algorithm for coastal waters. Proc. 22nd Asian conference on remote sensing; 2001. pp. 1–5. [Google Scholar]
- Littler, Littler & Taylor (1983).Littler M, Littler D, Taylor P. Evolutionary stragegies in a tropical barrier reef system: functional-form groups of marine macroalgae. Deep Sea Research Part B. Oceanographic Literature Review. 1983;30:935. doi: 10.1016/0198-0254(83)96598-6. [DOI] [Google Scholar]
- Liu, Newell & White (2015).Liu C, Newell G, White M. On the selection of thresholds for predicting species occurrence with presence-only data. Ecology and Evolution. 2015;6:337–348. doi: 10.1002/ece3.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longhurst (2007).Longhurst AR. Ecological gepgraphy of the sea. Academic Press; Amsterdam: 2007. [Google Scholar]
- Magalhães et al. (2015).Magalhães GM, Amado-Filho GM, Rosa MR, De Moura RL, Brasileiro PS, De Moraes FC, Francini-Filho RB, Pereira-Filho GH. Changes in benthic communities along a 0–60 m depth gradient in the remote St. Peter and St. Paul Archipelago (Mid-Atlantic Ridge, Brazil) Bulletin of Marine Science. 2015;91:377–396. doi: 10.5343/bms.2014.1044. [DOI] [Google Scholar]
- Meißner et al. (2014).Meißner K, Fiorentino D, Schnurr S, Martinez Arbizu P, Huettmann F, Holst S, Brix S, Svavarsson J. Distribution of benthic marine invertebrates at northern latitudes—an evaluation applying multi-algorithm species distribution models. Journal of Sea Research. 2014;85:241–254. doi: 10.1016/j.seares.2013.05.007. [DOI] [Google Scholar]
- Mendonça-Neto et al. (2008).Mendonça-Neto JP, Ferreira CEL, Chaves LCT, Pereira RC. Influence of Palythoa caribaeorum (Anthozoa, Cnidaria) zonation on site-attached reef fishes. Anais da Academia Brasileira de Ciencias. 2008;80:494–513. doi: 10.1590/S0001-37652008000300010. [DOI] [PubMed] [Google Scholar]
- Merow, Smith & Silander (2013).Merow C, Smith MJ, Silander JA. A practical guide to MaxEnt for modeling species’ distributions: what it does, and why inputs and settings matter. Ecography. 2013;36:1058–1069. doi: 10.1111/j.1600-0587.2013.07872.x. [DOI] [Google Scholar]
- Monteiro et al. (2008).Monteiro J, Almeida C, Freitas R, Delgado A, Porteiro F, Santos R. Coral assemblages of Cabo Verde : preliminary assessment and description. Proccedings of the 11th international coral reef symposium-2008; 2008. pp. 1416–1419. [Google Scholar]
- Okazaki et al. (2017).Okazaki RR, Towle EK, Van Hooidonk R, Mor C, Winter RN, Piggot AM, Cunning R, Baker AC, Klaus JS, Swart PK, Langdon C. Species-specific responses to climate change and community composition determine future calcification rates of Florida Keys reefs. Global Change Biology. 2017;23:1023–1035. doi: 10.1111/gcb.13481. [DOI] [PubMed] [Google Scholar]
- Onken (1994).Onken R. The asymmetry of western boundary currents in the upper Atlantic Ocean. Journal of Physical Oceanography. 1994;24:928–948. doi: 10.1175/1520-0485(1994)024<0928:TAOWBC>2.0.CO;2. [DOI] [Google Scholar]
- Pérez, Vila-Nova & Santos (2005).Pérez CD, Vila-Nova DA, Santos AM. Associated community with the zoanthid Palythoa caribaeorum (Duchassaing & Michelotti, 1860) (Cnidaria, Anthozoa) from littoral of Pernambuco, Brazil. Hydrobiologia. 2005;548:207–215. doi: 10.1007/s10750-005-5441-2. [DOI] [Google Scholar]
- Phillips, Anderson & Schapire (2006).Phillips SJ, Anderson RP, Schapire RE. Maximum entropy modeling of species geographic distributions. Ecological Modelling. 2006;190:231–259. doi: 10.1016/j.ecolmodel.2005.03.026. [DOI] [Google Scholar]
- Piola et al. (2000).Piola AR, Campos EJD, MÖller Jr OO, Charo M, Martinez C. Subtropical shelf front off eastern South America. Jornal of Geouphysical Research. 2000;105:6565–6578. doi: 10.1029/1999JC000300. [DOI] [Google Scholar]
- Polak et al. (2011).Polak O, Loya Y, Brickner I, Kramarski-Winter E, Benayahu Y. The widely-distributed Indo-Pacific Zoanthid Palythoa tuberculosa: a sexually conservative strategist. Bulletin of Marine Science. 2011;87:605–621. doi: 10.5343/bms.2010.1088. [DOI] [Google Scholar]
- R Core Team (2015).R Core Team . Vienna: R Foundation for Statistical Computing; 2015. R: a language and environment for statistical computing. [Google Scholar]
- Rabelo et al. (2015).Rabelo EF, Soares M de O, Bezerra LEA, Matthews-Cascon H. Distribution pattern of zoanthids (Cnidaria: Zoantharia) on a tropical reef. Marine Biology Research. 2015;11:584–592. doi: 10.1080/17451000.2014.962542. [DOI] [Google Scholar]
- Rasmussen, Gawarkiewicz & Owens (2005).Rasmussen LL, Gawarkiewicz G, Owens WB. Slope water, Gulf Stream, and seasonal influences on southern Mid-Atlantic Bight circulation during the fall-winter transition. Journal of Geophysical Research. 2005;110:1–16. doi: 10.1029/2004JC002311. [DOI] [Google Scholar]
- Reimer (2015).Reimer J. Palythoa caribaeorum (Duchassaing & Michelotti, 1860) 2015. [2016 September 26]. Accessed through: World Register of Marine Species. http://www.marinespecies.org/aphia.php?p=taxdetailsid=288456 .
- Reimer, Hirose & Wirtz (2010).Reimer JD, Hirose M, Wirtz P. Zoanthids of the Cape Verde Islands and their symbionts: previously unexamined diversity in the Northeastern Atlantic. Contributions to Zoology. 2010;79:147–163. [Google Scholar]
- Reiss et al. (2011).Reiss H, Cunze S, König K, Neumann H, Kröncke I. Species distribution modelling of marine benthos: a North Sea case study. Marine Ecology Progress Series. 2011;442:71–86. doi: 10.3354/meps09391. [DOI] [Google Scholar]
- Rocha et al. (2008).Rocha LA, Rocha CR, Robertson DR, Bowen BW. Comparative phylogeography of Atlantic reef fishes indicates both origin and accumulation of diversity in the Caribbean. BMC Evolutionary Biology. 2008;8:157. doi: 10.1186/1471-2148-8-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roff & Mumby (2012).Roff G, Mumby PJ. Global disparity in the resilience of coral reefs. Trends in Ecology and Evolution. 2012;27:404–413. doi: 10.1016/j.tree.2012.04.007. [DOI] [PubMed] [Google Scholar]
- Ryland (1997).Ryland JS. Reproduction in Zoanthidea (Anthozoa: Hexacorallia) Invertebrate Reproduction & Development. 1997;31:177–188. doi: 10.1080/07924259.1997.9672575. [DOI] [Google Scholar]
- Ryland et al. (2000).Ryland JS, De Putron S, Scheltema RS, Chimonides PJ, Zhadan DG. Semper’s (zoanthid) larvae: pelagic life, parentage and other problems. Hydrobiologia. 2000;440:191–198. doi: 10.1023/A:1004171127777. [DOI] [Google Scholar]
- Sandin & McNamara (2012).Sandin SA, McNamara DE. Spatial dynamics of benthic competition on coral reefs. Oecologia. 2012;168:1079–1090. doi: 10.1007/s00442-011-2156-0. [DOI] [PubMed] [Google Scholar]
- Santana et al. (2015).Santana EFC, Alves AL, Santos ADM, Cunha MDGGS, Perez CD, Gomes APB. Trophic ecology of the zoanthid Palythoa caribaeorum (Cnidaria: Anthozoa) on tropical reefs. Marine Biological Association of the United Kingdom. 2015;95:301–309. doi: 10.1017/S0025315414001726. [DOI] [Google Scholar]
- Santos et al. (2016).Santos MEA, Kitahara MV, Lindner A, Davis Reimer J. Overview of the order Zoantharia (Cnidaria: Anthozoa) in Brazil. Marine Biodiversity. 2016;46:547–559. doi: 10.1007/s12526-015-0396-7. [DOI] [Google Scholar]
- Sebens (1977).Sebens K. Autotrophic and heterotrophic nutrition of coral reef zoanthids. Proceedings of the 3rd international coral reef symposium; 1977. pp. 397–404. [Google Scholar]
- Sebens (1982).Sebens KP. Intertidal distribuition of Zoanthids on the Caribbean Coast of Panamá: effects of predation and desiccation. Bulletin of Marine Science. 1982;32:316–335. [Google Scholar]
- Segal & Castro (2011).Segal B, Castro CB. Coral community structure and sedimentation at different distances from the coast of the Abrolhos Bank, Brazil. Brazilian Journal of Oceanography. 2011;59:119–129. doi: 10.1590/S1679-87592011000200001. [DOI] [Google Scholar]
- Silva et al. (2015).Silva JF, Gomes PB, Santana EC, Silva JM, Lima ÉP, Santos AMM, Pérez CD. Growth of the tropical zoanthid Palythoa caribaeorum (Cnidaria: Anthozoa) on reefs in northeastern Brazil. Anais da Academia Brasileira de Ciências. 2015;87:985–996. doi: 10.1590/0001-3765201520140475. [DOI] [PubMed] [Google Scholar]
- Sorokin (1991).Sorokin YI. Biomass, metabolic rates and feeding of some common reef zoantharians and octocorals. Australian Journal of Marine and Freshwater Research. 1991;42:729–741. doi: 10.1071/MF9910729. [DOI] [Google Scholar]
- Spalding et al. (2007).Spalding MD, Fox HE, Allen GR, Davidson N, Ferdaña ZA, Finlayson M, Halpern BS, Jorge MA, Lombana A, Lourie SA, Martin KD, Mcmanus E, Molnar J, Recchia CA, Robertson J. Marine ecoregions of the world: a bioregionalization of coastal and shelf areas. BioScience. 2007;57:573–583. doi: 10.1641/B570707. [DOI] [Google Scholar]
- Stampar, Silva & Luiz Jr (2007).Stampar SN, Silva PF, Luiz Jr OJ. Predation on the Zoanthid Palythoa caribaeorum (Anthozoa, Cnidaria) by a Hawksbill Turtle (Eretmochelys imbricata) in Southeastern Brazil. Marine Turtle Newsletter. 2007;117:3–5. [Google Scholar]
- Steiner et al. (2015).Steiner AQ, Amaral FMD, Amaral JRBC, Sassi R, Barradas JI. Zonação de recifes emersos da Área de Proteção Ambiental Costa dos Corais, Nordeste do Brasil. Iheringia. Série Zoologia. 2015;105:184–192. doi: 10.1590/1678-476620151052184192. [DOI] [Google Scholar]
- Suchanek & Green (1981).Suchanek TH, Green DJ. Interspecific competition between Palythoa caribaeorum and other sessile invertebrates on St. Croix Reefs, U.S Virgin Islands. Proceedings of the 4th International Coral Reef Symposium. 1981;2:679–684. [Google Scholar]
- Swets (1988).Swets JA. Measuring the accuracy of diagnostic systems. Science. 1988;240:1285–1293. doi: 10.1126/science.3287615. [DOI] [PubMed] [Google Scholar]
- Talley et al. (2011).Talley LD, Pickard GL, Emery WJ, Swif JH. Descriptive physical oceanography. Amsterdam: Elsevier; 2011. Atlantic Ocean; p. 555. [Google Scholar]
- Tanner (1995).Tanner JE. Competition between scleractinian corals and macroalgae: an experimental investigation of coral growth, survival and reproduction. Journal of Experimental Marine Biology and Ecology. 1995;190:151–168. doi: 10.1016/0022-0981(95)00027-o. [DOI] [Google Scholar]
- Tester et al. (2013).Tester PA, Vandersea MW, Buckel CA, Kibler SR, Holland WC, Davenport ED, Clark RD, Edwards KF, Taylor JC, Pluym JL Vander, Hickerson EL, Litaker RW. Gambierdiscus (Dinophyceae) species diversity in the Flower Garden Banks National Marine Sanctuary, Northern Gulf of Mexico, USA. Harmful Algae. 2013;29:1–9. doi: 10.1016/j.hal.2013.07.001. [DOI] [Google Scholar]
- Thomas et al. (2017).Thomas L, Kennington WJ, Evans RD, Kendrick GA, Stat M. Restricted gene flow and local adaptation highlight the vulnerability of high-latitude reefs to rapid environmental change. Global Change Biology. 2017;23:2197–2205. doi: 10.1111/gcb.13639. [DOI] [PubMed] [Google Scholar]
- Tyberghein et al. (2012).Tyberghein L, Verbruggen H, Pauly K, Troupin C, Mineur F, De Clerck O. Bio-ORACLE: a global environmental dataset for marine species distribution modelling. Global Ecology and Biogeography. 2012;21:272–281. doi: 10.1111/j.1466-8238.2011.00656.x. [DOI] [Google Scholar]
- Van Vuuren et al. (2011).Van Vuuren DP, Edmonds J, Kainuma M, Riahi K, Thomson A, Hibbard K, Hurtt GC, Kram T, Krey V, Lamarque J-F, Masui T, Meinshausen M, Nakicenovic N, Smith SJ, Rose SK. The representative concentration pathways: an overview. Climatic Change. 2011;109:5–31. doi: 10.1007/s10584-011-0148-z. [DOI] [Google Scholar]
- Vazyulya et al. (2014).Vazyulya S, Khrapko A, Kopelevich O, Burenkov V, Eremina T, Isaev A. Regional algorithms for the estimation of chlorophyll and suspended matter concentration in the Gulf of Finland from MODIS-Aqua satellite data. Oceanologia. 2014;56:737–756. doi: 10.5697/oc.56-4.737. [DOI] [Google Scholar]
- Verbruggen (2012).Verbruggen H. OccurrenceThinner. Version 1.04http://phycoweb.net/software/OccurrenceThinner/ 2012
- Villaça & Pitombo (1997).Villaça R, Pitombo FB. Benthic communities of shallow-water reefs of Abrolhos, Brazil. Revista Brasileira de Oceanografia. 1997;45:35–43. doi: 10.1590/S1413-77391997000100004. [DOI] [Google Scholar]
- Villamizar et al. (2008).Villamizar E, Camisotti H, Rodríguez B, Pérez J, Romero M. Impacts of the 2005 Caribbean bleaching event at Archipiélago de Los Roques National Park, Venezuela. Revista de Biología Tropical. 2008;56:255–270. doi: 10.15517/rbt.v56i0.5591. [DOI] [Google Scholar]
- Walsh, Dieterle & Esaias (1987).Walsh JJ, Dieterle DA, Esaias WE. Satellite detection of phytoplankton export from the mid-Atlantic Bight during the 1979 spring bloom. Deep-Sea Research. 1987;34:675–703. doi: 10.1016/0198-0149(87)90030-6. [DOI] [Google Scholar]
- Warren & Seifert (2011).Warren DL, Seifert SN. Ecological niche modeling in Maxent: the importance of model complexity and the performance of model selection criteria. Ecological Applications. 2011;21:335–342. doi: 10.1890/10-1171.1. [DOI] [PubMed] [Google Scholar]
- Warren et al. (2014).Warren DL, Wright AN, Seifert SN, Shaffer HB. Incorporating model complexity and spatial sampling bias into ecological niche models of climate change risks faced by 90 California vertebrate species of concern. Diversity and Distributions. 2014;20:334–343. doi: 10.1111/ddi.12160. [DOI] [Google Scholar]
- Wood (1993).Wood R. Nutrients, predation and the History of Reef-Building. Society of Sedimentary Geology. 1993;8:526–543. doi: 10.2307/3515030. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Files contain all datasets in .csv format, along with the codes used for variable selection in R, and references list with original data.
Data Availability Statement
The following information was supplied regarding data availability:
All the data used, including matrices, maxent codes, and full list of references used to assemble the matrices, are provided in the Supplemental File.