Abstract
Background
There is uncertainty as to the appropriate follow up of patients who test positive on multimarker stool DNA (sDNA) testing and have a colonoscopy without neoplasia.
Aims
Determine the prevalence of missed colonic or occult upper gastrointestinal neoplasia in patients with an apparent false positive sDNA.
Methods
We prospectively identified 30 patients who tested positive with a commercially available sDNA followed by colonoscopy without neoplastic lesions. Patients were invited to undergo repeat sDNA at 11–29 months after the initial test followed by repeat colonoscopy and upper endoscopy. We determined the presence of neoplastic lesions on repeat evaluation stratified by results of repeat sDNA.
Results
Twelve patients were restudied. Seven patients had a negative second sDNA test and a normal second colonoscopy and upper endoscopy. In contrast, five of 12 subjects had a persistently positive second sDNA test, and 3 had positive findings, including a 3 cm sessile transverse colon adenoma with high grade dysplasia, a 2 cm right colon sessile serrated adenoma with dysplasia, and a nonadvanced colon adenoma (p=0.045). These corresponded to a positive predictive value of 0.60 (95% CI 0.17–1.00) and a negative predictive value of 1.00 (95% CI 1.00–1.00) for the second sDNA test. In addition, the medical records of all 30 subjects with apparent false positive testing were reviewed and no documented cases of malignant tumors were recorded.
Conclusions
Repeat positive sDNA testing may identify a subset of patients with missed or occult colorectal neoplasia after negative colonoscopy for an initially positive sDNA. High quality colonoscopy with careful attention to the right colon in patients with positive sDNA is critically important and may avoid false negative colonoscopy.
Keywords: Colorectal Neoplasms/diagnosis, Colon Polyps/diagnosis, Colonoscopy, DNA/analysis, Early Detection of Cancer
Introduction
Multimarker stool DNA (sDNA) testing is an approved technology for colorectal cancer screening that was recently endorsed as a noninvasive option as part of the US Preventive Services Task Force guidelines (Cologuard, Exact Sciences, Madison, WI) (1). The multimarker panel includes two aberrant methylation markers (NDRG4 and BMP3), KRAS mutations and a fecal immunochemical test (FIT), and uses an algorithm to determine a single composite qualitative positive or negative result. Current guidelines recommend colonoscopy to evaluate all patients with positive sDNA testing as a positive test could indicate the presence of colorectal neoplasia.
One of the stated clinical concerns of a positive sDNA is the absence of neoplasia on subsequent colonoscopy, and in the largest clinical trial of sDNA, the quoted specificity was 86.6–89.8%, depending on what definition of a positive result on colonoscopy was used (2). However, it is known that colonoscopy is an imperfect “gold standard”, with a quoted miss rate for advanced adenomas of up to 11% (3) and 2.6%–9.0% of cancers developing within 3 years of colonoscopy (interval cancers) (4). While neoplasms in the upper gastrointestinal tract or pancreas (5) are known to produce genetic markers that may survive passage into the stool and be detectable in stool assays, a recently published cohort study that followed more than 1000 patients with a false positive sDNA test found only 8 subsequent cancers (3 lung, 3 pancreas, 1 colon, 1 biliary) at an average 4 year follow up, which was not greater than expected in the general population (6). A similarly low estimate of extracolonic sources of sDNA test positivity was found in a case-control study (2 positive aerodigestive cancers for each 10,000 patients screened) (7). However, these studies did not ascertain the presence of premalignant colorectal lesions. Thus, the absence of neoplasia on colonoscopy poses a dilemma for clinicians, as they may elect to repeat a colonoscopy or sDNA sooner than recommended by guidelines as opposed to placing the patient back in an average risk screening program.
Therefore, we conducted a prospective study of patients with an apparent false positive sDNA result to determine if the findings represented missed colonic neoplasia or occult upper gastrointestinal neoplasia.
Methods
The study was conducted from 2012 to 2015 at three sites in metropolitan Cleveland, Ohio – an urban, tertiary care academic medical center, an affiliated suburban community hospital and an affiliated suburban ambulatory surgery center. Methods and results are reported in accordance with the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guidelines (8). Patients aged 40–80 who were referred for colonoscopy by their healthcare providers were eligible for enrollment. Exclusion criteria were a known history of any malignancy, a prior history of adenomatous polyps or serrated neoplasia in the colon, previous colon resection, prior colonoscopy within 5 years, overt gastrointestinal bleeding, a diagnosis of ulcerative colitis or Crohn’s disease, or inability to provide informed consent or understand English. Prior to undergoing colonoscopy and the bowel preparation, all patients collected stool for the sDNA panel that was processed according to standard protocol. Patients then underwent standard colonoscopy with the endoscopist unaware of the sDNA results. All visible lesions were removed or if not feasible, biopsied, and a positive test included findings of one or more adenomas, sessile serrated adenomas or carcinomas. Endoscopic examinations were performed by faculty endoscopists, all of whom exceeded benchmarks for quality metrics (9).
All patients with a positive result on sDNA and a negative colonoscopy were contacted approximately one year after the index colonoscopy to invite them to undergo repeat evaluation. Patients who agreed to be restudied underwent a second sDNA panel followed by repeat colonoscopy and upper endoscopy, the cost of which was paid by the study. As in the initial examination, all visible lesions were removed or biopsied. Differences in the findings on repeat endoscopic evaluation were compared according to repeat sDNA findings using the Fishers exact test.
Results
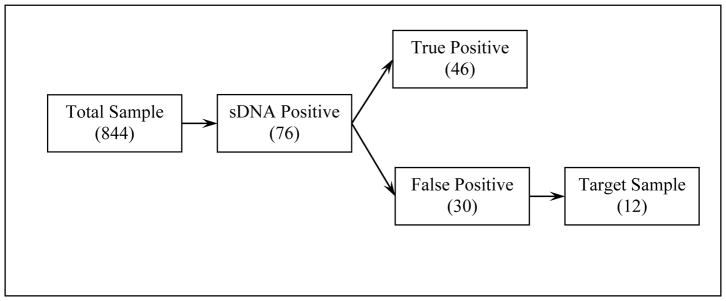
The study included 844 subjects with a mean age of 56.7 ± 8.0 years, 60.8% women and 31.9% African Americans. In this sample, a positive sDNA result was found in 76 patients, of whom 46 had one or more neoplasms on index colonoscopy. The remaining 30 patients were considered to have a false positive sDNA (Figure 1). Among these patients, we excluded 18 for the following reasons: competing comorbidities which would pose a high risk for repeat examination (n=1), finding of a large inflammatory polyp that was bleeding on the index colonoscopy (n=1), finding of a 12 mm sessile polyp that was removed but not recovered (n=1), poor bowel preparation on the index colonoscopy (n=3), the presence of visible rectal bleeding that followed a prostate biopsy at the time of sDNA collection (n=1), and refusal to undergo repeat evaluation (n=11).
Figure 1.
Flow Chart of Patient Follow Up
The results of evaluation of the 12 patients who were restudied are shown in Table 1. All female patients were postmenopausal except for the 40 year old woman, and she denied menstruation at the time of sDNA collection. In this sample, colonoscopy and upper endoscopy were performed between 11–29 months after the initial colonoscopy. Seven patients had a negative second sDNA test and a normal second colonoscopy and upper endoscopy. In contrast, five of 11 subjects had a persistently positive second sDNA test, and 3 had positive findings, including a 3 cm sessile transverse colon adenoma with high grade dysplasia, a 2 cm proximal colon sessile serrated adenoma with dysplasia, and a nonadvanced colon adenoma. The differences in repeat findings according to repeat sDNA result were statistically significant (p=0.045). A fourth patient was found to have atrophic gastritis with intestinal metaplasia on upper endoscopy but this was considered as a negative finding. These corresponded to a positive predictive value of 0.60 (95% CI 0.17–1.00) and a negative predictive value of 1.00 (95% CI 1.00–1.00) for the second sDNA test. In addition, the medical records of all 30 subjects with apparent false positive testing were reviewed and no documented cases of malignant tumors were reported.
Table 1.
Results of Repeat Evaluation of 12 Patients with Initially False Positive sDNA
| Age | Gender | Race | Months from Initial Colonoscopy | Repeat Examination Results |
|---|---|---|---|---|
| Repeat sDNA Positive | ||||
| 61 | F | B | 12 | Villous Adenoma with high grade dysplasia |
| 58 | F | W | 14 | Sessile serrated adenoma with dysplasia |
| 50 | M | W | 12 | Nonadvanced adenoma |
| 67 | F | W | 11 | Negative |
| 61 | F | W | 26 | Negative |
| Repeat sDNA Negative | ||||
| 51 | M | W | 11 | Negative |
| 40 | F | B | 11 | Negative |
| 51 | M | B | 25 | Negative |
| 54 | F | W | 13 | Negative |
| 58 | F | W | 14 | Negative |
| 63 | F | W | 12 | Negative |
| 43 | M | W | 21 | Negative |
Discussion
Based on the quoted specificity of 90%, the findings of no neoplasia on colonoscopy for evaluation of a positive sDNA is a commonly encountered clinical situation. The majority of these tests do in fact represent false positives and are an expected result of the test design which was optimized for sensitivity. However, there may be some degree of uncertainty as to their clinical significance. In this prospective study, we found that 42% (5/12) had a repeat positive sDNA and of these, 3/5 had positive colonoscopic findings on repeat evaluation, including two patients with advanced neoplasia that was not detected on initial colonoscopy. In contrast, none of seven patients with a subsequent negative test sDNA had findings on repeat colonoscopy. One of the missed lesions was serrated and these are associated with a greater variability in detection between endoscopists compared to traditional adenomas, suggesting a potentially higher miss rate (10,11). As both of the advanced lesions detected on repeat colonoscopy were in the proximal colon, careful attention to the right colon in patients with a positive sDNA is critically important.
Limitations of the study included the small number of patients with repeated sDNA tests and colonoscopies, and lack of knowledge as to which component(s) of the sDNA panel was positive on the initial and repeat evaluation. Although it would be of interest to know whether those with persistently positive testing had the same positive markers on both sets, the qualitative positive or negative test results were those that the FDA approved and reported clinically. In addition, the endoscopist who performed the second colonoscopy was aware that the initial sDNA was positive, and as shown in a recent study (12), may have deliberately performed a more careful examination. Also, as other diagnostic testing such as CT scans were not performed, we could not definitively exclude occult malignancy in the sample, though medical record review did not indicate such findings. We also used narrow band imaging for all follow up colonoscopies and although this technique has not been consistently shown to improve polyp detection, it may be helpful in differentiation of neoplastic from other lesions (13).
Current guidelines recommend repeat sDNA testing at a three year interval after a negative test and that colonoscopy be performed to evaluate positive tests (1). Recent guidelines from the US Multisociety Task Force (14) indicate that after a high quality colonoscopy, available evidence suggests that a repeat colonoscopy or evaluation of the remainder of the gastrointestinal tract are not needed. Conceivably, some of these patients may be triaged to repeat colonoscopy 10 years later, which is the recommended follow up for normal screening examinations, but this practice may lead to a higher than desired rate of interval cancers. Our data, though not guideline endorsed, may suggest that one should consider repeat sDNA testing at a one year interval and if persistently positive, repeat colonoscopy may be prudent. Although our study was performed at a tertiary referral center, this consideration might be even more applicable in broader practice and in instances of concerns over colonoscopy quality. If the repeat test is negative, then the patient would be placed back into an average risk screening program. However, further validation of these findings in larger samples would be needed before recommendations could be made.
In summary, we found that repeat positive sDNA testing may identify a subset of patients with missed or occult colorectal neoplasia after a negative colonoscopy for an initially positive sDNA. While this strategy may alleviate concerns from clinicians in dealing with this diagnostic dilemma, great care in the initial colonoscopic evaluation of sDNA positive patients, especially in the proximal colon is warranted.
Acknowledgments
Supported by the Case Gastrointestinal SPORE (P50CA150964), the Case Comprehensive Cancer Center (P30CA43703), the Cleveland Digestive Disease Research Core Center (P30DK097948), University of Michigan Gastrointestinal SPORE (P50CA130810), the University of Michigan Comprehensive Cancer Center (P30CA046592), the Early Detection Research Network (U01CA086400) and U01CA181770.
Footnotes
Conflict of Interest
Barry M. Berger, MD is a full-time employee of Exact Sciences, Inc, the manufacturer of the stool DNA technology used in this study. None of the other authors have conflicts of interest.
References
- 1.US Preventive Services Task Force. Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. JAMA. 2016;315:2564–75. doi: 10.1001/jama.2016.5989. [DOI] [PubMed] [Google Scholar]
- 2.Imperiale TF, Ransohoff DF, Itzkowitz SH, et al. Multi-taget stool DNA testing for colorectal cancer screening. N Engl J Med. 2014;370:1287–97. doi: 10.1056/NEJMoa1311194. [DOI] [PubMed] [Google Scholar]
- 3.Heresbach D, Barrioz T, Lapalus MG, et al. Miss rate for colorectal neoplastic polyps: A prospective multicenter study of back-to-back video colonoscopies. Endoscopy. 2008;40:284–90. doi: 10.1055/s-2007-995618. [DOI] [PubMed] [Google Scholar]
- 4.Singh S, Singh PP, Murad MH, Singh H, Samadder NJ. Prevalence, risk factors and outcomes of interval colorectal cancers: a systematic review and meta-analysis. Am J Gastroenterol. 2014;109:1375–89. doi: 10.1038/ajg.2014.171. [DOI] [PubMed] [Google Scholar]
- 5.Kisiel JB, Yab TC, Taylor WR, et al. Stool DNA testing for the detection of pancreatic cancer: assessment of methylation marker candidates. Cancer. 2012;118:2623–31. doi: 10.1002/cncr.26558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cotter TG, Burger KN, Devens ME, et al. Long-term follow-up of patients having false positive multi-target stool DNA tests after negative screening colonoscopy. The LONG-HAUL cohort study. Cancer Epidemiol Biomarkers Prev. 2017;26:614–21. doi: 10.1158/1055-9965.EPI-16-0800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. [accessed February 16, 2018];FDA Summary of Safety and Effectiveness, PMA P130017b. 2014 Aug;:9. at www.fda.cov.
- 8.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147:573–7. doi: 10.7326/0003-4819-147-8-200710160-00010. [DOI] [PubMed] [Google Scholar]
- 9.Rex DK, Schoenfeld PS, Cohen J, et al. Quality indicators for colonoscopy. Am J Gastroenterol. 2015;110:72–90. doi: 10.1038/ajg.2014.385. [DOI] [PubMed] [Google Scholar]
- 10.Hetzel JT, Huang CS, Coukos JA, et al. Variation in the detection of serrated polyps in an average risk colorectal cancer screening cohort. Am J Gastroenterol. 2010;105:2656–64. doi: 10.1038/ajg.2010.315. [DOI] [PubMed] [Google Scholar]
- 11.Kahi CJ, Hewett DG, Norton DL, et al. Prevalence and variable detection of proximal colon serrated polyps during screening colonoscopy. Clin Gastroenterol Hepatol. 2011;9:42–6. doi: 10.1016/j.cgh.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 12.Johnson DH, Kisiel JB, Burger KW, et al. Multitarget stool DNA test: clinical performance and impact on yield and quality of colonoscopy for colorectal cancer screening. Gastrointest Endosc. 2017;85:657–65. doi: 10.1016/j.gie.2016.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manfredi MA, Abu Dayyeh BK, Bhat YM, et al. Electronic chromoendoscopy. Gastrointest Endosc. 2015;81:249–61. doi: 10.1016/j.gie.2014.06.020. [DOI] [PubMed] [Google Scholar]
- 14.Rex DK, Boland CR, Dominitz JA, et al. Colorectal cancer screening: recommendations for physicians and patients from the U.S. Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2017;153:307–23. doi: 10.1053/j.gastro.2017.05.013. [DOI] [PubMed] [Google Scholar]