Abstract
Background
Virtual reality and augmented feedback have become more prevalent as training methods to improve balance. Few reports exist on the benefits of providing trunk motion visual feedback (VFB) during treadmill walking, and most of those reports only describe within session changes.
Research Question
To determine whether trunk motion VFB treadmill walking would improve over-ground balance for older adults with self-reported balance problems.
Methods
40 adults (75.8 years (SD 6.5)) with self-reported balance difficulties or a history of falling were randomized to a control or experimental group. Everyone walked on a treadmill at a comfortable speed 3x/week for 4 weeks in 2 minute bouts separated by a seated rest. The control group was instructed to look at a stationary bulls-eye target while the experimental group also saw a moving cursor superimposed on the stationary bulls-eye that represented VFB of their walking trunk motion. The experimental group was instructed to keep the cursor in the center of the bulls-eye. Somatosensory (monofilaments and joint position testing) and vestibular function (canal specific clinical head impulses) was evaluated prior to intervention. Balance and mobility were tested before and after the intervention using Berg Balance Test, BESTest, mini-BESTest, and Six Minute Walk.
Results
There were no significant differences between groups before the intervention. The experimental group significantly improved on the BESTest (p = 0.031) and the mini-BEST (p = 0.019). The control group did not improve significantly on any measure. Individuals with more profound sensory impairments had a larger improvement on dynamic balance subtests of the BESTest.
Significance
Older adults with self-reported balance problems improve their dynamic balance after training using trunk motion VFB treadmill walking. Individuals with worse sensory function may benefit more from trunk motion VFB during walking than individuals with intact sensory function.
This clinical trial was registered at www.clinicaltrials.gov Clinical Trial # 366151-1.
Keywords: Balance, Visual Biofeedback, Exercise Therapy, Gait
Introduction
Falls are the leading cause of fatal and nonfatal injuries in older adults [1,2]. Close to one third of the population over the age of 65 fall annually, with a half of those falls leading into injuries [2,3]. Aging is accompanied by an overall reduction in mobility and decrease in sensory integration which have been associated with falls [4–6]. Visual feedback/augmented reality for balance training has become more common method to reduce fall risk [7]. These technologies afford new avenues to enhance balance ability in a safe, controlled, and engaging environment [8].
Most visual feedback (VFB) balance interventions have focused on standing or weight-shifting tasks [9–16], however, falls primarily occur during locomotion [3,17,18]. While virtual/augmented reality (VR) training has recently been shifting to more dynamic activities like walking, the majority of the walking VFB training is based on foot or leg kinematics with an emphasis on normalizing the gait cycle [19–23]. Control of foot placement is important for controlling displacement of the whole body center of mass, but upright trunk orientation is degraded for individuals with balance problems [24,25]. Specifically, excessive forward trunk lean during walking has been associated with increased fall risk [26].
Training foot placement may contribute to enhanced control of center of mass translation [27], but may be insufficient to improve trunk on legs orientation as the legs and trunk respond differently and on different time scales to sensory perturbations [28,29]. VFB training involving trunk orientation and trunk translation would allow the individual to more flexibly solve the stability problem (trunk on legs, stepping, or both) by taking advantage of their many degrees of freedom [30]. Considering the benefits of improved trunk motion for balance [31], providing concurrent VFB of trunk motion during walking may be a beneficial training strategy to improve balance [32].
This study builds on responses to concurrent trunk motion VFB during treadmill walking [32,33] to investigate carry over and transfer to over-ground dynamic balance for older adults at risk of falling. The primary aim of this study was to determine whether training with trunk motion VFB for 4 weeks would result in improved balance for older adults with self-reported balance problems. We hypothesized that training with trunk motion VFB while walking on a treadmill will improve balance measured with clinical tests of dynamic balance.
Methods
Design Overview
This study was a 2 arm, assessor blinded experimental design with random assignment to the control and experimental arms.
Setting and Participants
40 older adults with self-reported balance difficulties or a history of falling completed this study (Clinical Trial # 366151-1, www.ClinicalTrials.gov). The average age of the control group was 75.8 years (SD 6.5, range 66–92 years) and 65% of them were female. The average age of the experimental group was 75.7 years (SD 5.3, range 68–87 years) and 80% were female. This study was approved by the Institutional Review boards at the University of Maryland and Temple University. All subjects provided written informed consent prior to participation. The experiment was performed at two locations: Temple University and Collington Episcopal Life Care Community. After providing informed consent and passing the Mini Mental Status Exam (scores > 23) subjects demonstrated they could safely and independently walk on a treadmill for at least 2 minutes at a self-selected speed. Subjects were excluded for not passing the Mini Mental Status Exam (n = 1) or not safely and independently walking on the treadmill for 2 minutes (n = 1). The assessors were blinded to group allocation until the study was completed. No attempt was made to blind the subjects, although they were not explicitly told whether they were in the control or VFB group.
Randomization and Interventions
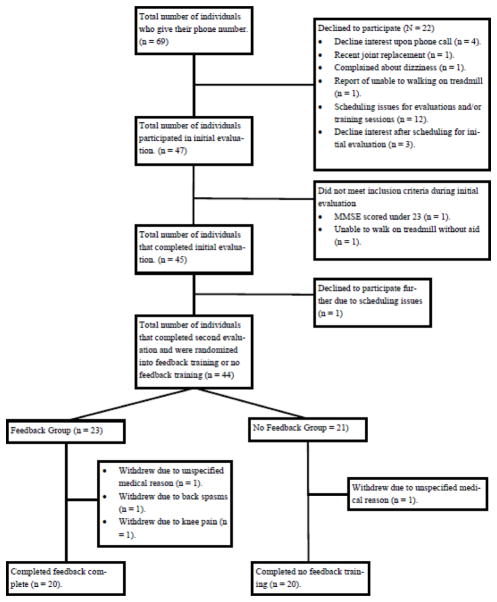
Individuals were randomized into either the experimental (n = 20) or control (n = 20) arms of the study. For each recruitment phase the study coordinator (LM) assigned participants a computer generated random number determining group allocation, see Figure 1.
Figure 1.
CONSORT flow chart diagram.
Trunk Motion Visual Feedback
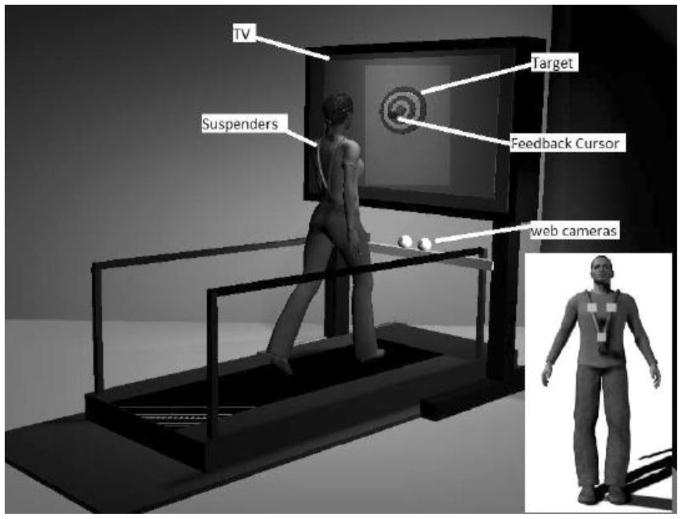
Subjects walked on a treadmilla approximately 24 inches in front of a 27” TVb, as shown in Figure 2. The VFB device has been described in detail and is briefly presented here [32]. Each of the 10 rings of the bull’s-eye was one inch wide and corresponded to one inch of physical space on the treadmill (translation) or 1 degree from vertical (orientation). Two webcamsc tracked the 3-D position of three markers (at the navel, and each shoulder, see the inset of Figure 2) attached to suspenders [32]. The subject’s virtual motion (translation vs. orientation) was displayed in the form of a moving cursor on the TV screen. Translation was defined as the 2-dimensional (anterior-posterior [AP] and mediolateral [ML]) displacement of the lower marker on the suspenders, see inset Figure 2. Orientation with respect to vertical was defined as the angular deviation of the trunk segment (defined by the lower marker and the midpoint of the two upper markers) from vertical [34]. Cursor motion was smoothed using a 5 point moving average filter and scaled to map subject motion to on screen cursor motion in a 1:1 manner.
Figure 2.
Illustration of the experimental set-up. Participants walked on a treadmill in front of a TV with a bulls-eye display. Participants wore a safety harness (not depicted) that did not provide support unless they fell. A moving cursor representing either their center of mass translation or trunk orientation motion was superimposed over the bullseye target only for the experimental group.
Intervention Procedures
The VFB training sessions lasted 30 minutes and were conducted 3 times per week for 4 weeks. Training sessions always consisted of the following: subjects were asked if they had fallen since their last session and donned a safety harness. Subjects were instructed to use the handrails only if they lost balance. Each session, the subject’s “comfortable speed” was determined: the treadmill speed was increased until the subject said “too fast” and then decreased speed until the subject said “too slow.” The midpoint of “too fast” and “too slow” was their “comfortable speed” for that session [35,36]. Subjects were blinded to their walking speed. During the first training session, subjects in the experimental group were instructed on how to interact with both types of trunk motion VFB (translation and orientation). They were briefly trained and demonstrated the ability to keep the cursor “as close to the center of the bull’s-eye as possible,” minimizing displacement or angular deviations. VFB sessions consisted of 2 minute walks with VFB, followed by a seated rest (30, 60, or 120 seconds at the subject’s request). That process was repeated for 30 minutes resulting in 8–12 walking bouts per session. The VFB (translation and orientation) order was randomized during each training session, each 2 minute walking bout provided either translation or orientation VFB. The experimental group was informed as to the type of VFB prior to each 2 minute bout.
The control group also attended the same training schedule. The procedures were the same except that they did not receive training in how to use the VFB, and they did not see or interact with the VFB. The bulls-eye was visible and the control group was instructed to look at the center of the bulls-eye while walking. Walking and seated rest timing was the same as for the experimental group.
Outcomes and Follow-up
The standardized gait and balance assessments were administered by blinded assessors (EA, ET, RR) at Pre-test 1 (week 1), Pre-test 2 (week 4) and the Post-test (week 8). The experimental timeline is provided in Table 2. No further follow up was provided. The BESTest, mini-BESTest (mBEST), Berg Balance Scale (BBS), Timed Up and Go (TUG), Activities-specific Balance Confidence (ABC) scale, and 6 minute walk test (6MWT) have excellent test-retest reliability (ICCs 0.84 – 0.99) and were used to characterize balance and walking ability [37–44]. The primary outcome measures were BESTest and mBEST scores, which may provide systems level mechanistic insight into clinical balance problems [42]. The BESTest is a physical performance test with 27 items distributed among six sub-systems of static and dynamic balance: 1) biomechanical constraints, 2) stability limits/verticality, 3) anticipatory postural adjustments, 4) postural responses, 5) sensory orientation, and 6) stability in gait [42]. The BESTest may allow for more targeted rehabilitation based on identified balance system impairments. The mBEST, a shortened version of the BESTest, more explicitly focuses on dynamic balance assessment [43,45]. Secondary outcome measures were the BBS, TUG, the ABC, and 6MWT. Fallers were classified using cut-off scores for the ABC, TUG, TUGc, BBS, and self-reported fall history in the previous 12 months [37,39]. Individuals with two or more positive scores after the first testing session were classified as fallers [40,41].
Table 2.
Experimental timeline. The treadmill training started within 48 hours of Pretest#2 and the Post-test occurred within 48 hours of the last treadmill training session. At the time the study was initiated the BESTest and mBEST were not validated for older fallers and the test retest reliability had not been reported. We used data from Pretest#1 and Pretest#2 to determine validity and test retest reliability which is reported elsewhere [37].
| Pretest#1 | 4 week control period with instructions not to modify physical activity | Pretest#2 | 4 weeks Treadmill Training either with VFB (experimental) or without VFB (control) | Post-test |
| BESTest | BESTest | BESTest | ||
| mBEST | mBEST | mBEST | ||
| BBS | BBS | BBS | ||
| ABC | ABC | ABC | ||
| TUG | TUG | TUG | ||
| 6MWT | 6MWT | 6MWT | ||
| Vestibular Test | ||||
| Monofilament Test | ||||
| Joint Position Test | ||||
| Vibration Test |
Vestibular and Somatosensory Assessment
Touch/pressure was measured using: Semmes Weinstein mono-filaments [46], passive tests of proprioception at the great toe, vibration testing using a 128 Hz tuning fork at the medial malleolus [47]. Canal specific clinical head impulse testing measured vestibular function [48]. Any plantar testing site requiring 2g or more force to identify monofilament touch was classified as impaired touch sensation [49]. Inability to discriminate tuning fork vibration was classified as impaired vibration sensation. Any incorrect identification of the great toe position during the proprioception test was classified as impaired proprioception. These classifications were coded (0/1) and summed (0–3) to indicate an overall sense of somatosensory impairment. Any positive head impulse test (observed re-fixation saccade) was classified as impaired vestibular function [48]. Some individuals refused specific tests (see Table 1).
Table 1.
Demographics and average balance and mobility scores before the visual feedback training. There were no significant differences between groups before the visual feedback training.
| Balance/Mobility Test | Control Group | Experimental Group | p value |
|---|---|---|---|
| Age | 75.8 (6.5) | 75.7 (5.3) | p = 0.937 |
| Gender (male/female) | 7/13 | 4/16 | |
| Impaired Touch (%) | 80%, n=20 | 80% n = 19† | |
| Impaired Vibration (%) | 26%, n = 19† | 37%, n = 19† | |
| Impaired Proprioception (%) | 18%, n = 17† | 22%, n = 18† | |
| Somatosensory Impairment | |||
| Impairment = 0 | 24%, n = 17† | 11%, n = 18† | |
| Impairment = 1 | 41%, n = 17† | 39%, n = 18† | |
| Impairment = 2 | 24%, n = 17† | 28%, n = 18† | |
| Impairment = 3 | 6%, n = 17† | 17%, n = 18† | |
| Impaired Vestibular (%) | 50%, n = 20 | 53%, n = 19 | |
| Fall History (n) | 14 | 16 | p = 0.478 |
| Classified Fallers (n) | 8 | 6 | p = 0.752 |
| BESTest (%) | 76 (7.6) | 75.9 (7.7) | p = 1.000 |
| Mini-BESTest | 20.6 (4.1) | 20.3 (3.1) | p = 0.309 |
| BBS | 51.2 (3.1) | 51.7 (3.8) | p = 0.188 |
| ABC (%) | 80.5 (14.3) | 77.7 (18.9) | p = 1.000 |
| TUG (sec) | 11.1 (3.3) | 10.6 (3.1) | p = 0.981 |
| 6MWT (m) | 370.8 (94.8) | 380.7 (74.2) | p = 0.174 |
- not all subjects were willing to complete the test
Sample Size
Sample size was estimated using G*Power© [50], with a conservative estimate of a medium effect size for the clinical tests a sample size of 14 subjects in each group (28 total) with α = .05 results in 95% power to detect a medium effect size with correlation r = 0.7. Sample size was inflated to 45 to allow for up to 10% loss to follow up. 20 individuals in each group should be more than sufficient to detect a significant difference in performance on the BESTest with 80% power at a significance level of 5%.
Statistical Analysis
Age was compared between groups with a t-test. Mixed-model repeated measures ANOVAs were used to investigate changes in balance and mobility, with post hoc comparisons for effects of time (within subjects) and group (between subjects) adjusting for multiple comparisons using Tukey’s method. Post-hoc exploratory linear regressions investigated whether prior sensory function impacted change in BESTest subtest scores. Statistical analysis was performed using STATA softwared. Hypothesis testing was conducted at α = 0.05.
Results
Only the 40 individuals who completed the intervention were included in the analysis. Each subject attended at least 10 of the 12 scheduled training sessions, see Figure 1. There were no adverse effects during the study. 75% reported at least one fall in the previous 12 months. Fourteen out of the 40 individuals were classified as fallers. Somatosensory impairments were identified in 76% (n = 17) of control subjects and 89% (n = 18) of experimental subjects who participated in the touch, vibration, and proprioception testing. Angular vestibular function was impaired in approximately 50% of subjects from each group.
There were no between group differences for age or balance and mobility scores before the four week training period, see Table 1. There was a main effect of time for the experimental group for the BESTest (F(2,76) = 10.97, p < 0.0001) and mBESTest (F(2,76) = 10.83, p < 0.0001). Tukey post hoc comparisons demonstrated that the experimental group showed significant improvement on the BESTest (t(3,76) = 3.11, p = 0.031) and the mBEST (t(3,76) = 3.28, p = 0.019) between Pre-Test 2 and Post-Test, see Table 3. There were no significant changes on any measures of balance and mobility for the control group, see Table 3.
Table 3.
Mean (SD) and [95% CI] balance and mobility scores before and after the visual feedback training. Significant within group effects of time indicated by an *, there were no significant between group differences over time.
| Group | Clinical Test | Pre- Test 1 | Pre- Test 2 | Post- Test | Pre1 – Pre2 p value |
Pre2 - Post p value |
|---|---|---|---|---|---|---|
| Experimental | BESTest (%) | 74.96(7.2) [71.6–78.3] |
75.9(7.7) [72.3–79.5] |
79.2(6.1) [76.4–82.1] |
p =0.944 | p =0.031* |
| Mini- BESTest | 20.2(2.9) [18.8–21.5] |
20.3(3.1) [18.8–21.7] |
21.9(2.7) [20.6–23.2] |
p =1.000 | p =0.019* | |
| BBS | 51.5(3.1) [50.0–52.9] |
51.7(3.8) [49.9–53.4] |
52.5(2.7) [51.2–53.8] |
p =0.999 | p =0.678 | |
| ABC (%) | 76.9(18.3) [68.3–85.4] |
77.7(18.9) [68.9–86.6] |
77.1(18.1) [68.6–85.6] |
p =1.000 | p =1.000 | |
| TUG(sec) | 10.9(2.8) [9.5–11.1] |
10.6(3.1) [9.2–10.9] |
10.1(2.9) [8.7–10.7] |
p =0.902 | p =0.646 | |
| 6MWT(m) | 369.7(67.9) [337.9–401.5] |
380.7(74.2) [346.0–415.4] |
387.8(70.8) [354.7–420.9] |
p =0.806 | p =0.964 | |
| Control | BESTest(%) | 75.22(7.3) [71.8–78.7] |
76 (7.6) [72.4– 79.6] |
77.7(7.0) [74.4–81.0] |
p =0.977 | p =0.580 |
| Mini- BESTest | 20.3(3.7) [18.6– 22.0] |
20.6(4.1) [18.7– 22.5] |
21.6(3.3) [20.0- 23.2] |
p =0.991 | p =0.360 | |
| BBS | 50.9(3.9) [49.0–52.7] |
51.2(3.1) [49.7–52.7] |
51.9(3.4) [50.3–53.4] |
p =0.990 | p =0.867 | |
| ABC (%) | 83.2(12.6) [77.3–89.0] |
80.5(14.3) [73.8–87.2] |
79.1(20.2) [69.6–88.5] |
p =0.939 | p =0.996 | |
| TUG(sec) | 11.3(3.2) [10.1–13.2] |
11.1(3.3) [9.7–12.4] |
11.2(2.9) [9.6–11.9] |
p =0.984 | p =1.000 | |
| 6MWT(m) | 374.5(76.1) [338.9–410.2] |
370.8(94.8) [326.5–415.2] |
377.6(88.9) [336.0–419.2] |
p =0.998 | p =0.971 |
Individuals with isolated vestibular impairment (n = 5) or multisensory (vestibular and somatosensory) impairment (n = 5) improved significantly more on stability limits and verticality ability (BESTest component 2) (β = 11.9, p = 0.011, 95% CI [3.2–20.6]) and (β = 10.2, p = 0.025, 95% CI [1.5–18.9]) respectively, compared to individuals with no sensory impairments (n = 4). Individuals with no sensory impairment (n = 4) improved significantly more on the BESTest component 4 which focuses on postural responses (β = 32.7, p = 0.031, 95% CI [3.5–61.8]) compared to individuals with multisensory impairment (n = 5). Individuals with multisensory impairment (n = 5) improved significantly more on stability in gait (BESTest component 6) (β = 14.2, p = 0.037, 95% CI [1.0–27.5]) compared to individuals with no sensory impairment (n = 4).
Discussion
Overall, training for four weeks with trunk motion VFB during treadmill walking resulted in a significant improvement in balance and mobility as measured by the BESTest and mBEST. No improvement was observed from treadmill walking without VFB. This is consistent with previous reports that walking alone was insufficient to improve balance [52,53], although a recent study reported that walking both forward and backward on a treadmill improved balance for older adults [54]. Overall, the magnitude of average improvement on the BESTest and mBEST was below the minimum detectable change (MDC) reported for these tests [44]. However, MDC values are intended to be applied to individual and not aggregate group change. Three individuals exceeded the MDC for the BESTest and another 3 exceeded the MDC for the mBEST. Approximately half of the cohort approached these clinical criteria for improvement, and those individuals who improved the most had lower scores before the training (data not shown). The current results add to the growing body of evidence suggesting that augmented reality or VFB may be an important tool for balance rehabilitation [10,55,56], by expanding those results specifically to balance training during walking.
VFB operates on a relatively slow time scale which suggests that changes in trunk motion may reflect voluntary changes in behavior [23,32,57]. These voluntary changes necessitate active participation, which may allow integration of the VFB error signal with internal sensory feedback leading to in an increased awareness of body motion during walking with respect to the external environment [58]. We previously demonstrated that the same visual input results in different responses for trunk translation and trunk orientation [34]. Therefore, the VFB in this study alternately represented either deviation from gravitational vertical, or a navigational cue of center of mass displacement. The external contextualization (gravity/path integration) may facilitate reweighting of visual, somatosensory, and vestibular signals for dynamic balance during walking [4,10,59]. Although the mechanism cannot be elucidated, trunk translation VFB was designed to enhance path integration [32], while the trunk orientation VFB was designed to facilitate balance while walking. Future studies are needed to characterize the mechanisms which mediate the balance improvement facilitated by trunk motion VFB reported here.
Partial vestibular loss is known to occur associated with the normal aging process [60]; however, it is unclear how impactful vestibular impairment is on walking balance ability [4,61]. In this cohort, approximately 75% had some form of somatosensory impairment and approximately 50% had some degree of rotational vestibular impairment (see Table 1). Interestingly, our preliminary results demonstrate that an individual’s response to VFB training may depend on the severity or complexity of their sensory impairment. Those with greater sensory impairment demonstrated significantly greater improvement on the BESTest sub-tests which emphasize “limits of stability/verticality” and “stability in gait” compared to their sensory healthy counterparts. Although the sample size is small, these preliminary results suggest that training trunk orientation and whole body translation during walking led to greater improvement in standing verticality ability and dynamic gait stability. This suggests individuals with sensory impairment would benefit from fall prevention programs using trunk feedback during walking. The sensory intact group improved to a greater extent on BESTest component evaluating reactive “postural responses.” It is unclear whether the group with impaired sensation responded more slowly (impaired detection of the loss of balance) or whether the corrective stepping was insufficient to correct the loss of balance (weak response or an underestimation of appropriate step size). The mechanisms driving improvement following trunk motion VFB training remain to be investigated. Future studies should evaluate sensory function before and after training to determine whether changes are driven by physiological adaptation or higher levels of sensory processing like perception.
Interestingly, the participants in this study only improved on balance tests including components of walking. The average BBS score before the intervention was 51 for this cohort; therefore, a ceiling effect may have contributed to the lack of change. However, it is also possible that the BBS is not capable of capturing changes in dynamic walking balance since the majority of BBS test items are performed with a fixed base of support. The “comfortable speed” walking VFB training paradigm was specifically designed not to introduce potentially confounding changes in walking ability [62]. This accounts for the lack of change in over-ground walking ability based on the 6MWT. The average ABC scores were above the threshold for elevated fall risk, which may make it more difficult to identify measureable change in balance confidence. Alternatively, the modest improvements observed on the BESTest and mBEST after only four weeks of training may not have been sufficient for subjects to recognize or attribute improvements in daily activities to improved balance ability [63]. Future work should identify appropriate dosage and training durations that will maximize balance improvements and retention.
Limitations
Recruiting individuals based on self-reported balance problems may have resulted in a biased sample, and cannot be generalized to more impaired clinical population. Many of the subjects were not classified as fallers and scored above the cut-off thresholds for identifying increased fall risk [37,39]. It is unknown whether any individuals experienced a change in sensory function or sensitivity following the training which may have contributed to our results. The results suggesting an increased effect for individuals with greater sensory impairment are preliminary, but may assist with determining which patients are appropriate for this type of intervention in clinical settings. It is unknown how long the beneficial effects of walking VFB balance training last.
Conclusion
Older adults with self-reported balance problems improved their over-ground dynamic walking balance after trunk motion VFB training while treadmill walking. Older adults with vestibular loss or mixed sensory impairments may benefit from trunk motion VFB training during walking more than individuals with normal sensory function. The effects of training with trunk motion VFB may be more beneficial for individuals with more severe balance impairment.
Highlights.
Trunk motion VFB while walking improved balance for older adults
Walking on a treadmill alone did improve balance
The degree of sensory impairment may impact balance training using VFB
Acknowledgments
This work was supported in part by NIH grant 7R21AG041714 (J Jeka, PI); NIH grant NIDCD T32 DC000023 (E Anson); PODS I and PODS II Scholarships from the Foundation for Physical Therapy, Inc. (E Anson, PI); and a Wylie Dissertation Fellowship from the University of Maryland Graduate School (E Anson, PI).
The authors would like to thank North City Congress (Philadelphia, PA), Dr. Grace Ma’s Center for Asian Health (Philadelphia, PA) and Collington Episcopal Life Care Community (Mitchellville, MD) for their help in the recruitment process. The authors would also like to thank: Lori Moore, Michelle Huang, Henry Khov, Aiste Cechaviciute, Holly Apeldorn, Yuzheng (Po) Liu, Yolanda Zhang, Xiaou Huang, and Jiajun He for their assistance during training and evaluation. A special thank you to all the participants.
Abbreviations
- VR
Virtual/augmented reality
- ML
Mediolateral
- AP
Anterior-posterior
- VFB
Visual feedback
- mBEST
mini-BESTest
- BBS
Berg Balance Scale
- TUG
Timed Up and Go
- ABC
Activities-specific Balance Confidence
- 6MWT
6 minute walk test
Footnotes
Suppliers list:
Cybex Trotter 900T, Cybex International, 10 Trotter Dr, Medway, MA
ViewSonic VA2703, Viewsonic Corporation, 10 Pointe Dr, Brea, CA
Logitech Orbit AF, Logitech Int., 7700 Gateway Blvd., Newark, CA
StataCorp LLC, 4905 Lakeway Drive, College Station, TX
Presentation
This work has been presented as a research platform talk at the Combined Sections Meeting of the APTA in 2017.
Clinical trial registration # NCT01690611
Disclosure of Interest
J Jeka, and E Anson disclose that they are listed as inventors of the sensory treadmill described in this experiment United States Patent# 8,900,165. All other authors have no conflict of interest.
Study sponsor
This work was supported in part by NIH grant 7R21AG041714 (J Jeka, PI); PODS I and PODS II Scholarships from the Foundation for Physical Therapy, Inc. (E Anson, PI); and a Wylie Dissertation Fellowship from the University of Maryland Graduate School (E Anson, PI).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.American College of Surgeons. Statement on older adult falls and falls prevention. Bull Am Coll Surg. 2014;99:47. [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention National Center for Injury Prevention and Control. [accessed June 30, 2012];Web–based Injury Statistics Query and Reporting System (WISQARS) 2015 http://www.cdc.gov/injury/wisqars/index.html.
- 3.Kenny RA, Romero-Ortuno R, Cogan L. Falls. Medicine (Baltimore) 2013;41:24–28. [Google Scholar]
- 4.Novak AC, Deshpande N. Effects of aging on whole body and segmental control while obstacle crossing under impaired sensory conditions. Hum Mov Sci. 2014;35:121–30. doi: 10.1016/j.humov.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 5.Callisaya ML, Blizzard L, Schmidt MD, Mcginley JL, Lord SR, Srikanth VK. A population-based study of sensorimotor factors affecting gait in older people. Age Ageing. 2009;38:290–295. doi: 10.1093/ageing/afp017. [DOI] [PubMed] [Google Scholar]
- 6.Mignardot JB, Deschamps T, Barrey E, Auvinet B, Berrut G, Cornu C, Constans T, De Decker L. Gait disturbances as specific predictive markers of the first fall onset in elderly people: A two-year prospective observational study. Front Aging Neurosci. 2014;6:1–13. doi: 10.3389/fnagi.2014.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lohse K, Shirzad N, Verster A, Hodges N, Van der Loos HFM. Video games and rehabilitation. J Neurol Phys Ther. 2013;37:166–175. doi: 10.1097/NPT.0000000000000017. [DOI] [PubMed] [Google Scholar]
- 8.Givon N, Zeilig G, Weingarden H, Rand D. Video-games used in a group setting is feasible and effective to improve indicators of physical activity in individuals with chronic stroke: A randomized controlled trial. Clin Rehabil. 2015:1–10. doi: 10.1177/0269215515584382. [DOI] [PubMed] [Google Scholar]
- 9.Liao YY, Yang YR, Cheng SJ, Wu YR, Fuh JL, Wang RY. Virtual reality-based training to improve obstacle-crossing performance and dynamic balance in patients with Parkinson’s Disease. Neurorehabil Neural Repair. 2015;29:658–667. doi: 10.1177/1545968314562111. [DOI] [PubMed] [Google Scholar]
- 10.Kang KY. Effects of visual biofeedback training for fall prevention in the elderly. J Phys Ther Sci. 2013;25:1393–5. doi: 10.1589/jpts.25.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Merriman NA, Whyatt C, Setti A, Craig C, Newell FN. Successful balance training is associated with improved multisensory function in fall-prone older adults. Comput Human Behav. 2015;45:192–203. [Google Scholar]
- 12.Sayenko DG, Masani K, Vette AH, Alekhina MI, Popovic MR, Nakazawa K. Effects of balance training with visual feedback during mechanically unperturbed standing on postural corrective responses. Gait Posture. 2012;35:339–344. doi: 10.1016/j.gaitpost.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 13.Hatzitaki V, Amiridis IG, Nikodelis T, Spiliopoulou S. Direction-induced effects of visually guided weight-shifting training on standing balance in the elderly. Gerontology. 2009;55:145–152. doi: 10.1159/000142386. [DOI] [PubMed] [Google Scholar]
- 14.Sihvonen SE, Sipilä S, Era PA. Changes in postural balance in frail elderly women during a 4-week visual feedback training: a randomized controlled trial. Gerontology. 2004;50:87–95. doi: 10.1159/000075559. [DOI] [PubMed] [Google Scholar]
- 15.Gschwind YJ, Schoene D, Lord SR, Ejupi A, Valenzuela Artaega T, Aal K, Woodbury A, Delbaere K. The effect of sensor-based exercise at home on functional performance associated with fall risk in older people - a comparison of two exergame interventions. Eur Rev Aging Phys Act. 2015:1–9. doi: 10.1186/s11556-015-0156-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ejupi A, Brodie M, Gschwind YJ, Lord SR, Delbaere K. Kinect-based choice reaching and stepping reaction time tests for clinical and in-home assessment of fall risk in older people: a prospective study. Eur Rev Aging Phys Act. 2016;13:1–7. doi: 10.1186/s11556-016-0162-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shimada H, Obuchi S, Kamide N, Shiba Y, Okamoto M, Kakurai S. Relationship with dynamic balance function during standing and walking. Am J Phys Med Rehabil. 2003;82:511–516. doi: 10.1097/01.PHM.0000064726.59036.CB. [DOI] [PubMed] [Google Scholar]
- 18.Voermans NC, Snijders AH, Schoon Y, Bloem BR. Why old people fall (and how to stop them) Pr Neurol. 2007;7:158–171. doi: 10.1136/jnnp.2007.120980. [DOI] [PubMed] [Google Scholar]
- 19.Mirelman A, Patritti BL, Bonato P, Deutsch JE. Effects of virtual reality training on gait biomechanics of individuals post-stroke. Gait Posture. 2010;31:433–437. doi: 10.1016/j.gaitpost.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 20.Mirelman A, Maidan I, Herman T, Deutsch JE, Giladi N, Hausdorff JM. Virtual reality for gait training: can it induce motor learning to enhance complex walking and reduce fall risk in patients with Parkinson’s disease? J Gerontol A Biol Sci Med Sci. 2011;66:234–240. doi: 10.1093/gerona/glq201. [DOI] [PubMed] [Google Scholar]
- 21.Jaffe DL, Brown Da, Pierson-Carey CD, Buckley EL, Lew HL. Stepping over obstacles to improve walking in individuals with poststroke hemiplegia. J Rehabil Res Dev. 2004;41:283–92. doi: 10.1682/jrrd.2004.03.0283. [DOI] [PubMed] [Google Scholar]
- 22.Levinger P, Zeina D, Teshome AK, Skinner E, Begg R, Abbott JH. A real time biofeedback using Kinect and Wii to improve gait for post-total knee replacement rehabilitation: a case study report. Disabil Rehabil Assist Technol. 2016;11:251–62. doi: 10.3109/17483107.2015.1080767. [DOI] [PubMed] [Google Scholar]
- 23.Halická Z, Lobotková J, Bučková K, Hlavačka F. Effectiveness of different visual biofeedback signals for human balance improvement. Gait Posture. 2014;39:410–4. doi: 10.1016/j.gaitpost.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 24.Gill J, Allum JH, Carpenter MG, Held-Ziolkowska M, Adkin AL, Honegger F, Pierchala K. Trunk sway measures of postural stability during clinical balance tests: effects of age. J Gerontol A Biol Sci Med Sci. 2001;56:M438–47. doi: 10.1093/gerona/56.7.m438. [DOI] [PubMed] [Google Scholar]
- 25.Borel L, Harlay F, Magnan J, Chays A, Lacour M. Deficits and recovery of head and trunk orientation and stabilization after unilateral vestibular loss. Brain. 2002;125:880–894. doi: 10.1093/brain/awf085. [DOI] [PubMed] [Google Scholar]
- 26.Mahoney JR, Oh-Park M, Ayers E, Verghese J. Quantitative trunk sway and prediction of incident falls in older adults. Gait Posture. 2017;58:183–187. doi: 10.1016/j.gaitpost.2017.07.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Winter D. Human balance and posture control during standing and walking. Gait Posture. 1995;3:193–214. [Google Scholar]
- 28.Logan D, Ivanenko YP, Kiemel T, Cappellini G, Sylos-Labini F, Lacquaniti F, Jeka JJ. Function dictates the phase dependence of vision during human locomotion. J Neurophysiol. 2014;112:165–80. doi: 10.1152/jn.01062.2012. [DOI] [PubMed] [Google Scholar]
- 29.Dakin CJ, Inglis JT, Chua R, Blouin J-S. Muscle-specific modulation of vestibular reflexes with increased locomotor velocity and cadence. J Neurophysiol. 2013;110:86–94. doi: 10.1152/jn.00843.2012. [DOI] [PubMed] [Google Scholar]
- 30.Latash ML. The bliss (not the problem) of motor abundance (not redundancy) Exp Brain Res. 2012;217:1–5. doi: 10.1007/s00221-012-3000-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Granacher U, Gollhofer A, Hortobágyi T, Kressig RW, Muehlbauer T. The importance of trunk muscle strength for balance, functional performance and fall prevention in seniors: A systematic review. Sport Med. 2013;43:627–641. doi: 10.1007/s40279-013-0041-1. [DOI] [PubMed] [Google Scholar]
- 32.Anson E, Rosenberg R, Agada P, Kiemel T, Jeka J. Does visual feedback during walking result in similar improvements in trunk control for young and older healthy adults? J Neuroeng Rehabil. 2013;10:110. doi: 10.1186/1743-0003-10-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Verhoeff LL, Horlings CGC, Janssen LJF, Bridenbaugh SA, Allum JHJ. Effects of biofeedback on trunk sway during dual tasking in the healthy young and elderly. Gait Posture. 2009;30:76–81. doi: 10.1016/j.gaitpost.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 34.Anson E, Agada P, Kiemel T, Ivanenko Y, Lacquaniti F, Jeka J. Visual control of trunk translation and orientation during locomotion. Exp Brain Res. 2014;232:1941–1951. doi: 10.1007/s00221-014-3885-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jordan K, Challis JH, Newell KM. Walking speed influences on gait cycle variability. Gait Posture. 2007;26:128–134. doi: 10.1016/j.gaitpost.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 36.Dal U, Erdogan T, Resitoglu B, Beydagi H. Determination of preferred walking speed on treadmill may lead to high oxygen cost on treadmill walking. Gait Posture. 2010;31:366–369. doi: 10.1016/j.gaitpost.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 37.Shumway-Cook A, Brauer S, Woollacott MH. Predicting the probability for falls in community-dwelling older adults using the Timed Up & Go Test. Phys Ther. 2000;80:896–903. [PubMed] [Google Scholar]
- 38.Powell LE, Myers AM. The Activities-specific Balance Confidence (ABC) Scale. J Gerontol A Biol Sci Med Sci. 1995;50A:M28–34. doi: 10.1093/gerona/50a.1.m28. [DOI] [PubMed] [Google Scholar]
- 39.Lajoie Y, Gallagher SP. Predicting falls within the elderly community: Comparison of postural sway, reaction time the Berg balance scale and the Activities-specific Balance Confidence (ABC) scale for comparing fallers and non-fallers. Arch Gerontol Geriatr. 2004;38:11–26. doi: 10.1016/s0167-4943(03)00082-7. [DOI] [PubMed] [Google Scholar]
- 40.Dibble LE, Christensen J, Ballard DJ, Foreman KB, Dibble LE, Christensen J, Ballard DJ, Foreman KB. Diagnosis of fall risk in Parkinson Disease: An analysis of individual test interpretation. Phys Ther. 2008;88:323–332. doi: 10.2522/ptj.20070082. [DOI] [PubMed] [Google Scholar]
- 41.Gates S, Smith LA, Fisher JD, Lamb SE. Systematic review of accuracy of screening instruments for predicting fall risk among independently living older adults. J Rehabil Res Dev. 2008;45:1105–1116. [PubMed] [Google Scholar]
- 42.Horak FB, Wrisley DM, Frank J. The Balance Evaluation Systems Test (BESTest) to differentiate balance deficits. Phys Ther. 2009;89:484–498. doi: 10.2522/ptj.20080071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.King L, Horak F. On the Mini-BESTest: scoring and the reporting of total scores. Phys Ther. 2013;93:571–575. doi: 10.2522/ptj.2013.93.4.571. [DOI] [PubMed] [Google Scholar]
- 44.Anson E, Thompson E, Ma L, Jeka J. Reliability and Fall Risk Detection for the BESTest and Mini-BESTest in Older Adults. J Geriatr Phys Ther. 2017:1. doi: 10.1519/JPT.0000000000000123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Franchignoni F, Horak F, Godi M, Nardone A, Giordano A. Using psychometric techniques to improve the Balance Evaluation Systems Test : The Mini-BESTest. J Rehabil Med. 2010;42:323–331. doi: 10.2340/16501977-0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Duffy JC, Patout CA., Jr Management of the insensitive foot in diabetes: lessons learned from Hansen’s disease. Mil Med. 1990;155:575–579. [PubMed] [Google Scholar]
- 47.Allison LK, Kiemel T, Jeka JJ. Multisensory reweighting of vision and touch is intact in healthy and fall-prone older adults. Exp Brain Res. 2006;175:342–352. doi: 10.1007/s00221-006-0559-7. [DOI] [PubMed] [Google Scholar]
- 48.Halmagyi GM, Curthoys IS. A clinical sign of canal paresis. Arch Neurol. 1988;45:737–739. doi: 10.1001/archneur.1988.00520310043015. [DOI] [PubMed] [Google Scholar]
- 49.Kamei N, Yamane K, Nakanishi S, Yamashita Y, Tamura T, Ohshita K, Watanabe H, Fujikawa R, Okubo M, Kohno N. Effectiveness of Semmes-Weinstein monofilament examination for diabetic peripheral neuropathy screening in Ahvaz Iran. J Diabetes Complications. 2005;19:47–53. doi: 10.1016/j.jdiacomp.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 50.Faul F, Erdfelder E, Lang A-G, Buchner A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–191. doi: 10.3758/BF03193146. [DOI] [PubMed] [Google Scholar]
- 51.Glass GV, Peckham PD, Sanders JR. Consequences of Failure to Meet Assumptions Underlying the Fixed Effects Analyses of Variance and Covariance. Rev Educ Res. 1972;42:237. doi: 10.2307/1169991. [DOI] [Google Scholar]
- 52.Sherrington C, Tiedemann A, Fairhall N, Close JCT, Lord SR. Exercise to prevent falls in older adults: an updated meta-analysis and best practice recommendations. N S W Public Health Bull. 2011;22:78–83. doi: 10.1071/NB10056. [DOI] [PubMed] [Google Scholar]
- 53.Lord S, Sherrington C, Menz H, Close J. Falls in Older People: Risk factors and strategies for prevention. 2. Cambridge University Press; New York: 2007. [Google Scholar]
- 54.Pirouzi S, Motealleh AR, Fallahzadeh F, Fallahzadeh MA. Effectiveness of treadmill training on balance control in elderly people: A randomized controlled clinical trial. Iran J Med Sci. 2014;39:565–570. [PMC free article] [PubMed] [Google Scholar]
- 55.Zijlstra GA, Mancini M, Chiari L, Zijlstra W. Biofeedback for training balance and mobility tasks in older populations: a systematic review. J Neuroeng Rehabil. 2010;7:58. doi: 10.1186/1743-0003-7-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gottshall KR, Sessoms PH, Bartlett JL. Vestibular physical therapy intervention: utilizing a computer assisted rehabilitation environment in lieu of traditional physical therapy. Conf Proc IEEE Eng Med Biol Soc. 2012;2012:6141–6144. doi: 10.1109/EMBC.2012.6347395. [DOI] [PubMed] [Google Scholar]
- 57.Fitzpatrick R, McCloskey DI. Proprioceptive, visual and vestibular thresholds for the perception of sway during standing in humans. J Physiol. 1994;478:173–186. doi: 10.1113/jphysiol.1994.sp020240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shea CH, Wulf G. Enhancing motor learning through external-focus instructions and feedback. Hum Mov Sci. 1999;18:553–571. [Google Scholar]
- 59.Yen SC, Landry JM, Wu M. Augmented multisensory feedback enhances locomotor adaptation in humans with incomplete spinal cord injury. Hum Mov Sci. 2014;35:80–93. doi: 10.1016/j.humov.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 60.Agrawal Y, Zuniga MG, Davalos-Bichara M, Schubert MC, Walston JD, Hughes J, Carey JP. Decline in semicircular canal and otolith function with age. Otol Neurotol. 2012;33:832–9. doi: 10.1097/MAO.0b013e3182545061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gadkaree SK, Sun DQ, Li C, Lin FR, Ferrucci L, Simonsick EM, Agrawal Y. Does sensory function decline independently or concomitantly with age? Data from the Baltimore Longitudinal Study of Aging. J Aging Res. 2016:1865038. doi: 10.1155/2016/1865038. epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang YR, Tsai MP, Chuang TY, Sung WH, Wang RY. Virtual reality-based training improves community ambulation in individuals with stroke: a randomized controlled trial. Gait Posture. 2008;28:201–206. doi: 10.1016/j.gaitpost.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 63.Burns KM, Burns NR, Ward L. Confidence-more a personality or ability trait? It depends on how it is measured: A comparison of young and older adults. Front Psychol. 2016;7:518. doi: 10.3389/fpsyg.2016.00518. [DOI] [PMC free article] [PubMed] [Google Scholar]