Abstract
Although didymin, a flavonoid-O-glycosides compound naturally found in the citrus fruits, has been reported to be a potent anticancer agent in the prevention of various cancers, its role in the prevention of cardiovascular complications is unclear. Most importantly, its effect in the prevention of endothelial dysfunction, a pathological process involved in the atherogenesis, is unknown. We have examined the efficacy of didymin in preventing the high glucose (HG; 25mM)-induced human umbilical vein endothelial cells (HUVECs) dysfunction. Our results indicate that incubation of HUVECs with HG resulted in the loss of cell viability, and pre-incubation of didymin prevented it. Further, didymin prevented the HG-induced generation of reactive oxygen species (ROS) as well as lipid peroxidation product, malondialdehyde. Pretreatment of HUVECs with didymin also prevented the HG-induced decrease in eNOS and increase in iNOS expressions. Further, didymin prevented the HG-induced monocytes cell adhesion to endothelial cells, expressions of ICAM-1 and VCAM-1and activation of NF-κB. Didymin also prevented the release of various inflammatory cytokines and chemokines in HG-treated HUVECs. In conclusion, our results demonstrate that didymin with its anti-oxidative and anti-inflammatory actions prevents hyperglycemia-induced endothelial dysfunction and death. Thus, it could be developed as a potential natural therapeutic agent for the prevention of cardiovascular complications in diabetes.
Keywords: Didymin, Endothelial cells, Hyperglycemia, Oxidative Stress, Inflammation
Graphical Abstract
1. Introduction
Several studies have shown that long-term diabetes is associated with the secondary vascular complications [1,2]. People with diabetes have a higher atherosclerotic disease burden and an increased risk of myocardial infarction [3]. In addition, in hyperglycemia increased inflammation and oxidative stress has been shown to be highly deleterious for endothelial cell dysfunction [4]. Vascular endothelial cells are crucial in maintaining the homeostasis of the cardiovascular system [5]. They provide a physical barrier between the vessel wall and lumen; the endothelium secretes a number of mediators such as inflammatory cytokines, adhesion molecules, nitric oxide (NO), and prostaglandins which regulate platelet aggregation, coagulation, fibrinolysis, and vascular tone [6–9]. The disruption of normal endothelial cell function has been implicated in the pathophysiology of different forms of cardiovascular diseases (CVD), including hypertension and atherogenesis [7,10]. A vast body of literature indicates that hyperglycemia-induced oxidative stress and inflammatory responses constitute major risk factors for the endothelial dysfunction [4,11]. Therefore, identification of novel therapeutic agents with potential anti-oxidative and anti-inflammatory activities will have greater clinical significance in controlling endothelial dysfunction and death.
From past few decades, flavonoids such as naringin, curcumin, hesperidin, diosmin, and rutin have provoked interest in drug discovery as they exhibited various biological and pharmacological effects including antioxidative, anti-allergic, anti-inflammatory, anti-mutagenic, and anti-carcinogenic [12–17]. Though most of the plant-derived flavonoids have been shown to prevent endothelial dysfunction in experimental cell culture and animal models, only a few of them have been shown to be effective in some clinical studies (18–20). Thus, there is a need to find more potent alternative treatment strategies to prevent endothelial dysfunction which could be developed further for the clinical use.
Didymin is a naturally occurring flavonoid (family; flavonoid O-Glycosides) found in various citrus fruits such as oranges, lemons, mandarin, and bergamot [21,22]. Recent studies showed that didymin has an antiproliferative effect as it prevents the growth of various cancer cells [21–23]. Specifically, it has been shown to cause cell death in non–small cell lung cancer cells in a p53-independent manner [22,24]. Further, it also inhibits proliferation of neuroblastomas cells by inhibiting N-Myc and up-regulating Raf kinase inhibitor protein (RKIP) [24–27]. Didymin also protects the hepatic cells from CCl4-induced hepatotoxicity by regulating the NF-κB signaling pathways [25]. Apart from its antiproliferative effect, didymin has shown some strong free radical scavenging activity which is mediated by inhibiting the CYP2E1 as well as preventing the lipid peroxidation [25,27]. Despite its well emerging role as an anti-mitogenic and anti-oxidative agent in preventing various cancers, its potential use as anti-inflammatory and anti-atherogenic is not well known.
Moreover, the mechanisms by which didymin prevents endothelial dysfunction and death is still unclear. Therefore, in the present study, we have examined the protective effects of didymin on high glucose-induced endothelial cells death and dysfunction. Our results suggest that didymin prevents high glucose-induced endothelial dysfunction via its anti-oxidative and anti-inflammatory properties. Thus, the results of this study provide evidence for a potential therapeutic use of didymin in endothelial dysfunction, a major cause of the cardiovascular complications in hyperglycemia.
2. Materials and methods
2.1 Chemicals and Reagents
Analytical grade Didymin, d-(+) -glucose and methyl-thiazolyl-diphenyl-tetrazolium bromide (MTT) were purchased from Sigma-Aldrich (St Louis, MO, USA). Endothelial cell medium (ECM) and ECGS supplements were purchased from the ScienCell Research Laboratory (Carlsbad, CA, USA). Fetal bovine serum (FBS) was obtained from Gemini Bio-Products (West Sacramento, CA, USA). RIPA buffer and IκB-α (sc-1643), VCAM-1 (SC1504) antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Antibodies against phospho-NF-κB (p65) (3033), Bcl2 (2872), Bax (2772), phospho-Erk1/2 (4370), Erk1/2 (4695), Poly-ADP-ribose polymerase (PARP) (9542), Caspase-3 (9662), ICAM-1 (4915), eNOS (9586), histone H3 (4499) and GAPDH (2118) were obtained from Cell Signaling Technologies (Danvers, MA, USA). Antibodies against iNOS (ab3523) were obtained from Abcam (Cambridge, MA, USA). Penicillin/streptomycin, trypsin/EDTA, Annexin V alexa fluor-488 conjugate, calcein AM, CM-H2DCFDA, hydroxyphenyl fluorescein (HPF) were purchased from Molecular Probes, Invitrogen (Eugene, OR, USA). Malondialdehyde (MDA) detection kit was obtained from OxisResearch (Foster City, CA, USA). Human Milliplex Angiogenic Cytokine Multiplex kit was obtained from EMD Millipore (Burlington, MA, USA). The structure of didymin is reported elsewhere [21].
2.2 Cell Culture
Human umbilical vein endothelial cells (HUVECs) and Human THP1 monocytic cells were obtained from the American Type Culture Collection (ATCC), Manassas, VA, USA. HUVEC cells were grown in complete Endothelial Cell Medium (ECM) containing 5.5mM glucose, Endothelial Cell Growth Supplement (ECGS) along with 5% FBS and 1% penicillin/streptomycin. All experiments were carried out with HUVECs in the passage of 6–8. THP1 cells were grown in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS) and penicillin/streptomycin at 37°C in a humidified atmosphere of 5% CO2. To examine the effect of high glucose (HG), 19.5mM glucose was added to the culture media already containing 5.5mM glucose to achieve a final concentration of 25mM. Didymin was dissolved in a DMSO to make a stock solution (10 mM) and further diluted in the culture media to obtain a final concentration.
2.3 Cell viability assays
Confluent HUVECs cells were growth-arrested with 0.1% serum containing ± didymin (10 & 20 μM) overnight, followed by stimulation with HG for another 24h. After the incubation period, cell viability was determined by MTT assay as well as cell counting using a hemocytometer as described earlier [28]. Live and dead cells were detected by using green-fluorescent cell-permeant dye calcein AM and propidium iodide (PI, 5.0μg/ml) solution. Further, in another set of experiments, equal number of treated HUVECs were suspended in 500μl 1×PBS solution in flow cytometry tubes. These cells were treated with PI (5.0μg/ml) and incubated at room temperature for 15 min in the dark. The stained cells were analyzed by FACS using BD LSRII Fortessa Flow Cytometry Analyzer.
2.4 Analysis of cellular apoptosis by flow cytometry
Growth-arrested HUVEC cells were treated with HG (25mM) without or with didymin (20μM) for 24h. After the incubation, the cells were washed and resuspended in 10mM HEPES-buffered saline containing 2.5mM CaCl2, then incubated with 0.5μg/ml FITC-Annexin V and 5μg/ml PI for 15 min on ice in the dark.
2.5 Determination of ROS levels in HUVECs
Intracellular ROS accumulation was measured by immunocytochemistry as well as flow cytometry by using CM-H2DCFDA. The cells were stimulated with HG (25mM) without or with didymin (20 μM) for 3h. The cells were stained with CM-H2DCFDA for 15 min and photographs (20×) were taken under the microscope (Nikon Eclipse E800 epifluorescence microscope) and fluorescence intensity was determined at 495/517 (excitation/emission) by using a florescence microplate reader (Biotek Synergy 2 modular multimode reader). In another set of experiments, cells were stained with CM-H2DCFDA for 15 min and analyzed immediately by a Flow Cytometer (BD LSRII Fortessa). Data analysis was performed using FlowJo (Treestar, Ashland, OR, USA). Further, HUVECs treated with HG without or with didymin were incubated with hydroxyphenyl fluorescein (HPF), which stain hydroxyl radical and peroxinitrite radicals, and fluorescence intensity was determined by flow cytometry.
2.6 Determination of nitric oxide levels
Nitrite is a stable metabolite of nitric oxide (NO), which was measured in culture media with the Griess reagent [29]. Briefly, upon completion of treatment, an equal amount of cell culture media of each treatment group was concentrated by freeze drying.100 μL concentrated culture media was added to a 96 well microtiter plate and mixed with 100 μL of Griess reagent (Sigma-Aldrich). The plate was then read on a microtiter plate reader using a 540 nm filter.
2.7 Determination of MDA Levels
Malondialdehyde (MDA) levels were determined to examine the effect of didymin on HG-induced oxidative stress in HUVECs using a kit from Oxis International Inc., as per manufacturer’s protocol. Briefly, cells were stimulated with HG (25mM) in the absence or presence of didymin (20 μM) for 24h. The cells were washed twice with 1× PBS and lysed in PBS by sonication and centrifuged at 3000×g for 10 mins at 4°C to remove cell debris. The supernatant was collected and used for determining the MDA levels. Total MDA levels (μM) were calculated based on the standard curve and normalized to protein levels.
2.8 Determination of NF-κB translocation
HUVECs were seeded in the chambered slide and allowed to form a monolayer. Cells were kept at 0.1% FBS containing media overnight in the absence and presence of didymin (20μM). Next day, the cells were treated with HG for 2h. HUVECs were then fixed with 4% paraformaldehyde for 15 min and washed with PBS. Cells were incubated with 5% goat serum in PBS for 1h at RT to block nonspecific binding. Primary antibodies against p65 were diluted 1:500 in 1% BSA contain 0.3% Triton X-100, and cells were incubated overnight with the diluted antibodies at 4°C. Cells were then washed with PBS followed by incubation with appropriate Alexa-488 secondary antibodies for 1 h at room temperature in the dark. Fluorescence in the cytoplasm as well as in the nucleus was evaluated by using a Nikon Eclipse E800 epifluorescence microscope.
2.9 Determination of monocyte adhesion to endothelial cells
In vitro monocyte cell adhesion assay was performed as described earlier by Tammali et al. [30]. Briefly, HUVECs were seeded in 96-well plates at a density of 4000cells/well. The cells were pretreated with didymin (20 μM) followed by HG without or with didymin for overnight. Subsequently, HUVECs were rinsed twice with serum-free media, and calcein AM pre-stained THP1 cells were added in a ratio of 1:3 (HUVEC: THP1) and incubated with HG ± didymin for another 6 h. After completion of the incubation period, cells were washed with PBS to remove un-attached THP-1 cells and photographs were taken under the EVOS epifluorescence microscope. In another set of experiments, at the end of treatment of HUVECs, 100μL of media containing MTT was added and incubated for another 3 h followed by the addition of DMSO. Absorbance was recorded at 570 nm by using an ELISA plate reader.
2.10 Western Blot Analysis
Growth-arrested HUVECs were stimulated with HG (25 mM) in the absence and presence of didymin (20μM) at different time points. Cells were lysed with RIPA buffer and protein extract was collected. Equal amounts of protein in the cell lysates were separated on 12% SDS-polyacrylamide gels and transferred to polyvinylidene difluoride (PVDF) membranes. Membranes were then blocked with 5% nonfat dried milk and incubated with the specific primary antibodies at 4°C overnight followed by incubating with the specific secondary antibodies. Immunolabeling was detected using SuperSignal West Pico Chemiluminescent Substrate (ECL) from Thermo Scientific (Waltham, MA, USA). The membranes were stripped with Restore PLUS stripping buffer from Thermo Scientific following manufacturer’s instructions and reprobed with other antibodies.
2.11 Determination of Inflammatory Cytokines Secreted by HG-induced HUVECs
Human Angiogenesis/Growth Factor Magnetic bead panel kit from Millipore was used to determine various inflammatory cytokines and chemokines. HUVECs were stimulated with HG (25mM) in the absence or presence of didymin (20μM) for 24 h. After completion of incubation period, equal amounts of cell culture media were collected and concentrated from each group. The concentrated media was incubated with the labeled magnetic beads overnight. Next day, the magnetic beads were counterstained with Streptavidin-phycoerythrin and cytokine levels were analyzed with a Luminex analyzer from Millipore. The results are expressed as pg/mL based on the standard curve generated with the standards.
2.12 Data availability
The data supporting the findings of this study are available within the article. All other relevant source data are available from the corresponding author upon request.
2.13 Statistical Analysis
Data presented as mean ± SD and p< 0.05 was considered as statistically significant. The statistical values were determined by Student’s “t” test for pair-wise and one-way ANNOVA for multiple comparisons using GraphPad Prism Software.
3 Results
3.1 Didymin protects HG-induced HUVECs death
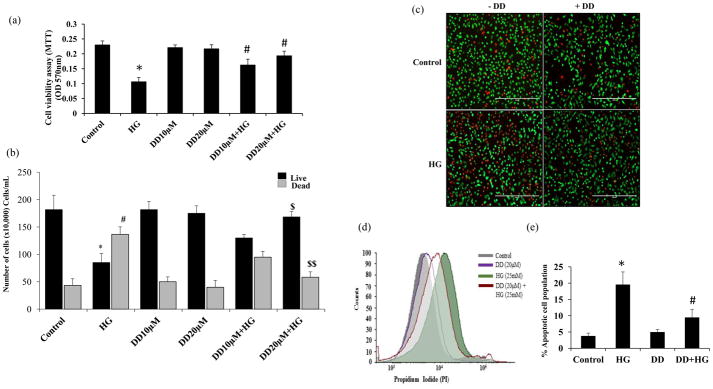
The effect of didymin on HG-induced cytotoxic signals in HUVECs is not known, therefore, we first examined the effect of didymin on HG-induced HUVEC’s viability. The growth arrested HUVECs were treated with HG in the absence and presence of didymin for 24 h, and cell viability was determined by MTT assay. The data shown in the Fig 1a indicate that the treatment of HUVECs with HG caused a significant decrease (54%) in the cell growth. However, pre-treatment of HUVECs with didymin (10 μM and 20 μM) prevented the HG-induced reduction in the cell growth. Didymin alone did not cause any effect on the cell growth, indicating that didymin by itself does not alter the HUVECs growth at the concentrations used (Fig 1a). Since 20 μM concentration of didymin almost restored the cells growth to normal as compared to 10 μM didymin. Therefore, in all our experiments, we have used 20 μM didymin as an optimal concentration. The results obtained from MTT were further confirmed by flow cytometry, immunostaining and counting the number of cells by hemocytometer. Similar to the data obtained from MTT assay, manual cell counting also demonstrated that didymin restored the HG-induced decrease in cell number (Fig 1b). Similar results were observed when HUVECs stained with FITC-calcein-AM and propidium iodide (PI) to determine the live and dead cells respectively using a fluorescence microscope (Fig 1c). Further, the FACS analysis data shown in the Fig 1d suggest that the treatment of HUVEC with HG increased the number of dead cells as compared to didymin+HG treated cells indicating didymin protects HG-induced cytotoxicity. However, treatment with didymin alone could not cause any significant effect on cell viability (Fig 1c & 1d). Similar results were also observed when HUVECs were stained with FITC-Annexin V and subjected to FACS for determination of apoptotic cells (Fig 1e). Thus, our studies demonstrate that didymin prevents HG-induced cytotoxicity in HUVECs.
Figure 1. Effect of didymin on HUVECs viability.
HUVECs were growth arrested in 0.1% serum containing media with indicated concentrations of didymin overnight followed by stimulation with HG (25 mM) for 24 h. (a) The cell viability was determined by MTT assay. Values are mean ± SD (n = 5).*p < 0.001 Vs. control and #p< 0.005 Vs. HG-treated group. (b) Cell number was determined using a hemocytometer. *p<0.01 Vs. control live; #p<0.01 Vs. control dead; $p<0.001 Vs. HG-live; $$p<0.001 Vs. HG-dead. (c) The cells were incubated with calcein-AM (green), and propidium iodide (PI; red) for 30 min to detect live and dead cells respectively, and photographs were taken under a fluorescence microscope. A representative figure was shown. (d& e) The cell were also stained with PI (d) and annexinV (e) and subjected to flow cytometry to determine the apoptosis. Values are mean ± SD (n = 5). *p < 0.001 Vs. control and #p< 0.005 Vs. HG-treated group.
3.2 Didymin prevents HG-induced ROS levels in HUVECs
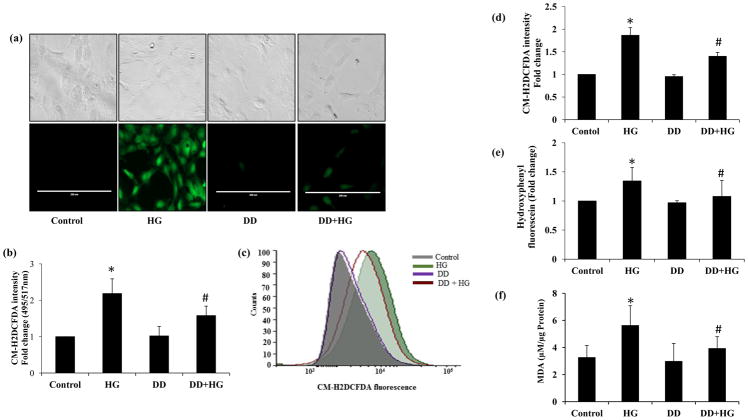
Since ROS play a significant role in HG-induced cytotoxic signals and the effect of didymin on HG-induced ROS generation is not known, therefore, we next examined the effect of didymin on the HG-induced ROS in HUVECs. HUVECs were treated with HG ± didymin for 3h followed by staining the cells with CM-H2DCFDA. Fluorescence microscopy was used to determine the green DCFDA fluorescence indicating the presence of ROS in the cells. A significantly increased green fluorescence was observed in the HG-treated cells only but not in control or didymin alone-treated cells. Further in HG+didymin-treated cells, the intensity was significantly reduced indicating that didymin prevents HG-induced ROS production in HUVECs (Fig 2a). Similar results were observed when we quantitatively measured ROS levels by fluorometrically. The results shown in the Fig 2b indicate that a ~2-fold increase in ROS level in the HUVECs treated with HG. Pretreatment of HUVECs with didymin significantly attenuated HG-induced ROS levels. However, didymin alone did not cause any significant change in the ROS levels and was comparable to the untreated control cells. Similarly, flow cytometry analysis also indicates that pre-incubation with didymin significantly attenuated HG-induced ROS production in HUVECs (Fig 2c and 2d). Furthermore, didymin also prevents HG-induced hydroxyl and peroxynitrite radicals as determined by staining the cells with hydroxyphenyl fluorescein (Fig 2e). Since during oxidative stress ROS is known to cause lipid peroxidation, we next examined the effect of didymin on HG-induced lipid peroxidation by measuring malondialdehyde (MDA) levels, a marker for oxidative stress. Our results shown in Fig 2f indicate that didymin significantly prevented HG-induced formation of MDA levels in HUVECs. Didymin alone does not affect the formation of MDA in HUVECs. Thus, our results suggest that didymin prevents HG-induced ROS as well as lipid peroxidation and thereby could prevent HG-induced cytotoxicity in the HUVECs.
Figure 2. Didymin attenuates HG-induced ROS production in HUVECs.
The HUVECs were treated HG without or with didymin for 3 h and further stained with CM-H2DCFDA for 30 min. (a) Fluorescence intensity in the live cells was captured by taking pictures (20×) under a fluorescence microscope (green channel). A representative image was shown from each group. (b) The fluorescence intensity (a) was quantified fluorometrically at 495/517 excitation/emission (c) The CM-H2DCFDA stained cells were also subjected to FACS analysis. (d) Graph bars are showing fold change in CM-H2DCFDA Mean Fluorescence Intensity (MFI) measured by flow cytometry in HUVECs. (e) The HUVECs were treated HG without or with didymin for 3h and further stained with hydroxyphenyl fluorescein (HPF) for 30 min. The fluorescence intensity of HPF was quantified fluorometrically. (f) The malondialdehyde (MDA) levels were determined by using a kit from Oxis International Inc. MDA values (μM/μg protein). Bars represent mean ± SD (n = 3).*p< 0.001 when compared with control and #p< 0.01, ## p<0.001 when compared with HG-treated group.
3.3. Didymin prevents HG-induced activation of caspase-3, ERK1/2, and Bcl2 family proteins
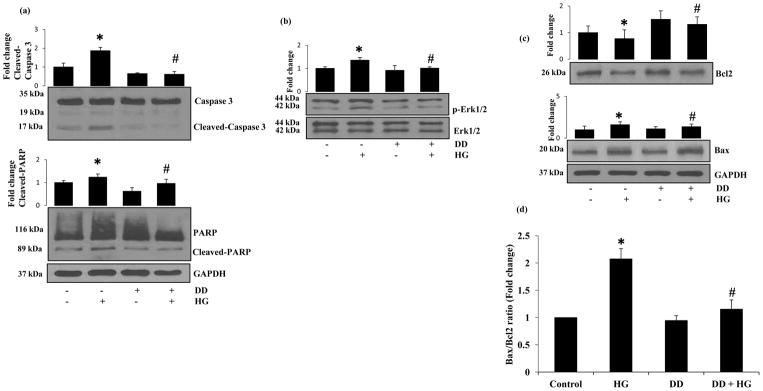
Since caspase-3 plays a critical role in apoptosis, we next examined the effect of didymin on HG-induced activation of caspase-3 in HUVECs. Incubation of HUVECs with HG for 24 h caused an approximately 2-fold increase in the cleaved caspase-3 expression and pre-incubation with didymin followed by stimulation with HG significantly attenuated activation of caspase-3 (Fig 3a). Caspase-3 activation was further confirmed by measuring the HG-induced PARP cleavage. Treatment of HUVECs with HG caused a significant increase in the cleavage of PARP and pre-incubation with didymin prevented it. These results thus suggest that didymin prevents HG-induced apoptosis by inhibiting the activation of caspase-3 in HUVECs. Since MAPKinases such as Erk1/2 have been shown to be involved in the activation of caspase-3 and induction of apoptosis [31], we next examined the effect of didymin on HG-induced activation of Erk1/2 in HUVECs. The data shown in the Figure 3b indicate that HG-induced the phosphorylation of Erk1/2 and preincubation of cells with didymin prevented it. However, didymin alone did not alter the expression of total ERK1/2 (Fig 3b). We next determined the effect of didymin on the expression of apoptotic markers such as Bcl-2 and Bax in HG-treated HUVECs. Stimulation of HUVECs with HG down-regulated the expression of Bcl2 and up-regulated the expression of Bax (Fig 3c). However, pretreatment with didymin significantly prevented the down-regulation of Bcl2 and up-regulation of Bax by HG in HUVECs.
Figure 3. Didymin prevents HG-induced Caspase-3 and PARP cleavage, phosphorylation of Erk1/2 and induces Bcl2 family protein expression in HUVECs.
Serum-starved HUVECs were either left untreated or pre-incubated with didymin (20 μM) for overnight followed by stimulation with HG (25 mM) for 24 h. (a–c) An equal amount of protein in the cell extracts were subjected to Western blot analysis by using specific antibodies against Caspase-3, PARP, p-Erk1/2, BCl2 and Bax proteins. A representative image was shown from 3 independent analysis. Fold changes were calculated by densitometry of Western blot using ImageJ software and normalized with loading controls. (d) The ratio Bax/Bcl2 was quantified. Bars represent mean ± SD (n = 3). *p< 0.001 when compared with control and #p< 0.001 when compared with HG-treated group.
3.4 Didymin inhibits HG-induced monocyte adhesion to endothelial cells
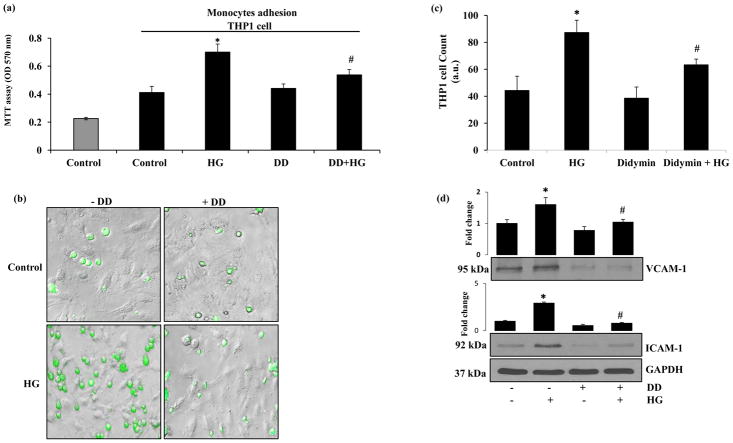
Since hyperglycemia is known to exacerbate endothelial dysfunction by increasing the monocyte adhesion to endothelial cells, we next examined whether didymin regulates HG-induced monocyte adhesion to HUVECs. The untreated control cells displayed a minimal adhesion of THP1 cells to HUVECs within 6h of incubation (Fig 4a–c). However, when HUVECs were stimulated with HG followed by addition of THP1 cells for 6 h monocyte adhesion was significantly (70%) increased. However, the addition of THP1 cells to the HG+didymin treated cells showed a significantly decreased adhesion of THP1 cells to endothelial cells (28%) as compared to HG-alone. These data indicate that didymin prevents endothelial-monocyte adhesion induced by hyperglycemia.
Figure 4. Didymin inhibits HG-induced adhesion of monocytes to HUVECs.
HUVECs (3000 cells/well) in 96-well plates were pretreated without or with didymin (20μM) followed by HG for overnight. Subsequently the HUVECs were incubated with calcein-AM stained THP1 cells for an additional 6 h. (a) Monocyte adhesion to HUVECs was determined by MTT absorbance. (b) The THP1 cells were pre-incubated with calcein-AM for 15 min before adding to HUVECs to examine the adhesion by microscopically. The photographs were taken under a fluorescence microscope in the green channel (10×). (c) The number of green fluorescent THP1 cells adhered to HUVECs were determined. (d) HUVECs were treated without or with didymin (20 μM) for overnight followed by incubation with HG (25 mM) for additional 6 h. Equal amounts of cell extracts were subjected to Western blot analysis using specific antibodies against VCAM-1 and ICAM-1. GAPDH was used as a loading control. A representative image was shown from 3 independent analysis. Fold changes were calculated by densitometry of Western blot using ImageJ software and normalized with loading controls. Bars represent mean ± SD (n = 5). *p< 0.001 when compared with control and #p< 0.005 when compared with HG-treated group.
Since expression of various cell surface adhesion molecules such as intracellular adhesion molecule (ICAM-1), vascular cell adhesion molecule (VCAM-1) on endothelial cells plays a critical role in endothelial dysfunction, we next examined the effect of didymin on the expression of HG-induced expression of adhesion molecules in HUVECs. Our results shown in the Fig 4d indicate that the treatment of HUVECs with HG significantly induced the expression of VCAM-1 and ICAM-1. However, pre-treatment of HUVECs with didymin followed by HG significantly prevented the HG-induced increase in the expression of ICAM-1 and VCAM-1. Didymin alone did not produce any significant change in the expression of these adhesion molecules. Thus, our results suggest that didymin could prevent HG-induced monocyte adhesion to endothelial cells by attenuating the expression of HG-induced adhesion molecules.
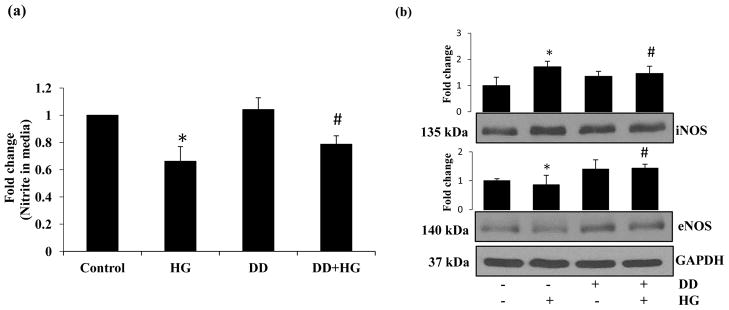
3.5 Didymin regulates HG-induced nitric oxide levels and expression of iNOS/eNOS in HUVECs
To further examine the protective effect of didymin on HG-induced endothelial dysfunction, we examined the HG-induced NO levels as well as expression of iNOS/eNOS proteins in HUVECs. We first measured the release of NO in the media of HUVECs treated with HG ± didymin for 24h. The data shown in Fig 5a indicate that HG-decreased the levels of nitrite/nitrate in the culture media of HUVECs treated with HG for 24 h. However, this decrease was not observed in the cell culture media obtained from HG+didymin treated cells. The levels of NO in HG+didymin -treated cells are almost similar to the untreated control and didymin alone-treated cells. Further, HG increased the expression of iNOS and decreased the expression of eNOS in HUVECs and pre-incubation of HUVECs with didymin reversed the HG-induced changes in the expression of eNOS and iNOS proteins (Fig 5b).
Figure 5. Didymin prevents HG regulated NO and eNOS/iNOS levels in HUVECs.
The HUVECs were treated with HG in the without or with didymin (20 μM) for 24 h. (a) NO levels were measured in culture media by using Griess reaction method as described in the methods. The data expressed as fold change when compared to control. Bars represent mean ± SD (n = 6). (b) An equal amount of protein in the cell extracts were subjected to Western blot analysis by using specific antibodies against eNOS and iNOS proteins. A representative image was shown from 3 independent analysis. Fold changes were calculated by densitometry of Western blot using ImageJ software and normalized with loading controls. *p< 0.05 when compared with control and #p< 0.01 when compared with HG-treated group.
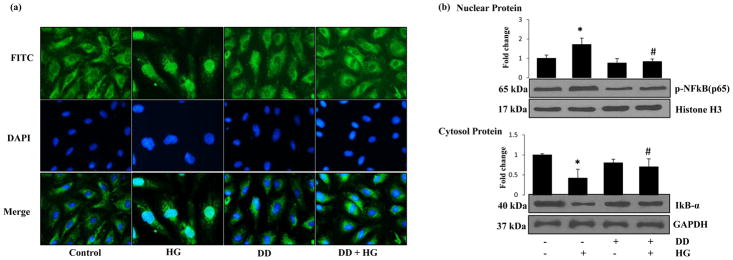
3.6 Didymin prevents HG-induced NF-κB activation in HUVECs
Since NF-κB is a redox-sensitive transcription factor known to be involved in the transcription of genes for iNOS, adhesion molecules, and inflammatory cytokines, we next examined if the cytoprotective effect of didymin is due to prevention of HG-induced activation of NF-κB. First, we examined the effect of didymin on the prevention of NF-κB nuclear translocation. HUVECs cells treated with HG for 2h in the absence and presence of didymin were immune-stained with p65 antibodies. Results shown in the Fig 6a indicate that HG-induced the nuclear translocation of p65 and didymin prevented it. Western blot analysis of nuclear protein further confirmed these results. The data shown in the Fig 6b suggests that HG increased the expression p65 in the nucleus and this increase was significantly reduced in didymin pretreated cells. Similarly, HG also caused degradation of IκB-α protein in the cytosolic extracts, but this decrease was not observed in the HG+didymin treated cells (Fig 6b). Thus, these results suggest that didymin prevents HG-induced IκB-α degradation and nuclear translocation and activation of NF-κB in HUVECs.
Figure 6. Didymin prevents the HG-induced activation of NF-κB.
Serum-starved HUVECs were either left untreated or pre-incubated with didymin (20 μM) for overnight followed by stimulation with HG (25mM) for 3 h. (a) NF-κB localization was determined by immunostaining the cells with antibodies against p65. The nuclear location was identified by staining the cells with DAPI. (b) An equal amount of protein in the nuclear extracts and cell extracts were subjected to Western blot analysis by using specific antibodies against phospho-p65, histone H3, IκB-α and GAPDH. A representative image was shown from 3 independent analysis. Fold changes were calculated by densitometry of Western blot using ImageJ software and normalized with loading controls. *p< 0.05 when compared with control and #p< 0.01 when compared with HG-treated group.
3.7 Didymin prevents HG-induced inflammatory markers release in HUVECs
Since NF-κB is known to increase the expression of various inflammatory markers responsible for endothelial dysfunction and death; we next examined the effect of didymin on the HG-induced expression of different inflammatory markers in HUVECs. Results shown in Table-1 indicate a significant increase in the expressions of EGF, TNF-α, Interferon-α2, G-CSF, MCP-1, IL-1β, IL-1α, IL-2, IL-5, IL-6, IL-8, and IL-10 was observed in the HG-treated HUVECs culture media. However, in the cells treated with HG+didymin, the expression of various inflammatory markers was significantly reduced. As compared to control and didymin alone treated cells do not show significant changes in the expression of inflammatory markers. Thus, these results suggest that didymin prevents HG-induced inflammatory marker expression which could be responsible for HG-endothelial dysfunction.
4.Discussion
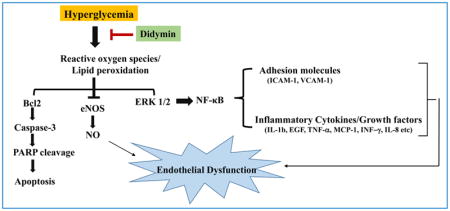
In this study, we demonstrate that didymin prevents HG-induced endothelial dysfunction and death, a major pathological event involved in the development of cardiovascular complications. Our results suggest that didymin prevents HG-induced oxidative stress that leads to activation of NF-κB-mediated inflammatory cytokines and growth factors responsible for endothelial dysfunction. Specifically, we have shown that didymin prevents endothelial death by preventing the HG-induced ROS/Caspase-3/Bcl2/MAPK and endothelial dysfunction by NO/eNOS/ICAM/VCAM/NF-κB pathways. To the best of our knowledge, this is the first report to demonstrate the effect of didymin in the prevention of endothelial dysfunction.
Over the last few decades, the development, as well as therapeutic use of plant-derived bioactive compounds, have been significantly increased due to changes in the current lifestyle. Most of the natural antioxidants, used as food supplements, have been shown to be effective in preventing some disease complications with no major side effects [32]. Several studies have shown that phytochemicals such as flavonoids, polyphenols, and quinones act as antioxidants and prevent oxidative stress-induced immune and inflammatory complications leading to various diseases including cancer [33,34]. Antioxidants such as curcumin, quercetin, NAC, Vitamin C, aspalatone, and resveratrol have been shown to be potent chemopreventive agents that can prevent cancer growth and metastasis [13, 35–39]. Further, these antioxidants have also shown to be effective in preventing endothelial dysfunction, hypertension, and cardiovascular complications in culture as well as in animal models [40,41]. Although didymin is a flavonoid compound isolated from citrus plants, only a few studies have shown its significance as an antimitogenic as well as anti-carcinogenic compound [22,23,42]. The neohesperidose containing glycosylated flavanones from citruses such as naringin and neoeriocitrin are bitter taste while rutinosides that contain flavanone and disaccharides such as didymin and narirutin have no taste [43]. Gattuso et al [44] have shown that orange (C.sinensis) juice contains 1.89 ± 0.92 mg/100 mL of didymin and 0.30± 0.04 mg/100 mL in grapefruit (C. paradisi) juice. Stuetz et al. [45] have shown that in Mandarin oranges (C.reticulata) peeled fruit had a high content (45–112 mg/kg) of didymin. Similarly, longlife orange juice contains 9.9 mg/L of didymin [43]. These studies indicate that some levels of didymin could be absorbed from the consumption of citrus fruits. Further, recent studies suggest that didymin could be developed for the cancer treatment [23, 24]. Singhal et al. [23] have treated mice with 2 mg/kg body wt of didymin and examined serum for didymin by HPLC analysis. They found that didymin has effective oral absorption with free didymin levels of 2.1 μM in serum. At the same time, the same amount of didymin prevents the growth of neuroblastoma xenograft tumors in mice. Although, these studies suggest that didymin is an excellent antioxidant with potential anti-carcinogenic activities, its efficacy in the prevention of vascular complications has not been explored. Therefore, in this study, we investigated the protective effects of didymin on HG-induced endothelial cell dysfunction and death.
Normal endothelial cells maintain the cardiovascular homeostasis by promoting vasodilation and inhibiting the platelet aggregation [9]. However, disruption of normal endothelial function by factors such as hyperglycemia, cigarette smoke, hypercholesterolemia and other oxidative stress conditions could lead to increase in the endothelial cell death, cause vasoconstriction and increases platelet aggregation [46,47]. Endothelial dysfunction leads to cardiovascular complications such as atherosclerosis, and restenosis [47]. Increased oxidative stress is one of the major cause of endothelial toxicity as it regulates various cellular processes leading to cell growth, differentiation, apoptosis, DNA damage, and dysfunction [48,49]. Several studies have shown that antioxidants protect and preserve endothelial cell integrity and function from oxidative stress by reducing the formation of ROS as well as preventing the redox signals [49,50]. Consistent with these results, didymin also significantly prevented HG-induced ROS generation and lipid peroxidation which could be involved in the endothelial cell apoptosis.
Caspase-3 is a well-known mediator of apoptotic cell death, and ROS has been shown to activate caspase-3 mediated cell death pathways [51]. Therefore, we examined the effect of didymin on HG-induced activation of caspase-3. Our data suggest that didymin prevents HG-induced caspase-3 activation as well as PARP cleavage. Since activation of MAPKinases such as p38MAPK and Erk1/2 has been shown to activate caspase-3 and participate in apoptotic pathways [31,52], therefore, we examined the effect of didymin on Erk1/2 activation. Several antioxidants such as curcumin, NAC, taurine, tetrahydroxy xanthone have also been shown to prevent activation of MAPKs and inhibits caspase-3–mediated apoptosis of endothelial cells [53–56]. Similar to those studies, our results also indicate that didymin prevents HG-induced activation of Erk1/2 and prevents apoptosis. It has been shown that Bcl2 family of proteins regulates cellular apoptosis as well as activation of caspase-3 [57]. Further, the ratio of Bax/Bcl2 has been demonstrated to be a significant regulator of apoptotic cell death [58]. We observed that HG-induces apoptosis of endothelial cells by downregulating the Bcl2 and upregulating the Bax proteins. Our results show that didymin restored HG-induced decrease in the Bcl-2 and inhibited the HG-induced Bax in HUVECs. Thus, by preventing the expression of Bcl2 proteins as well as activation of caspase-3 didymin could prevent HG-induced endothelial cells death.
Monocytes have been shown to be important regulators of the immune response and play a major role in angiogenesis [59,60]. Monocytes adhere to the endothelial cells and cause endothelial dysfunction, death as well as the proliferation of smooth muscle cells leading to the development of atherosclerosis [59,60]. The monocyte adhesion is mediated by the increased expression of adhesion molecules, such as ICAM-1 and VCAM-1, and increased secretion of chemokines such as MCP-1[61]. Several natural and synthetic agents have been shown to inhibit leukocyte adhesion to endothelial cells by preventing the expression of adhesion molecules such as ICAM-1, VCAM-1, and E-selectin [62]. In the current study, we found that didymin prevented HG-induced monocyte adhesion to endothelial cells as well as expression of VCAM-1 and ICAM-1 in HUVECs indicating that didymin prevents monocyte adhesion to endothelial cells by inhibiting the expression of adhesion molecules.
The decrease in the bioavailability of nitric oxide (NO), a critical regulator of vascular tone, could lead to endothelial dysfunction [9,63]. The eNOS but not iNOS is primarily responsible for the generation of NO in the vascular endothelium and eNOS is responsible for various vascular diseases [64,65]. It is known that persistent hyperglycemia disrupts normal endothelial function via production of reactive oxygen metabolites [54,66]. Impairment of endothelium-dependent vasorelaxation is also caused by a loss of NO activity in the vessel walls [63]. Antioxidants such as curcumin and naringin have been shown to restore NO levels and improve endothelial dysfunction in vitro and in vivo [67, 68]. We have found that treatment with didymin increased HG-depleted eNOS protein expression and restored NO levels, suggesting that by restoring NO levels didymin could improve endothelial dysfunction.
Several lines of evidence suggest that hyperglycemia-induced changes in metabolism and signaling have been linked to the increased formation of ROS and AGEs, which further induces the redox-sensitive transcription factors such as NF-κB that transcribe several cytokines, adhesion molecules, and growth factors and leads to endothelial cell activation [66–68]. Our data showed that pre-treatment of HUVECs with didymin decreased the HG-induced NF-κB activation, which was accompanied by decreased translocation of p65 to the nucleus. It has been shown that in atherosclerotic lesions, activated NF-κB localizes to sites expressing high levels of adhesion molecules, and inhibition of NF-κB prevents the monocyte adhesion by attenuating the expression of ICAM-1 and VCAM-1. Elevated levels of inflammatory markers such as interleukins (IL-1, IL-6, and IL-8 etc.), tumor necrosis factor (TNF), MCP-1, and interferons have been shown to be responsible for vascular complications during diabetic conditions. Several antioxidants, which can prevent NF-κB -mediated production of inflammatory markers have been shown to prevent endothelial dysfunction as well as atherosclerosis [69,70]. Our results have also demonstrated (Table-1) that treatment with didymin significantly prevented the HG-induced release of various cytokines and growth factors in endothelial cells suggesting that by preventing NF-κB -mediated release of inflammatory markers didymin could prevent endothelial dysfunction.
In conclusion, our results show that didymin could significantly inhibit the apoptosis of endothelial cells induced by HG via modulating the oxidative stress signals leading to the generation of ROS as well as activation of caspase-3, Erk1/2 and regulation of Bcl2 proteins. Further, didymin also prevents HG-induced endothelial dysfunction by preventing the monocyte adhesion to endothelial cells, restores eNOS and NO levels, prevents expression of iNOS, ICAM-1, VCAM-1, and NF-κB-mediated production of inflammatory cytokines. Thus, didymin with its potent anti-oxidant and anti-inflammatory properties has the potential for use in various clinical applications. Specifically, its use at low-dose when compared to various other flavonoids and plant-derived antioxidants along with its broad spectrum of effect on multiple cellular signaling pathways make didymin an exciting and potential candidate for further development as a therapeutic agent. Overall, our results suggest that didymin could be a potential novel compound to prevent endothelial dysfunction and related cardiovascular complications.
Table 1.
Didymin prevents HG-induced inflammatory cytokines and growth factor levels in HUVECs.
| Cytokines/Growth factors | Control | HG | DD | HG + DD |
|---|---|---|---|---|
| EGF (pg/mL) | 1218 ± 95.1 | 2700 ± 317.0** | 1270 ± 265.4 | 1717 ± 61.4# |
| FGF2 (pg/mL) | 2110 ± 147.2 | 3705 ± 149.1* | 2216 ± 225.2 | 2644 ± 323.7# |
| Flt-3L (pg/mL) | 8.88 ± 0.3 | 16.9 ± 0.7** | 9.0 ± 0.5 | 10.8 ± 0.7## |
| Fractalkine (pg/mL) | 230 ± 43.5 | 382 ± 47.1* | 237 ± 27.8 | 293 ± 30.1# |
| G-CSF (pg/mL) | 312.9 ± 38.7 | 513.5 ± 47.0* | 308.3 ± 13.4 | 429.2 ± 25.9# |
| GM-CSF (pg/mL) | 28.9 ± 2.3 | 48.0 ± 1.0* | 30.0 ± 0.9 | 33.8 ± 2.6# |
| IL-1α (pg/mL) | 12.6 ± 1.8 | 24.8 ± 0.4** | 12.4 ± 0.6 | 13.8 ± 0.9## |
| IL-1β (pg/mL) | 1.3 ± 0.2 | 3.7 ± 0.1** | 1.4 ± 0.1 | 1.8 ± 0.2# |
| IL-2 (pg/mL) | 0.41 ± 0.1 | 0.99 ± 0.2* | 0.34 ± 0.1 | 0.61 ± 0.1 # |
| IL-5 (pg/mL) | 0.25 ± 0.03 | 0.54 ± 0.06* | 0.29 ± 0.08 | 0.38 ± 0.08# |
| IL-6 (pg/mL) | 1629 ± 105 | 2938 ± 98* | 1743 ± 78 | 2196 ± 64# |
| IL-7 (pg/mL) | 14.7 ± 0.8 | 27.7 ± 1.2* | 14.2 ± 0.9 | 18.2 ± 1.7# |
| IL-8 (pg/mL) | 6082 ± 327 | 10567 ± 300** | 6091 ± 429 | 7337 ± 807## |
| IL-9 (pg/mL) | 0.71 ± 0.3 | 1.9 ± 0.2** | 0.9 ± 0.1 | 1.2 ± 0.1## |
| IL-10 (pg/mL) | 2.1 ± 0.3 | 5.0 ± 0.2** | 2.1 ± 0.2 | 3.4 ± 0.5# |
| IL-12p40 (pg/mL) | 26.1 ± 5.8 | 27.7 ± 1.3 | 26.7 ± 5.1 | 25.5 ± 0.9 |
| IL-12p70 (pg/mL) | 4.9 ± 1.0 | 5.5 ± 0.8 | 4.0 ± 0.4 | 4.6 ± 0.4 |
| IL-15 (pg/mL) | 41.5 ± 4.4 | 59.4 ± 1.8* | 47.3 ± 2.9 | 50.0 ± 4.9# |
| Interferon-α2 (pg/mL) | 28.5 ± 2.1 | 52.8 ± 2.7* | 30.6 ± 2.4 | 34.7 ± 4.1# |
| Interferon-γ (pg/mL) | 28.6 ± 2.1 | 62.8 ± 2.6** | 30.6 ± 2.4 | 34.7 ± 4.01## |
| TNF-α (pg/mL) | 1.7 ± 0.2 | 4.2 ± 0.2** | 1.6 ± 0.2 | 2.6 ± 0.1## |
| MCP-1 (pg/mL) | 6940 ± 352 | 12598 ± 477** | 7151 ± 270 | 8101 ± 436# |
The HUVECs were treated with HG in the absence and presence of didymin (20 μM) for 24 h. The levels of inflammatory markers in the culture media were determined by using a Multiplex ELISA kit from Millipore using a Milliplex Analyzer System (Luminex) as described in the methods. Bars represent mean ± SD (n = 5). *p< 0.05 & **p<0.001 compared with control and #< 0.01, ##p<0.001 when compared with HG-treated group.
Acknowledgments
Supported by funding from NIH/NIDDK grant DK104786.
Abbreviations
- DD
didymin
- eNOS
endothelial nitric oxide synthase
- FITC
Fluorescein isothiocyanate
- HG
high glucose
- HUVEC
human umbilical vein endothelial cells
- ICAM-1
intercellular adhesion molecule 1
- iNOS
inducible nitric oxide synthase
- IκB
inhibitor of kappa B
- MDA
Malondialdehyde
- NF-κB
nuclear factor-κB
- NO
nitric oxide
- ROS
reactive oxygen species
- VCAM
vascular cell adhesion molecule 1
Footnotes
Conflict of Interest: Authors declare no conflict of Interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.UK prospective diabetes study (UKPDS) Diabetologia. 1991;34(12):877–890. [PubMed] [Google Scholar]
- 2.Deshpande AD, Harris-Hayes M, Schootman M. Epidemiology of Diabetes and Diabetes-Related Complications. Physical Therapy. 2008;88(11):1254–1264. doi: 10.2522/ptj.20080020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chiha M, Njeim M, Chedrawy EG. Diabetes and Coronary Heart Disease: A Risk Factor for the Global Epidemic. International Journal of Hypertension. 2012;2012:7. doi: 10.1155/2012/697240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hadi HAR, Zubaid M, Mahmeed WA, El-Menyar AA, Ridha M, Alsheikh-Ali AA, et al. Prevalence and Prognosis of Chronic Obstructive Pulmonary Disease Among 8167 Middle Eastern Patients With Acute Coronary Syndrome. Clinical Cardiology. 2010;33(4):228–235. doi: 10.1002/clc.20751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Favero G, Paganelli C, Buffoli B, Rodella LF, Rezzani R. Endothelium and Its Alterations in Cardiovascular Diseases: Life Style Intervention. BioMed Research International. 2014;2014:28. doi: 10.1155/2014/801896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scallan J, Huxley VH, Korthuis RJ. Capillary Fluid Exchange: Regulation, Functions, and Pathology. Colloquium Series on Integrated Systems Physiology: From Molecule to Function. 2010;2(1):1–94. [PubMed] [Google Scholar]
- 7.Rajendran P, Rengarajan T, Thangavel J, Nishigaki Y, Sakthisekaran D, Sethi G, et al. The Vascular Endothelium and Human Diseases. Int J Biol Sci. 2013;9(10):1057–69. doi: 10.7150/ijbs.7502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yau JW, Teoh H, Verma S. Endothelial cell control of thrombosis. BMC Cardiovascular Disorders. 2015;15(1):130. doi: 10.1186/s12872-015-0124-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sandoo A, van Zanten J, Metsios GS, Carroll D, Kitas GD. The Endothelium and Its Role in Regulating Vascular Tone. The Open Cardiovascular Medicine Journal. 2010;4:302–12. doi: 10.2174/1874192401004010302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Widmer RJ, Lerman A. Endothelial dysfunction and cardiovascular disease. Global Cardiology Science & Practice. 2014;2014(3):291–308. doi: 10.5339/gcsp.2014.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sena CM, Pereira AM, Seiça R. Endothelial dysfunction — A major mediator of diabetic vascular disease. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 2013;1832(12):2216–2231. doi: 10.1016/j.bbadis.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 12.Alam MA, Subhan N, Rahman MM, Uddin SJ, Reza HM, Sarker SD. Effect of Citrus Flavonoids, Naringin and Naringenin, on Metabolic Syndrome and Their Mechanisms of Action. Advances in Nutrition. 2014;5(4):404–17. doi: 10.3945/an.113.005603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ali N, Soheil AE. A Review of Therapeutic Effects of Curcumin. Current Pharmaceutical Design. 2013;19(11):2032–2046. [PubMed] [Google Scholar]
- 14.Parhiz H, Roohbakhsh A, Soltani F, Rezaee R, Iranshahi M. Antioxidant and Anti-Inflammatory Properties of the Citrus Flavonoids Hesperidin and Hesperetin: An Updated Review of their Molecular Mechanisms and Experimental Models. Phytotherapy Research. 2015;29(3):323–331. doi: 10.1002/ptr.5256. [DOI] [PubMed] [Google Scholar]
- 15.Silambarasan T, Raja B. Diosmin, a bioflavonoid reverses alterations in blood pressure, nitric oxide, lipid peroxides and antioxidant status in DOCA-salt induced hypertensive rats. European Journal of Pharmacology. 2012;679(1):81–89. doi: 10.1016/j.ejphar.2011.12.040. [DOI] [PubMed] [Google Scholar]
- 16.Solomon H, Giovanni L. The Therapeutic Potential of Rutin for Diabetes: An Update. Mini-Reviews in Medicinal Chemistry. 2015;15(7):524–528. doi: 10.2174/138955751507150424103721. [DOI] [PubMed] [Google Scholar]
- 17.Sharma S, Ali A, Ali J, Sahni JK, Baboota S. Rutin: therapeutic potential and recent advances in drug delivery. Expert Opinion on Investigational Drugs. 2013;22(8):1063–1079. doi: 10.1517/13543784.2013.805744. [DOI] [PubMed] [Google Scholar]
- 18.Wang H, Khor TO, Shu L, Su ZY, Fuentes F, Lee JH, et al. Plants Against Cancer: A Review on Natural Phytochemicals in Preventing and Treating Cancers and Their Druggability. Anti-cancer agents in medicinal chemistry. 2012;12(10):1281–1305. doi: 10.2174/187152012803833026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Dam RM, Naidoo N, Landberg R. Dietary flavonoids and the development of type 2 diabetes and cardiovascular diseases: review of recent findings. Curr Opin Lipidol. 2013;24(1):25–33. doi: 10.1097/MOL.0b013e32835bcdff. [DOI] [PubMed] [Google Scholar]
- 20.Salvamani S, Gunasekaran B, Shaharuddin NA, Ahmad SA, Shukor MY. Antiartherosclerotic effects of plant flavonoids. Biomed Res Int. 2014;2014:480258. doi: 10.1155/2014/480258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hung J-Y, Hsu Y-L, Ko Y-C, Tsai Y-M, Yang C-J, Huang M-S, et al. Didymin, a dietary flavonoid glycoside from citrus fruits, induces Fas-mediated apoptotic pathway in human non-small-cell lung cancer cells in vitro and in vivo. Lung Cancer. 68(3):366–374. doi: 10.1016/j.lungcan.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 22.Wei J, Huang Q, Bai F, Lin J, Nie J, Lu S, et al. Didymin induces apoptosis through mitochondrial dysfunction and up-regulation of RKIP in human hepatoma cells. Chem Biol Interact. 2017;261:118–126. doi: 10.1016/j.cbi.2016.11.026. [DOI] [PubMed] [Google Scholar]
- 23.Singhal J, Nagaprashantha LD, Vatsyayan R, Singhal A, Awasthi S, Singhal SS. Didymin Induces Apoptosis by Inhibiting N-Myc and up regulating RKIP in Neuroblastoma. Cancer Prevention Research (Philadelphia, Pa) 2012;5(3):473–83. doi: 10.1158/1940-6207.CAPR-11-0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singhal SS, Singhal S, Singhal P, Singhal J, Horne D, Awasthi S. Didymin: an orally active citrus flavonoid for targeting neuroblastoma. Oncotarget. 2017;8(17):29428–41. doi: 10.18632/oncotarget.15204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang Q, Bai F, Nie J, Lu S, Lu C, Zhu X, et al. Didymin ameliorates hepatic injury through inhibition of MAPK and NF-κB pathways by up-regulating RKIP expression. International Immunopharmacology. 2017;42:130–138. doi: 10.1016/j.intimp.2016.11.028. [DOI] [PubMed] [Google Scholar]
- 26.Lin X, Bai F, Nie J, Lu S, Lu C, Zhu X, et al. Didymin Alleviates Hepatic Fibrosis Through Inhibiting ERK and PI3K/Akt Pathways via Regulation of Raf Kinase Inhibitor Protein. Cellular Physiology and Biochemistry. 2016;40(6):1422–1432. doi: 10.1159/000453194. [DOI] [PubMed] [Google Scholar]
- 27.Morelli S, Piscioneri A, Salerno S, Al-Fageeh MB, Drioli E, De Bartolo L. Neuroprotective Effect of Didymin on Hydrogen Peroxide-Induced Injury in the Neuronal Membrane System. Cells Tissues Organs. 2014;199(2–3):184–200. doi: 10.1159/000365072. [DOI] [PubMed] [Google Scholar]
- 28.Yadav UCS, Srivastava S, Ramana K. Prevention of VEGF-induced growth and tube formation in human retinal endothelial cell by aldose reductase inhibition. Journal of diabetes and its complications. 2012;26(5):369–77. doi: 10.1016/j.jdiacomp.2012.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Granger DL, Taintor RR, Boockvar KS, Hibbs JB. Measurement of nitrate and nitrite in biological samples using nitrate reductase and Griess reaction. Methods in Enzymology. 1996;268:142–151. doi: 10.1016/s0076-6879(96)68016-1. [DOI] [PubMed] [Google Scholar]
- 30.Tammali R, Reddy ABM, Saxena A, Rychahou PG, Evers BM, Qiu S, et al. Inhibition of aldose reductase prevents colon cancer metastasis. Carcinogenesis. 2011;32(8):1259–1267. doi: 10.1093/carcin/bgr102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cagnol S, Chambard JC. ERK and cell death: Mechanisms of ERK-induced cell death–apoptosis, autophagy and senescence. FEBS Journal. 2010;277(1):2–21. doi: 10.1111/j.1742-4658.2009.07366.x. [DOI] [PubMed] [Google Scholar]
- 32.Calixto JB. Efficacy, safety, quality control, marketing and regulatory guidelines for herbal medicines (phytotherapeutic agents) Brazilian Journal of Medical and Biological Research. 2000;33:179–189. doi: 10.1590/s0100-879x2000000200004. [DOI] [PubMed] [Google Scholar]
- 33.Pandey KB, Rizvi SI. Plant Polyphenols as Dietary Antioxidants in Human Health and Disease. Oxidative Medicine and Cellular Longevity. 2009;2(5) doi: 10.4161/oxim.2.5.9498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dai J, Mumper RJ. Plant Phenolics: Extraction, Analysis and Their Antioxidant and Anticancer Properties. Molecules. 2010;15(10):7313. doi: 10.3390/molecules15107313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sharmila G, Bhat FA, Arunkumar R, Elumalai P, Raja Singh P, Senthilkumar K, et al. Chemopreventive effect of quercetin, a natural dietary flavonoid on prostate cancer in in vivo model. Clinical Nutrition. 2014;33(4):718–726. doi: 10.1016/j.clnu.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 36.van Zandwijk N. N-Acetylcysteine (NAC) and glutathione (GSH): Antioxidant and chemopreventive properties, with special reference to lung cancer. Journal of Cellular Biochemistry. 1995;59(S22):24–32. doi: 10.1002/jcb.240590805. [DOI] [PubMed] [Google Scholar]
- 37.Lee KW, Lee HJ, Surh YJ, Lee CY. Vitamin C and cancer chemoprevention: reappraisal. The American Journal of Clinical Nutrition. 2003;78(6):1074–1078. doi: 10.1093/ajcn/78.6.1074. [DOI] [PubMed] [Google Scholar]
- 38.Sonowal H, Pal PB, Shukla K, Ramana KV. Aspalatone Prevents VEGF-Induced Lipid Peroxidation, Migration, Tube Formation, and Dysfunction of Human Aortic Endothelial Cells. Oxidative Medicine and Cellular Longevity. 2017;2017:11. doi: 10.1155/2017/2769347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dominique D, Allan L, Didier C, Brigitte J, Norbert L. Resveratrol as a Chemopreventive Agent: A Promising Molecule for Fighting Cancer. Current Drug Targets. 2006;7(4):423–442.A. doi: 10.2174/138945006776359331. [DOI] [PubMed] [Google Scholar]
- 40.Machha, Mustafa MR. Chronic Treatment with Flavonoids Prevents Endothelial Dysfunction in Spontaneously Hypertensive Rat Aorta. Journal of Cardiovascular Pharmacology. 2005;46(1):36–40. doi: 10.1097/01.fjc.0000162769.83324.c1. [DOI] [PubMed] [Google Scholar]
- 41.D’Andrea G. Quercetin: A flavonol with multifaceted therapeutic applications? Fitoterapia. 2015;106:256–271. doi: 10.1016/j.fitote.2015.09.018. [DOI] [PubMed] [Google Scholar]
- 42.Hsu Y, Hsieh C, Tsai E, Hung J, Chang W, Hou M, et al. Didymin reverses phthalate ester-associated breast cancer aggravation in the breast cancer tumor microenvironment. Oncology Letters. 2016;11(2):1035–42. doi: 10.3892/ol.2015.4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nkajima VM, Marcedo GA, Macedo JA. Citrus bioactive phenolics: Role in the obesity treatment. LWT-Food Science and Technology. 2014;59(2):1205–12. [Google Scholar]
- 44.Gattuso G, Barreca D, Gargiulli C, Leuzzi U, Caristi C. Flavonoid composition of Citrus juices. Molecules. 2007;12(8):1641–73. doi: 10.3390/12081641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stuetz W, Prapamontol T, Hongsibsong S, Biesalski HK. Polymethoxylated flavones, flavanone glycosides, carotenoids, and antioxidants in different cultivation types of tangerines ( Citrus reticulata Blanco cv. Sain ampueng) from Northern Thailand. J Agric Food Chem. 2010;58(10):6069–74. doi: 10.1021/jf904608h. [DOI] [PubMed] [Google Scholar]
- 46.Michael P. Cigarette smoking, endothelial injury and cardiovascular disease. International Journal of Experimental Pathology. 2000;81(4):219–230. doi: 10.1046/j.1365-2613.2000.00162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Matsuzawa Y, Lerman A. Endothelial dysfunction and coronary artery disease: assessment, prognosis, and treatment. Coronary Artery Disease. 2014;25(8):713–724. doi: 10.1097/MCA.0000000000000178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barrera G. Oxidative Stress and Lipid Peroxidation Products in Cancer Progression and Therapy. ISRN Oncology. 2012;2012:21. doi: 10.5402/2012/137289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dalleau S, Baradat M, Gueraud F, Huc L. Cell death and diseases related to oxidative stress:4-hydroxynonenal (HNE) in the balance. Cell Death Differ. 2013;20(12):1615–1630. doi: 10.1038/cdd.2013.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.He M, Siow RCM, Sugden D, Gao L, Cheng X, Mann GE. Induction of HO-1 and redox signaling in endothelial cells by advanced glycation end products: A role for Nrf2 in vascular protection in diabetes. Nutrition, Metabolism and Cardiovascular Diseases. 2011;21(4):277–285. doi: 10.1016/j.numecd.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 51.Porter AG, Janicke RU. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999;6(2):99–104. doi: 10.1038/sj.cdd.4400476. [DOI] [PubMed] [Google Scholar]
- 52.Kim EK, Choi EJ. Pathological roles of MAPK signaling pathways in human diseases. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 2010;1802(4):396–405. doi: 10.1016/j.bbadis.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 53.Wu Y, Wang F, Reece EA, Yang P. Curcumin ameliorates high glucose-induced neural tube defects by suppressing cellular stress and apoptosis. American Journal of Obstetrics and Gynecology. 2015;212(6):802.e1–802.e8. doi: 10.1016/j.ajog.2015.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Recchioni R, Marcheselli F, Moroni F, Pieri C. Apoptosis in human aortic endothelial cells induced by hyperglycemic condition involves mitochondrial depolarization and is prevented by N-acetyl-L-cysteine. Metabolism. 2002;51(11):1384–1388. doi: 10.1053/meta.2002.35579. [DOI] [PubMed] [Google Scholar]
- 55.di Wu Q, Wang JH, Fennessy F, Redmond HP, Bouchier-Hayes D. Taurine prevents high-glucose-induced human vascular endothelial cell apoptosis. American Journal of Physiology - Cell Physiology. 1999;277(6):C1229–C1238. doi: 10.1152/ajpcell.1999.277.6.C1229. [DOI] [PubMed] [Google Scholar]
- 56.Dai Z, Liao DF, Jiang DJ, Deng HW, Li YJ. 3,4,5,6-Tetrahydroxyxanthone prevents vascular endothelial cell apoptosis induced by high glucose. Naunyn-Schmiedeberg’s Archives of Pharmacology. 2004;370(4):314–319. doi: 10.1007/s00210-004-0973-y. [DOI] [PubMed] [Google Scholar]
- 57.van Delft MF, Huang DCS. How the Bcl-2 family of proteins interact to regulate apoptosis. Cell Res. 0000;16(2):203–213. doi: 10.1038/sj.cr.7310028. [DOI] [PubMed] [Google Scholar]
- 58.Perlman H, Zhang X, Chen MW, Walsh K, Buttyan R. An elevated bax/bcl-2 ratio corresponds with the onset of prostate epithelial cell apoptosis. Cell Death Differ. 1999;6(1):48–54. doi: 10.1038/sj.cdd.4400453. [DOI] [PubMed] [Google Scholar]
- 59.Chanmee T, Ontong P, Konno K, Itano N. Tumor-Associated Macrophages as Major Players in the Tumor Microenvironment. Cancers. 2014;6(3):1670. doi: 10.3390/cancers6031670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dalton HJ, Armaiz-Pena GN, Gonzalez-Villasana V, Lopez-Berestein G, Bar-Eli M, Sood AK. Monocyte Subpopulations in Angiogenesis. Cancer Research. 2014;74(5):1287–1293. doi: 10.1158/0008-5472.CAN-13-2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jaipersad AS, Lip GYH, Silverman S, Shantsila E. The Role of Monocytes in Angiogenesis and Atherosclerosis. Journal of the American College of Cardiology. 63(1):1–11. doi: 10.1016/j.jacc.2013.09.019. [DOI] [PubMed] [Google Scholar]
- 62.Panés J, Perry M, Granger DN. Leukocyte-endothelial cell adhesion: avenues for therapeutic intervention. British Journal of Pharmacology. 1999;126(3):537–550. doi: 10.1038/sj.bjp.0702328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Su JB. Vascular endothelial dysfunction and pharmacological treatment. World Journal of Cardiology. 2015;7(11):719–41. doi: 10.4330/wjc.v7.i11.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Förstermann U, Münzel T. Endothelial nitric oxide synthase in vascular disease: from marvel to menace. Circulation. 2006;113(13):1708–14. doi: 10.1161/CIRCULATIONAHA.105.602532. [DOI] [PubMed] [Google Scholar]
- 65.Fish JE, Marsden PA. Endothelial nitric oxide synthase: insight into cell-specific gene regulation in the vascular endothelium. Cell Mol Life Sci. 2006;63(2):144–62. doi: 10.1007/s00018-005-5421-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Popov D. Endothelial cell dysfunction in hyperglycemia: Phenotypic change, intracellular signaling modification, ultrastructural alteration, and potential clinical outcomes. International Journal of Diabetes Mellitus. 2010;2(3):189–195. [Google Scholar]
- 67.Soto-Urquieta MG, López-Briones S, Pérez-Vázquez V, Saavedra-Molina A, González-Hernández GA, Ramírez-Emiliano J. Curcumin restores mitochondrial functions and decreases lipid peroxidation in liver and kidneys of diabetic db/db mice. Biological Research. 2014;47(1):74. doi: 10.1186/0717-6287-47-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li G, Xu Y, Sheng X, Liu H, Guo J, Wang J, et al. Naringin Protects Against High Glucose-Induced Human Endothelial Cell Injury Via Antioxidation and CX3CL1 Downregulation. Cell Physiol Biochem. 2017;42(6):2540–51. doi: 10.1159/000480215. [DOI] [PubMed] [Google Scholar]
- 69.Ramana KV, Friedrich B, Srivastava S, Bhatnagar A, Srivastava SK. Activation of Nulcear Factor-κB by Hyperglycemia in Vascular Smooth Muscle Cells Is Regulated by Aldose Reductase. Diabetes. 2004;53(11):2910–2920. doi: 10.2337/diabetes.53.11.2910. [DOI] [PubMed] [Google Scholar]
- 70.Vinayagam R, Xu B. Antidiabetic properties of dietary flavonoids: a cellular mechanism review. Nutrition & Metabolism. 2015;12(1):60. doi: 10.1186/s12986-015-0057-7. [DOI] [PMC free article] [PubMed] [Google Scholar]