Abstract
Our capacity to flexibly shift between internally and externally directed attention is crucial for successful performance of activities in our daily lives. Neuroimaging studies have implicated the lateral prefrontal cortex (LPFC) in both internally directed processes, including autobiographical memory retrieval and future planning, and externally directed processes, including cognitive control and selective attention. However, the causal involvement of the LPFC in regulating internally directed attention states is unknown. The current study recorded scalp EEG from patients with LPFC lesions and healthy controls as they performed an attention task that instructed them to direct their attention either to the external environment or their internal milieu. We compared frontocentral midline theta and posterior alpha between externally and internally directed attention states. While healthy controls showed increased theta power during externally directed attention and increased alpha power during internally directed attention, LPFC patients revealed no differences between the two attention states in either electrophysiological measure in the analyzed time windows. These findings provide evidence that damage to the LPFC leads to dysregulation of both types of attention, establishing the important role of LPFC in supporting sustained periods of internally and externally directed attention.
Keywords: Internally directed attention, Externally directed attention, Lateral prefrontal cortex, Electrophysiology, Executive Control
Introduction
At any given moment during our waking hours, humans attend to either the external environment or internal milieu. The capacity to flexibly allocate neural resources between internally and externally directed attention states is essential for optimal cognitive performance (Buckner et al., 2008; Allen, 2013). Neuroimaging studies suggest the frontoparietal control network, which includes the lateral prefrontal cortex (LPFC), plays an important role in facilitating both attention states. In particular, the LPFC is recruited for numerous processes involving internally directed attention, which occupies as much as half of our waking state, including autobiographical memory retrieval (Addis et al., 2007), future planning (Spreng et al., 2010), and creative problem solving (Ellamil et al., 2012). The coordination of externally directed attention also draws upon the LPFC, including cognitive control (Miller and Cohen, 2001; Cole and Schneider, 2007) and selective attention (Desimone and Ducan, 1995; Corbetta and Shulman, 2002; Buschman and Miller, 2007). Human lesion studies have confirmed that the LPFC is necessary for externally directed attention (Barcelo et al., 2000; Voytek et al., 2010). Although the LPFC has been implicated in both attention states, its causal involvement in regulating internally directed attention has not been addressed. Here, we establish the critical role of LPFC in coordinating both internally and externally directed attention using a combination of electrophysiological and lesion approaches.
The LPFC’s involvement in regulating attention states draws support from both theoretical and empirical work. A theoretical framework of internally directed attention has proposed the importance of the LPFC, in cooperation with the Default Network, in producing an internal train of thought (Smallwood et al., 2012). More broadly, Dixon and colleagues highlight the role of intentionality and propose that the LPFC is recruited when either internally or externally directed attention is intentionally engaged (Dixon et al., 2014). Consistent with these theoretical suggestions, neuroimaging evidence reveals enhanced functional coupling between the LPFC and Default Network during an autobiographical planning task involving internally directed attention, and with the Dorsal Attention Network during a Tower of Hanoi task involving externally directed attention. These findings indicate that LPFC can flexibly couple with the relevant neural network to support goal-directed cognition (Spreng et al., 2010). In line with this finding, Vincent and colleagues reported that the frontoparietal control network, which includes the LPFC, is anatomically positioned between the Default Network implicated in internally directed attention and Dorsal Attention Network implicated in externally directed visuospatial attention. Its unique position allows it to flexibly integrate information from both networks and adjudicate between competing stimuli from the internal vs. external environments (Vincent et al., 2008). Together, both theoretical and empirical suggestions converge on the role of the LPFC in regulating various attentional states.
Electrophysiological studies have identified theta and alpha power as key markers of externally and internally directed attention, which may serve as mechanisms by which these attention states are regulated. Successful performance in various externally directed tasks involving high level cognitive processes often engages theta power. For example, frontocentral midline theta power has been associated with cognitive control (Cavanagh et al., 2009; Cavanagh and Frank, 2014), including working memory (Missonnier et al., 2006; Sauseng et al., 2010), conflict detection (Hanslmayr et al., 2008; Töllner et al., 2017), and target detection (Pennekamp et al., 1994; Cavanagh et al., 2012). In contrast, studies have focused on posterior alpha as an index of both internal and external attention. While externally directed processes involving selective attention have been associated with alpha decreases (Pfurtscheller, 1992; Pfurtscheller et al., 1996), internal attention has been primarily linked to alpha increases. For instance, O’Connell and colleagues reported increased alpha power over parietal sites during internally directed attention as indexed by missed targets in an externally directed steady state visual evoked potential (SSVEP) task (O’Connell et al., 2009). Further, simultaneous EEG and fMRI recordings of resting state – a prevalent form of internally directed attention in an experimental context in which no task constraints are placed on the subject and no stimuli are presented – reveal positive correlations between posterior alpha power and activation of the Default Network (Mantini et al., 2007; Jann et al., 2009). Of relevance to our experimental task, these findings converge on enhanced frontocentral midline theta as an electrophysiological signature of externally directed attention and posterior alpha increase as a signature of internally directed attention.
The present study used a combined electrophysiological and neuropsychological approach to determine whether LPFC is causally involved in facilitating internally and externally directed attention. We recorded EEG in patients with LPFC lesion and healthy controls as they performed an attention task that required them to direct their attention either to the external environment or internal milieu. We compared frontocentral midline theta and posterior alpha between externally and internally directed attention states. Given the novelty of the experimental paradigm, our first objective was to validate the task by examining these electrophysiological measures of the two attention states in healthy controls. We hypothesized increased frontocentral midline theta power in the externally directed attention state and increased posterior alpha power in the internally directed attention state. The main objective was to assess whether LPFC is a critical brain region for regulating sustained periods of internally vs. externally directed attention. In contrast to healthy controls, our hypothesis was that patients with lesion in the LPFC would be impaired in attentional regulation as indexed by reduced differences in electrophysiological measures between the two attention states.
Methods
Subjects
Nine patients with lesions in the lateral prefrontal cortex, and thirteen age and gender matched healthy controls participated in the experiment. Patients were recruited from and tested at two sites. Seven patients with LPFC lesions were tested at the University of California, Berkeley, and two were tested at Oslo University Hospital/University of Oslo. Exclusion criteria included a history of psychiatric disease, substance abuse requiring treatment, premorbid head injury, comorbid neurological disease, IQ < 85, or sensory impairment. Table S1 summarizes subjects’ demographic information and patient characteristics.
The LPFC group consisted of nine patients with a unilateral focal lesion in the dorsolateral PFC (2 right, 7 left). Their lesions were due to low-grade astrocytoma resection (n = 2 at Oslo), and ischemic or hemorrhagic stroke (n = 7 at Berkeley). Both patients with low-grade astrocytoma showed no evidence of recurrence in an MRI taken within a month of testing. Maximal lesion overlap was observed in Brodmann Areas 9 and 46. Fig. 1a shows the lesion overlap for LPFC patients. Individual lesion reconstructions for all patients are shown in Figure S1.
Fig. 1.
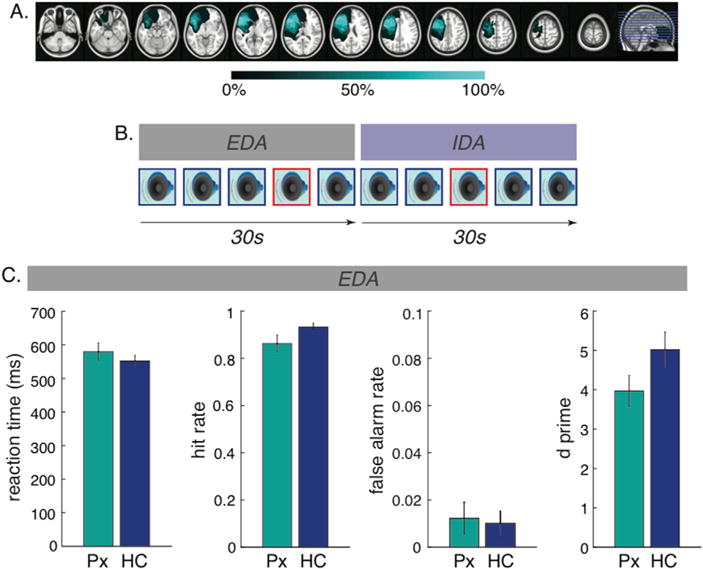
Lesion overlap, experimental paradigm, and behavioral performance. A) Lesion overlap for LPFC patients illustrated on the left hemisphere. Each horizontal line on the far right image reflects the location of each slice shown on the left. Colormap indicates percentage of overlap across patients. B) Experimental paradigm. Throughout each 30s block, subjects were presented with a series of standard (outlined in blue) and target (outlined in red) tones. In the externally directed attention (EDA) block, subjects were instructed to respond to the target tones, whereas in the internally directed attention (IDA) block, subjects were instructed to ignore all the tones. C) Behavioral performance during the EDA blocks for LPFC patients (Px, shown in teal) and healthy controls (HC, shown in blue). LPFC patients showed reduced hit rates, but comparable false alarm rate, d prime, and reaction time to targets, compared to healthy controls.
Healthy controls were recruited to match the patients by gender and age within 5 years. The two groups did not significantly differ in gender (χ(1) = .01, p = .94), age (t (20) = .01, p = .99), or education (t(20) = .21, p = .84). All provided subjects written informed consent, and were paid for their participation. This study was approved by the Institutional Review Boards at the University of California, Berkeley and Oslo University Hospital, and the Norwegian Regional Committee for Medical Research Ethics, Region South.
Lesion reconstruction
Lesion reconstructions were based on structural MRIs obtained after study inclusion. Lesions were outlined by manually drawing on Fluid Attenuated Inversion Recovery (FLAIR), T1 and T2 weighted images of each participant’s brain using MRIcron (www.mccauslandcenter.sc.edu/mricro/mricron/) and Adobe Photoshop CC 2015 (http://www.adobe. com/). T1, T2 and FLAIR images were first co-registered to a T1 MNI Template (normalized from 152 T1 scans), using Statistical Parametric Mapping software’s (SPM8: www.fil.ion.ucl.ac.uk/spm/) New Unified Segmentation routine. The manual delineation of the lesions was performed on axial mosaics of the normalized T1 scans. When available, high-resolution FLAIR and T2-weighted images were also used as aids to determine lesion borders. The resulting lesion masks were converted to three-dimensional MNI space using the Statistical Parametric Mapping software’s (SPM8: www.fil.ion.ucl.ac.uk/spm/) Mosaic to Volume routine. Lesions were reconstructed under the supervision of a neurologist (RTK). We calculated lesion sizes using the MRIcron descriptive statistics function after a lesion had been manually delineated.
Task paradigm and stimuli
Subjects were presented with a series of standard tones (800Hz) and target tones (1000Hz) presented in a random order with probabilities of 0.8 and 0.2, respectively. All auditory stimuli were pure tones presented through stereo speakers. The duration of each tone was 200ms, and the inter-trial interval was randomly jittered between 800 and 1200ms. Subjects were asked to keep their eyes fixated on the cross in the center of the screen throughout the task. Fig. 1b illustrates the experimental paradigm.
In the externally directed attention (EDA) condition, subjects were instructed to focus on the tones and press a button to target tones as quickly and accurately as possible. Mean reaction time and accuracy measures were computed. Accuracy measures included hit rate, false alarm rate, as well as d prime: d’ = Z (hit rate) − Z (false alarm rate). In the internally directed attention (IDA) condition, they were instructed to ignore the tones and to allow their minds to focus on any thoughts that come to mind (e.g. plans for the weekend). In other words, the stimulus set is identical across both conditions. The only difference is that attention is directed externally and a button press is required for target tones in the EDA condition whereas attention is directed internally in the IDA condition with no requirement of a button press. Each block began with 25 tones in the EDA condition followed by 25 tones in the IDA condition, or vice versa. At the beginning of each condition, the instructional words of EDA or IDA were presented for 3 s on screen to inform subjects of the upcoming condition. The order of these two conditions was counter-balanced within subjects. Subjects completed up to 28 blocks of EDA and IDA conditions (mean = 27.1, S.D. = 2.9), consisting of a maximum of 280 standard and 70 target tones in each condition. Stimuli presentation was operated by Eprime 2.0 (Psychology Software Tools, Inc., USA).
EEG data acquisition and analysis
EEG was recorded reference-free continuously from 64 active electrodes mounted on a cap according to the 10–20 system using the Bio-semi ActiveTwo system (www.Biosemi.com). Data were amplified and digitized at 1024Hz. Vertical and horizontal eye movements were recorded from electrodes above and below the right eye, and two electrodes placed at the right and left outer canthus.
EEG data were bandpass filtered between 1Hz and 50Hz. Ocular and muscle artifacts were corrected for using independent component analysis. Data decomposition was performed using the fastica toolbox in EEGLAB, and artifactual components were manually detected. Electrodes with excessively noisy signals were removed and replaced with an interpolation from neighboring electrodes using spherical spline interpolation (Perrin et al., 1989). Continuous EEG data were then segmented into 3000ms epochs, beginning at 1000ms prior to stimulus onset. Each trial was visually inspected for any remaining artifacts, which were further removed. The surface Laplacian transformation (i.e. current source density estimation) was applied to each subject’s data before analysis (Perrin et al., 1990). This transformation computes the second spatial derivative of voltage across nearby electrode sites, resulting in a reference-free signal that reduces volume conduction and emphasizes local activity (Perrin et al., 1989). Following this, since we had no predictions regarding hemispheric differences, for patients with lesions in the right hemisphere (n = 2), electrodes in the right hemisphere were exchanged so that left hemisphere electrodes are synonymous with lesioned hemisphere for both statistical analyses and illustrations. EEG data pre-processing and analysis were performed using EEGLAB (Delorme and Makeig, 2004), FieldTrip (Oostenveld et al., 2011), and custom Matlab scripts (Mathworks, Natick, MA, USA).
Only correct trials were considered for subsequent analyses. To ensure that conditional effects in EEG measures were not simply due to differences in signal-to-noise ratio resulting from a mismatched number of trials across conditions, the number of correct trials with standard and target tones between EDA and IDA conditions were matched (by random sampling) within subjects. Further, in order to ensure group differences in experimental effects are not attributable to varying trial numbers included in subsequent analyses between LPFC patients and healthy controls, we matched the number of trials included in subsequent analyses between the two groups. Given that the mean number of trials in healthy controls was slightly higher, we removed trials via random sampling in healthy controls to match the mean number of trials for LPFC patients.
Time-frequency decompositions of the EEG signal were quantified across a −1s pre-stimulus to 2s post-stimulus time window, using a sliding time window that advanced in steps of 50ms and lasted three cycles’ length for each frequency between 4 and 30Hz. The time series in each epoch were convolved with Morlet wavelets, after which spectral power was computed using fast Fourier transforms. Each epoch was then cut in length from 300ms pre-stimulus to 500ms post-stimulus and baseline corrected by subtracting and dividing by the average power in the prestimulus interval from −300 to condition −100ms. For each electrode within each and frequency, if the power value for a specific trial within the post-stimulus time window (0–500ms) exceeded 3 SDs beyond the mean power across trials, then that trial was considered an outlier and was excluded from subsequent analyses and figures. Low frequency power for the remaining standard and target trials in the EDA and IDA conditions were then averaged separately across subjects within each group for statistical comparisons.
This relative change in power was subsequently extracted for theta (4–8Hz) over the central region (C3, C1, Cz, C2, C4) and alpha (8–14Hz) over the posterior region (PO7, O1, Oz, O2, PO8), which have been associated with attention effects in aforementioned studies (Cavanagh et al., 2012; O’Connell et al., 2009). Topographic plots of mean theta and alpha power for LPFC patients and healthy controls are shown in Fig. 2.
Fig. 2.
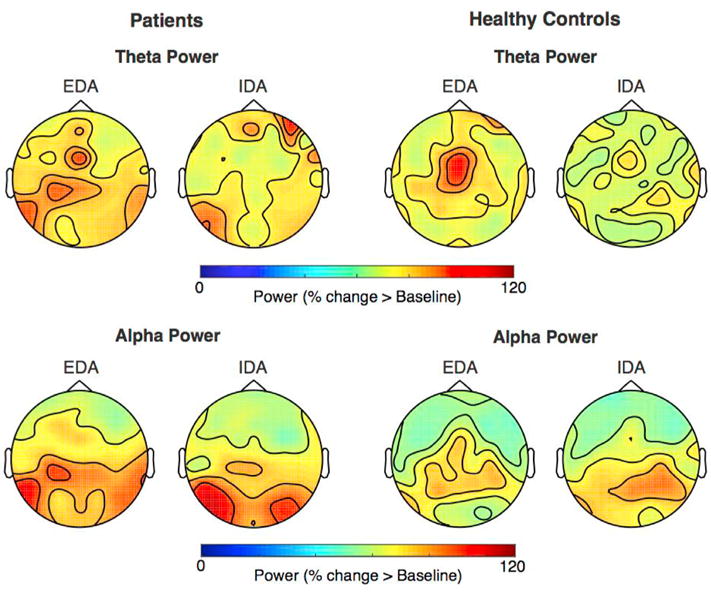
Topographic plots. Topography of theta (top panel) and alpha power (bottom panel) separately plotted for LPFC patients (left panel) and healthy controls (right panel) averaged across all trials within EDA and IDA blocks.
Statistical analyses
To assess group differences in behavioral measures, independent samples t-tests were conducted separately for mean reaction time and accuracy measures. As behavioral response was only required during the EDA blocks, the reported measures were only extracted from the EDA blocks. For EEG measures, we examined the relative change in anterior theta power and posterior alpha power (as described in Section 2.4). Our first step was to validate the task by examining these electrophysiological measures in the healthy controls using a repeated-measures ANOVA with attention (EDA vs. IDA) and tone (standard vs. target) as within-subject factors. We conducted separate ANOVAs for every 100ms time window up to 500ms post-stimulus to capture changes over time. Bonferroni correction was applied to account for multiple comparisons across the five time windows of analysis, yielding a critical α of 0.01. Significant interactions were further examined with post-hoc paired-samples t-tests. The same analyses were performed for the LPFC patients based on our a priori predictions, with an additional variable of Recording Site (Berkeley and Oslo) included as a covariate. To examine group differences, we also conducted an omnibus ANOVA including the aforementioned within-subject factors with an additional between-subject factor of group (patients vs. healthy controls). For reasons of brevity, we only report effects involving attention and group below. Finally, a Spearman’s two-tailed correlation was implemented to assess the relationship between the behavioral and electrophysiological measures. All statistical analyses were performed using SPSS (IBM, Armonk, NY, USA).
Results
Behavioral performance
Mean reaction time and accuracy measures for each group are shown in Fig. 1c. The LPFC patients had a lower hit rate relative to healthy controls (t (20) = −2.07, p = .05), whereas the false alarm rate was comparable between groups (t (20) = 0.37, p = .72). There was a trend towards patients showing reduced d prime compared to healthy controls (t (20) = −1.66, p = .11). Mean reaction time did not differ between patients and healthy controls (t (20) = 0.94, p = .36).
Electrophysiological measures
Theta power (4–8 Hz)
Repeated-measures ANOVAs were performed to examine attentional differences in frontocentral theta power and posterior alpha power. In order to validate the task, we first tested our hypothesis of increased theta power during EDA and increased alpha power during IDA in the healthy controls. Across all time windows, we observed a main effect of attention (F (1,12) >= 17.65, p ≤ .001) with more theta power during EDA relative to IDA. There was also a main effect of tone (F (1,12) >= 9.47 p ≤ .01) across the 200–400ms time windows, driven by higher theta power for target tones than standard tones. These main effects were modified by an attention by tone interaction (F (1,12) >= 16.37, p < .005) throughout all time windows. Paired-samples t-tests were used to follow up this interaction, and revealed increased theta power during EDA compared to IDA for target tones across all time windows (t (12) >= 4.92, p < .001). A similar attention effect was observed for standard tones during the 0–400ms post stimulus time windows, but it did not survive Bonferroni correction (t (12) >= 2.21, p ≤ .05). In LPFC patients, neither the main effect of attention (F (1,7) = 0.02–1.33, p = .29–.89) nor the attention by tone interaction (F (1,7) = 0.03–0.98, p = .35–.87) were significant across any time windows.
An omnibus ANOVA was also conducted to examine group differences. We observed a main effect of attention throughout the 0–400ms post stimulus time windows (F (1,20) > = 13.65, p ≤ .001), indicating greater theta power during EDA relative to IDA. The main effect of group did not survive Bonferroni correction (F (1,20) >= 7.97, p ≤ .05). However, consistent with aforementioned analyses, the pattern suggests LPFC patients showed reduced theta power relative to healthy controls during the 0–400ms post stimulus time windows. There was also an attention by group interaction throughout the 200–400ms post stimulus time windows (F (1,20) >= 9.23, p < .01), and a three-way interaction (attention × tone × group) during the same post stimulus time windows (F (1,20) >= 9.40, p < .01). The aforementioned analyses within groups suggest these interaction effects were driven by greater theta power during EDA relative to IDA in the healthy controls only. Fig. 3 displays the mean theta power traces as well as time frequency representations as a function of attention and tones separately for patients and healthy controls.
Fig. 3.
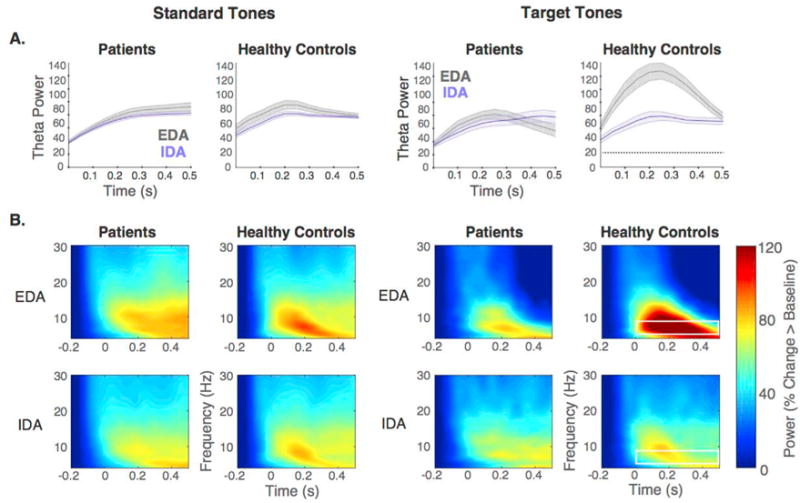
LPFC patients did not show increased theta power during EDA as observed in healthy controls. A) Mean theta power (shading indicates SEM) throughout the 500ms post-stimulus period for EDA and IDA are plotted separately for standard (left panel) and target tones (right panel) in patients and healthy controls. While healthy controls displayed increased theta power during EDA relative to IDA, this difference was absent in patients. Significant attention effects are indicated by dotted black lines. B) Time frequency representations of low frequency power aggregated across central electrodes (C3,C1,Cz,C2,C4) in response to standard tones (left panel) and target tones (right panel), averaged separately for EDA (top) and IDA (bottom) for LPFC patients (left) and healthy controls (right). White box outline indicates frequency band of interest and time window showing significant effects.
Alpha power (8–14 Hz)
In the healthy controls, a main effect of attention across the 200–500 ms post-stimulus time windows (F (1,12) >= 10.72, p < .01) revealed that alpha power was greater during IDA relative to EDA. The attention by tone interaction was not significant throughout the post-stimulus time windows (F (1,12) >= 0.14, p ≤ .72). In the LPFC patients, there were no significant differences between EDA and IDA (F (1,8) = 0.04–1.29, p = .29–.85) and no significant interactions between attention and tone (F (1,8) = 0.17–3.56, p = .10 - .69) across any time windows.
For the omnibus ANOVA, a main effect of attention was observed across all time windows, driven by higher alpha power during IDA compared to EDA (F (1,20) >= 12.20, p < .005). Neither the two way (attention × group) nor three way (attention × tone × group) interactions were significant across any time windows (F (1,20) = 0.00–0.87, p = .36–.99). Given the main effect of attention, Fig. 4 shows the mean alpha power traces as well as time frequency representations averaged across both tones separately for each attention condition.
Fig. 4.
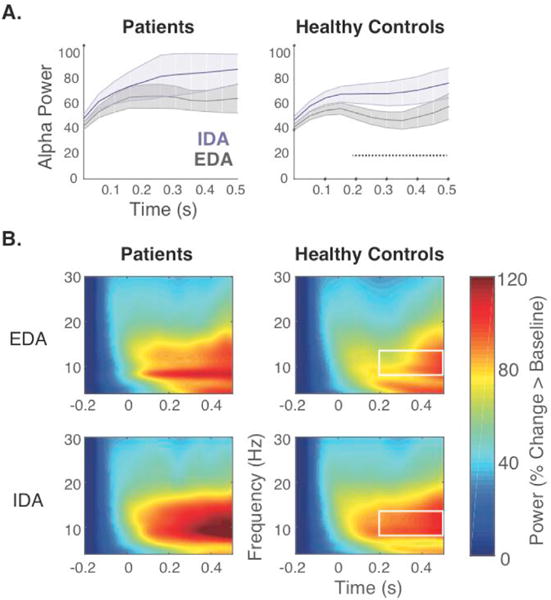
LPFC patients did not show increased alpha power in IDA as observed in healthy controls. A) Mean alpha power (shading indicates SEM) averaged across tones throughout the 500ms post-stimulus period for EDA and IDA plotted separately for patients and healthy controls. Healthy controls displayed increased alpha power during IDA relative to EDA, whereas patients failed to show any differences between attention states. Significant attention effects are indicated by dotted black line. B) Time frequency representations of low frequency power aggregated across posterior electrodes (PO7,O1,Oz,O2,PO8) averaged across tones, plotted separately for EDA (top) and IDA (bottom) for LPFC patients (left) and healthy controls (right). White box outline indicates frequency band of interest and time window showing significant effects.
Control analyses
In light of the group differences observed in theta and alpha power, we compared the signal-to-noise ratio (SNR) between groups separately for theta and alpha power to ensure that these group differences are not attributable to SNR differences. Specifically, we computed the SNR for each trial by dividing the maximum power value across the trial epoch (i.e. signal) by the standard deviation across the entire trial epoch (i.e. noise; Hu et al., 2010). These SNR values per trial were then averaged within each individual, and statistically compared between LPFC patients and healthy controls via an independent samples t-test. We implemented separate comparisons for theta power and alpha power. The SNR did not significantly differ between groups for both theta power (t (20) = −1.33, p = .20) and alpha power (t (20) = −0.68, p = .50).
Further, in order to ensure our results from the power analyses were not driven by the target-elicited posterior P3b event-related potential (ERP) component, we performed control analyses examining the P3b as a function of attention and tone across LPFC patients and healthy controls. If the pattern of results for P3b is similar to the pattern observed for theta or alpha power, then the results from the power analyses may have been driven by the P3b. However, if the two patterns differed, then this disparity suggests the power results occurred independently of the P3b. We found that the P3b amplitude was greater for target tones during EDA relative to IDA, and comparable for standard tones, for both LPFC patients and healthy controls. This pattern contrasts with our findings of theta and alpha power, suggesting the P3b results were unlikely to be the primary driving force of the power findings. Importantly, given that the P3b reflects allocation of attentional resources (Polich, 2007), these results suggest that both groups were indeed attending to the tones during EDA and not paying attention to them during IDA. The ANOVA results, as well as details regarding the preprocessing steps and statistical analyses of the P3b component, are reported in the Supplementary Information. Figure S2 illustrates the ERP waveforms separately for patients and controls.
Finally, we examined the possibility that the observed group differences in the power measures may be attributable to effects of fatigue. Although subjects were granted as much time as necessary to rest throughout the experiment in order to ensure they were able to perform the task to the best of their ability, it is possible that patients became more exhausted near the end of the experiment. To test this possibility, we examined global changes in theta and alpha power throughout the duration of the task by computing the slope of these power changes over time. If patients’ fatigue was indeed the source of changes in EEG measures, then we would expect a non-zero slope for theta and alpha power in LPFC patients. Further, if fatigue contributed to group differences in EEG measures, we also anticipate that the slope values significantly differ between groups. Contrary to this possibility, we found that the slope in theta and alpha power were not different from zero for LPFC patients, and their slopes do not differ from the slopes of healthy controls. A detailed description of the methods and results of statistical analyses are reported in the Supplementary Information. These findings indicate that fatigue effects did not contribute to the observed power differences between groups.
Effects of lesion laterality
We performed the above repeated-measures ANOVA with an additional variable of laterality to assess whether the lesioned and intact hemispheres showed differences in the electrophysiological measures in LPFC patients. Therefore, only electrodes in the lesioned versus intact hemisphere were included in the analyses as a within-subject factor of laterality, with all midline electrodes discarded. We also tested for hemispheric differences in healthy controls to ensure the task itself did not elicit asymmetry in low frequency power. In order to be more conservative in our control analyses, theta power and alpha power were averaged across all time windows to avoid correction for multiple comparisons. Given that the auditory stimuli in our experimental task were binaurally presented and that previous evidence indicates the LPFC was bilaterally recruited during attention states (Corbetta and Shulman, 2002; Spreng et al., 2010), we did not anticipate seeing significant effects of laterality. Indeed, there were no significant differences between hemispheres for theta (p > .10) and alpha power (p > .15) in either patients or controls.
Impact of brain lesion volume
We also examined the impact of lesion volume on behavioral and electrophysiological measures for the LPFC patients. In terms of behavioral measures, there were no significant correlations between lesion volume and reaction time (rs (9) = 0.20, p = .61), hit rate (rs (9) = −0.14, p = .72), false alarm rate (rs (9) = −0.09, p = .81), nor d prime (rs (9) = −0.01, p = .98). For EEG measures, lesion volume also did not correlate with overall theta power (rs (9) = 0.03, p = .93) or alpha power (rs (9) = −0.57, p = .11) averaged across time windows.
Selectivity of lesion location
To assess whether the observed effects in LPFC patients reflect specific contributions of the LPFC in EDA and IDA, we tested a clinical control group consisting of nine patients with lesions in the orbitofrontal cortex (OFC). These patients performed the same attention task and the results of the P3b analyses have been reported in another study that focused on ERP components (Kam et al., in press). Here, we implemented the same time frequency and statistical analyses as in LPFC patients and healthy controls. Figure S3 displays the mean theta and alpha power traces as well as time frequency representations for each attention condition. OFC patients showed patterns in these measures similar to those observed in healthy controls: greater fronto-central theta power during EDA relative to IDA, and greater posterior power during IDA relative to EDA. However, statistical analyses only revealed trends for attentional modulations in both theta and alpha power after correcting for multiple comparisons. Although we are unable to make firm conclusions about the selectivity of the role of lateral PFC, these trends in the OFC patients suggest that observed patterns in LPFC patients were less influenced by the lesion per se and more impacted by the particular location of the lesion. A detailed description of the analyses and results are reported in the Supplementary Information.
Correlation between behavioral and EEG measures
Separate Spearman correlations were implemented to examine the relationship between behavioral measures (i.e. reaction time, hit rate, false alarm rate and d prime) and electrophysiological measures (i.e. theta and alpha power during EDA averaged across the 500ms post-stimulus time window) across both groups. There was a positive correlation between theta power and d prime (rs (22) = 0.44, p = .040), indicating higher accuracy was linked to greater theta power. No other correlations were significant (p > .07). Figure S4 illustrates this relationship.
Discussion
The current study examined whether the LPFC is necessary for the regulation of internally and externally directed attention using a combined neuropsychological and electrophysiological approach. Our data provides key evidence demonstrating that lesions in the LPFC lead to impairment in coordinating both internally and externally directed attention. Unlike the healthy controls who displayed increased fronto-central theta power during externally directed attention and increased posterior alpha power during internally directed attention, patients with LPFC damage showed minimal differences in theta power between the two attention states and larger, but still non-significant, differences in alpha power within the analyzed time windows. This study provides causal evidence that LPFC is critical for regulating sustained periods of externally directed attention and plays an important role in maintaining periods of internally directed attention.
LPFC is important for coordinating sustained attention
Patients with LPFC lesions displayed reduced differences in fronto-central midline theta power and posterior alpha power between internally and externally directed attention compared to healthy controls in the analyzed time windows, indicating their lesions led to a failure in the regulation of attention states. The patients also showed disrupted behavioral performance during externally directed attention. We observed that patients with OFC lesions performed similarly to healthy controls, suggesting that the observed effects in LPFC patients were less influenced by the lesion per se and more by the specific location of the lesion. Notably, these attentional differences in the electrophysiological measures were not completely absent in LPFC patients. Given that sustained external attention recruits bilateral frontal cortex (Coull et al., 1996), compensatory mechanism may be in place by the intact hemisphere. Moreover, it is conceivable that the intact LPFC, alongside with more posterior areas of the frontoparietal control network, suffice to partially modulate posterior alpha during internally directed attention. Previous work has indeed shown that PFC modulation of posterior alpha occurs bilaterally (Helfrich et al., 2017).
Our results are in line with Vincent and colleagues’ study using fMRI-based functional connectivity measures during rest to illustrate the LPFC is uniquely positioned to adjudicate the competition between internally oriented stimuli recruiting the Default Network and externally oriented stimuli recruiting the Dorsal Attention Network (Vincent et al., 2008). Similarly, LPFC also flexibly couples with the Default Network and the Dorsal Attention Network depending on the task-at-hand in order to facilitate goal-directed cognition (Spreng et al., 2010). Although our experiment did not allow for the direct examination of the specific instance of switching between attention states due to insufficient occurrences of switching, these past studies along with our findings provide evidence for a causal role of LPFC in flexibly engaging either internally and externally directed attention to adapt our behavior to the current goal.
Neuroimaging research in executive functions involving healthy subjects has implicated the LPFC in switching between abstract rules or task sets. For example, LPFC activation is observed during switch trials in which attention is shifted between stimuli within the same perceptual modality (Larson and Lee, 2013; Leong et al., 2017). High-order switching between abstract task rules also draws upon the LPFC (Konishi et al., 1998; Monchi et al., 2001; Cools, 2004). In accordance with these neuroimaging findings, neurophysiological evidence indicates that neurons in the LPFC encode both simple stimulus-response mappings as well as abstract task rules (Assad et al., 1998; Wallis et al., 2001). These neurons have the capacity to represent a wide range of information and determine their relevance to the current task, enabling the adaptive coding of task-relevant information (Duncan, 2001; Freedman et al., 2001; Miller and Cohen, 2001), and implementation of task demands by flexibly coordinating task-relevant brain regions. Our study extends this notion by demonstrating that the LPFC is also critical for coordinating between large-scale networks responsible for internally and externally directed attention. In other words, the goal-driven decision to attend to one of many stimuli, and the implementation of that decision, is not restricted to competing stimuli in the external environment. Rather, internally and externally oriented processes both rely on the LPFC.
Intentionally engaged attention recruits the LPFC
In the current experimental paradigm, subjects were instructed to direct their attention internally or externally; therefore they had to intentionally orient their attention. Our finding that the LPFC is necessary to facilitate these intentionally guided attention states is consistent with the framework put forward by Dixon and colleagues emphasizing the role of intentionality (Dixon et al., 2014). This framework purports that the LPFC would only be recruited during intentional processes, whether it be internally or externally oriented, and that processes occurring spontaneously would not engage the LPFC. Their model draws support from past studies demonstrating that both attention states can be engaged spontaneously or intentionally. In the context of internally directed attention, this distinction between intentionally and spontaneously engaged processes has been shown in both the laboratory setting and in daily life (Seli et al., 2016). In the context of externally directed attention, this distinction is often characterized as voluntary shifts of attention, which involves higher order regions such as the LPFC, and reflexive shifts of attention, which involves lower order regions such as primary sensory cortices (Corbetta and Shulman, 2002; Petersen and Posner, 2012). These previous findings illustrate the LPFC is recruited only when attention is intentionally engaged, as was the case in this study.
Theta and alpha as signatures of externally and internally directed attention
As hypothesized, our healthy controls showed increased frontocentral midline theta power during externally directed attention. This electrophysiological measure also correlated with behavioral performance across subjects. Given the target detection component of the task, our observation of theta power during externally directed attention is consistent with previous findings of increased theta during target detection (Pennekamp et al., 1994; Cavanagh et al., 2012). More broadly, there is clear evidence implicating frontocentral midline theta power in cognitive control (Cavanagh et al., 2009; Cavanagh and Frank, 2014). The robust attenuation of theta power during internally vs. externally directed attention in our data is also in line with reports of disrupted theta phase-locking during an off-task state in which subjects’ attention is directed away from an ongoing visual target detection task (Baird et al., 2014). Together, these findings indicate frontocentral midline theta serves as an electrophysiological marker of externally directed attention in the current task.
The observed increase in posterior alpha power during internally directed attention in healthy controls is consistent with two lines of research. First, electrophysiological studies of selective attention have demonstrated that auditory tasks can modulate posterior alpha (Foxe et al., 1998; Wöstmann et al., 2016), as was observed in our data. Furthermore, alpha activity has been theorized as a gating mechanism of task-irrelevant information (Jensen and Mazaheri, 2010). Given that subjects were asked to engage in their own thoughts and instructed to ignore the tones in the internally directed attention condition, the observed posterior alpha increase may reflect an inhibitory mechanism in which the task-irrelevant tones are actively being suppressed (Foxe and Snyder, 2011). Second, the enhanced posterior alpha power during internally relative to externally directed attention is consistent with studies of attentional lapses over a longer time scale. In particular, O’Connell and colleagues reported increased alpha power over parietal sites for up to 20 s prior to an error in a SSVEP task, suggesting posterior alpha as a signature of lapses in sustained attention (O’Connell et al., 2009). Similar conclusions are derived from simultaneous EEG and fMRI recordings during long periods of rest. These studies revealed that alpha power is positively correlated with activity in the Default Network (Mantini et al., 2007; Jann et al., 2009), which has been implicated in various types of internally directed cognition, including mind wandering (Mason et al., 2007; Christoff et al., 2009), autobiographical memory retrieval (Addis et al., 2007; Spreng et al., 2010), and theory of mind tasks (Saxe and Powell, 2006; Buckner and Carroll, 2007; Spreng et al., 2009). Whether posterior alpha reflects the inhibition of external inputs during internally directed attention or the actual engagement with internal inputs remains to be determined. Importantly, these two lines of research indicate alpha power can serve as an electrophysiological signature of internally directed attention.
Limitations and conclusion
One potential limitation is that while the accuracy of target detection can be used as a proxy for the participant’s level of engagement during externally directed attention, there is no corresponding behavioral measure during the internally directed attention condition. We also have no knowledge about the specific content of the subjects’ thoughts during this condition. This reflects a trade-off between a lack of manipulation of thought content and the ecological validity of the task. The current experimental design prioritized higher ecological validity by allowing subjects to think about whatever comes to mind to imitate periods of time throughout the day in which we freely engage in our own thoughts at the cost of reduced experimental manipulation. Critically, to ensure that subjects ignored the tones as instructed in the internally directed attention condition, we examined an independent measure of stimulus-evoked activity, the P3 ERP component. We found that both groups showed smaller P3 amplitude, indicating reduced processing of tones, during the internally relative to externally directed attention condition. This provides evidence that the subjects did indeed ignore the tones during the internally directed attention condition.
In conclusion, this study provides key evidence for the important role of the LPFC in facilitating both internally and externally directed attention. We demonstrated that lesions in this frontal region lead to impairments in regulating externally directed attention and mild impairments in maintaining internally directed attention. Future studies involving patients with lesions in the posterior regions of the frontoparietal control network can inform whether other parts of the network play a similar role in regulating sustained periods of internally and externally directed attention.
Supplementary Material
Acknowledgments
We would like to thank our patients and control subjects for participating. We also want to thank Alejandro Blenkmann, Maya Dyhre Foldal, Ingrid Funderud, Sandon Griffin, Randolph Helfrich, Richard Jimenez, Anaïs Llorens, James Lubell, and Anat Perry for their help with data collection, lesion reconstructions, enhancing figure quality, and useful discussions. This work was supported by the Natural Sciences and Engineering Research Council of Canada and the James S. MacDonald Foundation for JWYK, Research Council of Norway 240389/F20 and Internal Funding from the University of Oslo for AKS, TRM, and TE, and NINDS R3721135 to RTK. The funding sources have no involvement in the conduct of the research and preparation of the article.
Appendix A. Supplementary data
Supplementary data related to this article can be found at https://doi.org/10.1016/j.neuroimage.2018.03.063.
Footnotes
Declarations of interest
None.
References
- Addis DR, Wong AT, Schacter DL. Remembering the past and imagining the future: common and distinct neural substrates during event construction and elaboration. Neuropsychologia. 2007;45:1363–1377. doi: 10.1016/j.neuropsychologia.2006.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen M. The balanced mind: the variability of task-unrelated thoughts predicts error monitoring. Front Hum Neurosci. 2013;7:1–15. doi: 10.3389/fnhum.2013.00743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assad WF, Rainer G, Miller EK. Neural activity in the primate pre- frontal cortex during associative learning. Neuron. 1998;21:1399–1407. doi: 10.1016/s0896-6273(00)80658-3. [DOI] [PubMed] [Google Scholar]
- Baird B, Smallwood J, Lutz A, Schooler JW. The decoupled mind: mind wandering disrupts cortical phase-locking to perceptual events. J Cogn Neurosci. 2014;26:2596–2607. doi: 10.1162/jocn_a_00656. [DOI] [PubMed] [Google Scholar]
- Barcelo F, Suwazono S, Knight RT. Prefrontal modulation of visual processing in humans. Nat Neurosci. 2000;3:399–403. doi: 10.1038/73975. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Carroll DC. Self-projection and the brain. Trends Cogn Sci. 2007;11:49–57. doi: 10.1016/j.tics.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Buschman TJ, Miller EK. Top-down versus bottom-up control of attention in the prefrontal and posterior parietal cortices. Science. 2007;315:1860–1862. doi: 10.1126/science.1138071. [DOI] [PubMed] [Google Scholar]
- Cavanagh JF, Cohen MX, Allen JJB. Prelude to and resolution of an error: EEG phase synchrony reveals cognitive control dynamics during action monitoring. J Neurosci. 2009;29:98–105. doi: 10.1523/JNEUROSCI.4137-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh JF, Frank MJ. Frontal theta as a mechanism for cognitive control. Trends Cogn Sci. 2014;18:414–421. doi: 10.1016/j.tics.2014.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh JF, Zambrano-Vazquez L, Allen JJB. Theta lingua franca: a common mid-frontal substrate for action monitoring processes. Psychophysiology. 2012;49:220–238. doi: 10.1111/j.1469-8986.2011.01293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoff K, Gordon AM, Smallwood J, Smith R, Schooler W, Posner MI, et al. fMRI reveals default Experience sampling during and executive network contributions system to mind wandering. Proc Natl Acad Sci U S A. 2009;106:8719–8724. doi: 10.1073/pnas.0900234106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole MW, Schneider W. The cognitive control network: integrated cortical regions with dissociable functions. Neuroimage. 2007;37:343–360. doi: 10.1016/j.neuroimage.2007.03.071. [DOI] [PubMed] [Google Scholar]
- Cools R. Differential responses in human striatum and prefrontal cortex to changes in object and rule relevance. J Neurosci. 2004;24:1129–1135. doi: 10.1523/JNEUROSCI.4312-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Coull JT, Frith CD, Frackowiak RSJ, Grasby PM. A Fronto-parietal Network for Rapid Visual Information Processing: a PET Study of Sustained Attention and Working Memory. Neuropsychologia. 1996;34:1085–1095. doi: 10.1016/0028-3932(96)00029-2. [DOI] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Desimone R, Ducan J. Neural mechanisms of selective visual attention. Annu Rev Neurosci. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- Dixon ML, Fox KCR, Christoff K. A Framework for Understanding the Relationship between Externally and Internally Directed Cognition. Neuropsychologia. 2014;62:321–330. doi: 10.1016/j.neuropsychologia.2014.05.024. [DOI] [PubMed] [Google Scholar]
- Duncan J. An adaptive coding model of neural function in prefrontal cortex. Nat Rev Neurosci. 2001;2:820–829. doi: 10.1038/35097575. [DOI] [PubMed] [Google Scholar]
- Ellamil M, Dobson C, Beeman M, Christoff K. Evaluative and generative modes of thought during the creative process. Neuroimage. 2012;59:1783–1794. doi: 10.1016/j.neuroimage.2011.08.008. [DOI] [PubMed] [Google Scholar]
- Foxe JJ, Simpson GV, Ahlfors SP. Parieto-occipital approximately 10Hz activity reflects anticipatory state of visual attention mechanisms. Neuroreport. 1998;9:3929–3933. doi: 10.1097/00001756-199812010-00030. [DOI] [PubMed] [Google Scholar]
- Foxe JJ, Snyder AC. The role of alpha-band brain oscillations as a sensory suppression mechanism during selective attention. Front Psychol. 2011;2:1–13. doi: 10.3389/fpsyg.2011.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman DJ, Riesenhuber M, Poggio T, Miller EK. Categorical representation of visual stimuli in the primate prefrontal cortex. Science. 2001;291:312–316. doi: 10.1126/science.291.5502.312. [DOI] [PubMed] [Google Scholar]
- Hanslmayr S, Pastötter B, Bäuml KH, Gruber S, Wimber M, Klimesch W. The electrophysiological dynamics of interference during the stroop task. J Cogn Neurosci. 2008;20:215–225. doi: 10.1162/jocn.2008.20020. [DOI] [PubMed] [Google Scholar]
- Helfrich R, Huang M, Wilson G, Knight R. Prefrontal cortex modulates posterior alpha oscillations during top-down guided visual perception. Proc Natl Acad Sci. 2017;114:9457–9462. doi: 10.1073/pnas.1705965114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L, Mouraux A, Hu Y, Iannetti GD. A Novel Approach for Enhancing the Signal-to-noise Ratio and Detecting Automatically Event-related Potentials (ERPs) in Single Trials. Neuroimage. 2010;50:99–111. doi: 10.1016/j.neuroimage.2009.12.010. [DOI] [PubMed] [Google Scholar]
- Jann K, Dierks T, Boesch C, Kottlow M, Strik W, Koenig T. BOLD Correlates of EEG Alpha Phase-locking and the FMRI Default Mode Network. Neuroimage. 2009;45:903–916. doi: 10.1016/j.neuroimage.2009.01.001. [DOI] [PubMed] [Google Scholar]
- Jensen O, Mazaheri A. Shaping functional architecture by oscillatory alpha activity: gating by inhibition front. Hum Neurosci. 2010;4:1–8. doi: 10.3389/fnhum.2010.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kam JWY, Solbakk AK, Funderud I, Endestad T, Meling TR, Knight RT, et al. Orbitofrontal damage reduces auditory sensory response in humans. Cortex. 2018;101:309–312. doi: 10.1016/j.cortex.2017.12.023. https://doi.org/10.1016/j.cortex.2017.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi S, Nakajima K, Uchida I, Kameyama M, Nakahara K, Sekihara K, et al. Transient activation of inferior prefrontal cortex during cognitive set shifting. Nat Neurosci. 1998;1:80–84. doi: 10.1038/283. [DOI] [PubMed] [Google Scholar]
- Larson E, Lee AKC. The cortical dynamics underlying effective switching of auditory spatial attention. Neuroimage. 2013;64:365–370. doi: 10.1016/j.neuroimage.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong YC, Radulescu A, Daniel R, DeWoskin V, Niv Y. Dynamic Interaction between Reinforcement Learning and Attention in Multidimensional Environments. Neuron. 2017;93:451–463. doi: 10.1016/j.neuron.2016.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantini D, Perrucci MG, Del Gratta C, Romani GL, Corbetta M. Electrophysiological signatures of resting state networks in the human brain. Proc Natl Acad Sci U S A. 2007;104:13170–13175. doi: 10.1073/pnas.0700668104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN, et al. Wandering minds: stimulus-independent thought. Science. 2007;315:393–395. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Missonnier P, Deiber MP, Gold G, Millet P, Gex-Fabry Pun M, Fazio-Costa L, et al. Frontal theta event-related synchronization: comparison of directed attention and working memory load effects. J Neural Transm. 2006;113:1477–1486. doi: 10.1007/s00702-005-0443-9. [DOI] [PubMed] [Google Scholar]
- Monchi O, Petrides M, Petre V, Worsley K, Dagher A. Wisconsin card sorting revisited: distinct neural circuits participating in different stages of the task identified by event-related functional magnetic resonance imaging. J Neurosci. 2001;21:7733–7741. doi: 10.1523/JNEUROSCI.21-19-07733.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell RG, Dockree PM, Robertson IH, Bellgrove MA, Foxe JJ, Kelly SP. Uncovering the neural signature of lapsing attention: electrophysiological signals predict errors up to 20 s before they occur. J Neurosci. 2009;29:8604–8611. doi: 10.1523/JNEUROSCI.5967-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oostenveld R, Fries P, Maris E, Schoffelen JM. FieldTrip: open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput Intell Neurosci. 2011;2011 doi: 10.1155/2011/156869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennekamp P, Bosel R, Mecklinger A, Ott H. Differences in EEG-theta for Responded and Omitted Targets in a Sustained Attention Task 1994 [Google Scholar]
- Perrin F, Pernier J, Bertrand O, Echallier JF, Corrigenda EEG. Electroencephalogr. Clin Neurophysiol. 1990;76:565. 02274. [Google Scholar]
- Perrin P, Pernier J, Bertrand O, Echallier JF. Spherical splines for scalp potential and current density mapping. Electroencephalogr Clin Neurophysiol. 1989;72:184–187. doi: 10.1016/0013-4694(89)90180-6. [DOI] [PubMed] [Google Scholar]
- Petersen S, Posner M. The attention system of the human brain: 20 years after. Annu Rev Neurosci. 2012;21:73–89. doi: 10.1146/annurev-neuro-062111-150525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfurtscheller G, Stancak AJ, Neuper C. Event-related synchronization (ERS) in the alpha band-an electrophysiological correlate of cortical idling: a review. Int J Psychophysiol. 1996;24:39–46. doi: 10.1016/s0167-8760(96)00066-9. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G. Event-related synchronization (ERS): an electro- physiological correlate of cortical areas at rest. Electroencephalogr Clin Neurophysiol. 1992;83:62–69. doi: 10.1016/0013-4694(92)90133-3. [DOI] [PubMed] [Google Scholar]
- Polich J. Updating P300: an integrative theory of P3a and P3b. Clin Neurophysiol. 2007;118:2128–2148. doi: 10.1016/j.clinph.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauseng P, Griesmayr B, Freunberger R, Klimesch W. Control mechanisms in working memory: a possible function of EEG theta oscillations Neurosci. Biobehav Rev. 2010;34:1015–1022. doi: 10.1016/j.neubiorev.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Saxe R, Powell LJ. It’s the thought that counts: specific brain regions for one component of theory of mind. Psychol Sci. 2006;17:692–699. doi: 10.1111/j.1467-9280.2006.01768.x. [DOI] [PubMed] [Google Scholar]
- Seli P, Risko EF, Smilek D, Schacter DL. Mind-wandering with and without intention. Trends Cogn Sci. 2016;20:605–617. doi: 10.1016/j.tics.2016.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smallwood J, Brown K, Baird B, Schooler JW. Cooperation between the default mode network and the frontal-parietal network in the production of an internal train of thought. Brain Res. 2012;1428:60–70. doi: 10.1016/j.brainres.2011.03.072. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Mar R, Kim ASN. The common neural basis of autobiographical memory, prospection, navigation, theory of mind and the default mode: a quantitative meta-analysis. J Cogn Neurosci. 2009;21:489–510. doi: 10.1162/jocn.2008.21029. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Stevens WD, Chamberlain JP, Gilmore AW, Schacter DL. Default Network Activity, Coupled with the Frontoparietal Control Network, Supports Goal-directed Cognition. Neuroimage. 2010;53:303–317. doi: 10.1016/j.neuroimage.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Töllner T, Wang Y, Makeig S, Müller HJ, Jung TP, Gramann K. Two independent frontal midline theta oscillations during conflict detection and adaptation in a simon-type manual reaching task. J Neurosci. 2017;37:2504–2515. doi: 10.1523/JNEUROSCI.1752-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J Neurophysiol. 2008;100:3328–3342. doi: 10.1152/jn.90355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voytek B, Davis M, Yago E, Barcelo F, Vogel EK, Knight RT. Dynamic neuroplasticity in human prefrontal cortex. Neuron. 2010;68:401–408. doi: 10.1016/j.neuron.2010.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis J, Anderson K, Miller EK. Single neurons in prefrontal cortex encode abstract rules. Nature. 2001;411:953–956. doi: 10.1038/35082081. [DOI] [PubMed] [Google Scholar]
- Wöstmann M, Herrmann B, Maess B, Obleser J. Spatiotemporal dynamics of auditory attention synchronize with speech. Proc Natl Acad Sci. 2016;113:3873–3878. doi: 10.1073/pnas.1523357113. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


