Abstract
Background
Estimates of adiposity in evaluating the metabolic syndrome (MetS) have traditionally utilized measures of waist circumference (WC), whereas body mass index (BMI) is more commonly used clinically. Our objective was to determine if a MetS severity Z-score employing BMI as its measure of adiposity (MetS-Z-BMI) would perform similarly to a WC-based score (MetS-Z-WC) in predicting future disease.
Methods
To formulate the MetS-Z-BMI, we performed confirmatory factor analysis on a sex- and race/ethnicity-specific basis on MetS-related data for 6,870 adult participants of the National Health and Nutrition Survey 1999–2010. We then validated this score and compared it to MetS-Z-WC in assessing correlations with future coronary heart disease (CHD) and Type 2 diabetes mellitus (T2DM) using Cox proportional hazard analysis of 13,094 participants of the Atherosclerosis Risk in Communities study and Jackson Heart Study.
Results
Loading factors, which represent the relative contribution of each component to the latent MetS factor, were lower for BMI than for WC in formulating the two respective scores (MetS-Z-BMI and MetS-Z-WC). Nevertheless, MetS-Z-BMI and MetS-Z-WC exhibited similar hazard ratios (HR) toward future disease. For each one standard-deviation-unit increase in MetS-Z-BMI, HR for CHD was 1.76 (95% confidence interval [CI]: 1.65, 1.88) and HR for T2DM was 3.39 (CI 3.16, 3.63) (both p<0.0001). There were no meaningful differences between the MetS-Z-WC and MetS-Z-BMI scores in their associations with future CHD and T2DM.
Conclusions
A MetS severity Z-score utilizing BMI as its measure of adiposity operated similarly to a WC-based score in predicting future CHD and T2DM, suggesting overall similarity in MetS-based risk as estimated by both measures of adiposity. This indicates potential clinical usefulness of MetS-Z-BMI in assessing and following MetS-related risk over time.
Keywords: metabolic syndrome, cardiovascular disease risk, type 2 diabetes, obesity
INTRODUCTION
The metabolic syndrome (MetS) is a constellation of cardiovascular disease (CVD) risk factors that cluster together, likely based on underlying pathology related to cellular dysfunction and pathway-selective insulin resistance.1–3 These clinical risk factors include central obesity, high blood pressure, high fasting triglycerides, low HDL-cholesterol and high fasting glucose. We used confirmatory factor analysis to study how the usual MetS components correlate with a single MetS “factor”, and how these correlations vary by sex and race/ethnicity. This analysis then directly provides a way to formulate a sex- and race/ethnicity-specific MetS severity Z-score (http://mets.health-outcomes-policy.ufl.edu/) based on how measurements for these MetS components cluster together among population sub-groups.4, 5 We demonstrated that baseline levels of this MetS severity score correlated with risk of future type 2 diabetes (T2DM)6–8 and CVD.7, 9, 10 Moreover, changes in MetS severity score can be tracked over time11, 12 and confer added risk for future disease,6, 8, 9 raising the potential for such a score to be used clinically to assess and track MetS-related risk and potentially trigger intervention.
The adult version of the MetS severity score uses measurement of waist circumference (WC) as an estimate of central obesity.4 Use of WC is frequently employed as an estimate of visceral adiposity to minimize misclassification of subcutaneous fat and lean body mass as visceral fat, which may occur when measures of body mass index (BMI) are used.13, 14 However, assessment of WC requires a more rigorous technique and is not as frequently performed in clinical settings, potentially limiting clinical application of such a MetS severity score.15 Use of height and WC together may provide an even better estimate16 but faces the same potential difficulties in clinical application. By contrast, BMI is commonly measured clinically.17 Because it is a measure of body mass and not body fat, BMI has clear potential limitations as an estimate of central adiposity.15 Nevertheless, among a sample of US adults 20–79, BMI correlated reasonably well with both WC (Pearson’s r values 0.88–0.94 based on age groups and sex) and percent body fat (Pearson’s r values 0.7–0.86).18 In formulating the adolescent version of the MetS severity Z-score, we utilized BMI because of a lack of standardized WC values by age; this BMI-based score correlated strongly with additional CVD risk factors (such as insulin,5, 7 hsCRP,5 uric acid5 and adiponectin7) and with long-term risk of T2DM6, 7 and CVD.7, 9, 10 This suggested that BMI may serve as a reasonable estimate of central obesity in the context of MetS severity.
The goals of this study were, using NHANES, 1) to perform a confirmatory factor analysis (CFA) in adults for MetS components similarly to what we have done previously,4 but using BMI as the estimate of central adiposity instead of WC; 2) to compare the CFA using BMI to our prior CFA using WC; and 3) to evaluate the agreement between the two resulting factor (MetS severity) scores (MetS-Z-BMI and MetS-Z-WC, respectively). Then, our fourth and final goal was to use separate existing epidemiologic cohorts to compare MetS-Z-BMI and MetS-Z-WC with respect to their associations with future disease, specifically coronary heart disease (CHD) and T2DM. We hypothesized that when compared to the MetS severity score utilizing WC, a score employing BMI would yield similar correlation with other MetS associated CVD risk factors and with future disease. Such a score would be expected to be more useful clinically given the widespread availability of BMI.
METHODS AND MATERIALS
NHANES
For the initial goals of the study, we used the same analyses on the same dataset used to derive our adult MetS severity score using WC,4 but substituting BMI for WC. These methods are described in detail elsewhere.4 Specifically, we used combined two-year cycles from NHANES (1999–2010), a complex, multistage probability sample of the US population19 conducted by the National Center for Health Statistics (NCHS) of the Centers for Disease Control (CDC). WC, SBP, and laboratory measures of triglycerides, HDL-cholesterol, and fasting glucose were obtained using standardized protocols and calibrated equipment.19 For SBP, the mean of up to four readings taken on each individual was used. All blood samples used for analyses were obtained following a fast ≥8 hours prior to the blood draw.
Data from non-Hispanic-white, non-Hispanic-black, or Hispanic (Mexican-American/other Hispanic) participants 20–64 years old were analyzed (using race/ethnicity terminology from NHANES). For the initial CFA done previously4 as well as here, participants were excluded if they were pregnant, had known diabetes or unknown diabetes (fasting plasma glucose >125 mg/dL), or were taking antihyperlipidemic or anti-diabetic medications, as we sought an unbiased setting of metabolic disarray and all of these situations are likely to alter lipid and insulin levels. We did not exclude those on antihypertensives, given that many of these medications are used for indications other than treatment of high blood pressure. Individuals who reported having congestive heart failure (CHF) or CHD, or ever having had a myocardial infarction (MI) or a stroke, were excluded.
Confirmatory factor analysis (CFA) is a statistical approach that analyzes how multiple individual variables correlate together in their contribution to a latent “factor.” This factor can be thought of as operating behind the scenes to influence the levels of its components, with loading factors assigned based on the strength of association between each component and the latent factor. Here, a series of one-factor CFA were performed on the five identified MetS components in adults: BMI, SBP, HDL, triglycerides, and glucose. In our previous MetS severity derivation, we used SBP rather than both SBP and diastolic blood pressure given the two are highly correlated with each other.20 We chose SBP given it is more strongly associated with insulin resistance21 and other outcomes.22 We used SBP here as well given our goal to replicate our previous analysis using BMI instead of WC. Triglycerides were log-transformed, and all variables were standardized (mean=0, SD=1) over the entire sample. The inverse of HDL was used when standardizing, so a higher factor loading score would be similar in interpretation to the other measures in the model. As in our previous study, we performed this CFA both overall and on a sex- and race/ethnicity-specific basis (because of apparent differences in traditional MetS criteria by race/ethnicity23–29) using SAS PROC CALIS. The variables were not standardized within groups to allow for potential overall higher standardized scores within the six individual sex- and race/ethnic-specific groups. Chi-square tests of the equality of the factor loadings across the six groups were performed. Models were compared using various fit statistics, both overall and by group. Chi-square and Akaike’s Information Criteria (AIC) were used for model comparisons; smaller chi-square and AIC values indicated a better fit. Other goodness of fit indices included the Root Mean Square Error of Approximation (RMSEA; >0.06 indicates a poor fit), the Standardized Root Mean Square Residual (SRMR; >0.08 poor fit), the Goodness of Fit Index (GFI; <0.90 poor fit), and the Bentler-Bonett Normed Fit Index (NFI; <0.90 poor fit).30 Results from the previous CFA analysis using WC were compared to results from this new CFA analysis on BMI.
The standardized factor coefficients from the BMI CFA (by group) were used to calculate the MetS factor score on each individual. This score can be interpreted as a Z-score (mean 0, SD=1), with higher scores representing an increased risk, or severity, of MetS. As was done with our MetS severity score based on WC (MetS-Z-WC), the linear association between MetS severity score based on BMI (MetS-Z-BMI) and various biomarkers associated with MetS (fasting insulin, adiponectin, hsCRP, and uric acid4, 7, 31, 32) was assessed via simple Pearson correlations in the 1999–2010 NHANES dataset.
Using newer NHANES data from 2011–2014, the agreement between the new MetS-Z-BMI score and the established MetS-Z-WC score was assessed via intraclass correlation coefficients (ICC’s), with a value of 1 indicating perfect agreement. Bland-Altman figures were also produced, in which differences between the two scores were plotted against MetS-Z-WC.33
Validation: Atherosclerosis Risk in Communities (ARIC) Study & the Jackson Heart Study (JHS)
We next set out to validate this score by assessing its correlation with later risk for CHD and T2DM compared to the WC-based score in a combined cohort of the Atherosclerotic Risk in Communities (ARIC) study and Jackson Heart Study (JHS). ARIC is a large community-based epidemiological cohort study beginning in 1987–89 across 4 field centers in the US. Further details regarding study design and objectives are published elsewhere.34 A total of 15,397 mostly white and African-American participants ages 45–64 years old were enrolled. We utilized data through Visit 5 (2011–2013), with further adjudicated CHD outcomes as described below. JHS began as an extension of African-American participants in the Jackson, MS site of ARIC and similar methodologies were utilized. Starting in 2000–04, 5,306 participants age 21–95 years were recruited; this included 1,626 participants who had been followed as part of ARIC and for whom data from ARIC and not JHS were utilized for the present analysis.35 For the remainder of JHS participants, we utilized data through Visit 3 (2009–2013) and further adjudicated CHD outcomes.
After combining the two cohorts (n=19,026), we excluded participants with baseline T2DM (n=2485), CHD (n=973), or stroke (n=393), and participants who were missing baseline data on MetS components (n=792), who had non-fasting labs (n=507), and/or those without follow-up data regarding outcomes (n=2,992).
MetS components were tested using similar approaches for both cohorts as described previously,35, 36 and MetS severity Z-scores were calculated using both WC and BMI. Incident CHD was ascertained using standard ARIC and JHS protocols37, 38 and included fatal or nonfatal hospitalized myocardial infarction, fatal CHD, silent myocardial infarction identified by electrocardiography, or coronary revascularization. Follow-up time for incident CHD events was the minimum number of days between the baseline visit and either the first event, death from other causes, last contact, or Dec 31, 2011 (JHS).37, 38 In ARIC, participants were defined as having T2DM if they reported that a physician had told them they had diabetes, had a fasting glucose ≧126 mg/dL or a non-fasting glucose ≥ 200 mg/dL, or if they reported they were taking insulin or oral hypoglycemic medications.39, 40 In JHS, participants were defined as having 2DM if they had a fasting glucose ≧ 126 mg/dL or an HbA1c ≥ 6.5% or if they took a diabetic medication within two weeks prior to the clinic visit. This definition of T2DM was used at Visits 1–4 for ARIC participants and at Visits 1–3 each for JHS participants. As primary interest was incident T2DM, for those individuals without T2DM at Visit 1, time to T2DM was defined as the number of years between Visit 1 and the first visit where T2DM was reported, regardless of T2DM status at subsequent visits.
Cox proportional hazards regression (via SAS PROC PHREG), adjusted for baseline age and stratified by site (4 ARIC sites + JHS), was used to model the relationship between MetS severity, both measured by MetS-Z-WC and MetS-Z-BMI, and time to incident CHD and T2DM. Hazard ratios and 95% CI’s were reported, both overall and by sex and race. The ability to discriminate outcomes was quantified by the C-statistic for survival models41 using programs developed elsewhere (http://ncook.bwh.harvard.edu/sas-macros.html); estimates and 95% Noether confidence intervals are reported. The C statistic is a measure of discrimination, which is a model’s ability to distinguish individuals with and without disease. A C-statistic with a value of 1 indicates perfect discrimination. We did not account for the interval censoring associated with incident T2DM; however, given our primary goal of comparing two different MetS severity scores, any bias associated with ignoring the interval censoring would equally impact inferences made on both severity scores. We further examined and compared the ability of the two scores to predict future disease when the two scores disagreed in ARIC/JHS. We calculated the difference between the two scores and categorized the level of disagreement into four categories: 1) Differences less than 1 SD below the mean difference (when MetS-Z-BMI was much lower than MetS-Z-WC); 2) Differences greater than −1 SD but less than the mean difference (when MetS-Z-BMI was marginally lower than its WC counterpart); 3) Differences greater than the mean difference but less than 1 SD above the mean difference (MetS-Z-BMI marginally greater than MetS-Z-WC); and 4) Differences greater than 1 SD above the mean difference (MetS-Z-BMI much greater than its WC counterpart).
RESULTS
Score Derivation: NHANES
To assess the potential for utilizing BMI as a component of the MetS severity score, we utilized data from 6,870 non-Hispanic-white, non-Hispanic-black and Hispanic adult participants of NHANES. Participant characteristics for this derivation population were published previously.4 Table 1 displays results from the CFA comparing the MetS severity score using WC (previously published4) and BMI, including loading factors for each of the MetS components by sex- and racial/ethnic group. With the exception of Hispanic females, all sex and racial/ethnic subgroups had lower loading factors for the obesity component of the MetS severity score when using BMI compared to WC. These lower loading factors indicate that relative to the other MetS components, BMI had a lower contribution to the latent MetS factor than did WC. This was most striking among non-Hispanic-black males, who exhibited a decrease in loading factor from 0.67 to 0.50. This decreased loading for the obesity component of the MetS severity was likely countered by increased factors related to HDL cholesterol in all groups except non-Hispanic-black females. A sensitivity analysis performing the derivation CFA’s excluding individuals on antihypertensive medications yielded similar loading factors for all components (data not shown).
Table 1.
Confirmatory Factor Analysis Results (Adults age 20–64, n = 6,870)*
| Measure of Obesity: Waist Circumference
|
Measure of Obesity: BMI
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Males | Females | Males | Females | |||||||||||
|
|
|
|||||||||||||
| Model Fit Indices | Overall | NHW | NHB | Hisp | NHW | NHB | Hisp | Overall | NHW | NHB | Hisp | NHW | NHB | Hisp |
|
|
|
|
||||||||||||
| Chi-square (df) | 783.0 (30) | 764.6 (30) | ||||||||||||
| Akaike’s Information Criteria (AIC) | 903.0 | 884.6 | ||||||||||||
| Root Mean Square Error of Approximation (RMSEA) | 0.148 | 0.146 | ||||||||||||
| Standardized Root Mean Square Residual (SRMR) | 0.071 | 0.075 | 0.069 | 0.080 | 0.065 | 0.080 | 0.056 | 0.070 | 0.070 | 0.063 | 0.076 | 0.067 | 0.083 | 0.059 |
| Goodness of Fit Index (GFI) | 0.956 | 0.952 | 0.957 | 0.950 | 0.958 | 0.948 | 0.969 | 0.958 | 0.958 | 0.967 | 0.954 | 0.956 | 0.945 | 0.965 |
| Bentler-Bonett Normed Fit Index (NFI) | 0.817 | 0.810 | 0.785 | 0.792 | 0.845 | 0.729 | 0.862 | 0.809 | 0.824 | 0.809 | 0.802 | 0.824 | 0.654 | 0.843 |
| Factor Loadings | p-value* | p-value* | ||||||||||||
|
|
|
|||||||||||||
| Measure of Obesity | < 0.001 | 0.49 | 0.67 | 0.36 | 0.71 | 0.77 | 0.45 | < 0.001 | 0.41 | 0.50 | 0.32 | 0.69 | 0.73 | 0.48 |
| SBP | < 0.001 | 0.17 | 0.16 | 0.19 | 0.33 | 0.31 | 0.38 | < 0.001 | 0.15 | 0.08 | 0.18 | 0.34 | 0.34 | 0.38 |
| HDL | < 0.001 | 0.60 | 0.64 | 0.57 | 0.52 | 0.40 | 0.45 | < 0.001 | 0.62 | 0.74 | 0.59 | 0.54 | 0.38 | 0.46 |
| Triglycerides | < 0.001 | 0.73 | 0.45 | 0.70 | 0.56 | 0.37 | 0.59 | < 0.001 | 0.72 | 0.48 | 0.69 | 0.55 | 0.36 | 0.58 |
| Glucose | < 0.001 | 0.26 | 0.37 | 0.27 | 0.46 | 0.46 | 0.44 | < 0.001 | 0.25 | 0.31 | 0.27 | 0.45 | 0.47 | 0.45 |
Chi-square test (5 df) p-value of the equivalency of the factor loadings across the six groups
NHW = Non-Hispanic White; NHB = Non-Hispanic Black; Hisp = Hispanic
Equations for score generation and internal validation in NHANES
Table 2 provides the equations generated from the CFA for calculating the MetS severity score by sex- and racial/ethnic group using BMI, but including our original WC equations for comparison4. Among NHANES 1999–2010 participants, the BMI-based MetS severity score correlated with additional CVD risk factors including insulin, hsCRP, and uric acid (Pearson’s R values: 0.61, 0.38, 0.42, respectively; all p<0.0001), as had similarly been noted for the WC-based score.4
Table 2.
Equations for Sex and Race/Ethnic-Specific Metabolic Syndrome Risk Z-Score
| Using WC |
| Males |
| Non-Hispanic White = −5.4559 + 0.0125 * WC − 0.0251 * HDL + 0.0047 * SBP + 0.8244 * ln(Tri) + 0.0106 * Glu |
| Non-Hispanic Black = −6.3767 + 0.0232 * WC − 0.0175 * HDL + 0.0040 * SBP + 0.5400 * ln(Tri) + 0.0203 * Glu |
| Hispanic = −5.5541 + 0.0135 * WC − 0.0278 * HDL + 0.0054 * SBP + 0.8340 * ln(Tri) + 0.0105 * Glu |
| Females |
| Non-Hispanic White = −7.2591 + 0.0254 * WC − 0.0120 * HDL + 0.0075 * SBP + 0.5800 * ln(Tri) + 0.0203 * Glu |
| Non-Hispanic Black = −7.1913 + 0.0304 * WC − 0.0095 * HDL + 0.0054 * SBP + 0.4455 * ln(Tri) + 0.0225 * Glu |
| Hispanic = −7.7641 + 0.0162 * WC − 0.0157 * HDL + 0.0084 * SBP + 0.8872 * ln(Tri) + 0.0206 * Glu |
| Using BMI |
| Males |
| Non-Hispanic White = −4.8316 + 0.0315 * BMI − 0.0272 * HDL + 0.0044 * SBP + 0.8018 * ln(Tri) + 0.0101 * Glu |
| Non-Hispanic Black = −4.8134 + 0.0460 * BMI − 0.0233 * HDL + 0.0020 * SBP + 0.5983 * ln(Tri) + 0.0166 * Glu |
| Hispanic = −4.8198 + 0.0355 * BMI − 0.0303 * HDL + 0.0051 * SBP + 0.7835 * ln(Tri) + 0.0104 * Glu |
| Females |
| Non-Hispanic White = −6.5231 + 0.0523 * BMI − 0.0138 * HDL + 0.0081 * SBP + 0.6125 * ln(Tri) + 0.0208 * Glu |
| Non-Hispanic Black = −6.7982 + 0.0484 * BMI − 0.0108 * HDL + 0.0073 * SBP + 0.5278 * ln(Tri) + 0.0281 * Glu |
| Hispanic = −7.1844 + 0.0333 * BMI − 0.0166 * HDL + 0.0085 * SBP + 0.8625 * ln(Tri) + 0.0221 * Glu |
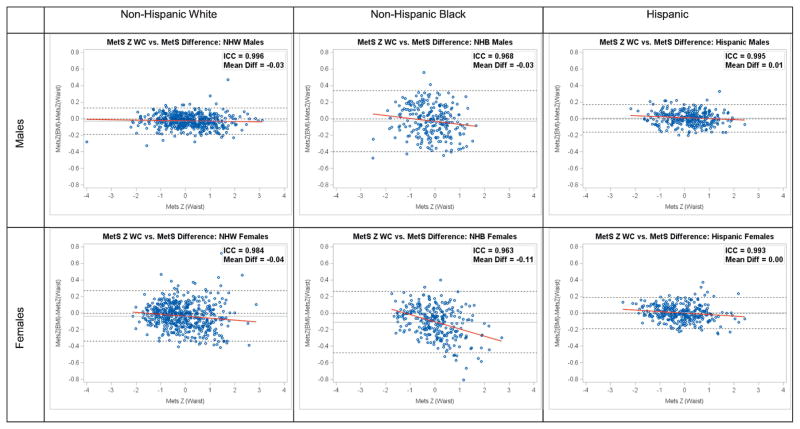
Figure 1 displays Bland-Altman plots from separate subsequent cycles of NHANES (2011–2014; n = 2,211) after calculating the two MetS severity scores using the equations listed in Table 2. These plots, as well as the ICC values, demonstrate a high degree of agreement between the two scores by sex and racial/ethnic group, with ICC values all 0.963–0.996. There appears to be a systematic decrease in agreement between the two scores for non-Hispanic blacks, particularly among females, with the MetS score based on BMI tending to underestimate MetS severity based on WC for larger values of the WC-based score.
Figure 1.
Bland Altman Plots MetS Difference by Race/Ethnicity and Gender Groups
Disease outcomes score validation: ARIC and JHS
As an essential step of validation, we subsequently assessed the validity of the BMI-based MetS severity score by comparing to the WC-based score for prediction of future CHD and T2DM in Cox regression models from a combined cohort of participants of ARIC and JHS (n = 13,094). Further details regarding participant characteristics and disease outcome incidence have been published previously.8, 10 Table 3 displays HRs for disease incidence for every 1 standard deviation increment of the BMI- and WC-based MetS severity scores overall and by sex- and racial/ethnic group. The BMI-based score was significantly correlated to both future CHD and T2DM overall, with each increase of 1 standard-deviation unit in the MetS Severity score associated with significantly (p<0.001) increased risk (HR, 95%CI) for CHD [1.78, (1.67, 1.90)] and for T2DM [3.37 (3.15, 3.61) The HRs by sex- and racial subgroup for the BMI-based score were similar to those seen for the WC-based score (Table 3). C-statistics confirmed similar discriminatory ability between the two MetS severity scores with respect to both outcomes. For incident CHD, the C-statistic was equal to 0.63 for both versions of the MetS severity score; for incident T2DM, the C-statistic was equal to 0.75 and 0.74 for MetS-Z-WC and MetS-Z-BMI, respectively. We then examined and compared performance of these two scores for various levels of disagreement (Supplementary Table 1). When there was a strong negative disagreement between scores (i.e., the BMI-based score was lower than the WC-based score), the scores remained similar in predicting CVD (overall HR 1.33 and 1.36 for the WC- and BMI-based scores, respectively), while there was a tendency for the BMI-based score to perform better for T2DM prediction (overall HR 3.00 and 3.31 for the WC- and BMI-based scores, respectively). When there was a strong positive disagreement between scores (i.e., the BMI-based score was higher than the WC-based score), the scores were again similar in predicting CVD (overall HR 1.68 and 1.66 for the WC- and BMI-based scores, respectively), while there was a tendency for the WC-based score to perform better for T2DM prediction (overall HR 4.34 and 3.78 for the WC- and BMI-based scores, respectively). When there was only weak disagreement between the scores, the HR’s were nearly identical between scores.
Table 3.
MetS-WC vs. MetS-BMI: Predicting Incident CHD and T2DM in ARIC and JHS
| MetS Z-score (Waist Circumference) | MetS Z-Score (BMI) | |||
|---|---|---|---|---|
|
|
|
|||
| Hazard Ratio (95% CI)* | C-Statistic (95% CI)** | Hazard Ratio (95% CI)* | C-Statistic (95% CI)** | |
| Incident CVD | ||||
| Overall | 1.72 (1.61, 1.83) | 0.63 (0.62, 0.64) | 1.78 (1.67, 1.90) | 0.63 (0.62, 0.64) |
| Non-Hispanic White Males | 1.50 (1.37, 1.64) | 0.58 (0.57, 0.60) | 1.52 (1.39, 1.66) | 0.58 (0.57, 0.60) |
| Non-Hispanic White Females | 1.73 (1.54, 1.95) | 0.64 (0.61, 0.66) | 1.81 (1.60, 2.05) | 0.64 (0.61, 0.66) |
| Non-Hispanic Black Males | 1.65 (1.33, 2.04) | 0.60 (0.56, 0.65) | 1.60 (1.29, 1.99) | 0.59 (0.55, 0.64) |
| Non-Hispanic Black Females | 1.55 (1.26, 1.90) | 0.62 (0.57, 0.67) | 1.63 (1.29, 2.05) | 0.61 (0.56, 0.66) |
| Incident T2DM | ||||
| Overall | 3.27 (3.06, 3.50) | 0.75 (0.73, 0.76) | 3.37 (3.15, 3.61) | 0.74 (0.73, 0.76) |
| Non-Hispanic White Males | 2.71 (2.37, 3.10) | 0.70 (0.68, 0.73) | 2.68 (2.35, 3.06) | 0.70 (0.67, 0.72) |
| Non-Hispanic White Females | 4.61 (4.04, 5.27) | 0.81 (0.79, 0.84) | 4.86 (4.24, 5.57) | 0.82 (0.80, 0.84) |
| Non-Hispanic Black Males | 2.89 (2.48, 3.38) | 0.72 (0.69, 0.75) | 2.74 (2.30, 3.25) | 0.69 (0.66, 0.72) |
| Non-Hispanic Black Females | 3.14 (2.78, 3.56) | 0.76 (0.74, 0.78) | 3.50 (3.08, 3.98) | 0.76 (0.74, 0.78) |
Adjusted for baseline age and stratified by center
10-Year Risk; Noether Confidence Intervals (unadjusted)
DISCUSSION
We demonstrated that a MetS severity scoring system using BMI as an indicator of adiposity provided similar predictive power for future disease as did the score based on WC. Whereas assessment of WC is technically more difficult and time-consuming and is rarely a codified field in electronic health record (EHR) systems, BMI is routinely measured on a clinical basis and is a codified value in the EHR, increasing the opportunity for MetS severity to be automatically calculated by an EHR system.17 Thus, the availability and comparable performance of the BMI-based score may increase the potential for such a score to be used as a clinical tool that represents a single metric of metabolic disarray and assists in risk assessment—and could be used as an indicator of particularly high risk and need for further intervention. For example, Cefalu at al suggested recommending bariatric surgery on pre-diabetes patients with high BMI.42 Given difficulties in determining metabolic risk from obesity alone, a MetS severity score above a given cut-off could be used to divide patients in greater need of bariatric surgery in these settings. Because risk exists on a spectrum, such a score could also identify individuals for intervention at earlier degrees of risk, including initiation of metformin or aspirin—though clearly, validation studies would be necessary.
It was perhaps not surprising that the loading weights for BMI were all lower than for WC, leading to a lower relative contribution of the obesity component for the BMI-based MetS severity equations. This may relate to WC being an overall better indicator of visceral obesity, which is considered a key etiologic component of MetS.13 The widest differences in loading factors between BMI and WC and the lowest ICC values were among black males, potentially due to a greater misattribution of higher BMI to fat vs. lean mass.43 Nevertheless, the differences in loading factors between BMI and WC did not appear to be a limitation of the score. Indeed, despite changes in the factor loadings using BMI, the relative order of the obesity measures between racial/ethnic groups remained the same, supporting that both adiposity estimates provided similar information—but that in its contribution to the latent MetS factor, BMI was less dominant among all of the MetS components. Overall, the lower contribution of BMI to the MetS severity score did not significantly affect the association of MetS severity with future disease, emphasizing the importance of the combination of each of the components. The BMI-based score instead had higher factor loadings for HDL for most sex- and racial/ethnic subgroups. This is notable given recent skepticism as to the role of HDL in modifying disease outcomes.44 Nevertheless, this increased emphasis on HDL in the BMI-based score is consistent with HDL being a component of multiple risk scores for CVD45, 46 and T2DM39, 47 and may have contributed to the non-significant elevations in HR’s for future CHD and T2DM using the BMI-based score.
Unfortunately, NHANES investigators only performed a more direct measure of body fat—dual energy X-ray absorptiometry—in a subset of participants, limiting our ability to perform a similar CFA using body fat alone. Nevertheless, studies that have been performed comparing DXA-measured body fat, BMI and WC in the sample population have shown relatively close correlations.18 Even more specific tests of visceral fat such as CT-based assessment of truncal fat may have provided greater precision regarding central adiposity48—but would further limit clinical application.
This set of experiments had several limitations. For the derivation of the MetS severity score we used cross-sectional data and assumed one latent “MetS” factor with all 5 traditional MetS components instead of using a more exploratory approach that allowed for multiple factors. Nevertheless, our assumption of a single factor was supported by a large body of prior research.21, 49, 50 [[[Remove #48 to stay at 50 REFS]]] For the outcomes-based validation, we utilized cohorts that were followed from 1987 for ARIC and 2000 for JHS; interval advances in CHD prevention may render these results different from current studies of predictive risk. The definitions of incident diabetes in this study could have applied equally to Type 1 diabetes and T2DM. While it was T2DM that we were predominantly interested in, inclusion of Type 1 diabetes may have been expected to biased us against associations between the score and new Type 1 diabetes. While we excluded participants on antidiabetic and antihyperlipidemic medications in deriving these scores, we lacked adequate data regarding treatment with MetS components. This study only included white and black participants, while studies of this score in Hispanics and other racial/ethnic groups remains needed. In particular, assessment of differences in contribution of WC and BMI to such a score would be instructive, given that lipid abnormalities occur at a much lower BMI among South Asian individuals.28 Finally, future validation will be needed alongside other predictive scores such as the Framingham calculator,46 the American Heart Association Atherosclerotic Cardiovascular Disease score 45 and the American Diabetes Association prediction score.47 However, this study had several strengths, including use of separate derivation and validation cohorts and comparison of the new BMI-based MetS severity score to the WC-based score in the prediction of long-term disease outcomes.
In conclusion, we used CFA to generate MetS severity scores that are based not on WC but on BMI—a much more clinically available measure. This score varied by sex and race/ethnicity and strongly correlated with future CHD and T2DM. These data support MetS severity as a tool that could potentially be used in the EHR to follow risk within individuals over time, both as a motivator to change and a way to track response to preventative treatments.
Highlights.
Waist circumference is often used in classifying metabolic syndrome (MetS), but BMI is more often collected and recorded in clinical settings.
We developed a measure of MetS severity that uses BMI instead of WC.
We show that MetS severity using BMI predicts future disease as well as the measure that uses WC.
MetS severity using BMI has much greater clinical potential than the measure using WC.
Acknowledgments
Funding Sources: This work was supported by NIH grants 1R01HL120960 (MJG and MDD). The Jackson Heart Study is supported by contracts HHSN268201300046C, HHSN268201300047C, HHSN268201300048C, HHSN268201300049C, HHSN268201300050C from the National Heart, Lung, and Blood Institute and the National Institute on Minority Health and Health Disparities. The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C).
The authors thank the staff and participants of the ARIC study and JHS for their important contributions.
Footnotes
Conflict of Interest Statement: None of the authors has any competing interests to declare.
AUTHOR CONTRIBUTIONS
Matthew J. Gurka and Mark D. DeBoer designed the study and planned the analysis. Matthew J. Gurka and Stephanie L. Filipp performed the analysis. Mark D. DeBoer and Matthew J. Gurka wrote the manuscript. Solomon K. Musani and Mario Sims provided editing assistance for the manuscript. Matthew J. Gurka is the guarantor of this work and had full access to the data in the study and takes final responsibility for the decision to submit for publication. All authors have read and given final approval of the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.de Ferranti S, Mozaffarian D. The perfect storm: obesity, adipocyte dysfunction, and metabolic consequences. Clin Chem. 2008;54:945–955. doi: 10.1373/clinchem.2007.100156. [DOI] [PubMed] [Google Scholar]
- 2.DeBoer MD. Obesity, systemic inflammation, and increased risk for cardiovascular disease and diabetes among adolescents: A need for screening tools to target interventions. Nutrition. 2013;29:379–386. doi: 10.1016/j.nut.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williams KJ, Wu X. Imbalanced insulin action in chronic over nutrition: Clinical harm, molecular mechanisms, and a way forward. Atherosclerosis. 2016;247:225–282. doi: 10.1016/j.atherosclerosis.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 4.Gurka MJ, Lilly CL, Norman OM, et al. An Examination of Sex and Racial/Ethnic Differences in the Metabolic Syndrome among Adults: A Confirmatory Factor Analysis and a Resulting Continuous Severity Score. Metabolism. 2014;63:218–225. doi: 10.1016/j.metabol.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gurka MJ, Ice CL, Sun SS, et al. A confirmatory factor analysis of the metabolic syndrome in adolescents: an examination of sex and racial/ethnic differences. Cardiovascular Diabetology. 2012:11. doi: 10.1186/1475-2840-11-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeBoer MD, Gurka MJ, Woo JG, et al. Severity of the metabolic syndrome as a predictor of type 2 diabetes between childhood and adulthood: the Princeton Lipid Research Cohort Study. Diabetologia. 2015;58:2745–2752. doi: 10.1007/s00125-015-3759-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeBoer MD, Gurka MJ, Morrison JA, et al. Inter-relationships between the severity of metabolic syndrome, insulin and adiponectin and their relationship to future type 2 diabetes and cardiovascular disease. Int J Obes (Lond) 2016;40:1353–1359. doi: 10.1038/ijo.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gurka MJ, Golden SH, Musani SK, et al. Independent associations between a metabolic syndrome severity score and future diabetes by sex and race: the Atherosclerosis Risk In Communities Study and Jackson Heart Study. Diabetologia. 2017;60:1261–1270. doi: 10.1007/s00125-017-4267-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeBoer MD, Gurka MJ, Woo JG, et al. Severity of Metabolic Syndrome as a Predictor of Cardiovascular Disease Between Childhood and Adulthood: The Princeton Lipid Research Cohort Study. J Amer Coll Card. 2015;66:755–757. doi: 10.1016/j.jacc.2015.05.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeBoer MD, Gurka MJ, Hill Golden S, et al. Independent Associations between Metabolic Syndrome Severity & Future Coronary Heart Disease by Sex and Race. J Am Coll Card. 2017;69:1204–1205. doi: 10.1016/j.jacc.2016.10.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vishnu A, Gurka MJ, DeBoer MD. The severity of the metabolic syndrome increases over time within individuals, independent of baseline metabolic syndrome status and medication use: The Atherosclerosis Risk in Communities Study. Atherosclerosis. 2015;243:278–285. doi: 10.1016/j.atherosclerosis.2015.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gurka MJ, Vishnu A, Santen RJ, et al. Progression of Metabolic Syndrome Severity During the Menopausal Transition. J Am Heart Assoc. 2016:5. doi: 10.1161/JAHA.116.003609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huxley R, Mendis S, Zheleznyakov E, et al. Body mass index, waist circumference and waist:hip ratio as predictors of cardiovascular risk--a review of the literature. Eur J Clin Nutr. 2010;64:16–22. doi: 10.1038/ejcn.2009.68. [DOI] [PubMed] [Google Scholar]
- 14.Bogl LH, Kaye SM, Rämö JT, et al. Abdominal obesity and circulating metabolites: A twin study approach. Metabolism. 2016;65:111–121. doi: 10.1016/j.metabol.2015.10.027. [DOI] [PubMed] [Google Scholar]
- 15.Bauduceau B, Vachey E, Mayaudon H, et al. Should we have more definitions of metabolic syndrome or simply take waist measurement? Diabetes Metab. 2007;33:333–339. doi: 10.1016/j.diabet.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 16.Ashwell M, Gunn P, Gibson S. Waist-to-height ratio is a better screening tool than waist circumference and BMI for adult cardiometabolic risk factors: systematic review and meta-analysis. Obes Rev. 2012;13:275–286. doi: 10.1111/j.1467-789X.2011.00952.x. [DOI] [PubMed] [Google Scholar]
- 17.Abbasi F, Blasey C, Reaven GM. Cardiometabolic risk factors and obesity: does it matter whether BMI or waist circumference is the index of obesity? Am J Clin Nutr. 2013;98:637–640. doi: 10.3945/ajcn.112.047506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flegal KM, Shepherd JA, Looker AC, et al. Comparisons of percentage body fat, body mass index, waist circumference, and waist-stature ratio in adults. Am J Clin Nutr. 2009;89:500–508. doi: 10.3945/ajcn.2008.26847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ford ES, Li C, Cook S, et al. Serum concentrations of uric acid and the metabolic syndrome among US children and adolescents. Circulation. 2007;115:2526–2532. doi: 10.1161/CIRCULATIONAHA.106.657627. [DOI] [PubMed] [Google Scholar]
- 20.Fraser A, Longnecker MP, Lawlor DA. Prevalence of elevated alanine aminotransferase among US adolescents and associated factors: NHANES 1999–2004. Gastroenterology. 2007;133:1814–1820. doi: 10.1053/j.gastro.2007.08.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li C, Ford ES. Is there a single underlying factor for the metabolic syndrome in adolescents? A confirmatory factor analysis. Diabetes Care. 2007;30:1556–1561. doi: 10.2337/dc06-2481. [DOI] [PubMed] [Google Scholar]
- 22.Kannel WB. Elevated systolic blood pressure as a cardiovascular risk factor. Am J Cardiol. 2000;85:251–255. doi: 10.1016/s0002-9149(99)00635-9. [DOI] [PubMed] [Google Scholar]
- 23.Sumner AE, Cowie CC. Ethnic differences in the ability of triglyceride levels to identify insulin resistance. Atherosclerosis. 2008;196:696–703. doi: 10.1016/j.atherosclerosis.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 24.DeBoer MD, Gurka MJ, Sumner AE. Diagnosis of the Metabolic Syndrome Is Associated With Disproportionately High Levels of High-Sensitivity C-Reactive Protein in Non-Hispanic Black Adolescents: An analysis of NHANES 1999–2008. Diabetes Care. 2011;34:734–740. doi: 10.2337/dc10-1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DeBoer MD, Dong L, Gurka MJ. Racial/Ethnic and Sex Differences in the Ability of Metabolic Syndrome Criteria to Predict Elevations in Fasting Insulin Levels in Adolescents. Journal of Pediatrics. 2011;159:975–981. doi: 10.1016/j.jpeds.2011.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DeBoer MD, Gurka MJ. Low sensitivity for the metabolic syndrome to detect uric acid elevations in females and non-Hispanic-black male adolescents: An analysis of NHANES 1999–2006. Atherosclerosis. 2012;220:575–580. doi: 10.1016/j.atherosclerosis.2011.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DeBoer MD. Underdiagnosis of Metabolic Syndrome in Non-Hispanic Black Adolescents: A Call for Ethnic-Specific Criteria. Curr Cardiovasc Risk Rep. 2010;4:302–310. doi: 10.1007/s12170-010-0104-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Razak F, Anand SS, Shannon H, et al. Defining obesity cut points in a multiethnic population. Circulation. 2007;115:2111–2118. doi: 10.1161/CIRCULATIONAHA.106.635011. [DOI] [PubMed] [Google Scholar]
- 29.Walker SE, Gurka MJ, Oliver MN, et al. Racial/ethnic discrepancies in the metabolic syndrome begin in childhood and persist after adjustment for environmental factors. Nutrition Metabolism and Cardiovascular Diseases. 2012;22:141–148. doi: 10.1016/j.numecd.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu LT, Bentler PM. Cut-off criteria for fit indexes in covariance structure analysis: Conventional criteria vs. new alternatives. Structural Equation Modeling. 1999;6:1–55. [Google Scholar]
- 31.Lee AM, Gurka MJ, DeBoer MD. Correlation of metabolic syndrome severity with cardiovascular health markers in adolescents. Metabolism. 2017;69:87–95. doi: 10.1016/j.metabol.2017.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu TY, Jee JH, Bae JC, et al. Serum uric acid: A strong and independent predictor of metabolic syndrome after adjusting for body composition. Metabolism. 2016;65:432–440. doi: 10.1016/j.metabol.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 33.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- 34.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 35.Taylor HA, Wilson JG, Jones DW, et al. Toward resolution of cardiovascular health disparities in African Americans: design and methods of the Jackson Heart Study. Ethn Dis. 2005;15:S6-4-17. [PubMed] [Google Scholar]
- 36.McNeill AM, Schmidt MI, Rosamond WD, et al. The metabolic syndrome and 11-year risk of incident cardiovascular disease in the atherosclerosis risk in communities study. Diabetes Care. 2005;28:385–390. doi: 10.2337/diacare.28.2.385. [DOI] [PubMed] [Google Scholar]
- 37.Folsom AR, Wu KK, Rosamond WD, et al. Prospective study of hemostatic factors and incidence of coronary heart disease: the Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 1997;96:1102–1108. doi: 10.1161/01.cir.96.4.1102. [DOI] [PubMed] [Google Scholar]
- 38.Keku E, Rosamond W, Taylor HA, et al. Cardiovascular disease event classification in the Jackson Heart Study: methods and procedures. Ethn Dis. 2005;15:S6-62-70. [PubMed] [Google Scholar]
- 39.Schmidt MI, Duncan BB, Bang H, et al. Identifying individuals at high risk for diabetes: The Atherosclerosis Risk in Communities study. Diabetes Care. 2005;28:2013–2018. doi: 10.2337/diacare.28.8.2013. [DOI] [PubMed] [Google Scholar]
- 40.Effoe VS, Correa A, Chen H, et al. High-Sensitivity C-Reactive Protein Is Associated With Incident Type 2 Diabetes Among African Americans: The Jackson Heart Study. Diabetes Care. 2015;38:1694–1700. doi: 10.2337/dc15-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pencina MJ, D’Agostino RB. Overall C as a measure of discrimination in survival analysis: model specific population value and confidence interval estimation. Stat Med. 2004;23:2109–2123. doi: 10.1002/sim.1802. [DOI] [PubMed] [Google Scholar]
- 42.Cefalu WT, Rubino F, Cummings DE. Metabolic Surgery for Type 2 Diabetes: Changing the Landscape of Diabetes Care. Diabetes Care. 2016;39:857–860. doi: 10.2337/dc16-0686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Deurenberg P, Yap M, van Staveren WA. Body mass index and percent body fat: a meta analysis among different ethnic groups. Int J Obes Relat Metab Disord. 1998;22:1164–1171. doi: 10.1038/sj.ijo.0800741. [DOI] [PubMed] [Google Scholar]
- 44.von Eckardstein A, Rohrer L. HDLs in crises. Curr Opin Lipidol. 2016;27:264–273. doi: 10.1097/MOL.0000000000000294. [DOI] [PubMed] [Google Scholar]
- 45.Goff DC, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S49–73. doi: 10.1161/01.cir.0000437741.48606.98. [DOI] [PubMed] [Google Scholar]
- 46.D’Agostino RB, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117:743–753. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 47.Bang H, Edwards AM, Bomback AS, et al. Development and validation of a patient self-assessment score for diabetes risk. Ann Intern Med. 2009;151:775–783. doi: 10.1059/0003-4819-151-11-200912010-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rosito GA, Massaro JM, Hoffmann U, et al. Pericardial fat, visceral abdominal fat, cardiovascular disease risk factors, and vascular calcification in a community-based sample: the Framingham Heart Study. Circulation. 2008;117:605–613. doi: 10.1161/CIRCULATIONAHA.107.743062. [DOI] [PubMed] [Google Scholar]
- 49.Eisenmann JC. On the use of a continuous metabolic syndrome score in pediatric research. Cardiovascular Diabetology. 2008;7:17. doi: 10.1186/1475-2840-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Andersen LB, Harro M, Sardinha LB, et al. Physical activity and clustered cardiovascular risk in children: a cross-sectional study (The European Youth Heart Study) Lancet. 2006;368:299–304. doi: 10.1016/S0140-6736(06)69075-2. [DOI] [PubMed] [Google Scholar]