Abstract
Purpose
To determine the value of novel whole tumor metrics in DWI-MRI and DCE-MRI of rectal cancer treatment assessment.
Materials and Methods
This retrospective study included 24 uniformly-treated patients with rectal adenocarcinoma who underwent MRI including diffusion-weighted (DW) and dynamic contrast-enhanced (DCE) sequences, before and after chemoradiotherapy. Two experienced readers independently measured tumor volume and apparent diffusion coefficient (ADC) on DWI-MRI and tumor volume and transfer constant Ktrans on DCE-MRI. In addition, we explored and defined Total Lesion Diffusion (TLD) as Total DWI tumor volume multiplied by mean volumetric ADC and Total Lesion Perfusion (TLP) as the total DCE tumor volume multiplied by the mean volumetric Ktrans. These metrics were correlated with histopathologic percent tumor regression in the resected specimen (%TR). Inter-reader agreement was assessed using the concordance correlation coefficient (CCC).
Results
For both readers, post-treatment TLP revealed comparable correlations with %TR compared with Ktrans (reader 1; Spearman’s rho = −0.36 vs. −0.32, reader 2; Spearman’s rho = −0.32 vs. −0.28). In addition, TLP afforded the highest inter-reader agreement at post treatment among TLP, DCE vol and Ktrans (CCC: 0.64 vs. 0.36 vs. 0.35). Post-treatment TLD showed similar correlation with %TR as DWI volume in reader 1 and superior correlation with %TR for reader 2 (reader 1; Spearman’s rho −0.56 vs. −0.57, reader 2; Spearman’s rho −0.59 vs. −0.45).
Conclusion
The novel tumor metrics TLD and TLP revealed similar results to established metrics for correlation with tumor response with equivalent or superior inter-reader agreements and we recommend that these be studied in larger trials.
Keywords: Rectal Cancer, Tumor response, Diffusion-weighted MRI, Dynamic contrast-enhanced MRI
Introduction
Assessment of rectal cancer tumor response at MRI is markedly limited due to the effects of chemotherapy and radiation such as fibrosis, necrosis and edema in the tumor bed. However, a recent meta-analysis indicates that interrogating tumor biology with DWI-MRI significantly increases the sensitivity to detect residual tumor compared with anatomical imaging [1]. The restricted motion of water protons in the tumor bed, measured at DWI-MRI, occurs to varying degrees throughout the tumor and the metric describing this water proton motion, the apparent diffusion coefficient (ADC) will depend on how the radiologist places their regions of interest (ROI) among full cross-sectional area in a single-slice, within-tumor sub-regions in a single-slice or full volumetric coverage [2]. Another biologic aspect of tumors interrogated by MRI is malignant neovascularity and its associated altered flow and permeability compared with normal tissues. During DCE-MRI, the most reliable pharmacokinetic model-based parameter to capture this is Ktrans, the permeability transfer constant. Our own recent investigation concurs with prior literature which has shown some promise using this parameter with regard to tumor response [3]. Since there may be intrinsic tumor heterogeneity of neovascularity, as with water proton mobility, the method of measurement of the tumor ROI by the radiologist will affect this metric as well [4]. For both DWI and DCE-MRI, we hypothesize that the interrogation of the entire lesion would reflect tumor biology, including tumor environment heterogeneity, if present and would be reproducible and show correlation with pathologic response.
Although volumetric changes in rectal tumor between pre- and post-treatment MRI scans have been correlated with tumor response at pathology, revealing superiority of DWI over T2-weighted images, this approach has always been analyzed separately from the bio-specific sequences like DWI and DCE-MRI, even though many publications will compare one against the other. For example in the studies of Gollub [3], Intven [5], Beets-Tan [6] and Kim [7], both morphological (e.g. size, volume and signal intensity) and biological (water mobility and vascular permeability) metrics were variably investigated in the same set of tumors with correlation to a gold standard to see which is best for predicting tumor response. However, the next logical step, now that quantitative metrics derived from DWI and DCE are widely available and being increasingly used in the realm of “molecular imaging”, is to follow the example of our Nuclear Medicine colleagues in their efforts to quantitate FDG uptake in cancers. At the time of the newly available whole-body PET technology, quantitation of tumor uptake required refinement and discovery of reproducible, pragmatic metrics that could be widely applied. Applying logic and approaching a “lesion” as a metabolizing entity, it became clear that quantitation of the entire lesion, rather than only a portion, especially due to potential heterogeneity within the lesion, might offer some advantages. In that vein, Total Lesion Glycolysis (TLG) was founded [8–10].
Given the ongoing active area of investigation into how best to radiologically assess rectal tumor response after neoadjuvant chemoradiotherapy, and the limitations that have been encountered, we aimed to test the concept that total lesion biology could also be interrogated with the MR bio-specific sequences DWI-MRI and DCE-MRI to derive the quantities of Total Lesion Diffusion and Total Lesion Perfusion from simple multiplication of the model-based quantities (e.g. Ktrans for DCE and ADC for DWI) with the whole tumor volume as captured from ROI of the whole tumor on every slice. As such, the purpose of this study was to compare the correlation of Total Lesion Diffusion (TLD) and Total Lesion Perfusion (TLP) of rectal tumors after treatment with the separate components of DCE tumor volume, DWI tumor volume, ADC and Ktrans, and also to see which metric is more reproducible between readers.
Materials and Methods
Patient selection
After institutional review board approval for this retrospective, HIPAA-compliant study and issuance of a waiver of informed consent, our institution’s PACS and electronic medical records were searched between the years 2007 to 2013 for patients who had biopsy-proven primary rectal adenocarcinoma that underwent long-course neoadjuvant chemoradiotherapy and underwent MRI examinations at the start and after the completion of neoadjuvant treatment including diffusion-weighted and/or dynamic contrast-enhanced sequences. All patients at our institution undergo contrast-enhanced static and dynamic (DCE-MRI) imaging routinely. All patients had surgical tumor resection performed at our institution.
This study expands upon a prior study by the authors in which we assessed the value of multiple MRI parameters in predicting response to treatment: the patients in the present study were selected with the same detailed search criteria and exclusions used in the prior study [11]. All patients underwent standard long-course chemoradiation with 50.4 Gy in 25–28 fractions and administration of 5- fluorouracil between the first and the second MRI, according to National Comprehensive Cancer Network (NCCN) guidelines [12].
MRI Methodology
MRI examinations were performed on different MRI scanners manufactured by GE Healthcare (Waukesha, WI, USA) at a field strength of 1.5 Tesla (n=20) or 3 Tesla (n=4) using a standardized MRI protocol that included standard high-resolution T2-weighted imaging in axial, sagittal, coronal and oblique orientation (TR: 4400–5000; TE: 90–110; echo train length: 12–24; slice thickness: 3–4 mm; interslice gap: 1 mm; FOV: 20 cm; matrix: 320 × 160; NEX: 2), an axial 2D diffusion-weighted sequence (single-shot spin-echo EPI sequence, b-values: 0 and 750–1000 s/mm2; TR: 1800–5550 ms; TE: 60–112 ms; slice thickness: 3–5 mm; interslice gap: 1 mm; FOV: 18–40 cm; matrix: 96–256 × 96–128; NEX: 3–6; mean acquisition time: 2.4 min, typically 20–30 slices) and a sagittal 3D DCE-MRI sequence (TR: 3.1–7.9 ms; TE: 0.9–4.2 ms; slice thickness: 4–10 mm; no interslice gap; FOV: 20–34 cm; matrix: 256–320 × 128–192; mean temporal resolution: 8.3 (5 – 11.5) s; 30 - 40 phases; mean acquisition time: 5.2 min, typically 12 locations and 40 slices per location). A bolus of Gd- DTPA (Magnevist, Bayer Schering, Germany) at a constant dose of 0.1 mmol/kg was power injected at the rate of 2 mL/s followed by a saline flush for all patients.
Image Analysis
Two readers with experience reading rectal MRI (reader 1: with 5 years, reader 2: with 10 years of experience) independently assessed each pre- and post-treatment MRI, blinded to clinical and histopathological information. Each reader assessed the following metrics: (1) Tumor volume on diffusion-weighted imaging (measured on the high b-value diffusion-weighted sequence, using the formula Volume = sum of the area on each slice × [slice thickness + gap]) and (2) the ADC values for each voxel within the tumor volume were calculated using a monoexponential model. The voxel-wise ADC values were subsequently used to calculate volumetric slice ADC [13, 14, 15]; (3) Tumor volume from all dynamic contrast-enhanced (DCE) frames (using the formula Volume = sum of the area on each slice × [slice thickness + gap]) and the (4) transfer constant Ktrans of the generalized Tofts model [16], measured by drawing regions of interest on all slices containing tumor including all visible tumor on every slice at the phase of maximal enhancement [17]. A population-derived arterial input function and T1 reference times (1317ms at 1.5T and 1597ms at 3T) were used [18].
For volumetric tumor assessments (diffusion-weighted or dynamic contrast-enhanced MRI sequences), each radiologist drew a region of interest (ROI) encircling the entire tumor on every slice where it appeared, using the software Image J (version 1.47m, National Institutes of Health, Bethesda, MD, USA [19]). All assessments of DWI-MRI and DCE-MRI were done with reference to the T2-weighted images as needed. The data from these ROIs were then analyzed by in-house software written in Matlab (Matlab R2014b, The MathWorks, Inc, Natick, MA, USA) to calculate the volume, apparent diffusion coefficients (mean value for the whole tumor volume) and transfer constant Ktrans values, respectively. We defined two new composite metrics: (1) total lesion diffusion (TLD) defined as the volume ADCmean [sum of all volumetric slice ADC’s divided by the number of slices] multiplied by the DWI volume of the entire tumor [added from each slice volume] and (2) total lesion perfusion (TLP) defined as the volume Ktrans mean [sum of all volumetric slice Ktrans values divided by the number of slices] multiplied by the DCE volume of the entire tumor (Figure 1).
Fig. 1.
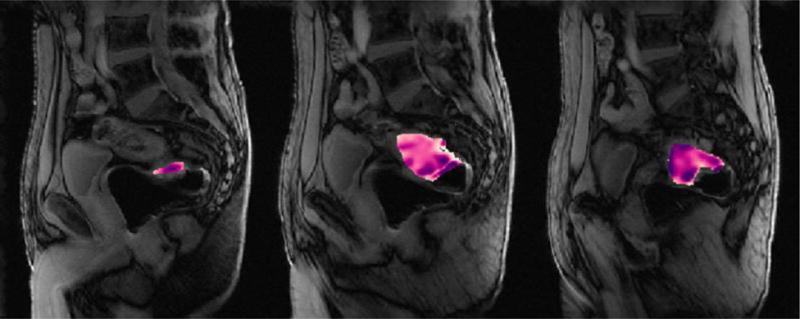
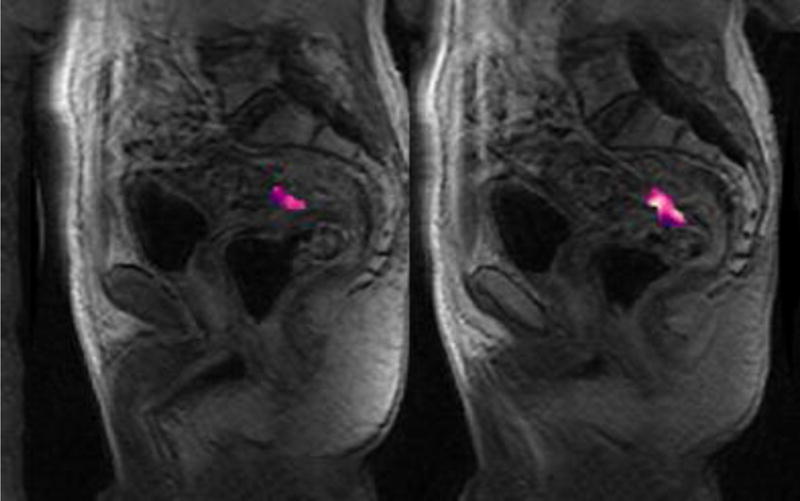
71-year-old male with rectal adenocarcinoma undergoing treatment with chemoradiotherapy prior to surgery.
Part A – Pre-treatment sagittal dynamic contrast-enhanced images depicting regions of interest (color) at all locations of tumor on contiguous slices. Average Ktrans = 1.151; Volume = 53187.01;
Part B - Post-treatment sagittal dynamic contrast-enhanced images depicting regions of interest (color) at all locations of tumor on contiguous slices. Average Ktrans = 0.367008; Volume =6275.177;
Histopathology
The surgical resection of tumors and the histopathological workup of the specimens were performed as previously described [11]. In brief, each patient underwent either abdominoperineal resection (APR) or low anterior resection (LAR) as performed by our colorectal surgeons, all of whom had specialty training and were certified in the performance of total mesorectal excision (TME). Tumors were resected 6–8 weeks following the end of chemoradiation per NCCN guidelines. Routine histopathological assessment was done, which included the evaluation of surgical margins, tumor type and differentiation, involvement of the perineural (PN) or lymphovascular (LV) space by tumor, T stage, and N stage (AJCC 7th edition). In addition, the percent tumor response was estimated by assessing the amount of fibrosis and inflammatory tissue in relation to the amount of residual viable carcinoma in the lesion [11, 20, 21].
Statistical analysis
To analyze agreement between the two readers, the concordance correlation coefficient was used [22]. This coefficient combines measures of both precision and accuracy to determine how far the observed data deviate from the line of perfect concordance (i.e., the line at 45 degrees on a square scatterplot). The closer the coefficient is to 1, the better the agreement between the two readers for that imaging parameter. Spearman’s rank correlation was used to analyze the correlation of the imaging parameters with percent tumor response. A test with a p-value < 0.05 was considered statistically significant. R version 3.1.1 was used for all analyses, including the epiR and irr packages.
Results
Patient Characteristics
Twenty-four patients had a mean age of 57 (range: 37–86) years; 19 were male and 5 were female. The pre-treatment clinical stage was cT2 in 3 patients (all were, as well, cN+), T3 in 17 patients and T4 in 4 patients. Histopathological tumor stage after resection was ypT0 in 4 (percentage tumor regression %TR: 100%), yp T1 in 2 (%TR: 80–90%), ypT2 in 7 (all N0, %TR: 30–95%), ypT3N0 in 4 (%TR: 60–95%) and ypT3N1 in 7 (%TR: 25–99%) patients.
Median values for each MRI parameter on pre- and post-treatment MRI are given in Table 1, as are the median absolute changes in the parameters from pre- to post-treatment MRI.
Table 1.
Median (range) for MRI parameters in pre- and post-treatment measurements, including the absolute change through therapy (units: DWI volume, mm3; ADC, ×10−3 mm2/s; DCE volume, mm3; Ktrans, min−1).
| Reader 1 | Reader 2 | |||||
|---|---|---|---|---|---|---|
|
|
||||||
| Pre-treatment | Post-treatment | Change | Pre-treatment | Post-treatment | Change | |
| TLP (n=24) | 23540 | 2314 | −17330 | 8086 | 1645 | −5298 |
| DCE vol | 22640 | 350 | −19940 | 9075 | 4240 | −6407 |
| Ktrans | 0.94 (0.11, 3.16) | 0.81 (0.14, 2.14) | −0.04 (−1.80, 1.02) | 0.80 (0.19, 1.80) | 0.42 (0.10, 1.99) | −0.23 (−1.56, 0.75) |
| TLD (n=21*) | 36.24 | 6.39 | −24.22 | 59.07 | 10.96 | −42.69 |
| DWI vol | 45890 | 8443 | −28160 | 66160 | 10520 | −50590 |
| ADC* | 0.94 (0.33, 1.47) | 0.98 (0.44, 1.85) | 0.12 (−0.78, 0.68) | 1.02(0.29, 1.48) | 0.92 (0.43, 1.69) | 0.10 (−1.00, 1.12) |
Total lesion perfusion (TLP) = DCE volume × Ktrans
Total lesion diffusion (TLD) = DWI × ADC
Change = post-pre
Note: 3 patients missing DWI volume and ADC for both pre and post time points
x 10−3mm2/s
Inter-reader agreement (Table 2, Figure 2)
Table 2.
Inter-reader agreement. Values are Concordance Correlation Coefficient (95% CI) for all measured MRI parameters in pre- and post-therapeutic assessment.
| Pre-treatment | Post treatment | |
|---|---|---|
| TLP (n=24) | 0.17 (0.02, 0.31) | 0.64 (0.34, 0.83) |
| DCE vol | 0.15 (0.05, 0.25) | 0.36 (0.05, 0.60) |
| Ktrans | 0.44 (0.09, 0.69) | 0.35 (−0.003, 0.62) |
| TLD (n=21*) | 0.88 (0.77, 0.94) | 0.30 (−0.001, 0.56) |
| DWI vol | 0.89 (0.80, 0.95) | 0.29 (−0.10, 0.61) |
| ADC | 0.97 (0.94, 0.99) | 0.77 (0.51, 0.90) |
Fig. 2.
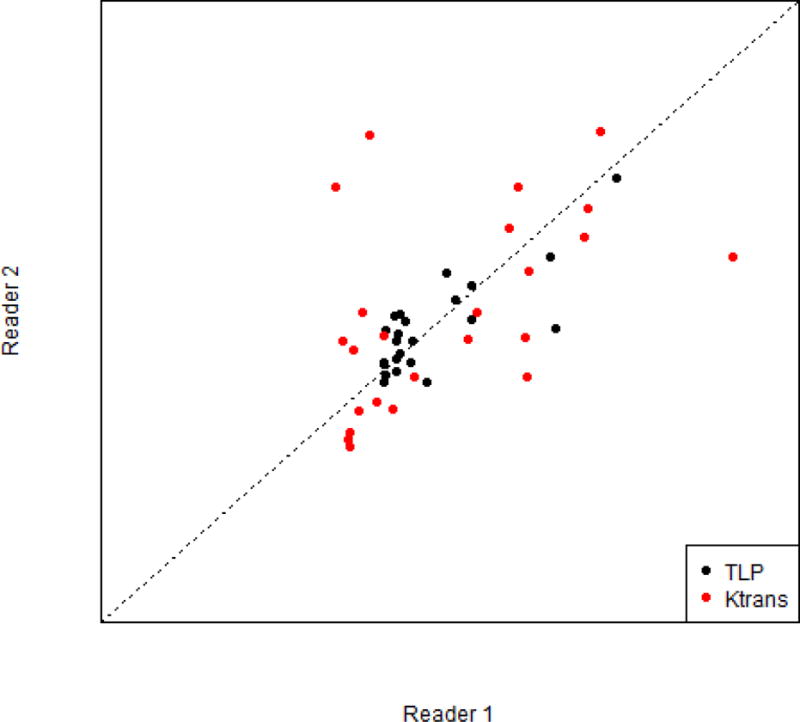
Inter-reader agreement (concordance correlation coefficient) comparison between Ktrans and total lesion perfusion (TLP) post-treatment.
Inter-reader agreement varied substantially among the MRI parameters assessed; in general, agreement was greater for pre-treatment values than for post-treatment values (Table 2). In the context of response assessment after therapy, the best inter-reader agreement was found for ADC (CCC: 0.77). Among DCE metrics, however, TLP had the highest inter-reader agreement (CCC: 0.64) when assessing absolute post-treatment values. For agreement prior to therapy, DWI based assessments were superior to DCE assessments and were quite high and comparable to one another.
Correlation between MRI parameters and histopathological tumor regression (Table 3)
Table 3.
Spearman’s Rank Correlations for absolute post-therapeutic values for MRI parameters with percentage tumor response in histopathology and 95% CI.
| Reader 1 | ||||||
| Pre-treatment values | Post-treatment values | Delta (post-pre) values | ||||
| Parameter | Rho | p-value | Rho | p-value | Rho | p-value |
| DWI volume | −0.25 (−0.61, 0.21) | 0.28 | −0.57(−0.79, −0.22) | 0.007 | −0.58 (−0.80, −0.23) | 0.009 |
| ADC | −0.09 (−0.50, 0.36) | 0.71 | −0.12 (−0.50, 0.30) | 0.60 | −0.08 (−0.34, 0.47) | 0.60 |
| DCE volume | −0.18 (−0.54, 0.24) | 0.40 | −0.55 (−0.78, −0.18) | 0.006 | 0.07 (−0.88, 0.49) | 0.86 |
| Ktrans | −0.18 (−0.54, 0.24) | 0.40 | −0.32 (−0.64, 0.09) | 0.12 | −0.36 (−0.04, 0.67) | 0.08 |
| TLP (n=24) | −0.28 (0.62, 0.14) | 0.18 | −0.36 (−0.66, 0.05) | 0.09 | 0.13 (−0.29, 0.51) | 0.55 |
| TLD (n=21*) | −0.25 (−0.62, 0.20) | 0.28 | −0.55 (−0.80, −0.16) | 0.009 | 0.14 (−0.31, −0.54) | 0.56 |
|
| ||||||
| Reader 2 | ||||||
| Pre-treatment values | Post-treatment values | Delta (post-pre) values | ||||
| Parameter | Rho | p-value | Rho | p-value | Rho | p-value |
| DWI volume | −0.36 (−0.68, 0.09) | 0.11 | −0.45 (-0.72, −0.06) | 0.04 | −0.20 (−0.58, 0.25) | 0.39 |
| ADC | 0.03 (−0.40, 0.46) | 0.89 | 0.02 (−0.39, 0.42) | 0.94 | −0.06 (−0.48, 0.38) | 0.79 |
| DCE volume | −0.18(−0.55, 0.24) | 0.39 | −0.52 (−0.76, −0.15) | 0.009 | −0.02 (−0.42, 0.39) | 0.93 |
| Ktrans | 0.07 (−0.34, 0.46) | 0.75 | −0.28 (−0.61, 0.14) | 0.19 | 0.28 (−0.18, 0.63) | 0.19 |
| TLP (n=24) | −0.35 (−0.66, 0.06) | 0.09 | −0.32 (−0.64, 0.09) | 0.12 | −0.02 (−0.39, 0.42) | 0.94 |
| TLD (n=21*) | −0.35 (−0.68, 0.10 | 0.12 | −0.59 (−0.81, −0.21) | 0.005 | 0.24 (−0.21, 0.61) | 0.29 |
Note: 3 patients missing DWI volume and ADC for both pre and post time points.
Among DCE-MRI metrics, post treatment DCE volumetry showed moderate correlation with histopathological tumor regression for both readers (Spearman’s rho = −0.55 and −0.52, p =0.006 and 0.009). Of note, the new metric TLP showed similar correlation at post-treatment with tumor regression compared to Ktrans for both readers (reader 1; −0.36 vs −0.32 and reader 2 −0.32 vs. −0.28).
Among DWI-MRI metrics, the new metric TLD showed comparable strengths of correlations at post-treatment to DWI volumetry in correlation with tumor response (reader 1 Spearman’s rho = −0.55 vs −0.57, [p = 0.009 and 0.007] and reader 2 −0.59 vs. −0.45, [p = 0.005 and 0.04]), and these were stronger correlations than between ADC and response.
Changes in TLD and TLP due to therapy
Pre-treatment metrics of tumor showed weak correlations with %TR, none of which were significant. The three strongest were TLP, TLD and DWI volume (Spearman’s rho −0.28, −0.25 and −0.25 respectively). Changes between pre- and post-treatment metrics indicated only one significant parameter, DWI volume (Spearman’s rho −0.58), with most other parameters revealing weak correlations with %TR.
Discussion
In our study, the new composite metric called total lesion diffusion (TLD), performed as well or better than DWI and DCE volumetry in terms of correlation of histopathological tumor response after chemoradiotherapy in patients with rectal cancer. For one reader, TLD had a stronger correlation with response and for the other reader it was similar to DWI and DCE volumetry. In addition, TLD inter-reader agreement was comparable to DWI and DCE volumetry pre- and post treatment. Although total lesion perfusion (TLP) achieved comparable inter-reader agreement to pretreatment DCE-volumetry and superior inter-reader agreement to post treatment DCE-volumetry and post treatment Ktrans, the correlation with tumor response was weak. As we have noted in a companion publication [11]; the purely volumetric tumor assessments on post-therapeutic DWI and DCE MRI sequences were found to be significantly associated with percentage tumor regression on histopathology for both readers.
Quantitating diffusion of the entire volume of a lesion post-chemoradiotherapy, as expressed by TLD, although a new metric, is not a new concept. Up until now either the volume of DWI or the apparent diffusion coefficient of lesions has been investigated and been shown to have some utility [1, 5, 6, 13, 14]. In this study we have simply chosen to explore this composite measure for global diffusion assessment and compare it with its parent metrics. For one reader, the incorporation of ADC information to DWI volume information (TLD) did not affect tumor response correlation, while for another reader it improved the strength of the association. This suggests that there is some added value in combining the strengths of morphologic assessment of tumor extent with actual quantitation of tumor biology. Although it would be comforting to be able to offer a simplistic explanation as it has been applied in FDG-PET scanning metrics for the superiority of total lesion glycolysis compared with SUV (“An overestimation of tumor volume will be associated with an underestimation of SUV because of inclusion of non-tumor tissue in the tumor volume estimate. The errors… will tend to cancel each other out”) [9], it is too soon to speculate on this in this small series. It is of interest that the two components of TLD trend in the opposite direction namely, volume decreases and ADC increases with successful response. Unlike the general principle that surrounding non-tumor tissue has a higher or lower ADC, we suspect it may vary for the particular tissue and for the particular degree of necrosis in the tumor.
In contradistinction, total lesion perfusion “lost” significant association with tumor response when Ktrans was multiplied by the remaining tumor volume (TLP). Although it was shown that DCE-volumetry may be a viable metric for response assessment in our companion results published on this data set [11], it’s possible that the potential over- or under- inclusion of tissue when drawing the regions of interest happened more often since the conspicuity of (perfusing) tumor, in our experience, is lower compared with the conspicuity of tumor as displayed at DWI-MRI. This is due to the lower spatial resolution of the spoiled GRE sequence (in-plane voxel resolution =0.83–1.3mm) as well as due to the decreased contrast resolution with adjacent normal rectal wall, in particular due to possible increased permeability from radiation treatment [23]. The contrast resolution in DWI-MRI is a bimodal display (white-black for DWI or black-white for ADC), possibly making it easier to perceive borders than on the grey-scale appearance of the DCE-MRI type image.
To our knowledge, total lesion diffusion and total lesion perfusion have not yet been reported, and represent new and incremental information regarding rectal cancer response assessment. Since TLD performed well in this study and both TLD and TLP demonstrated reproducibility across the two readers, the authors believe that these parameters would be of interest in future prospective investigations. The parallels between total diffusivity and total perfusion in a lesion with total lesion glycolysis are evident: each of these composite measures assesses a unique biological process in an entire tumor, albeit the processes are different, and each of these measures accounts for size and function of the given lesion. In fact, a prior study from our institution correlated total diffusivity of a lesion and TLG during FDG-PET in a population of rectal cancer patients. First proposed by Gu J. et al. in 2011, our TLD was called the “total diffusion index” and was shown to have a significant positive correlation with TLG in 33 patients (Pearson’s r=0.634 with p<0.001) [24]. That study did not, however, attempt to correlate those metrics with tumor response.
The clinical implications of our study relate to the challenge radiologists have when trying to assess the response of rectal cancer to neoadjuvant treatment, specifically chemoradiation. Non-volumetric, pure morphologic response quantitation remains difficult on routine T2-weighted rectal MRI, in part due to scarring which may still contain tumor [25, 26]. Some success and reproducibility is claimed in studies performed by the Pelican Foundation using mrTRG, a system which correlates MR degrees of fibrosis with histopathological degrees of fibrosis similar to the tumor regression schemes of Dworak and Mandard [27–29].
Quantitative methods based on pharmacokinetic modeling are also limited and show controversial results in the available literature [30]. A similar heterogeneity in study results can also be found when investigating the use of pharmacokinetic parameters of dynamic contrast-enhanced MRI (e.g., Ktrans) and their usefulness in response assessment, with some authors reporting associations between post-treatment values for Ktrans and pCR status [17], while others cannot find the same correlation [31]. Therefore, given the varying strengths and limitations of each of these broad strategies to evaluate tumor response, effectively size and function, the future incorporation of combined or composite metrics as investigated here may offer some advantage. Further investigations in larger populations using more standardized DCE-MRI sequences with uniform temporal resolutions and more uniform DWI-MRI sequences with uniform b-value quantities and ranges may well strengthen the robustness of these composite measures. Despite the variations in underlying exam parameters in this retrospective study, the positive associations still found here are encouraging.
Our study is limited due to the small number of patients included. Only 4 patients had complete pathological response, a clinically significant cohort of interest but in this investigation, too small to allow for more detailed statistical subanalysis. Inter-reader agreement for DCE volumes was lower than expected, possibly due to the effects of radiation and/or our relatively low temporal resolution. Finally, due to our retrospective study design, minor deviations in MRI protocols could not be accounted for and might have influenced our results.
In conclusion, our study demonstrated that total lesion diffusion (TLD) on post-therapeutic examinations showed moderate correlation with histopathological tumor regression and comparably with both DWI and DCE volumetry. We therefore encourage further study of these new parameters in future prospective studies to assess their value in larger samples, based on these preliminary results.
Acknowledgments
We would like to thank our editor, Ada Muellner, MS, for her help with manuscript preparation.
Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Number R25CA020449. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This research was conducted under a Waiver of authorization by the Institutional Review Board of MSKCC. Patient consent was deemed unnecessary for this retrospective research.
Funding: Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Number R25CA020449. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
No disclosures to make.
Authors A–E declare that they have no conflict of interest.
Investigation of Whole Lesion Tumor Biology in Rectal Cancer MRI using Newly Defined Metrics: Total Lesion Diffusion and Total Lesion Perfusion.
Compliance with Ethical Standards
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent: The need for informed consent was deemed unnecessary by the Memorial Sloan Kettering IRB and was thus waived for this retrospective study.
References
- 1.van der Paardt MP, Zagers MB, Beets-Tan RGH, Stoker J, Bipat S. Patients Who Undergo Preoperative Chemoradiotherapy for Locally Advanced Rectal Cancer Restaged by Using Diagnostic MR Imaging: A Systematic Review and Meta-Analysis. Radiology. 2013;269(1):101–112. doi: 10.1148/radiol.13122833. [DOI] [PubMed] [Google Scholar]
- 2.Lambregts DM, Beets GL, Maas M, et al. Tumor ADC measurements in rectal cancer: effect of ROI methods on ADC values and interobserver variability. Eur Radiol. 2011 Dec;21(12):2567–74. doi: 10.1007/s00330-011-2220-5. Epub 2011 Aug 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gollub MJ, Tong T, Weiser M, Zheng J, Gonen M, Zakian K. Limited accuracy of DCE-MRI in identification of pathological complete responders after chemoradiotherapy treatment for rectal cancer. European Radiology. 2016 doi: 10.1007/s00330-016-4493-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hötker AM, Schmidtmann I, Oberholzer K, Düber C. Dynamic contrast enhanced-MRI in rectal cancer: Inter- and intraobserver reproducibility and the effect of slice selection on pharmacokinetic analysis. J Magn Reson Imaging. 2014 Sep;40(3):715–22. doi: 10.1002/jmri.24385. Epub 2013 Oct 11. [DOI] [PubMed] [Google Scholar]
- 5.Intven M, Monninkhof EM, Reerink O, Philippens ME. Combined T2w volumetry, DW-MRI and DCE-MRI for response assessment after neo-adjuvant chemoradiation in locally advanced rectal cancer. Acta Oncol. 2015 Nov;54(10):1729–36. doi: 10.3109/0284186X.2015.1037010. Epub 2015 Apr 27. [DOI] [PubMed] [Google Scholar]
- 6.Curvo-Semedo L, Lambregts DM, Maas M, et al. Rectal cancer: assessment of complete response to preoperative combined radiation therapy with chemotherapy–conventional MR volumetry versus diffusion- weighted MR imaging. Radiology. 2011;260(3):734–743. doi: 10.1148/radiol.11102467. [DOI] [PubMed] [Google Scholar]
- 7.Kim SH, Lee JM, Hong SH, et al. Locally advanced rectal cancer: added value of diffusion-weighted MR imaging in the evaluation of tumor response to neoadjuvant chemo- and radiation therapy. Radiology. 2009;253(1):116–125. doi: 10.1148/radiol.2532090027. [DOI] [PubMed] [Google Scholar]
- 8.Larson SM, Erdi Y, Akhurst T, Mazumdar M, et al. Tumor Treatment Response Based on Visual and Quantitative Changes in Global Tumor Glycolysis Using PET-FDG Imaging: The Visual Response Score and the Change in Total Lesion Glycolysis. Clin Pos Imag. 1999;2:159–171. doi: 10.1016/s1095-0397(99)00016-3. [DOI] [PubMed] [Google Scholar]
- 9.Akhurst T, Ng V, Larson SM, O’Donoghue JA, O’Neel J, Erdi Y, Divgi CR. Tumor Burden Assessment with Positron Emission Tomography with [18-F] 2-fluoro 2-deoxyglucose (FDG PET) Modeled in Metastatic Renal Cell Cancer. Clin Pos Imag. 2000;3:57–65. doi: 10.1016/s1095-0397(00)00041-8. [DOI] [PubMed] [Google Scholar]
- 10.Guillem JG, Puig-La Calle J, Jr, Akhurst T, et al. Prospective assessment of primary rectal cancer response to preoperative radiation and chemotherapy using 18-fluorodeoxyglucose positron emission tomography. Dis Colon Rectum. 2000 Jan;43(1):18–24. doi: 10.1007/BF02237238. [DOI] [PubMed] [Google Scholar]
- 11.Hötker AM, Tarlinton L, Mazaheri Y, et al. Multiparametric MRI in the assessment of response of rectal cancer to neoadjuvant chemoradiotherapy: A comparison of morphological, volumetric and functional MRI parameters. Eur Radiol. 2016 Dec;12(26):4303–4312. doi: 10.1007/s00330-016-4283-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benson AB, Bekaii-Saab T, Chan E, et al. Rectal Cancer. J Natl Compr Cancer Netw. 2012;10(12):1528–1564. doi: 10.6004/jnccn.2012.0158. [DOI] [PubMed] [Google Scholar]
- 13.Curvo-Semedo L, Lambregts DM, Maas M, Beets GL, Caseiro-Alves F, Beets-Tan RG. Diffusion-weighted MRI in rectal cancer: apparent diffusion coefficient as a potential noninvasive marker of tumor aggressiveness. J Magn Reson Imaging. 2012;35(6):1365–1371. doi: 10.1002/jmri.23589. [DOI] [PubMed] [Google Scholar]
- 14.Curvo-Semedo L, Lambregts DM, Maas M, Thywissen T, Mehsen RT, Lammering G, et al. Rectal cancer: assessment of complete response to preoperative combined radiation therapy with chemotherapy–conventional MR volumetry versus diffusion-weighted MR imaging. Radiology. 2011;260(3):734–743. doi: 10.1148/radiol.11102467. [DOI] [PubMed] [Google Scholar]
- 15.Kim SH, Lee JY, Lee JM, Han JK, Choi BI. Apparent diffusion coefficient for evaluating tumor response to neoadjuvant chemoradiation therapy for locally advanced rectal cancer. Eur Radiol. 2011;21(5):987–995. doi: 10.1007/s00330-010-1989-y. [DOI] [PubMed] [Google Scholar]
- 16.Tofts PS, Brix G, Buckley DL, et al. Estimating kinetic parameters from dynamic contrast-enhanced T1-weighted MRI of a diffusable tracer: standardized quantities and symbols. J Magn Reson Imaging. 1999;10(3):223–232. doi: 10.1002/(sici)1522-2586(199909)10:3<223::aid-jmri2>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 17.Gollub MJ, Gultekin DH, Akin O, et al. Dynamic contrast enhanced-MRI for the detection of pathological complete response to neoadjuvant chemotherapy for locally advanced rectal cancer. Eur Radiol. 2012;22(4):821–831. doi: 10.1007/s00330-011-2321-1. [DOI] [PubMed] [Google Scholar]
- 18.Stanisz GJ, Odrobina EE, Pun J, et al. T1, T2 relaxation and magnetization transfer in tissue at 3T. Magnetic resonance in medicine: official journal of the Society of Magnetic Resonance in Medicine/Society of Magnetic Resonance in Medicine. 2005;54(3):507–512. doi: 10.1002/mrm.20605. [DOI] [PubMed] [Google Scholar]
- 19.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9(7):671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shia J, Guillem JG, Moore HG, et al. Patterns of morphologic alteration in residual rectal carcinoma following preoperative chemoradiation and their association with long-term outcome. Am J Surg Pathol. 2004;28(2):215–223. doi: 10.1097/00000478-200402000-00009. [DOI] [PubMed] [Google Scholar]
- 21.Trakarnsanga A, Gönen M, Shia J, et al. Comparison of tumor regression grade systems for locally advanced rectal cancer after multimodality treatment. J Natl Cancer Inst. 2014;106(10) doi: 10.1093/jnci/dju248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin LI. A concordance correlation coefficient to evaluate reproducibility. Biometrics. 1989;45(1):255–268. [PubMed] [Google Scholar]
- 23.Harvey C, Dooher A, Morgan J, et al. Imaging of tumor therapy response by dynamic CT. Eur J Radiol. 1999;30:221–226. doi: 10.1016/s0720-048x(99)00015-7. [DOI] [PubMed] [Google Scholar]
- 24.Gu J, Khong P-L, Wang S, Chan Q, Law W, Zhang J. Quantitative Assessment of Diffusion-Weighted MR Imaging in Patients with Primary Rectal Cancer: Correlation with FDG-PET/CT. Mol Imaging Biol. 2011;13:1020–1028. doi: 10.1007/s11307-010-0433-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barbaro B, Fiorucci C, Tebala C, et al. Locally advanced rectal cancer: MR imaging in prediction of response after preoperative chemotherapy and radiation therapy. Radiology. 2009;250(3):730–739. doi: 10.1148/radiol.2503080310. [DOI] [PubMed] [Google Scholar]
- 26.Kuo LJ, Chern MC, Tsou MH, et al. Interpretation of magnetic resonance imaging for locally advanced rectal carcinoma after preoperative chemoradiation therapy. Dis Colon Rectum. 2005;48(1):23–28. doi: 10.1007/s10350-004-0787-5. [DOI] [PubMed] [Google Scholar]
- 27.Dworak O, Keilholz L, Hoffmann A. Pathological features of rectal cancer after preoperative radiochemotherapy. Int J Colorectal Dis. 1997;12(1):19–23. doi: 10.1007/s003840050072. [DOI] [PubMed] [Google Scholar]
- 28.Mandard AM, Dalibard F, Mandard JC, et al. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations Cancer. 1994 Jun 1;73(11):2680–6. doi: 10.1002/1097-0142(19940601)73:11<2680::aid-cncr2820731105>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 29.Patel UB, Taylor F, et al. Magnetic resonance imaging-detected tumor response for locally advanced rectal cancer predicts survival outcomes: MERCURY experience. Clin Oncol. 2011 Oct 1;29(28):3753–60. doi: 10.1200/JCO.2011.34.9068. Epub 2011 Aug 29. [DOI] [PubMed] [Google Scholar]
- 30.Hötker AM, Garcia-Aguilar J, Gollub MJ. Multiparametric MRI of rectal cancer in the assessment of response to therapy: a systematic review. Dis Colon Rectum. 2014;57(6):790–799. doi: 10.1097/DCR.0000000000000127. [DOI] [PubMed] [Google Scholar]
- 31.Lim JS, Kim D, Baek SE, et al. Perfusion MRI for the prediction of treatment response after preoperative chemoradiotherapy in locally advanced rectal cancer. Eur Radiol. 2012;22(8):1693–1700. doi: 10.1007/s00330-012-2416-3. [DOI] [PubMed] [Google Scholar]


