Abstract
Background
Reduced expression of the serotonin transporter (SERT) promotes anxiety and cocaine intake in both humans and rats. We tested the hypothesis that median raphe nucleus (MRN) and dorsal raphe nucleus (DRN) serotonergic projections differentially mediate these phenotypes.
Methods
We used virally-mediated RNA interference to locally down-regulate SERT expression and compared the results to constitutive SERT knockout. Rats were allowed either short access (ShA, 1 h) or long access (LgA, 6 h) to cocaine self-administration to model moderate versus compulsive-like cocaine taking.
Results
SERT knockdown in the MRN increased cocaine intake selectively under ShA conditions and, like ShA cocaine self-administration, reduced CRF immunodensity in the paraventricular nucleus of the hypothalamus. In contrast, SERT knockdown in the DRN increased cocaine intake selectively under LgA conditions and, like LgA cocaine self-administration, reduced CRF immunodensity in the central nucleus of the amygdala. SERT knockdown in the MRN or DRN produced anxiety-like behavior, as did withdrawal from ShA or LgA cocaine self-administration. The phenotype of SERT knockout rats was a summation of the phenotypes generated by MRN- and DRN-specific SERT knockdown.
Conclusions
Our results highlight a differential role of serotonergic projections arising from the MRN and DRN in the regulation of cocaine intake. We propose that a cocaine-induced shift from MRN-driven serotonergic control of CRF levels in the hypothalamus to DRN-driven serotonergic control of CRF levels in the amygdala may contribute to the transition from moderate to compulsive intake of cocaine.
Keywords: serotonin transporter (SERT), knockout, knockdown and gene silencing, dorsal and median raphe nuclei, corticotropin-releasing factor (CRF), anxiety-related behavior, short and long access to cocaine self-administration
Introduction
In humans, reduced SERT expression and function (1–4) predicts an increased presentation of negative emotional states (1, 5) and increased intake of psychostimulants (6–8). These clinical findings are consistent with studies performed in SERT knockout (KO) rats showing that genetic inactivation of SERT increases not only anxiety-related behavior (5, 9), but also intravenous self-administration of ecstasy and cocaine (10, 11). In addition, cocaine increases locomotor activity and conditioned place preference more strongly in SERT KO than wild-type (WT) animals (10, 12).
Cocaine is known to inhibit the neuronal re-uptake of serotonin by blocking SERT. In the present study, we sought to determine the respective contribution of serotonergic projections from the median raphe nucleus (MRN) and the dorsal raphe nucleus (DRN) to cocaine intake behavior using virally-mediated local SERT knockdown (KD), and compare the results with constitutive SERT deletion in KO rats. Animals were exposed to either short or long access (ShA or LgA) cocaine self-administration (13) in order to evaluate the contribution of MRN and DRN serotonergic neurons to the moderate and compulsive intake of this drug (14). Extended access to cocaine self-administration recapitulates key elements of substance use disorders diagnosis, including gradual escalation of drug intake, enhanced motivation for the drug despite increased cost or negative consequences, and increased sensitivity to craving (for review: 15).
A second purpose of the present study was to determine how SERT deficiency may drive an increase in cocaine intake. It has been suggested that the increased psychostimulant intake observed in animals with a reduction of SERT is due to serotonin-induced changes in the synaptic levels of dopamine and/or noradrenaline (11). However, basal and cocaine-induced levels of these two catecholamines do not differ between SERT KO and WT animals (16, 17). We therefore hypothesize that the increased intake of cocaine in SERT-deficient subjects is due to serotonin-induced changes in non-catecholaminergic systems (16). Numerous studies have shown that corticotropin-releasing factor (CRF) signaling can produce anxiety-related behavior (18–22), which, in turn, may drive the escalation of cocaine intake and stress-induced reinstatement of drug seeking behavior (for review: 23, 24–26). CRF is highly expressed in both the central nucleus of the amygdala (CeA) and the paraventricular nucleus of the hypothalamus (PVN) (27–30), and both regions are innervated by serotonergic neurons (31–33). Moreover, serotonin and various serotonergic modulators can alter the activity of CRF-expressing cells (34–39). These data suggest that CRF changes in the CeA and PVN may contribute to the increase in anxiety-related behavior and enhanced cocaine intake observed in humans expressing low levels of SERT. To further explore this possibility, we compared the effect of local and constitutive SERT down-regulation on baseline and cocaine withdrawal-induced CRF levels in the CeA and PVN, as well as on anxiety-like behavior in cocaine-naïve and cocaine-withdrawn rats.
Methods and Materials
A summary of the experimental design can be found in Supplementary Figure S1. Male Wistar rats (weighing 250–300 g) were first implanted with a catheter into the jugular vein (40) and were then trained to self-administer cocaine (0.5 mg/kg/infusion) under a fixed ratio 1 (FR1) schedule of reinforcement in daily 1-h sessions (41). An additional cocaine-naïve group of rats also underwent intravenous catheterization, but was not exposed to cocaine (41). For local knockdown experiments (approach A in Supplementary Figure S1), rats were infused with adeno-associated viral (AAV) vectors serotype 2, encoding either a short-hairpin RNA (shRNA) sequence designed to silence the expression of SERT (shSERT1 or shSERT2) or a scrambled control sequence (shSCR) into the MRN or DRN (Supplementary Figure S2). AAV vector serotype 2 infusion typically resulted in transduction volumes not exceeding 1 mm3 (Supplementary Figure S3). Cocaine self-administration sessions were resumed 2 weeks after viral vector infusion in daily sessions of 1 h (ShA) or 6 h (LgA) (41). For constitutive KO experiments (approach B in Supplementary Figure S1), SERT KO rats (for details: 42, 43) and their WT counterparts were also trained to self-administer cocaine in 1-h sessions and were then split in ShA and LgA groups as well (41). For both approaches, once cocaine intake had successfully escalated under LgA conditions, rats were allowed to self-administer cocaine under a progressive ratio (PR) schedule of reinforcement in order to test the effects of reduced SERT expression on the motivation to work for cocaine (44, 45). On the following day, the rats were subjected to a regular ShA or LgA cocaine self-administration session, and another 24 h later, they were tested on the elevated plus-maze (EPM) to measure anxiety-related behavior (46). On the following day, rats were again subjected to a regular ShA or LgA cocaine self-administration session, and another 24 hours later, they were transcardially perfused with paraformaldehyde. Coronal brain sections containing the PVN, CeA, and bed nucleus of the stria terminalis (BNST) were then processed for CRF immunohistochemistry. Sections were incubated overnight in polyclonal rabbit CRF antiserum (1:2000, provided by Dr. Vale, 47), followed by a secondary antibody (Biotin-SP-conjugated donkey anti rabbit IgG, 1:200) for 60 min, and immunostaining was visualized using diaminobenzidine and H2O2 (for details: 48). Image-J (NIH) was used to measure the mean optical immunodensity in the brain regions of interest corrected for the optical immunodensity of the background. More details can be found in the Supplementary Information file.
Results
Effect of access duration on cocaine self-administration in control rats
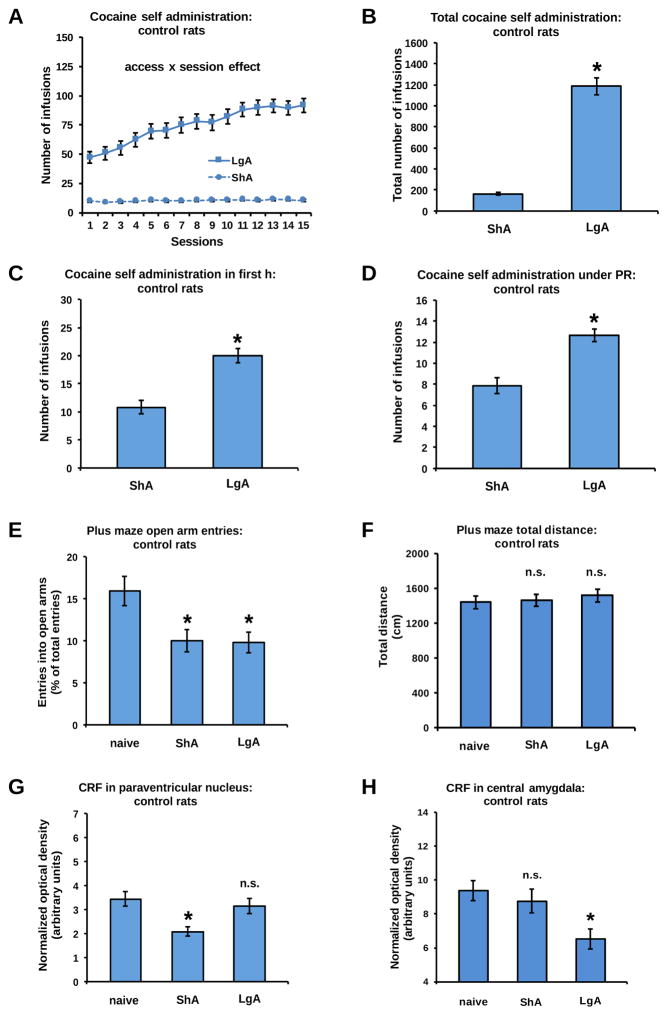
The cocaine intake of control rats was equivalent across the 3 cohorts of rats that were used in this study (see Supplementary Figure S4). As expected, analysis of the pooled data from these three control subgroups showed that ShA cocaine self-administration remained stable over the testing days whereas LgA cocaine self-administration resulted in an escalation of the daily intake of cocaine (Figure 1A: access x session interaction: F(14,1064)=27.72, P<0.001; session effect in LgA rats: P<0.001, session effect in ShA rats: n.s.). The cumulative cocaine intake was 7.5-fold larger in LgA than in ShA rats (Figure 1B: access effect: F(1,76)=170.89, P<0.001). LgA sessions also increased cocaine intake during the first hour (h) of the final self-administration session by 2-fold compared to the 1-h intake of ShA sessions (Figure 1C: access effect: F(1,76)=26.10, P<0.001), as well as the motivation to work for the drug under a progressive ratio (PR) schedule of reinforcement (Figure 1D: access effect: F(1,76)=26.88, P<0.001).
Figure 1.
In control rats, daily LgA to cocaine self-administration produced a gradual escalation of cocaine intake (A), reflected by a higher cumulative intake (B), a higher intake during the first h of the final self-administration session (C), and a higher intake during a PR schedule of reinforcement (D), when compared to daily ShA self-administration. Withdrawal from both ShA and LgA to cocaine reduced the number of open arm entries on the EPM (E), without affecting locomotor activity (F). ShA cocaine self-administration reduced CRF immunodensity levels in the PVN (G), but not in the CeA (H). In contrast, LgA cocaine self-administration reduced CRF in the CeA (H), but not the PVN (G). *Significant change (P<0.05) vs ShA or naive, n.s: no significant change. Given their similar total intake of cocaine (see supplementary Figure S4), data of SERT WT and shSCR rats were pooled and each graph shows the mean ± S.E.M. of this combined group of animals. Naive (blue): n=37 (SERT WT: n=13, MRN shSCR: n=9, DRN shSCR: n=15), ShA (blue): n=41 (SERT WT: n=14, MRN shSCR: n=13, DRN shSCR: n=14), LgA (blue): n=37 (SERT WT: n=10, MRN shSCR: n=15, DRN shSCR: n=12).
Effect of cocaine self-administration on anxiety-like behavior and CRF levels in control rats
To analyze the withdrawal-induced changes in CRF and anxiety levels, a cocaine-naive control group was included (see Supplementary Methods and Materials). Twenty-four h after self-administration, open arm entries on the EPM were reduced after both ShA and LgA of cocaine (Figure 1E: access effect: F(2,112)=6.18, P<0.01; naive vs ShA: P<0.01, naive vs LgA: P<0.01, LgA vs ShA: n.s.) while the total distance traveled on the maze was not affected (Figure 1F: access effect: n.s.). Withdrawal from ShA, but not LgA, to cocaine significantly reduced CRF immunodensity levels in the PVN (Figure 1G: access effect: F(2,112)=7.23, P=0.001; naive vs ShA: P<0.001, naive vs LgA: n.s., LgA vs ShA: P<0.01) whereas withdrawal from LgA, but not ShA, of cocaine reduced CRF immunodensity levels in the CeA (Figure 1H: access effect: F(2,112)=4.08, P<0.05; naive vs ShA: n.s., naive vs LgA: P<0.01; LgA vs ShA: P<0.05) and the BNST (Supplementary Figure S5: access effect: F(2,34)=3.88, P<0.05; naive vs ShA: n.s., naive vs LgA: P<0.05; LgA vs ShA: P<0.05).
Validation of constitutive SERT KO and local SERT KD
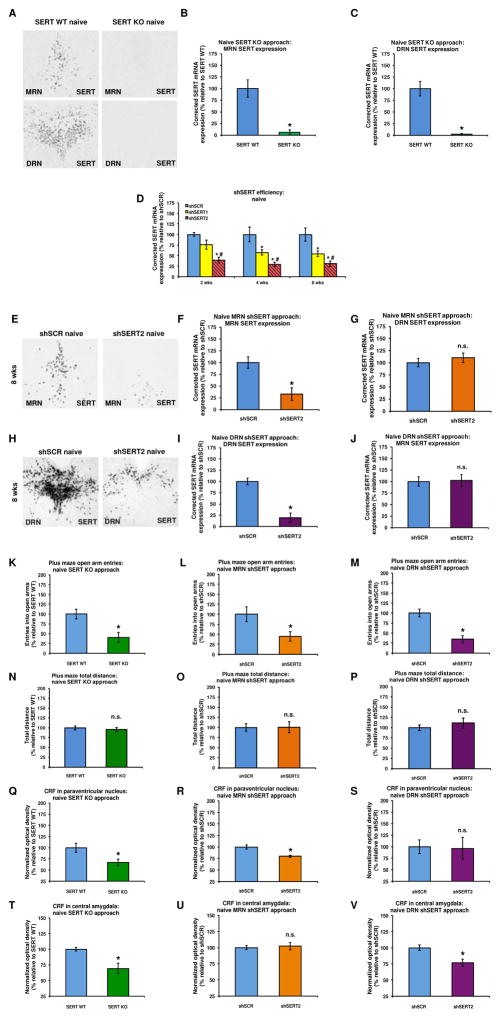
No SERT mRNA was expressed in the raphe nuclei of constitutive SERT KO rats (Figures 2A–C), consistent with the complete abolition of SERT binding throughout the brain of these rats (43). Intra-raphe infusion of a viral vector expressing either of two shRNA sequences (shSERT1 or shSERT2) directed against the SERT transcript resulted in a reduction of SERT expression in transduced neurons (Figure 2D: shRNA effect: F(2,42)=32.21, P<0.001) that reached statistical significance 4 weeks post-infusion for shSERT1 and 2 weeks post-infusion for shSERT2, compared to a non-targeting shRNA (shSCR). shSERT2 was more effective than shSERT1 in reducing local SERT expression (Figure 2D (LSD): shSCR vs shSERT1: P<0.001, shSCR vs shSERT2: P<0.001 and shSERT2 vs shSERT1: P<0.001). Eight weeks following intra-MRN injection of shSERT2, SERT expression levels in the MRN were reduced by ~75% (Figures 2E–F: shRNA effect: F(1,16)= 13.07, P<0.01), whereas SERT expression in the DRN was unaffected (Figure 2G: shRNA effect: n.s.). Eight weeks following intra-DRN injection of shSERT2, DRN SERT expression levels were reduced to a similar extent (Figures 2H–I: shRNA effect: F(1,28)=44.53, P<0.001), whereas SERT expression in the MRN was not affected (Figure 2J: shRNA effect: n.s.).
Figure 2.
In situ hybridization revealed no SERT mRNA expression in the MRN (A and B) and DRN (A and C) of SERT KO rats. Intra-raphe infusion of a viral vector encoding either of two shRNA sequences targeting SERT mRNA (shSERT1 or shSERT2) reduced the local expression of SERT mRNA (D). shSERT2 was more effective than shSERT1 in reducing local SERT expression. Eight weeks following intra-MRN injection of shSERT2, SERT expression levels in the MRN, but not DRN, were reduced (E, F and G). Eight weeks following intra-DRN injection of shSERT2, DRN, but not MRN, SERT expression levels were reduced to a similar extent (H, I and J). Both constitutive SERT KO and local SERT KD reduced the number of open arm entries on the EPM (K, L and M) without affecting locomotor activity (N, O and P) in cocaine-naïve rats. The increase in anxiety-related behavior in cocaine-naïve SERT KO rats (K) was accompanied by a decrease in CRF immunodensity in both the PVN (Q) and CeA (T). In contrast, the increase in anxiety-related behavior in cocaine naïve MRN shSERT rats (L) was accompanied by a decrease in CRF immunodensity in the PVN (R), but not the CeA (U), whereas the increase in anxiety-related behavior in cocaine-naïve DRN shSERT rats (M) was accompanied by a decrease in CRF immunodensity in the CeA (V), but not the PVN (S). All data are normalized to values obtained in SERT WT or shSCR control rats (see Supplementary Table S4 for absolute values of SERT expression and Supplementary Tables S5–S7 for absolute values of cocaine intake, EPM behavior and CRF levels). *Significant decrease (P<0.05) relative to SERT WT or shSCR, #significant difference (P<0.05) between shSERT2 and shSERT1, n.s: no difference relative to control. SERT WT (blue): n=13, SERT KO (green): n=10; MRN shSCR (blue): n=9, MRN shSERT2 (orange): n=9; DRN shSCR (blue): n=15, DRN shSERT2 (purple): n=15.
Effect of constitutive SERT KO and local SERT KD on anxiety-related behavior and CRF levels in cocaine-naïve rats
In the EPM, open arm entries were reduced in cocaine-naive SERT KO rats (Figure 2K: genotype effect: F(1,21)=10.90, P<0.01) as well as in cocaine-naive rats infused with shSERT2 in either the MRN (Figure 2L: shRNA effect: F(1,16)=6.12, P<0.05) or DRN (Figure 2M: shRNA effect: F(1,28)=24.54, P<0.001). Neither constitutive nor local SERT downregulation affected the total distance traveled (Figure 2N, genotype effect: n.s. and Figures 2O–P, shRNA effect: n.s.). Constitutive SERT KO decreased CRF immunodensity in both the PVN (Figure 2Q: genotype effect: F(1,21)=6.39, P<0.05) and CeA (Figure 2T: genotype effect: F(1,21)=14.32, P<0.01). MRN-specific SERT KD reduced CRF immunodensity in the PVN (Figure 2R: shRNA effect: F(1,16)=14.85, P=0.001), but not in the CeA (Figure 2U: shRNA effect: n.s.), whereas DRN-specific SERT KD reduced CRF immunodensity in the CeA (Figure 2V: shRNA effect: F(1,28)=11.25, P<0.01), but not in the PVN (Figure 2S: shRNA effect: n.s.). CRF immunodensity in the BNST was not affected by constitutive SERT KO (Supplementary Figure S5) and was therefore not examined in SERT KD rats. In MRN SERT KD animals, both open arm entries and CRF levels in the PVN, but not CeA, were positively correlated with SERT levels in the MRN, but not DRN (Supplementary Table S1). In contrast, in the DRN SERT KD animals, open arm entries and CRF levels in the CeA, but not PVN, were positively correlated with SERT levels in the DRN, but not MRN (Supplementary Table S1).
Effect of constitutive SERT KO on cocaine intake
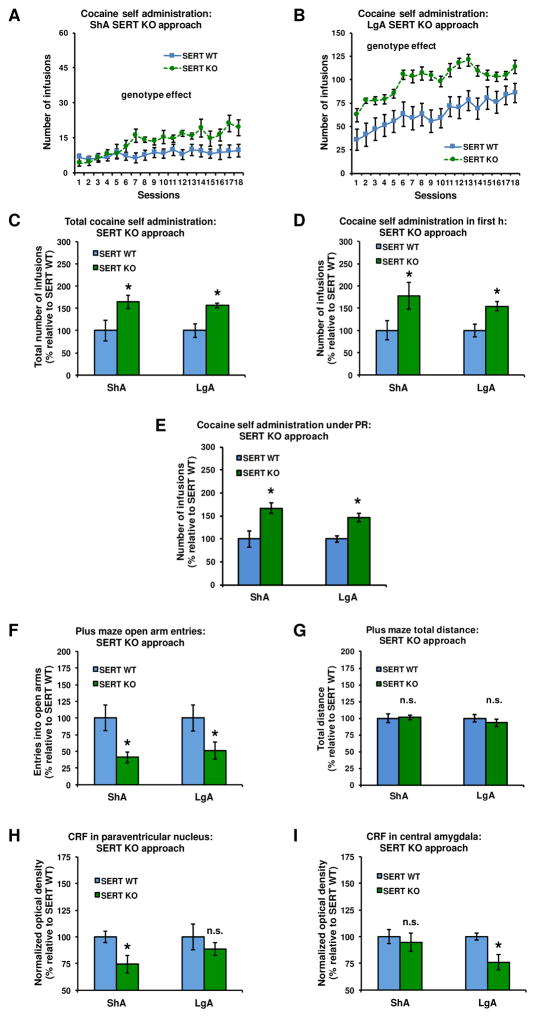
SERT constitutive KO increased the daily intake of cocaine in both ShA and LgA rats (Figures 3A–B: genotype effect: F(1,43)=19.38, P<0.001; genotype x access interaction: n.s.). This daily increase resulted in a larger cumulative intake of cocaine under both access conditions in SERT KO vs SERT WT rats (Figure 3C: genotype effect: F(1,43)=11.48, P<0.01, genotype x access interaction: n.s.). In addition, SERT constitutive KO increased the intake of cocaine during the first h of the final self-administration session (Figure 3D: genotype effect: F(1,43)=9.43, P<0.01, genotype x access interaction: n.s.), as well as under a PR schedule of reinforcement (Figure 3E: genotype effect: F(1,43)=18.13, P<0.001; genotype x access interaction: n.s.). Inactive lever responding was low and not affected by constitutive SERT KO (Supplementary Figure S6).
Figure 3.
Constitutive SERT KO increased daily cocaine intake (A–B), cumulative cocaine intake (C), intake during the first h of the final self-administration session (D), and intake during a PR schedule of reinforcement (E) in rats exposed to either ShA or LgA cocaine self-administration. SERT KO also reduced open arm entries, but not locomotor activity, on the EPM after both durations of cocaine access (F and G). The SERT KO-induced increase in ShA cocaine intake (A) was accompanied by a reduction of CRF immunodensity levels in the PVN (H), but not CeA (I). In contrast, the SERT KO-induced increase in LgA intake of cocaine (B) was accompanied by a reduction of CRF in the CeA (I), but not PVN (H) (see Supplementary Figure S8 for representative pictures). In panels C–I, data are normalized to values obtained in SERT WT counterparts (see Supplementary Table S5 for absolute values). *Significant change (P<0.05) vs SERT WT, n.s: no significant change. ShA: SERT WT (blue): n=14, SERT KO (green): n=12; LgA: SERT WT (blue): n=10, SERT KO (green): n=11.
Effect of constitutive SERT KO on cocaine-induced anxiety-related behavior and CRF levels
Twenty-four h after ShA or LgA self-administration, open-arm entries, but not the total distance traveled, on the EPM were significantly reduced in SERT KO vs SERT WT rats (Figure 3F: genotype effect: F(1,43)=11.19, P<0.01; genotype x access interaction: n.s; Figure 3G: genotype effect and genotype x access interaction: n.s.). Under ShA conditions, SERT constitutive KO decreased CRF immunodensity in the PVN (Figure 3H: genotype effect: F(1,43)=5.38, P<0.05; genotype x access interaction: F(1,43)=5.59, P<0.05; genotype effect in ShA rats: F(1,24)=6.95, P<0.05; genotype effect in LgA rats: n.s.), but not CeA (Figure 3I). In contrast, under LgA conditions, SERT constitutive KO decreased CRF immunodensity in the CeA (Figure 3I: genotype effect: F(1,43)=5.52, P<0.05; genotype x access interaction: F(1,43)=5.74, P<0.05; genotype effect in LgA rats: F(1,19)=8.97, P<0.01; genotype effect in ShA rats: n.s.), but not PVN (Figure 3H). The cumulative cocaine intake of SERT WT and KO rats was negatively correlated with both CRF immunodensity in the PVN under ShA conditions and CRF immunodensity in the CeA under LgA conditions (Supplementary Table S2). SERT constitutive KO did not affect CRF immunodensity in the BNST under either ShA or LgA conditions (Supplementary Figure S5).
Effect of MRN-specific SERT KD on cocaine intake
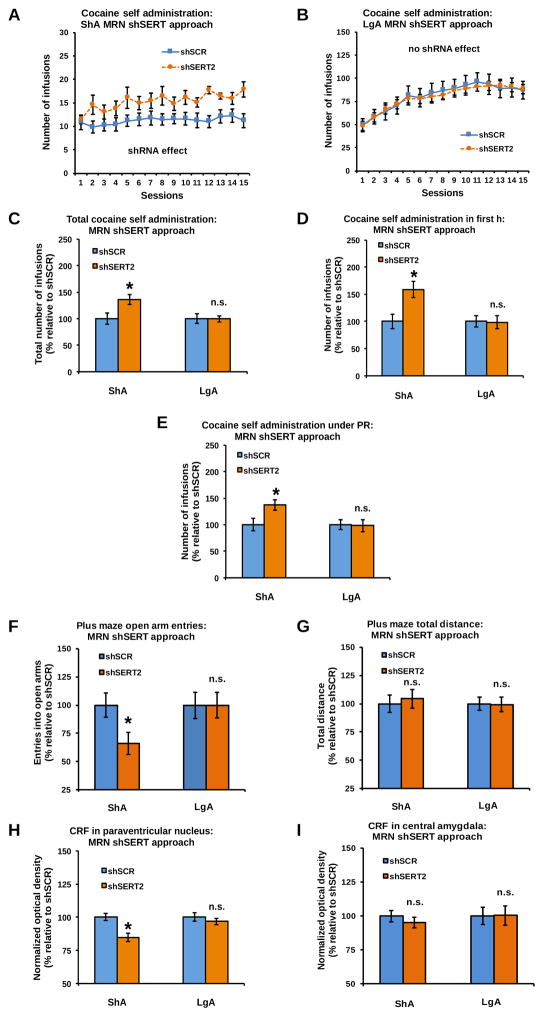
MRN-specific SERT KD increased the daily intake of cocaine in ShA, but not in LgA, rats (Figures 4A–B: shRNA x access interaction: F(1,50)=4.87, P<0.05; shRNA effect in ShA rats: F(1,24)=7.19, P<0.05; shRNA effect in LgA rats: n.s.). This increased daily self-administration resulted in a larger cumulative intake of cocaine in shSERT2 vs shSCR rats under ShA, but not LgA, conditions (Figure 4C: shRNA x access interaction: F(1,50)=9.93, P<0.01; shRNA effect in ShA rats: F(1,24)=7.18, P<0.05; shRNA effect in LgA rats: n.s.). MRN-specific SERT KD increased the first-h cocaine intake of ShA, but not LgA, rats (Figure 4D: shRNA x access interaction: F(1,50)=17.15, P<0.001; shRNA effect in ShA rats: F(1,24)=9.48, P<0.01; shRNA effect in LgA rats: n.s.). MRN-specific SERT KD also increased PR responding under ShA, but not LgA, conditions (Figure 4E: shRNA x access interaction: F(1,50)=10.57, P<0.01; shRNA effect in ShA rats: F(1,24)=6.42, P<0.05; shRNA effect in LgA rats: n.s.). Inactive lever responding was low and not affected by MRN-specific SERT KD (Supplementary Figure S6).
Figure 4.
SERT KD in the MRN increased daily cocaine intake (A–B), cumulative cocaine intake (C), intake during the first h of the final self-administration session (D), and intake during a PR schedule of reinforcement (E) in rats exposed to ShA, but not LgA, cocaine self-administration. MRN-specific SERT KD also reduced open arm entries, but not locomotor activity, on the EPM after ShA, but not LgA, to cocaine (F and G). The lack of shSERT2 effects on LgA cocaine intake (B) is accompanied by a lack of effect on CRF immunodensity in the PVN (H) and CeA (I) of LgA rats. In contrast, the shSERT2-induced increase in ShA cocaine intake (A) was accompanied by a reduction of CRF immunodensity in the PVN (H), but not CeA (I), of ShA rats (see Supplementary Figure S8 for representative pictures). In panels C–I, data are normalized to values obtained in shSCR counterparts (see Supplementary Table S6 for absolute values). *Significant change (P<0.05) vs shSCR, n.s: no significant change. ShA: shSCR (blue): n=13, shSERT2 (orange): n=13; LgA: shSCR (blue): n=15, shSERT2 (orange): n=13.
Effect of MRN-specific SERT KD on cocaine-induced anxiety-related behavior and CRF levels
MRN-specific SERT KD reduced open arm exploration on the EPM in ShA, but not LgA, rats (Figure 4F: shRNA x access interaction: F(1,50)=7.06, P<0.05; shRNA effect in ShA rats: F(1,24)=5.56, P<0.05; shRNA effect in LgA rats: n.s.), without affecting the total distance traveled (Figure 4G: shRNA effect and shRNA x access interaction: n.s.). MRN-specific SERT KD decreased CRF immunodensity in the PVN in ShA, but not LgA, rats (Figure 4H: shRNA x access interaction: F(1,50)=11.92, P<0.01; shRNA effect in ShA rats: F(1,24)=8.49, P<0.01; shRNA effect in LgA rats: n.s.). There was no effect of MRN-specific SERT KD on CRF immunodensity in the CeA (Figure 4I: shRNA effect and shRNA x access interaction: n.s.). The cumulative cocaine intake of MRN shSCR and shSERT2 rats was negatively correlated with CRF immunodensity in the PVN under ShA conditions (Supplementary Table S2).
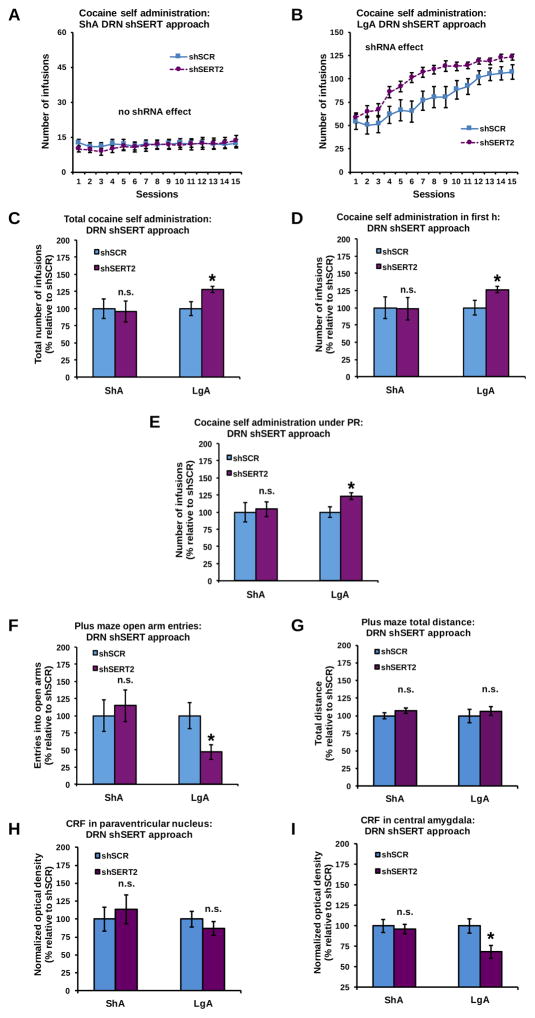
Effect of DRN-specific SERT KD on cocaine intake
DRN-specific SERT KD increased the daily intake of cocaine in LgA, but not in ShA, rats (Figures 5A–B: shRNA x access interaction: F(1,56)=4.13, P<0.05; shRNA effect in LgA rats: F(1,28)=7.29, P<0.05; shRNA effect in ShA rats: n.s.). This increased daily self-administration resulted in a larger cumulative intake of cocaine in shSERT2 vs shSCR rats under LgA, but not ShA, conditions (Figure 5C: shRNA x access interaction: F(1,56)=5.23, P<0.05; shRNA effect in LgA rats: F(1,28)=7.20, P<0.05; shRNA effect in ShA rats: n.s.). DRN-specific SERT KD increased the first-h cocaine intake of LgA, but not ShA, rats (Figure 5D: shRNA x access interaction: F(1,56)=3.82, P=0.05; shRNA effect in LgA rats: F(1,28)=6.79, P<0.05; shRNA effect in ShA rats: n.s.). DRN-specific SERT KD also increased PR responding under LgA, but not ShA, conditions (Figure 5E: shRNA x access interaction: F(1,56)=4.09, P<0.05; shRNA effect in LgA rats: F(1,28)=7.25, P<0.05; shRNA effect in ShA rats: n.s.). Inactive lever responding was low and not affected by DRN-specific SERT KD (Supplementary Figure S6).
Figure 5.
SERT KD in the DRN increased daily cocaine intake (A–B), cumulative cocaine intake (C), intake during the first h of the final self-administration session (D), and intake during a PR schedule of reinforcement (E) in rats exposed to LgA, but not ShA, cocaine self-administration. DRN-specific SERT KD also reduced open arm entries, but not locomotor activity, on the EPM after LgA, but not ShA, to the cocaine (F and G). The lack of shSERT2 effects on ShA cocaine intake (A) is accompanied by a lack of effect on CRF immunodensity in the PVN (H) and CeA (I) of ShA rats. In contrast, the shSERT2-induced increase in LgA cocaine intake (B) was accompanied by a reduction of CRF immunodensity in the CeA (I), but not PVN (H), of LgA rats (see Supplementary Figure S8 for representative pictures). In panels C–I, data are normalized to values obtained in shSCR counterparts (see Supplementary Table S7 for absolute values). *Significant change (P<0.05) vs shSCR, n.s: no significant change. ShA: shSCR (blue): n=14, shSERT2 (purple): n=16; LgA: shSCR (blue): n=12, shSERT2 (purple): n=18. Changes in cocaine self-administration following intra-DRN infusion of shSERT1 are presented in Supplementary Figure S7.
Effect of DRN-specific SERT KD on cocaine-induced anxiety-related behavior and CRF levels
DRN-specific SERT KD reduced open arm exploration on the EPM in LgA, but not ShA, rats (Figure 5F: shRNA x access interaction: F(1,56)=8.00, P<0.01; shRNA effect in LgA rats: F(1,28)=6.79, P<0.05; shRNA effect in ShA rats: n.s.), without affecting the total distance traveled (Figure 5G: shRNA effect and shRNA x access interaction: n.s.). There was no effect of DRN-specific SERT KD on CRF immunodensity in the PVN (Figure 5H: shRNA effect and shRNA x access interaction: n.s.). However, DRN-specific SERT KD decreased CRF immunodensity in the CeA in LgA, but not ShA, rats (Figure 5I: shRNA x access interaction: F(1,56)=13.73, P<0.001; shRNA effect in LgA rats: F(1,28)=7.34, P<0.05; shRNA effect in ShA rats: n.s.). The cumulative cocaine intake of DRN shSCR and shSERT2 rats was negatively correlated with CRF immunodensity in the CeA under LgA conditions (Supplementary Table S2).
The quantitative relationship between DRN-specific SERT KD and cocaine intake was evaluated using the shSERT1 sequence, which induces a less severe down-regulation of SERT transcript expression compared to shSERT2 (see Figure 2D). A gene-dosage effect was observed, whereby the phenotype generated by shSERT1 was intermediate between the phenotypes generated by shSCR and shSERT2 (Supplementary Figures S7C–E). Despite being milder than with shSERT2, the increase in cocaine self-administration elicited by shSERT1 was statistically significant (Supplementary Figures S7A–B).
Correlations between CRF levels, anxiety-related behavior and motivation for cocaine self-administration
A Pearson analysis revealed moderate, but significant, correlations between CRF levels, open arm exploration on the EPM, and motivation to take cocaine, depending on the duration of access to cocaine self-administration. CRF immunodensity in the PVN, but not the CeA, significantly correlated with anxiety-like behavior under ShA conditions. Changes in anxiety-like behavior and PVN CRF levels also correlated with an increased motivation to press for cocaine (i.e., increased PR responding) under ShA, but not LgA, conditions (Supplementary Table S3). In contrast, CRF immunodensity in the CeA, but not in the PVN, significantly correlated with anxiety-like behavior under LgA conditions. Changes in anxiety-like behavior and CeA CRF levels also correlated with an increased motivation to press for cocaine (i.e., increased PR responding) under LgA, but not ShA, conditions (Supplementary Table S3).
Discussion
This study shows that central reduction of SERT has profound effects on both the daily intake of cocaine and the motivation to take the drug. The phenotype of SERT KO rats, in which SERT expression is abolished in neurons arising from both the MRN and DRN, could be dissociated in two complementary and non-overlapping components by local KD of SERT expression in either the MRN or the DRN. MRN-specific KD recapitulated the increased cocaine intake of SERT KO rats under ShA conditions, while DRN-specific KD recapitulated the increased cocaine intake of SERT KO rats under LgA conditions. A first implication of these findings is that the observed phenotype of SERT KO rats does not result from neurodevelopmental alterations, since SERT KD was initiated in adulthood. Furthermore, considering that ShA self-administration models moderate cocaine use whereas LgA self-administration models compulsive cocaine use (for review: 14, 49), we propose that the transition from moderate to compulsive cocaine intake is accompanied by a shift from MRN- to DRN-driven serotonergic control over behavior. The effect of SERT downregulation on cocaine intake may be hypothesized to result from local desensitization of postsynaptic 5-HT1A receptors in projection areas of MRN and DRN serotonergic neurons, which was previously described in SERT KO rats, and proposed as a potential mechanism for the facilitation of psychostimulant self-administration (10, 50). Unknown is whether the cocaine intake increase elicited by SERT reduction results from increased extracellular baseline levels of serotonin (16, 17, 43) or reduced cocaine-induced serotonin release (16, 17).
Based on our finding that SERT deletion does not alter basal or cocaine-stimulated dopamine and noradrenaline release, we previously proposed that serotonin-mediated changes in non-catecholaminergic systems may underlie the increased cocaine intake of SERT KO rats (16). The current data obtained in both SERT KO and SERT KD rats show that 24 h after ShA and LgA cocaine self-administration, CRF immunodensity levels are lower in the PVN and CeA, respectively. These brain region-specific reductions in CRF immunodensity levels correlated with cumulative cocaine intake, suggesting that the exacerbation of CRF effects by SERT downregulation may result from increased cocaine exposure. In vivo microdialysis previously demonstrated that extracellular levels of CRF in the amygdala strongly increase during withdrawal from cocaine (51). Accordingly, a decrease in CRF immunoreactivity may reflect an increased release of CRF from intracellular vesicles into the extracellular space. This interpretation has been proposed in earlier studies to explain decreases in CRF immunoreactivity associated with pharmacological evidence of increased CRF signaling (52, 53).
In addition to various studies showing that CRF transmission alters the activity of serotonergic neurons (for review: 54), the results of the present study indicate that serotonin also regulates the levels of CRF. This finding is consistent with previous reports showing that serotonin or serotonergic agents lead to changes in the CRF system (34–39). The observed changes in CRF immunodensity levels in the present study may result from serotonergic afferents controlling the release of CRF by local CRF-containing neurons located in the PVN and CeA (55, 56), or by extrinsic CRF afferents (57, 58). Changes in CRF levels were accompanied by, and correlated with, a decrease in open-arm exploration on the EPM. In turn, this increase in anxiety-related behavior was accompanied by, and correlated with, an increase in the motivation to take cocaine. Together these data support various studies indicating that CRF-induced changes in emotional behavior can promote cocaine intake and seeking (24, 25, 53, 59–61).
The present study reveals the neuroanatomical specificity of the relationship that ties central serotonergic projections, local CRF levels, anxiety-related behavior, and the motivation for cocaine taking. ShA cocaine intake changed CRF levels in the PVN, but not CeA, whereas LgA cocaine intake changed CRF levels in the CeA, but not PVN. Serotonergic signaling in the BNST activates local CRF-expressing neurons (22) and mediates the anxiety-like state associated with withdrawal from chronic intermittent ethanol exposure (62, 63). We found that LgA cocaine self-administration reduced CRF levels in the BNST, consistent with the contribution of CRF signaling in the BNST to cocaine seeking (64, 65). However, BNST CRF levels were not further affected by SERT KO, indicating that CRF signaling in the BNST is likely not involved in the behavioral effects of SERT reduction reported in the present study.
Our local KD approach suggests that cocaine-induced changes in the levels of CRF in the PVN and CeA, and the concomitant anxiogenic-like effects, are produced by cocaine-induced changes in serotonergic neurons of the MRN and DRN, respectively. This hypothesis is supported by various anatomical studies (31–33) showing that neurons arising from the MRN project to brain regions that are located along the brain midline (e.g., PVN) whereas neurons arising from the DRN project to laterally located brain regions (e.g., CeA, see also: 57, 66, 67, 68).
Conclusions
Altogether, the present study supports the novel hypothesis that a cocaine-induced shift from MRN-driven serotonergic control of CRF levels in the hypothalamus to DRN-driven serotonergic control of CRF levels in the amygdala plays an important role in the transition from moderate to compulsive intake of cocaine in dependent individuals. Moderate cocaine intake is primarily driven by the positive incentive properties of cocaine, while compulsive cocaine intake is, at least in part, driven by the negative emotional states associated with withdrawal (69). The dual role of serotonergic neurons from the MRN and DRN in the regulation of cocaine self-administration under ShA versus LgA conditions provides a neurobiological basis for the contribution of serotonin signaling to both positive and negative reinforcement associated with the development of compulsive cocaine seeking (70).
Implications
The finding that CRF levels in the CeA and PVN as well as open-arm entries on the EPM were reduced in cocaine-naive SERT KO and SERT KD rats indicates that individuals carrying an allelic variant of the serotonin transporter polymorphic region decreasing SERT gene transcription are not only vulnerable to the transition from moderate cocaine intake to drug dependence, but may also be at risk for the initiation of cocaine use. In addition, serotonin-induced changes in CRF and anxiety may contribute to the previously reported increased intake of non-psychostimulant drugs of abuse (e.g., alcohol (71–73), opiates (8, 74, 75) and nicotine (76–78)) in these individuals. Finally, our study suggests that restoring the balance between MRN and DRN serotonergic activity may reverse the dysregulation of brain emotional systems that promote cocaine use, as was previously proposed for mood disorders (79, 80). We propose that during the early stages of cocaine dependence, MRN-targeted treatment may be beneficial, whereas at later stages DRN-targeted treatment may be more effective. In this respect, it is worth mentioning that the DRN and MRN display unique neurochemical features (e.g., differential serotonin receptor subtypes and densities), which could be leveraged for selective manipulation via systemic pharmacological interventions (for review: 80).
Supplementary Material
Supplementary Figure S1: Experimental timeline. The SERT knockout (KO) approach (B) was similar to the local SERT knockdown (KD) approach (A) except for the fact that there was no stereotactic surgery necessary in these animals and the time-out period during self-administration was increased from 20 to 40 s (see Supplementary Methods and Materials for details). Cocaine-naive animals were not exposed to the self-administration chambers during training and the various short and long access sessions.
Supplementary Figure S2: Schematic representation of the tips of all stereotactically implanted and correctly placed needles that were used to knockdown SERT in DRN (black dots) and MRN (red dots) neurons. Coronal diagrams are taken from Paxinos and Watson, 2007 (1).
Supplementary Figure S3: Regional selectivity of virally-mediated SERT knockdown. Chromogenic in situ hybridization of GFP (A) and SERT (B) mRNA on adjacent serial sections from the brain of a rat injected in the DRN with shSERT2 and euthanized 4 weeks later. SERT expression is virtually ablated from the area transduced with the viral vector (dashed outline) while SERT expression outside of the transduced area (including the MRN) is intact.
Supplementary Figure S4: LgA, but not ShA, to cocaine self-administration produced an escalation of the daily cocaine intake in the control rats of the SERT KO approach (A: access x session interaction: F(17,374)=6.08, P<0.001; session effect LgA: P<0.001, session effect ShA: n.s.), the local SERT KD in the MRN (B: access x session interaction: F(14,364)=12.42, P<0.001; session effect LgA: P<0.001, session effect ShA: n.s.), and the local SERT KD in the DRN (C: access x session interaction: F(14,336)=18.29, P<0.001; session effect LgA: P<0.001, session effect ShA: n.s.). SERT WT rats required three more self-administration sessions than MRN and DRN shSCR animals to reach an equivalent total cocaine intake (D–E: approach effect: n.s.). The control groups did also not differ in their cocaine intake during the first h of the final self-administration session (F–G: approach effect: n.s.) and their intake under a progressive ratio (PR) schedule of reinforcement (H–I: approach effect: n.s.). Each graph shows mean ± S.E.M. of values obtained in SERT WT, MRN shSCR or DRN shSCR rats. n.s: no significant approach effect. ShA: SERT WT (blue): n=14, MRN shSCR (blue): n=13, DRN shSCR (blue): n=14; LgA: SERT WT (blue): n=10, MRN shSCR (blue): n=15, DRN shSCR (blue): n= 12.
Supplementary Figure S5: LgA, but not ShA, to cocaine self-administration reduced CRF immunodensity levels in the BNST of SERT WT rats (A), and constitutive SERT KO had no effects on CRF levels in the BNST of animals that had either short (ShA), long (LgA) or no access (naive) to cocaine (B–C). *Significant decrease relative to cocaine naive, n.s: no difference relative to control. Data have been obtained from SERT KO rats, lacking SERT in neurons arising from both the MRN and DRN (see main Figure 2: naive: SERT WT (blue): n=13, SERT KO (green): n=10; and main Figure 3: ShA: SERT WT (blue): n=14, SERT KO (green): n=12; LgA: SERT WT (blue): n=10, SERT KO (green): n=11).
Supplementary Figure S6: No effects of constitutive SERT KO (A–B), MRN-specific SERT KD (C–D), or DRN-specific SERT KD (E–F) on the number of inactive lever presses during both ShA (left) and LgA (right) to cocaine. n.s: no significant change (no genotype/shRNA effect or no genotype/shRNA x session effect). The SERT-deletion-induced changes in active lever pressing can be found in main Figures 3–5. ShA: SERT WT (blue): n=14, SERT KO (green): n=12, MRN shSCR (blue): n=13, MRN shSERT2 (orange): n=13, DRN shSCR (blue): n=14, DRN shSERT2 (purple): n=16; LgA: SERT WT (blue): n=10, SERT KO (green): n=11, MRN shSCR (blue): n=15, MRN shSERT2 (orange): n=13, DRN shSCR (blue): n=12, DRN shSERT2 (purple): n=18.
Supplementary Figure S7: Intra-DRN infusion of shSERT1 increased the daily intake of cocaine in LgA, but not ShA, rats (A–B: shRNA x session interaction in LgA rats: F(14,308)=1.76, P<0.05, shRNA x session interaction in ShA rats: n.s.). To allow direct comparison of cocaine self-administration in these rats with cocaine self-administration in rats marked by a lower or higher expression of SERT, the cocaine self-administration data of the rats infused with respectively shSCR or shSERT2 into the DRN have also been included (data reproduced from main Figure 5). When compared to the effect of shSERT2 (which reduced local SERT expression by ~75%), intra-DRN infusion of shSERT1 (which reduced local SERT expression by ~50%) resulted in an intermediate increase in cumulative cocaine intake (C: shRNA effect in LgA rats: F(2,39)=4.09, P<0.05, shRNA effect in ShA rats: n.s.), intake during the first h of the final self-administration session (D: shRNA effect in LgA rats: F(2,39)=3.67, P<0.05, shRNA effect in ShA rats: n.s.), and intake during a PR schedule of reinforcement (E: shRNA effect in LgA rats: F(2,39)=3.83, P<0.05, shRNA effect in ShA rats: n.s.) in rats exposed to LgA, but not ShA, cocaine self-administration. In panels C–E, data are normalized to values obtained in shSCR counterparts. *Intake significantly higher than shSCR (LSD: P<0.05). ^Intake higher than shSCR and lower than shSERT2 (LSD: P<0.075), n.s: no significant change vs shSCR or shSERT2. ShA: shSCR (blue): n=14, shSERT1 (yellow): n=15, shSERT2 (purple): n=16; LgA: shSCR (blue): n=12, shSERT1 (yellow): n=12, shSERT2 (purple): n=18. The effects of shSERT1 and shSERT2 infusions on SERT expression in the DRN can be found in main Figure 2.
Supplementary Figure S8: Representative pictures illustrating the reported SERT reduction-induced changes in CRF immunodensity levels after ShA and LgA to cocaine self-administration (see also Main figures 3–5). The levels of CRF in the PVN (first and second rows) were reduced after ShA to cocaine in SERT KO (left column) and MRN (middle column), but not DRN (right column), SERT KD rats, whereas the levels of CRF in the CeA (third and fourth rows) were reduced after LgA to cocaine in SERT KO and DRN, but not MRN, SERT KD rats.
Supplementary Table S1: Results of a Pearson’s analysis to study the correlation between SERT levels, local levels of CRF and anxiety-related behavior in the cocaine-naïve animals of the SERT KD approach. A positive Pearson’s correlation coefficient denotes a positive correlation. n.s: no significant correlation. MRN SERT KD approach: n=18 (see Main Figure 2, shSCR: n=9, shSERT2: n=9), DRN SERT KD approach: n=30 (see Main Figure 2: shSCR: n=15, shSERT2: n=15).
Supplementary Table S2: Results of a Pearson’s analysis to study the correlation between total cocaine intake and CRF levels. A negative Pearson’s correlation coefficient denotes a negative correlation. n.s: no significant correlation. ShA (see Main Figures 3 and 4): SERT KO approach: n=26 (SERT WT: n=14, SERT KO: n=12), MRN SERT KD approach: n=26 (shSCR: n=13, shSERT2: n=13), LgA (see Main Figures 3 and 5): SERT KO approach: n=21 (SERT WT: n=10, SERT KO: n=11), DRN SERT KD approach: n=30 (shSCR: n=12, shSERT2: n=18).
Supplementary Table S3: Results of a Pearson’s analysis to study the correlation between the observed SERT-deletion-induced local reductions of CRF and the increases in anxiety-related behavior (as illustrated by a decrease in open arm entries on the EPM) and the motivation to take cocaine (as illustrated by an increase in PR intake). A positive Pearson’s correlation coefficient denotes a positive correlation, and a negative Pearson’s correlation coefficient denotes a negative correlation. n.s: no significant correlation. The ShA correlation analysis was performed in ShA rats in which SERT reduction increased PR responding (main Figure 3 (SERT WT+SERT KO: n=14+12) and main Figure 4 (shSCR+shSERT2: n=13+13), main Figure 3 + main Figure 4: n=52). The LgA correlation analysis was performed in LgA rats in which SERT reduction increased PR responding (main Figure 3 (SERT WT+SERT KO: n=10+11) and main Figure 5 (shSCR+shSERT2: n=12+18), main Figure 3 + main Figure 5: n=51).
Supplementary Table S4: Absolute values (± sem) of SERT mRNA expression in the MRN and DRN after constitutive SERT KO (measure 1) and raphe-specific SERT KD (measures 2 and 3). Data are obtained from the cocaine-naive rats depicted in Main Figure 2. Arbitrary units represent SERT optical density corrected for background density.
Supplementary Table S5: Absolute values (± sem) of the various measures of cocaine self-administration (SA), anxiety-related behavior on the elevated plus-maze (EPM) and CRF expression in the paraventricular nucleus (PVN) and central amygdala (CeA) depicted in main Figures 2 and 3, as well as results of pairwise comparisons between SERT WT and SERT KO rats and between the different types of access to cocaine (no access: naive, short access: ShA and long access: LgA).
Supplementary Table S6: Absolute values (± sem) of the various measures of cocaine self-administration (SA), anxiety-related behavior on the elevated plus-maze (EPM) and CRF expression in the paraventricular nucleus (PVN) and central amygdala (CeA) depicted in main Figures 2 and 4, as well as results of pairwise comparisons between MRN shSCR and MRN shSERT2 rats and between the different types of access to cocaine (no access: naive, short access: ShA and long access: LgA).
Supplementary Table S7: Absolute values (± sem) of the various measures of cocaine self-administration (SA), anxiety-related behavior on the elevated plus-maze (EPM) and CRF expression in the paraventricular nucleus (PVN) and central amygdala (CeA) depicted in main Figures 2 and 5, as well as results of pairwise comparisons between DRN shSCR and DRN shSERT2 rats and between the different types of access to cocaine (no access: naive, short access: ShA and long access: LgA).
Acknowledgments
The authors would like to thank Julian Hanisch, Rosan Weber, Boyd van Reijmersdal, Stephanie Seeger, Stefano Indaco, Lourens Nonkes, Jos Dederen, Leandro Vendruscolo, Scott Edwards and Elena Crawford for advice and/or technical assistance. Michel Verheij, Candice Contet, Judith Homberg and George Koob were supported by a joint research grant (no. 31180005) awarded by the Netherlands Organisation for Health Research and Development (ZonMW) and the USA National Institute on Drug Abuse (NIDA). Michel Verheij was also awarded a NIDA international program INVEST fellowship and a European College of Neuropsychopharmacology (ECNP) Research Grant for Young Scientists. This work was also supported by National Institute on Alcohol Abuse and Alcoholism (NIAAA) grants to Candice Contet (AA006420, AA024198, AA021491), ALW Grant 819.02.022 from the Netherlands Organisation for Scientific Research to Tamas Kozicz and Judith Homberg, and Era-Net NEURON grant “RESPOND” and VIDI grant 864.10.003 awarded to Judith Homberg.
Footnotes
Financial Disclosures
The authors declare no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary information is available at Biological Psychiatry’s website.
References
- 1.Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, et al. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- 2.Heils A, Teufel A, Petri S, Stober G, Riederer P, Bengel D, et al. Allelic variation of human serotonin transporter gene expression. J Neurochem. 1996;66:2621–2624. doi: 10.1046/j.1471-4159.1996.66062621.x. [DOI] [PubMed] [Google Scholar]
- 3.Heinz A, Jones DW, Mazzanti C, Goldman D, Ragan P, Hommer D, et al. A relationship between serotonin transporter genotype and in vivo protein expression and alcohol neurotoxicity. Biol Psychiatry. 2000;47:643–649. doi: 10.1016/s0006-3223(99)00171-7. [DOI] [PubMed] [Google Scholar]
- 4.Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS, et al. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nat Neurosci. 2005;8:828–834. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- 5.Osher Y, Hamer D, Benjamin J. Association and linkage of anxiety-related traits with a functional polymorphism of the serotonin transporter gene regulatory region in Israeli sibling pairs. Mol Psychiatry. 2000;5:216–219. doi: 10.1038/sj.mp.4000660. [DOI] [PubMed] [Google Scholar]
- 6.Gerra G, Zaimovic A, Garofano L, Ciusa F, Moi G, Avanzini P, et al. Perceived parenting behavior in the childhood of cocaine users: relationship with genotype and personality traits. Am J Med Genet. 2007;144B:52–57. doi: 10.1002/ajmg.b.30388. [DOI] [PubMed] [Google Scholar]
- 7.Martin-Santos R, Torrens M, Poudevida S, Langohr K, Cuyas E, Pacifici R, et al. 5-HTTLPR polymorphism, mood disorders and MDMA use in a 3-year follow-up study. Addict Biol. 2010;15:15–22. doi: 10.1111/j.1369-1600.2009.00180.x. [DOI] [PubMed] [Google Scholar]
- 8.Enoch MA, Gorodetsky E, Hodgkinson C, Roy A, Goldman D. Functional genetic variants that increase synaptic serotonin and 5-HT3 receptor sensitivity predict alcohol and drug dependence. Mol Psychiatry. 2011;16:1139–1146. doi: 10.1038/mp.2010.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kalueff AV, Olivier JD, Nonkes LJ, Homberg JR. Conserved role for the serotonin transporter gene in rat and mouse neurobehavioral endophenotypes. Neurosci Biobehav Rev. 2010;34:373–386. doi: 10.1016/j.neubiorev.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 10.Homberg JR, De Boer SF, Raaso HS, Olivier JD, Verheul M, Ronken E, et al. Adaptations in pre- and postsynaptic 5-HT1A receptor function and cocaine supersensitivity in serotonin transporter knockout rats. Psychopharmacology (Berl) 2008;200:367–380. doi: 10.1007/s00213-008-1212-x. [DOI] [PubMed] [Google Scholar]
- 11.Oakly AC, Brox BW, Schenk S, Ellenbroek BA. A genetic deletion of the serotonin transporter greatly enhances the reinforcing properties of MDMA in rats. Mol Psychiatry. 2014;19:534–535. doi: 10.1038/mp.2013.75. [DOI] [PubMed] [Google Scholar]
- 12.Sora I, Wichems C, Takahashi N, Li XF, Zeng Z, Revay R, et al. Cocaine reward models: conditioned place preference can be established in dopamine- and in serotonin-transporter knockout mice. Proc Natl Acad Sci USA. 1998;95:7699–7704. doi: 10.1073/pnas.95.13.7699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998;282:298–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- 14.Le Moal M, Koob GF. Drug addiction: pathways to the disease and pathophysiological perspectives. Eur Neuropsychopharmacol. 2007;17:377–393. doi: 10.1016/j.euroneuro.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 15.Ahmed SH, Kenny PJ. Cracking the molecular code of cocaine addiction. Ilar j. 2011;52:309–320. doi: 10.1093/ilar.52.3.309. [DOI] [PubMed] [Google Scholar]
- 16.Verheij MM, Karel P, Cools AR, Homberg JR. Reduced cocaine-induced serotonin, but not dopamine and noradrenaline, release in rats with a genetic deletion of serotonin transporters. Eur Neuropsychopharmacol. 2014;24:1850–1854. doi: 10.1016/j.euroneuro.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 17.Shen HW, Hagino Y, Kobayashi H, Shinohara-Tanaka K, Ikeda K, Yamamoto H, et al. Regional differences in extracellular dopamine and serotonin assessed by in vivo microdialysis in mice lacking dopamine and/or serotonin transporters. Neuropsychopharmacology. 2004;29:1790–1799. doi: 10.1038/sj.npp.1300476. [DOI] [PubMed] [Google Scholar]
- 18.Koob GF. Corticotropin-releasing factor, norepinephrine, and stress. Biol Psychiatry. 1999;46:1167–1180. doi: 10.1016/s0006-3223(99)00164-x. [DOI] [PubMed] [Google Scholar]
- 19.Binder EB, Nemeroff CB. The CRF system, stress, depression and anxiety-insights from human genetic studies. Mol Psychiatry. 2010;15:574–588. doi: 10.1038/mp.2009.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elliott E, Ezra-Nevo G, Regev L, Neufeld-Cohen A, Chen A. Resilience to social stress coincides with functional DNA methylation of the Crf gene in adult mice. Nat Neurosci. 2010;13:1351–1353. doi: 10.1038/nn.2642. [DOI] [PubMed] [Google Scholar]
- 21.Regev L, Tsoory M, Gil S, Chen A. Site-specific genetic manipulation of amygdala corticotropin-releasing factor reveals its imperative role in mediating behavioral response to challenge. Biol Psychiatry. 2012;71:317–326. doi: 10.1016/j.biopsych.2011.05.036. [DOI] [PubMed] [Google Scholar]
- 22.Marcinkiewcz CA, Mazzone CM, D’Agostino G, Halladay LR, Hardaway JA, DiBerto JF, et al. Serotonin engages an anxiety and fear-promoting circuit in the extended amygdala. Nature. 2016;537:97–101. doi: 10.1038/nature19318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sarnyai Z, Shaham Y, Heinrichs SC. The role of corticotropin-releasing factor in drug addiction. Pharmacol Rev. 2001;53:209–243. [PubMed] [Google Scholar]
- 24.Koob GF. A role for brain stress systems in addiction. Neuron. 2008;59:11–34. doi: 10.1016/j.neuron.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koob GF. The role of CRF and CRF-related peptides in the dark side of addiction. Brain Res. 2010;1314:3–14. doi: 10.1016/j.brainres.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Silberman Y, Winder DG. Emerging role for corticotropin releasing factor signaling in the bed nucleus of the stria terminalis at the intersection of stress and reward. Front Psychiatry. 2013;4:42. doi: 10.3389/fpsyt.2013.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cummings S, Elde R, Ells J, Lindall A. Corticotropin-releasing factor immunoreactivity is widely distributed within the central nervous system of the rat: an immunohistochemical study. J Neurosci. 1983;3:1355–1368. doi: 10.1523/JNEUROSCI.03-07-01355.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Swanson LW, Sawchenko PE, Rivier J, Vale WW. Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: an immunohistochemical study. Neuroendocrinology. 1983;36:165–186. doi: 10.1159/000123454. [DOI] [PubMed] [Google Scholar]
- 29.Treweek JB, Jaferi A, Colago EE, Zhou P, Pickel VM. Electron microscopic localization of corticotropin-releasing factor (CRF) and CRF receptor in rat and mouse central nucleus of the amygdala. J Comp Neurol. 2009;512:323–335. doi: 10.1002/cne.21884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kawano H, Daikoku S, Shibasaki T. CRF-containing neuron systems in the rat hypothalamus: retrograde tracing and immunohistochemical studies. J Comp Neurol. 1988;272:260–268. doi: 10.1002/cne.902720208. [DOI] [PubMed] [Google Scholar]
- 31.Vertes RP. A PHA-L analysis of ascending projections of the dorsal raphe nucleus in the rat. J Comp Neurol. 1991;313:643–668. doi: 10.1002/cne.903130409. [DOI] [PubMed] [Google Scholar]
- 32.Vertes RP, Fortin WJ, Crane AM. Projections of the median raphe nucleus in the rat. J Comp Neurol. 1999;407:555–582. [PubMed] [Google Scholar]
- 33.Hensler JG. Serotonergic modulation of the limbic system. Neurosci Biobehav Rev. 2006;30:203–214. doi: 10.1016/j.neubiorev.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 34.Fernandez Macedo GV, Cladouchos ML, Sifonios L, Cassanelli PM, Wikinski S. Effects of fluoxetine on CRF and CRF1 expression in rats exposed to the learned helplessness paradigm. Psychopharmacology (Berl) 2013;225:647–659. doi: 10.1007/s00213-012-2859-x. [DOI] [PubMed] [Google Scholar]
- 35.Choi S, Blake V, Cole S, Fernstrom JD. Effects of chronic fenfluramine administration on hypothalamic neuropeptide mRNA expression. Brain Res. 2006;1087:83–86. doi: 10.1016/j.brainres.2006.02.129. [DOI] [PubMed] [Google Scholar]
- 36.Owens MJ, Edwards E, Nemeroff CB. Effects of 5-HT1A receptor agonists on hypothalamo-pituitary-adrenal axis activity and corticotropin-releasing factor containing neurons in the rat brain. Eur J Pharmacol. 1990;190:113–122. doi: 10.1016/0014-2999(90)94118-h. [DOI] [PubMed] [Google Scholar]
- 37.Fuller RW. Serotonin receptors and neuroendocrine responses. Neuropsychopharmacology. 1990;3:495–502. [PubMed] [Google Scholar]
- 38.Nakagami Y, Suda T, Yajima F, Ushiyama T, Tomori N, Sumitomo T, et al. Effects of serotonin, cyproheptadine and reserpine on corticotropin-releasing factor release from the rat hypothalamus in vitro. Brain Res. 1986;386:232–236. doi: 10.1016/0006-8993(86)90159-9. [DOI] [PubMed] [Google Scholar]
- 39.Van de Kar LD, Javed A, Zhang Y, Serres F, Raap DK, Gray TS. 5-HT2A receptors stimulate ACTH, corticosterone, oxytocin, renin, and prolactin release and activate hypothalamic CRF and oxytocin-expressing cells. J Neurosci. 2001;21:3572–3579. doi: 10.1523/JNEUROSCI.21-10-03572.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wee S, Specio SE, Koob GF. Effects of dose and session duration on cocaine self-administration in rats. J Pharmacol Exp Ther. 2007;320:1134–1143. doi: 10.1124/jpet.106.113340. [DOI] [PubMed] [Google Scholar]
- 41.Verheij MM, Vendruscolo LF, Caffino L, Giannotti G, Cazorla M, Fumagalli F, et al. Systemic Delivery of a Brain-Penetrant TrkB Antagonist Reduces Cocaine Self-Administration and Normalizes TrkB Signaling in the Nucleus Accumbens and Prefrontal Cortex. J Neurosci. 2016;36:8149–8159. doi: 10.1523/JNEUROSCI.2711-14.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smits BM, Mudde JB, van de Belt J, Verheul M, Olivier J, Homberg J, et al. Generation of gene knockouts and mutant models in the laboratory rat by ENU-driven target-selected mutagenesis. Pharmacogenet Genomics. 2006;16:159–169. doi: 10.1097/01.fpc.0000184960.82903.8f. [DOI] [PubMed] [Google Scholar]
- 43.Homberg JR, Olivier JD, Smits BM, Mul JD, Mudde J, Verheul M, et al. Characterization of the serotonin transporter knockout rat: a selective change in the functioning of the serotonergic system. Neuroscience. 2007;146:1662–1676. doi: 10.1016/j.neuroscience.2007.03.030. [DOI] [PubMed] [Google Scholar]
- 44.Richardson NR, Roberts DC. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- 45.Hodos W. Progressive ratio as a measure of reward strength. Science. 1961;134:943–944. doi: 10.1126/science.134.3483.943. [DOI] [PubMed] [Google Scholar]
- 46.Pellow S, Chopin P, File SE, Briley M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14:149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- 47.De Souza EB, Whitehouse PJ, Kuhar MJ, Price DL, Vale WW. Reciprocal changes in corticotropin-releasing factor (CRF)-like immunoreactivity and CRF receptors in cerebral cortex of Alzheimer’s disease. Nature. 1986;319:593–595. doi: 10.1038/319593a0. [DOI] [PubMed] [Google Scholar]
- 48.Rouwette T, Vanelderen P, de Reus M, Loohuis NO, Giele J, van Egmond J, et al. Experimental neuropathy increases limbic forebrain CRF. Eur J Pain. 2012;16:61–71. doi: 10.1016/j.ejpain.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 49.Vanderschuren LJ, Everitt BJ. Drug seeking becomes compulsive after prolonged cocaine self-administration. Science. 2004;305:1017–1019. doi: 10.1126/science.1098975. [DOI] [PubMed] [Google Scholar]
- 50.Muller CP, Carey RJ, Huston JP, De Souza Silva MA. Serotonin and psychostimulant addiction: focus on 5-HT1A-receptors. Prog Neurobiol. 2007;81:133–178. doi: 10.1016/j.pneurobio.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 51.Richter RM, Weiss F. In vivo CRF release in rat amygdala is increased during cocaine withdrawal in self-administering rats. Synapse. 1999;32:254–261. doi: 10.1002/(SICI)1098-2396(19990615)32:4<254::AID-SYN2>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 52.Funk CK, O’Dell LE, Crawford EF, Koob GF. Corticotropin-releasing factor within the central nucleus of the amygdala mediates enhanced ethanol self-administration in withdrawn, ethanol-dependent rats. J Neurosci. 2006;26:11324–11332. doi: 10.1523/JNEUROSCI.3096-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sarnyai Z, Biro E, Gardi J, Vecsernyes M, Julesz J, Telegdy G. Brain corticotropin-releasing factor mediates ‘anxiety-like’ behavior induced by cocaine withdrawal in rats. Brain Res. 1995;675:89–97. doi: 10.1016/0006-8993(95)00043-p. [DOI] [PubMed] [Google Scholar]
- 54.Homberg JR, Contet C. Deciphering the interaction of the corticotropin-releasing factor and serotonin brain systems in anxiety-related disorders. J Neurosci. 2009;29:13743–13745. doi: 10.1523/JNEUROSCI.4362-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liposits Z, Phelix C, Paull WK. Synaptic interaction of serotonergic axons and corticotropin releasing factor (CRF) synthesizing neurons in the hypothalamic paraventricular nucleus of the rat. A light and electron microscopic immunocytochemical study. Histochemistry. 1987;86:541–549. doi: 10.1007/BF00489545. [DOI] [PubMed] [Google Scholar]
- 56.Asan E, Yilmazer-Hanke DM, Eliava M, Hantsch M, Lesch KP, Schmitt A. The corticotropin-releasing factor (CRF)-system and monoaminergic afferents in the central amygdala: investigations in different mouse strains and comparison with the rat. Neuroscience. 2005;131:953–967. doi: 10.1016/j.neuroscience.2004.11.040. [DOI] [PubMed] [Google Scholar]
- 57.Commons KG, Connolley KR, Valentino RJ. A neurochemically distinct dorsal raphe-limbic circuit with a potential role in affective disorders. Neuropsychopharmacology. 2003;28:206–215. doi: 10.1038/sj.npp.1300045. [DOI] [PubMed] [Google Scholar]
- 58.Uryu K, Okumura T, Shibasaki T, Sakanaka M. Fine structure and possible origins of nerve fibers with corticotropin-releasing factor-like immunoreactivity in the rat central amygdaloid nucleus. Brain Res. 1992;577:175–179. doi: 10.1016/0006-8993(92)90554-m. [DOI] [PubMed] [Google Scholar]
- 59.Homberg JR, Karel P, Verheij MM. Individual differences in cocaine addiction: maladaptive behavioural traits. Addict Biol. 2014;19:517–528. doi: 10.1111/adb.12036. [DOI] [PubMed] [Google Scholar]
- 60.Specio SE, Wee S, O’Dell LE, Boutrel B, Zorrilla EP, Koob GF. CRF(1) receptor antagonists attenuate escalated cocaine self-administration in rats. Psychopharmacology (Berl) 2008;196:473–482. doi: 10.1007/s00213-007-0983-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Basso AM, Spina M, Rivier J, Vale W, Koob GF. Corticotropin-releasing factor antagonist attenuates the “anxiogenic-like” effect in the defensive burying paradigm but not in the elevated plus-maze following chronic cocaine in rats. Psychopharmacology (Berl) 1999;145:21–30. doi: 10.1007/s002130051028. [DOI] [PubMed] [Google Scholar]
- 62.Marcinkiewcz CA, Dorrier CE, Lopez AJ, Kash TL. Ethanol induced adaptations in 5-HT2c receptor signaling in the bed nucleus of the stria terminalis: implications for anxiety during ethanol withdrawal. Neuropharmacology. 2015;89:157–167. doi: 10.1016/j.neuropharm.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kash TL. The role of biogenic amine signaling in the bed nucleus of the stria terminals in alcohol abuse. Alcohol. 2012;46:303–308. doi: 10.1016/j.alcohol.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Erb S, Stewart J. A role for the bed nucleus of the stria terminalis, but not the amygdala, in the effects of corticotropin-releasing factor on stress-induced reinstatement of cocaine seeking. J Neurosci. 1999;19:Rc35. doi: 10.1523/JNEUROSCI.19-20-j0006.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Erb S, Salmaso N, Rodaros D, Stewart J. A role for the CRF-containing pathway from central nucleus of the amygdala to bed nucleus of the stria terminalis in the stress-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2001;158:360–365. doi: 10.1007/s002130000642. [DOI] [PubMed] [Google Scholar]
- 66.Rizvi TA, Ennis M, Behbehani MM, Shipley MT. Connections between the central nucleus of the amygdala and the midbrain periaqueductal gray: topography and reciprocity. J Comp Neurol. 1991;303:121–131. doi: 10.1002/cne.903030111. [DOI] [PubMed] [Google Scholar]
- 67.Fernandez SP, Cauli B, Cabezas C, Muzerelle A, Poncer JC, Gaspar P. Multiscale single-cell analysis reveals unique phenotypes of raphe 5-HT neurons projecting to the forebrain. Brain Struct Funct. 2016;221:4007–4025. doi: 10.1007/s00429-015-1142-4. [DOI] [PubMed] [Google Scholar]
- 68.Muzerelle A, Scotto-Lomassese S, Bernard JF, Soiza-Reilly M, Gaspar P. Conditional anterograde tracing reveals distinct targeting of individual serotonin cell groups (B5-B9) to the forebrain and brainstem. Brain Struct Funct. 2016;221:535–561. doi: 10.1007/s00429-014-0924-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Uhl GR, Hall FS, Sora I. Cocaine, reward, movement and monoamine transporters. Mol Psychiatry. 2002;7:21–26. doi: 10.1038/sj.mp.4000964. [DOI] [PubMed] [Google Scholar]
- 71.Lichtermann D, Hranilovic D, Trixler M, Franke P, Jernej B, Delmo CD, et al. Support for allelic association of a polymorphic site in the promoter region of the serotonin transporter gene with risk for alcohol dependence. Am J Psychiatry. 2000;157:2045–2047. doi: 10.1176/appi.ajp.157.12.2045. [DOI] [PubMed] [Google Scholar]
- 72.Hallikainen T, Saito T, Lachman HM, Volavka J, Pohjalainen T, Ryynanen OP, et al. Association between low activity serotonin transporter promoter genotype and early onset alcoholism with habitual impulsive violent behavior. Mol Psychiatry. 1999;4:385–388. doi: 10.1038/sj.mp.4000526. [DOI] [PubMed] [Google Scholar]
- 73.Covault J, Tennen H, Armeli S, Conner TS, Herman AI, Cillessen AH, et al. Interactive effects of the serotonin transporter 5-HTTLPR polymorphism and stressful life events on college student drinking and drug use. Biol Psychiatry. 2007;61:609–616. doi: 10.1016/j.biopsych.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 74.Tan EC, Yeo BK, Ho BK, Tay AH, Tan CH. Evidence for an association between heroin dependence and a VNTR polymorphism at the serotonin transporter locus. Mol Psychiatry. 1999;4:215–217. doi: 10.1038/sj.mp.4000541. [DOI] [PubMed] [Google Scholar]
- 75.Gerra G, Garofano L, Santoro G, Bosari S, Pellegrini C, Zaimovic A, et al. Association between low-activity serotonin transporter genotype and heroin dependence: behavioral and personality correlates. Am J Med Genet B Neuropsychiatr Genet. 2004;126b:37–42. doi: 10.1002/ajmg.b.20111. [DOI] [PubMed] [Google Scholar]
- 76.Lerman C, Caporaso NE, Audrain J, Main D, Boyd NR, Shields PG. Interacting effects of the serotonin transporter gene and neuroticism in smoking practices and nicotine dependence. Mol Psychiatry. 2000;5:189–192. doi: 10.1038/sj.mp.4000672. [DOI] [PubMed] [Google Scholar]
- 77.Gerra G, Garofano L, Zaimovic A, Moi G, Branchi B, Bussandri M, et al. Association of the serotonin transporter promoter polymorphism with smoking behavior among adolescents. Am J Med Genet B Neuropsychiatr Genet. 2005;135b:73–78. doi: 10.1002/ajmg.b.30173. [DOI] [PubMed] [Google Scholar]
- 78.Hu S, Brody CL, Fisher C, Gunzerath L, Nelson ML, Sabol SZ, et al. Interaction between the serotonin transporter gene and neuroticism in cigarette smoking behavior. Mol Psychiatry. 2000;5:181–188. doi: 10.1038/sj.mp.4000690. [DOI] [PubMed] [Google Scholar]
- 79.Teissier A, Chemiakine A, Inbar B, Bagchi S, Ray RS, Palmiter RD, et al. Activity of Raphe Serotonergic Neurons Controls Emotional Behaviors. Cell Rep. 2015;13:1965–1976. doi: 10.1016/j.celrep.2015.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lechin F, van der Dijs B, Hernandez-Adrian G. Dorsal raphe vs. median raphe serotonergic antagonism. Anatomical, physiological, behavioral, neuroendocrinological, neuropharmacological and clinical evidences: relevance for neuropharmacological therapy. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:565–585. doi: 10.1016/j.pnpbp.2005.11.025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1: Experimental timeline. The SERT knockout (KO) approach (B) was similar to the local SERT knockdown (KD) approach (A) except for the fact that there was no stereotactic surgery necessary in these animals and the time-out period during self-administration was increased from 20 to 40 s (see Supplementary Methods and Materials for details). Cocaine-naive animals were not exposed to the self-administration chambers during training and the various short and long access sessions.
Supplementary Figure S2: Schematic representation of the tips of all stereotactically implanted and correctly placed needles that were used to knockdown SERT in DRN (black dots) and MRN (red dots) neurons. Coronal diagrams are taken from Paxinos and Watson, 2007 (1).
Supplementary Figure S3: Regional selectivity of virally-mediated SERT knockdown. Chromogenic in situ hybridization of GFP (A) and SERT (B) mRNA on adjacent serial sections from the brain of a rat injected in the DRN with shSERT2 and euthanized 4 weeks later. SERT expression is virtually ablated from the area transduced with the viral vector (dashed outline) while SERT expression outside of the transduced area (including the MRN) is intact.
Supplementary Figure S4: LgA, but not ShA, to cocaine self-administration produced an escalation of the daily cocaine intake in the control rats of the SERT KO approach (A: access x session interaction: F(17,374)=6.08, P<0.001; session effect LgA: P<0.001, session effect ShA: n.s.), the local SERT KD in the MRN (B: access x session interaction: F(14,364)=12.42, P<0.001; session effect LgA: P<0.001, session effect ShA: n.s.), and the local SERT KD in the DRN (C: access x session interaction: F(14,336)=18.29, P<0.001; session effect LgA: P<0.001, session effect ShA: n.s.). SERT WT rats required three more self-administration sessions than MRN and DRN shSCR animals to reach an equivalent total cocaine intake (D–E: approach effect: n.s.). The control groups did also not differ in their cocaine intake during the first h of the final self-administration session (F–G: approach effect: n.s.) and their intake under a progressive ratio (PR) schedule of reinforcement (H–I: approach effect: n.s.). Each graph shows mean ± S.E.M. of values obtained in SERT WT, MRN shSCR or DRN shSCR rats. n.s: no significant approach effect. ShA: SERT WT (blue): n=14, MRN shSCR (blue): n=13, DRN shSCR (blue): n=14; LgA: SERT WT (blue): n=10, MRN shSCR (blue): n=15, DRN shSCR (blue): n= 12.
Supplementary Figure S5: LgA, but not ShA, to cocaine self-administration reduced CRF immunodensity levels in the BNST of SERT WT rats (A), and constitutive SERT KO had no effects on CRF levels in the BNST of animals that had either short (ShA), long (LgA) or no access (naive) to cocaine (B–C). *Significant decrease relative to cocaine naive, n.s: no difference relative to control. Data have been obtained from SERT KO rats, lacking SERT in neurons arising from both the MRN and DRN (see main Figure 2: naive: SERT WT (blue): n=13, SERT KO (green): n=10; and main Figure 3: ShA: SERT WT (blue): n=14, SERT KO (green): n=12; LgA: SERT WT (blue): n=10, SERT KO (green): n=11).
Supplementary Figure S6: No effects of constitutive SERT KO (A–B), MRN-specific SERT KD (C–D), or DRN-specific SERT KD (E–F) on the number of inactive lever presses during both ShA (left) and LgA (right) to cocaine. n.s: no significant change (no genotype/shRNA effect or no genotype/shRNA x session effect). The SERT-deletion-induced changes in active lever pressing can be found in main Figures 3–5. ShA: SERT WT (blue): n=14, SERT KO (green): n=12, MRN shSCR (blue): n=13, MRN shSERT2 (orange): n=13, DRN shSCR (blue): n=14, DRN shSERT2 (purple): n=16; LgA: SERT WT (blue): n=10, SERT KO (green): n=11, MRN shSCR (blue): n=15, MRN shSERT2 (orange): n=13, DRN shSCR (blue): n=12, DRN shSERT2 (purple): n=18.
Supplementary Figure S7: Intra-DRN infusion of shSERT1 increased the daily intake of cocaine in LgA, but not ShA, rats (A–B: shRNA x session interaction in LgA rats: F(14,308)=1.76, P<0.05, shRNA x session interaction in ShA rats: n.s.). To allow direct comparison of cocaine self-administration in these rats with cocaine self-administration in rats marked by a lower or higher expression of SERT, the cocaine self-administration data of the rats infused with respectively shSCR or shSERT2 into the DRN have also been included (data reproduced from main Figure 5). When compared to the effect of shSERT2 (which reduced local SERT expression by ~75%), intra-DRN infusion of shSERT1 (which reduced local SERT expression by ~50%) resulted in an intermediate increase in cumulative cocaine intake (C: shRNA effect in LgA rats: F(2,39)=4.09, P<0.05, shRNA effect in ShA rats: n.s.), intake during the first h of the final self-administration session (D: shRNA effect in LgA rats: F(2,39)=3.67, P<0.05, shRNA effect in ShA rats: n.s.), and intake during a PR schedule of reinforcement (E: shRNA effect in LgA rats: F(2,39)=3.83, P<0.05, shRNA effect in ShA rats: n.s.) in rats exposed to LgA, but not ShA, cocaine self-administration. In panels C–E, data are normalized to values obtained in shSCR counterparts. *Intake significantly higher than shSCR (LSD: P<0.05). ^Intake higher than shSCR and lower than shSERT2 (LSD: P<0.075), n.s: no significant change vs shSCR or shSERT2. ShA: shSCR (blue): n=14, shSERT1 (yellow): n=15, shSERT2 (purple): n=16; LgA: shSCR (blue): n=12, shSERT1 (yellow): n=12, shSERT2 (purple): n=18. The effects of shSERT1 and shSERT2 infusions on SERT expression in the DRN can be found in main Figure 2.
Supplementary Figure S8: Representative pictures illustrating the reported SERT reduction-induced changes in CRF immunodensity levels after ShA and LgA to cocaine self-administration (see also Main figures 3–5). The levels of CRF in the PVN (first and second rows) were reduced after ShA to cocaine in SERT KO (left column) and MRN (middle column), but not DRN (right column), SERT KD rats, whereas the levels of CRF in the CeA (third and fourth rows) were reduced after LgA to cocaine in SERT KO and DRN, but not MRN, SERT KD rats.
Supplementary Table S1: Results of a Pearson’s analysis to study the correlation between SERT levels, local levels of CRF and anxiety-related behavior in the cocaine-naïve animals of the SERT KD approach. A positive Pearson’s correlation coefficient denotes a positive correlation. n.s: no significant correlation. MRN SERT KD approach: n=18 (see Main Figure 2, shSCR: n=9, shSERT2: n=9), DRN SERT KD approach: n=30 (see Main Figure 2: shSCR: n=15, shSERT2: n=15).
Supplementary Table S2: Results of a Pearson’s analysis to study the correlation between total cocaine intake and CRF levels. A negative Pearson’s correlation coefficient denotes a negative correlation. n.s: no significant correlation. ShA (see Main Figures 3 and 4): SERT KO approach: n=26 (SERT WT: n=14, SERT KO: n=12), MRN SERT KD approach: n=26 (shSCR: n=13, shSERT2: n=13), LgA (see Main Figures 3 and 5): SERT KO approach: n=21 (SERT WT: n=10, SERT KO: n=11), DRN SERT KD approach: n=30 (shSCR: n=12, shSERT2: n=18).
Supplementary Table S3: Results of a Pearson’s analysis to study the correlation between the observed SERT-deletion-induced local reductions of CRF and the increases in anxiety-related behavior (as illustrated by a decrease in open arm entries on the EPM) and the motivation to take cocaine (as illustrated by an increase in PR intake). A positive Pearson’s correlation coefficient denotes a positive correlation, and a negative Pearson’s correlation coefficient denotes a negative correlation. n.s: no significant correlation. The ShA correlation analysis was performed in ShA rats in which SERT reduction increased PR responding (main Figure 3 (SERT WT+SERT KO: n=14+12) and main Figure 4 (shSCR+shSERT2: n=13+13), main Figure 3 + main Figure 4: n=52). The LgA correlation analysis was performed in LgA rats in which SERT reduction increased PR responding (main Figure 3 (SERT WT+SERT KO: n=10+11) and main Figure 5 (shSCR+shSERT2: n=12+18), main Figure 3 + main Figure 5: n=51).
Supplementary Table S4: Absolute values (± sem) of SERT mRNA expression in the MRN and DRN after constitutive SERT KO (measure 1) and raphe-specific SERT KD (measures 2 and 3). Data are obtained from the cocaine-naive rats depicted in Main Figure 2. Arbitrary units represent SERT optical density corrected for background density.
Supplementary Table S5: Absolute values (± sem) of the various measures of cocaine self-administration (SA), anxiety-related behavior on the elevated plus-maze (EPM) and CRF expression in the paraventricular nucleus (PVN) and central amygdala (CeA) depicted in main Figures 2 and 3, as well as results of pairwise comparisons between SERT WT and SERT KO rats and between the different types of access to cocaine (no access: naive, short access: ShA and long access: LgA).
Supplementary Table S6: Absolute values (± sem) of the various measures of cocaine self-administration (SA), anxiety-related behavior on the elevated plus-maze (EPM) and CRF expression in the paraventricular nucleus (PVN) and central amygdala (CeA) depicted in main Figures 2 and 4, as well as results of pairwise comparisons between MRN shSCR and MRN shSERT2 rats and between the different types of access to cocaine (no access: naive, short access: ShA and long access: LgA).
Supplementary Table S7: Absolute values (± sem) of the various measures of cocaine self-administration (SA), anxiety-related behavior on the elevated plus-maze (EPM) and CRF expression in the paraventricular nucleus (PVN) and central amygdala (CeA) depicted in main Figures 2 and 5, as well as results of pairwise comparisons between DRN shSCR and DRN shSERT2 rats and between the different types of access to cocaine (no access: naive, short access: ShA and long access: LgA).