Abstract
Background
Predominantly antibody deficiencies (PADs) are the most common type of primary immunodeficiency in adults. PADs frequently pass undetected leading to delayed diagnosis, delayed treatment, and the potential for end-organ damage including bronchiectasis. In addition, PADs are frequently accompanied by comorbid autoimmune disease, and an increased risk of malignancy.
Objectives
To characterize the diagnostic and clinical features of adult PAD patients in Victoria, Australia.
Methods
We identified adult patients receiving, or having previously received immunoglobulin replacement therapy for a PAD at four hospitals in metropolitan Melbourne, and retrospectively characterized their clinical and diagnostic features.
Results
179 patients from The Royal Melbourne, Alfred and Austin Hospitals, and Monash Medical Centre were included in the study with a median age of 49.7 years (range: 16–87 years), of whom 98 (54.7%) were female. The majority of patients (116; 64.8%) met diagnostic criteria for common variable immunodeficiency (CVID), and 21 (11.7%) were diagnosed with X-linked agammaglobulinemia (XLA). Unclassified hypogammaglobulinemia (HGG) was described in 22 patients (12.3%), IgG subclass deficiency (IGSCD) in 12 (6.7%), and specific antibody deficiency (SpAD) in 4 individuals (2.2%). The remaining four patients had a diagnosis of Good syndrome (thymoma with immunodeficiency). There was no significant difference between the age at diagnosis of the disorders, with the exception of XLA, with a median age at diagnosis of less than 1 year. The median age of reported symptom onset was 20 years for those with a diagnosis of CVID, with a median age at diagnosis of 35 years. CVID patients experienced significantly more non-infectious complications, such as autoimmune cytopenias and lymphoproliferative disease, than the other antibody deficiency disorders. The presence of non-infectious complications was associated with significantly reduced survival in the cohort.
Conclusion
Our data are largely consistent with the experience of other centers internationally, with clear areas for improvement, including reducing diagnostic delay for patients with PADs. It is likely that these challenges will be in part overcome by continued advances in implementation of genomic sequencing for diagnosis of PADs, and with that opportunities for targeted treatment of non-infectious complications.
Keywords: predominantly antibody deficiency, primary immunodeficiency, diagnostic delay, common variable immunodeficiency, X-linked agammaglobulinemia, immunoglobulin subclass deficiency, specific antibody deficiency
Introduction
Primary immunodeficiencies (PIDs) are a heterogeneous group of diseases, characterized by an impaired immune response to pathogens, predisposing to more frequent and severe infection, in some instances to a single pathogen, and dysregulated immune function, which may result in autoimmune disease or inflammatory conditions (1). Knowledge of the key molecular processes underpinning these varied disorders continues to evolve with advances in genomic technology. The archetypal classification of immunologic disorders has recognized predominantly antibody deficiency (PAD) as the most prevalent PID, and these patients require lifelong antibody replacement therapy (2, 3). In comparison with other countries, the prevalence of PADs in Australia has not been clearly established. There are only two reports over the last two decades with varied results that are likely to represent significant under reporting, due to ascertainment bias (4, 5).
Diagnosis is challenging because PADs have varied clinical presentations and may present from infancy to late adulthood. The hallmark clinical feature is a history of recurrent sino-pulmonary bacterial infections, resulting from an ineffective antibody response, however, in many cases, non-infectious complications, such as autoimmune disease, or malignancy may complicate the clinical presentation.
The most profound PAD is agammaglobulinemia with a complete or near-complete absence of serum Ig and absence of mature B cells in blood. Most of these individuals suffer from X-linked agammaglobulinemia (XLA) which occurs due to mutations in the gene encoding Bruton’s tyrosine kinase (BTK), an enzyme essential for B-cell development (6). Defects in other genes crucial for B-cell development and survival have been identified in a smaller proportion of patients (7–10), currently leaving only 5–10% without a genetic diagnosis.
Common variable immunodeficiency (CVID) constitutes the majority of PAD cases which require ongoing treatment, with a global incidence of approximately 1:25,000 (11) although this varies according to the population studied. A recent Finnish study found the prevalence to be as high as 6.9 per 100,000 (12). CVID has complex clinical features and pathophysiology, underpinned by identifiable monogenic defects in a minority of patients (13–15). Patients may present with a spectrum of manifestations, including recurrent infections and autoimmune cytopenias. Several diagnostic criteria, based upon a combination of clinical and laboratory features, may be used to aid in diagnosis and classification of patients with these complex presentations (16–18). A diagnosis may be made from 2 years of age (19).
The spectrum of PAD also includes milder syndromes, albeit with variable clinical features that include selective IgA deficiency; unclassified hypogammaglobulinemia (HGG), characterized by reduced levels of IgG in the presence of normal IgA and IgM; IgG subclass deficiency (IGSCD), characterized by a reduction in one or more IgG subclasses with normal total IgG; and specific antibody deficiency (SpAD), characterized by normal serum immunoglobulins with an apparent failure to produce antibody in response to vaccines. Thymoma with immunodeficiency, otherwise eponymously known as Good syndrome, is a rare PID associated with the presence of a thymoma, although the mechanism is not well understood (1, 20–22).
We sought to identify the diagnostic approaches that had been used for PADs and potential obstacles to diagnosis. In addition we aimed to characterize the infectious and non-infectious manifestations in our patients, such as organ-specific or systemic autoimmune disease as well as malignancies such as lymphomas and gastrointestinal (GI) tumors.
Materials and Methods
Patients
We performed a comprehensive cross-sectional clinical analysis of adult patients with PADs managed under clinical immunology services in Victoria from January 2001 to February 2017. Patients who were currently receiving, or had previously received replacement immunoglobulin for PADs were identified by physicians specializing in Clinical Immunology at four major teaching hospitals in metropolitan Melbourne, Victoria: The Royal Melbourne Hospital, The Alfred Hospital, Monash Medical Centre and Austin Health. Patients with secondary hypogammaglobulinemia were excluded, as were individuals with a defined combined immunodeficiency, or a history of hematopoietic stem-cell transplantation. To ensure that the cohort included patients with clinically significant disease, and receiving regular follow-up, we did not include patients who were not deemed to require replacement immunoglobulin therapy. The study was carried out according to the principles of the Declaration of Helsinki and was approved by local human research ethics committees (Melbourne Health projects 2009.162, 2013.245; Walter and Eliza Hall Research Institute projects 10/02, 14/01; Alfred Health 109/15, 277/17). All living patients, or their next of kin, consented to the collection of their medical information. For those individuals who were deceased at the time of the data collection, ethical approval was obtained to review the medical records without the consent of the next of kin.
Data Collection
Data were retrospectively collected from the clinical case notes using a pre-specified template to capture age of reported onset of symptoms, age at diagnosis and organ-specific manifestations that included respiratory, GI, neurological, musculoskeletal, organomegaly, and malignancy. We defined immune dysregulation on clinical notes reporting any of the following diagnoses: autoimmune disease, lymphoproliferative disease, organomegaly, granulomatous inflammation, and/or enteropathy.
Statistical Analysis
We examined differences between groups using one-way ANOVA for comparison of continuous variables; Chi square analysis for comparison of categorical variables with a high frequency, and Fisher’s exact test for categorical variables with a frequency of less than 5; and Mantel–Cox log rank for comparing differences in survival between disease groups. As the data for age of onset, diagnosis and diagnostic delay were non-parametrically distributed, we have reported median values for these measures. A p value of less than 0.05 was considered statistically significant. Statistical analyses were performed using GraphPad Prism 7 software.
Results
Patient Demographics
179 patients with PAD were identified from the four centers. 98 patients were females, and 81 were males, with median age of 49 years (Table 1). CVID was the most frequent antibody deficiency disorder requiring clinical follow-up with 116 patients. XLA was diagnosed in 21 male patients. 22 patients had HGG, 4 had SpAD, and 12 were diagnosed with IGSCD. We also included four patients with thymoma and immunodeficiency, with a diagnosis of Good syndrome (Table 1).
Table 1.
Diagnostic and demographic data of the Victorian adult predominantly antibody deficiency cohort.
| Immunologic diagnosis | Number of patients (%) | Male:female ratio | Current age (years; range) | Median age at diagnosis (years) | Median age at symptom onseta (years; range) | Median diagnostic delaya (years; range) |
|---|---|---|---|---|---|---|
| All | 179 | 0.82 | 49 (16–87) | 36 (0–87) | 20 (0–68) | 7.5 (0–63) |
| Common variable immunodeficiency (CVID) | 116 (64.8) | 0.78 | 48.5 (16–80) | 35 (2–80) | 20 (1–65) | 9 (1–63) |
| X-linked agammaglobulinemia (XLA) | 21 (11.7) | N/A | 31.5 (23–65) | 0 (0–15) | 0 (0–2) | 1 (0–15) |
| Hypogammaglobulinemia (unclassified) (HGG) | 22 (12.29) | 0.16 | 54 (18–87) | 43 (5–80) | 19.5 (1–50) | 4 (1–59) |
| Specific antibody deficiency (SpAD) | 4 (2.23) | 1.00 | 65.5 (43–81) | 68 (33–72) | N/A | N/A |
| IgG subclass deficiency (IGSCD) | 12 (6.7) | 0.20 | 56 (30–81) | 36 (7–74) | 20 (2–68) | 9 (6–16) |
| Good syndrome | 4 (2.23) | 3.0 | 69 (46–77) | 60 (40–71) | 58 (38–68) | 2 (0–3) |
aData for age at symptom onset and diagnostic delay available for the following numbers of patients in each group.
CVID, 83/116; XLA, 16/21; HGG, 7/22; SpAD, 1/4; IGSCD, 7/12; Good syndrome 2/4.
Diagnostic Features
Age at Diagnosis and Diagnostic Delay
The median age at diagnosis for the entire cohort was 36 years, which ranged from diagnoses at birth to 80 years old. Patients with XLA were diagnosed at significantly younger ages than all other antibody deficiency disorders (Figure 1A; Table 1) and experienced significantly shorter delay from symptom onset to diagnosis (Table 1; Table S1 in Supplementary Material). With regards to CVID specifically, the median age of reported onset of symptoms was 20 years, and the median age of diagnosis was 35 years (Table 1; Figures 1A,B). The median diagnostic delay was available for only 83/116 CVID patients and was 9 years. The four patients with Good syndrome were diagnosed at a median age of 60.75 years (interquartile range 44.3–74), which was significantly older than XLA (p < 0.0001) and CVID (p < 0.05) diagnoses, but not the other antibody deficiency syndromes (Table 1; Figures 1A,B).
Figure 1.
Age at diagnosis and diagnostic features differ between disease groups in Victorian adult patients with predominantly antibody deficiencies. (A) Box–whisker plot of age at diagnosis according to disease classification with median and 5–95% percentiles indicated. (B) Histogram demonstrating age at reported symptom onset, light red, and age at diagnosis (dark red) in common variable immunodeficiency (CVID) patients. (C) Qualitative vaccine responses to pneumococcal polysaccharide vaccine responses measured IgG binding against PPS23 polysaccharides in CVID patients (red), unclassified hypogammaglobulinemia (HGG) patients (green), IgG subclass deficiency (IGSCD) (purple), and specific antibody deficiency (SpAD) (orange). Darker colored columns indicate protective responses, P, and lighter colored columns indicate unprotective responses, U (χ2 analysis: **p < 0.01 and ****p < 0.0001). (D) Serum IgG and IgA (g/L, mean ± SD) at diagnosis according to disease classification (**p < 0.01, ***p < 0.001, and ****p < 0.0001, using one-way ANOVA).
Serum Immunoglobulin Levels
The serum IgG levels at time of diagnosis of CVID were significantly lower than those recorded for patients with HGG (3.10 ± 1.55 versus 6.32 ± 4.67 g/L, respectively, p < 0.0001; Figure 1D). However, serum IgG levels for CVID and HGG were both significantly lower than in patients with a diagnosis of IgGSCD and SpAD (p < 0.0001; Figure 1D). Serum IgA levels were also significantly reduced in CVID patients, compared with other PAD, consistent with the diagnostic criteria (0.23 ± 0.28 versus 1.16 ± 1.05 g/L, p = < 0.0001; Figure 1D). Serum IgM levels in CVID (0.3 ± 0.36 g/L) were significantly lower compared with HGG (0.97 ± 0.6 g/L; p < 0.0001), and IGSCD (1.15 ± 0.66 g/L; p < 0.0001) (Figure 1D). Serum Ig levels at time of diagnosis were not available for most of the XLA patients, due to the historic nature of their diagnoses.
Specific Antigen Responses
The results of pneumococcal polysaccharide vaccine responses were available for 55 patients with PAD reported here. For CVID, specific pneumococcal polysaccharide antibody titers were available for 30 of 116 patients. From these 30 records, 29 CVID patients were reported to have unprotective vaccine responses (96.7%). Reflecting the heterogeneity of these conditions, the single patient with protective vaccine responses, in retrospect had an atypical presentation for CVID with early-onset chronic mucocutaneous candidiasis, and subsequent adult-onset hypogammaglobulinemia, was identified to have a gain-of-function (GOF) STAT1 mutation, which is not typically associated with PAD. Furthermore, consistent with the diagnostic criteria for CVID, a higher proportion of HGG and IGSCD patients demonstrated protective vaccine responses compared with the CVID and SpAD groups, who consistently demonstrated suboptimal vaccine responses (χ2; p < 0.001 and p < 0.01 respectively; Figure 1C).
Genetic Diagnoses
Although genetic testing was not uniformly used in the diagnosis of these patients, 40/178 had an identified genetic cause for PAD (Table S1 in Supplementary Material). This included 21 XLA patients with hemizygous BTK mutations. Of the remaining 19 patients with PAD and an identified genetic contribution, 17 had CVID, and 2 had IGSCD. Of the CVID patients, 4 had NFκB1 deficiency, and 3 were heterozygous for the (C104R) variant in TNFSRF13B, encoding TACI, a known risk-factor for the development of CVID (23). One of these patients also harbored a pathogenic mutation in TCF3 (E555K), which causes an autosomal dominant form of agammaglobulinemia (24). In addition, we identified NFKB2 mutations in three CVID and two IgGSCD patients, who had marked autoimmune clinical manifestations. Two patients initially diagnosed with CVID were found to have CXCR4 mutations and three patients with CVID were rediagnosed with CTLA4 haploinsufficiency.
Clinical Features and Disease Complications
All patients presented with infections; however, most patients developed complications in addition; only 28% demonstrated an infection-only clinical phenotype (Table 2; Figure 2A). Respiratory tract involvement was the most common clinical manifestation followed by disease of the GI tract, skin and musculoskeletal system (Table 2; Figure 2A). Pneumonia and sinusitis were the most common infectious manifestations of the respiratory tract, present in 78% of all PAD patients, and GI infections were present in 19% of patients (data not shown). CVID patients were disproportionately affected and appeared susceptible to a broad range of infections that included Giardia in eight patients, Campylobacter and Salmonella in five, candidiasis in three, Helicobacter pylori in three and Cryptosporidia spp. in two patients. Bronchiectatic structural lung disease was most common in XLA with 66.7% patients affected compared with 27% of CVID patients (Table 2; Figure 2A). Four of these patients with chronic lung disease underwent lung transplantation; one female with CVID, and three males with XLA. One of four patients with Good syndrome also developed bronchiectasis.
Table 2.
Complications in Victorian adults with predominantly antibody deficiency.
| CVID, n(%) | XLA | HGG | IGSCD | SpAD | Good syndrome | Total, n(%) | |
|---|---|---|---|---|---|---|---|
| Total number of patients | 116 | 21 | 22 | 12 | 4 | 4 | 179 |
| Infections onlya | 33 (28) | 7 (33) | 12 (55) | 4 (33) | 1 (25)c | 0 | 57 (32) |
| Bronchiectasis | 31 (27) | 14 (67) | 4 (18) | 4 (33) | 2 (50) | 1 (25) | 28 (16) |
| GLILD | 5 (4) | 0 | 0 | 0 | 0 | 0 | 5 (3) |
| Autoimmunity (total) | 44 (38) | 0 | 3 (14) | 4 (33) | 1 (25) | 2 (50) | 54 (30) |
| Musculoskeletal | 11 (9) | 0 | 0 | 2 (17) | 0 | 1 (25) | 14 (8) |
| Cytopeniab | 24 (21) | 0 | 1 (5) | 0 | 0 | 1 (25)b | 26 (15) |
| Endocrine | 9 (8) | 0 | 0 | 2 (17) | 1 | 0 | 12 (7) |
| Gastrointestinal disease (total) | 27 (23) | 0 | 2 (9) | 1 (8) | 0 | 0 | 30 (17) |
| Enteropathy | 16 (14) | 0 | 0 | 1 (8) | 0 | 0 | 17 (9) |
| Colitis | 11 (9) | 0 | 2 (9) | 0 | 0 | 1 (25)c | 14 (8) |
| Granulomatous disease | 8 (7) | 0 | 0 | 0 | 0 | 1 (25)c | 9 (5) |
| Autoimmune liver disease | 3 (3) | 0 | 1 (5) | 0 | 0 | 0 | 4 (2) |
| Neuropathy | 4 (3) | 0 | 0 | 1 (8) | 0 | 0 | 5 (3) |
| Malignancyc | 20 (17) | 0 | 2 (9) | 3 (25) | 0 | 2 (50) | 27 (15) |
| Solid organ | 14 (12) | 0 | 2 (9) | 2 (17) | 0 | 1 (25)c | 19 (11) |
| Hematological | 6 (5) | 0 | 0 | 1 (8) | 0 | 1 (25)c | 8 (4) |
CVID, common variable immunodeficiency; XLA, X-linked agammaglobulinemia; HGG, unclassified hypogammaglobulinemia; IGSCD, immunoglobulin subclass deficiency; GLILD, granulomatous lymphocytic interstitial lung disease; SpAD, specific antibody deficiency.
aExcludes autoimmunity, gastrointestinal disease, granulomatous disease, liver disease, malignancy, and GLILD.
bExcluding lymphopenia.
cExcluding thymoma.
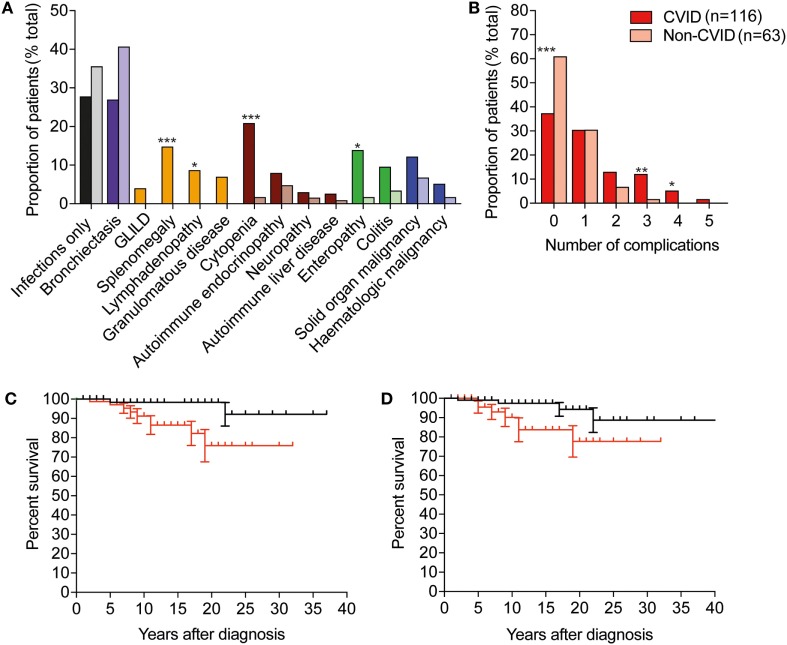
Figure 2.
CVID is associated with more frequent non-infectious complications than other predominantly antibody deficiencies, and these complications are associated with reduced survival. (A) Histogram depicting frequency of complications in CVID, darker-colored columns, compared with non-CVID cases in lighter-colored columns. Abbreviations: GLILD, granulomatous lymphocytic interstitial lung disease; CVID, common variable immunodeficiency. Purple represents bronchiectasis; yellow represents non-malignant lymphoproliferative complications; brown, autoimmune complications; green, gastrointestinal disease, and blue, malignancies. (Fisher’s exact test: ***p < 0.001; **p < 0.01; and *p < 0.05). (B) Histogram depicting percentage of CVID and non-CVID patients with 0, 1, or more non-infectious complications of disease. (C) Survival after diagnosis in individuals with immune dysregulation is significantly reduced compared with patients with infections only (p < 0.05, Mantel–Cox log rank). Black line indicates survival of individuals without features of immune dysregulation, red line indicates survival of individuals with immune dysregulation. (D) Survival after diagnosis in individuals with bronchiectasis is significantly reduced compared with patients with infections only (p < 0.05, Mantel–Cox log rank). Black line indicates survival of individuals without bronchiectasis, and red indicates survival of individuals with bronchiectasis.
Neurologic manifestations were also evident in this cohort. There were five cases of meningitis, which were enterovirus-related in two patients, one of whom succumbed to a fatal progressive meningoencephalitis (Table 3). Neuropathy was reported in four CVID patients and a single patient with IGSCD (Table 2). The features of the neuropathy were not specified as to severity, pattern or progression. Seizures, optic neuritis, autoimmune hypophysitis were also recognized in individual patients from this cohort. Endocrine manifestations that were recorded included autoimmune thyroid disease in eight CVID patients, one IGSCD and one SpAD, while autoimmune adrenal insufficiency was reported in one CVID and one IGSCD patient.
Table 3.
Causes of death among antibody deficient patients in Victoria (2000–2017).
| Patient no. | Diagnosis | Sex | Clinical phenotype | Age at diagnosis | Age deceased | Cause of death |
|---|---|---|---|---|---|---|
| 46 | IGSCDa | F | Immune dysregulation | 9 | 28 | Enteroviral encephalitis |
| 90 | CVID | F | Infections only | 62 | 70 | Breast cancer |
| 91 | Good syndrome | M | Infections only | 75 | 77 | Thymoma |
| 92 | CVID | F | Immune dysregulation | 69 | 74 | Serous ovarian cancer |
| 93 | CVID | F | Infections only | 47 | 74 | Squamous esophageal cancer |
| 113 | CVIDb | F | Immune dysregulation | 67 | 81 | Lung adenocarcinoma |
| 123 | CVID | F | Immune dysregulation | 40 | 65 | Intracerebral hemorrhage |
| 156 | CVID | M | Immune dysregulation | 42 | 62 | Diffuse large B cell lymphoma |
| 157 | CVID | F | Immune dysregulation | ? | 48 | Sepsis |
| 158 | CVID | M | Immune dysregulation | 71 | 78 | Respiratory failure—ILD |
| 159 | XLA | M | Infections/bronchiectasis | ? | 58 | Infection post lung transplant |
| 160 | CVID | F | Infections only | 68 | 73 | Not available |
| 161 | XLA | M | Infections/bronchiectasis | ? | 35 | Lung transplant complication |
| 162 | CVID | M | Immune dysregulation | 27 | 32 | Enteropathy/cachexia/sepsis |
| 163 | XLA | M | Infections/bronchiectasis | 13 | 59 | Respiratory failure |
| 164 | XLA | M | Infections/bronchiectasis | ? | 39 | Infection post lung transplant (CMV) |
| 165 | XLA | M | Infections/bronchiectasis/cirrhosis | ? | 36 | HCV-related cirrhosis |
| 166 | CVID | M | Immune dysregulation | 36 | 53 | Respiratory failure and refractory enteropathy |
| 167 | CVID | F | Infections/bronchiectasis | 71 | 80 | Respiratory failure |
IGSCD, immunoglobulin subclass deficiency; CVID, common variable immunodeficiency; XLA, X-linked agammaglobulinemia; ILD, interstitial lung disease; CMV, cytomegalovirus; HCV, hepatitis C virus.
? indicates unknown.
aNFKB2 mutation.
bNFKB1 mutation.
Overall, manifestations of immune dysregulation were most prevalent in CVID and IGSCD patients (72 and 67%, respectively), compared with only 5/22 hypogammaglobulinemic patients, and 1/4 SpAD (Table 2). The proportion of patients with all non-infectious manifestations was significantly higher in the CVID group, compared with the aggregated non-CVID cohort of patients (59/116 versus 12/38, respectively; χ2, p < 0.05).
We reviewed the incidence of specific clinical manifestations in the cohort according to diagnostic group (Figure 2A). Compared with the non-CVID antibody deficient patients, the 116 CVID patients more frequently developed cytopenia (p < 0.001), enteropathy (p < 0.01), splenomegaly (p < 0.01), lymphadenopathy (p < 0.05), and granulomatous infiltration (p < 0.05), but there were no significant differences between the proportions of patients with bronchiectasis, colitis, solid organ or hematological neoplasia and autoimmune liver disease.
To determine if organ complications were cumulative, we assessed the presence or absence of bronchiectasis, autoimmune liver disease, enteropathy, colitis, organomegaly, granulomas and malignancy across the key antibody deficient disease groups. Most patients suffered from <2 complications of disease; however, CVID patients were more likely to have >2 complications (Figure 2B).
Management of PAD
Patients were identified on the basis of current or previous immunoglobulin replacement therapy. At the time of data analysis all patients with a diagnosis of XLA, CVID and Good syndrome were receiving replacement immunoglobulin and maintaining IgG mean trough levels of 8.8 g/L (data not shown). Antibiotics were used prophylactically in 10 of 20 CVID patients with available data while systemic immunosuppression was used in 17 CVID patients. These included nine individual patients treated with prednisolone, four patients with azathioprine or methotrexate in combination with prednisolone, whereas rituximab (anti-CD20) was administered in six CVID patients for cytopenias. One patient with CVID and granulomatous lymphocytic interstitial lung disease was treated with rituximab and mycophenolate. Splenectomy was performed on two patients for refractory cytopenia. One patient had undergone liver transplantation due to cirrhosis secondary to nodular regenerative hyperplasia and has tolerated tacrolimus well. One other patient had undergone renal transplantation. Three XLA patients and one CVID patient received lung transplants and were treated with combination immunosuppression including mycophenolate, tacrolimus and prednisolone as prophylaxis against rejection.
Mortality
From this retrospective analysis, we identified 19 PAD patients who had died, including 4 from infections (cytomegalovirus, enteroviral encephalitis, sepsis-unspecified, and respiratory infection) and 6 patients died as a result of malignant disease (breast cancer, malignant thymoma, serous ovarian cancer, squamous esophageal cancer, lung adenocarcinoma, and diffuse large B cell lymphoma; Table 3). Of those, five had CVID and one had a diagnosis of Good syndrome, with a malignant thymoma. One patient died from an intracerebral hemorrhage, which was thought to be unrelated to PAD. Survival following diagnosis was significantly reduced in patients with immune dysregulation, with a 75% survival at 20 years after diagnosis compared with 98% for those with an infection-only phenotype (p < 0.05; Figure 2C). Significantly reduced survival was also noted in PAD patients with bronchiectasis as early as 10 years post-diagnosis (Figure 2D).
Discussion
Primary immunodeficiencies are a heterogeneous mix of disorders, with >300 defined conditions. The most prevalent of these are PADs, which may present with a broad range of clinical features, age of onset, and population-dependent frequency. As a result, the underlying immunodeficiency diagnosis is typically significantly delayed or missed.
We aimed to characterize how PAD patients have been diagnosed, managed and manifested disease and sought to identify areas for improvement in clinical practice in Victoria, the second most populous state in Australia, with a population aged over 15 years of approximately 4.9 million at the end of 2016 (Australian Bureau of Statistics). Australia is a geographically expansive country, with a relatively small population; posing unique challenges for diagnosis and treatment of rare diseases. The most recent Australian study demonstrated incomplete capture of all PIDs, with the finding of 5.6 cases per 100,000 (5). We found a prevalence of PAD in Victorian adults of approximately 1 in 25,000, which is similar to other population frequencies internationally (11). The prevalence of PAD varies geographically, with a very high prevalence in Finland, a non-diverse population, in contrast to that of Australia, which is more heterogeneous (25).
As with any retrospective study, there are some limitations to this report. Data collection may be incomplete, and inconsistency may arise due to differences in practice between individual physicians and centers. Under-diagnosis is likely to relate to the relative infrequency of these conditions, and therefore lack of suspicion among primary care providers, particularly as the clinical features are varied (25). There is also a significant delay in diagnosis observed in the majority of these disorders, which is associated with an increased risk of mortality, and significant morbidity (26). Thus, increased awareness among medical practitioners and improvements in diagnostic tools are of great clinical importance. Implementation of increased awareness programs is likely to raise the number of patients to be diagnosed and potentially this will lead to identification of a higher prevalence of PAD than 1 in 25,000.
While this cohort represents a small group of patients, our median diagnostic delay of 9 years in the CVID group is longer than other recently reported cohorts. European data from more than 2,000 patients with CVID identified a median diagnostic delay overall of 5 years when the diagnosis was made prior to the year 2000, and 4.2 years when the diagnosis was made during or after the year 2000 (27). However, those patients comprised a large proportion of children, whereas our cohort only included adults, which may introduce a bias to a shorter diagnostic delay in that study. Indeed, in other smaller European adult cohorts a diagnostic delay of 7 years has been reported (28, 29).
Predominantly antibody deficiency remains a diagnostic challenge unless specifically considered in light of the varied non-infectious manifestations, and involvement with physicians with expertise in diagnosis and treatment of primary immunodeficiency. Our data demonstrate that distinguishing primary hypogammaglobulinemia from CVID cannot be readily made using laboratory parameters in isolation, as there is overlap in these parameters between the two conditions. Furthermore, vaccination studies, which are used for diagnosis of PAD, are complicated by the need for paired serum analyses, and are confounded by increasing community use of pneumococcal vaccines and variability in the measurement, and interpretation, of polysaccharide responses. Taken together, there is a need to improve PAD diagnosis, which may entail the use of molecular tests and dialog between internal medicine specialists and clinical immunologists.
Molecular tests that identify pathogenic mutations will improve diagnostic precision in PAD. In this cohort, patients who were later identified to have a genetic contribution to their disease, had a shorter time to a clinical diagnosis (Table 2). This may be influenced by the severity of presenting illnesses and/or a positive family history pre-empting screening for antibody deficiency. In addition, molecular tests may help identify patients-at-risk for certain disease complications, as in the case of NFKB2 mutations which are associated with a high risk of central adrenal insufficiency. Further highlighting the complexities of clinical presentation in PID, we have described a patient who was initially misdiagnosed with CVID, but later found to have a STAT1 GOF mutation after his diagnosis was reconsidered in light of early-onset mucocutaneous candidiasis.
We also identified one individual with digenic disease due to a pathogenic E555K variant in TCF3 and C104R variant in TNFRSF13B. We have previously reported another kindred affected by PAD and autoimmune disease; the proband harbored a novel non-sense TCF3 mutation and the C104R variant in TNFRSF13B, with a resultant CVID-like disorder and systemic lupus erythematosus (30). We are not aware of other reports of digenic PAD due to the more common pathogenic TCF3 E555K variant in combination with an additional genetic risk-factor.
Our findings support improved access to diagnostic genomic studies in these patients, although currently the majority of CVID cases are unlikely to be readily diagnosed due to their complex pathogenesis (31, 32). Nevertheless diagnostic rates could increase to 30% for CVID patients selected for sequencing on the basis of certain clinical or laboratory features (14). Other studies have identified XLA in adult patients previously misdiagnosed as CVID, which has significant implications regarding missed opportunities for genetic counseling (33). The genetic diagnoses we have reported here may have been biased toward patients with more extreme phenotypes, and further work is ongoing to determine the clinical utility of genomic sequencing to aid diagnosis in the cohort more broadly.
Earlier diagnosis of PAD will facilitate earlier IgG replacement therapy and/or antibiotic therapy to mitigate the susceptibility to infections, and delay or prevent end-organ changes from infections such as bronchiectasis. However, optimal management of infections alone may not prevent complications that arise from immune dysregulation such as autoimmune disease, granulomatous inflammation and enteropathy, which affected 68% of our cohort. This is a higher proportion than reported in other cohorts and may reflect a referral bias toward patients with more severe disease, and a failure to include stable PID patients with infection-only phenotypes controlled on immunoglobulin replacement managed by non-Immunology services, or indeed those not receiving immunoglobulin at all.
Prediction of risk for the non-infectious complications of PAD is a major challenge. In a recent US study, unbiased network clustering using two large CVID patient datasets has identified novel biochemical markers of patients with lymphoproliferative, autoimmune and allergic disease (34). Whether these associations apply to the other PADs is unclear. In our cohort CVID patients suffered the most non-infectious complications, but we also identified non-infectious complications in other PADs. Non-infectious complications in IGSCD have also been recognized in other cohorts, although a recent Dutch study did not identify any non-infectious complications in a cohort of patients with HGG (35, 36). Importantly, two IGSCD patients in our cohort harbored NFKB2 mutations, which may have predisposed these patients to the more severe clinical phenotype.
Conclusion
Predominantly antibody deficiencies are the most common of the primary immune deficiency diseases, yet are still likely to be underdiagnosed, or diagnosed after the development of life-threatening complications. Individuals with PADs suffer from diverse and severe complications including infection, bronchiectasis and disorders of immune dysregulation, which are associated with reduced survival. These complications may be averted by earlier and more precise diagnosis, and targeted therapeutic interventions. Early diagnosis relies upon improved knowledge and appropriate clinical suspicion among internal medical physicians and primary care providers, as well as equitable access to genetic diagnosis and the benefits of personalized medicine.
Ethics Statement
The study was carried out according to the principles of the Declaration of Helsinki and was approved by local human research ethics committees (Melbourne Health projects 2009.162, 2013.245; Walter and Eliza Hall Research Institute projects 10/02, 14/01; Alfred Health 109/15, 277/17). All living patients, or their next of kin, consented to the collection of their medical information. For those individuals who were deceased at the time of the data collection, ethical approval was obtained to review the medical records without the consent of the next of kin.
Author Contributions
CS and JB designed the study, collected data, interpreted data, and wrote the manuscript. MZ and VB designed the study, interpreted the data, and wrote the manuscript; TG and EK collected and interpreted data. RS, PC, FH-L, MS, SH, SO, SB, GU, JL, MP, JM, KS, YT, PA, KN, RO, JD, and PH contributed data and assisted writing the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. The authors acknowledge the Melbourne Genomics Health Alliance, supported by the Victorian Government and Alliance members, the Australian National Health and Medical Research Council (NHMRC, Project Grant 1127198 for VB; Postgraduate Scholarship 1075666 for CS; Senior Research Fellowship 1117687 for MZ), The Jeffrey Modell Foundation, and Independent Research Institutes Infrastructure Support Scheme Grant 361646 for fellowship and grant funding support. VB also receives support from the Victorian State Government Operational Infrastructure Scheme and Australian Government NHMRC IRIISS, Holmes Grant Charitable Trust, Rae Grant, Bloody Long Way (BLW), and Walter and Eliza Hall Institute Innovation Grant.
Supplementary Material
The Supplementary Material for this article can be found online at https://www.frontiersin.org/articles/10.3389/fimmu.2018.00694/full#supplementary-material.
References
- 1.Bousfiha A, Jeddane L, Picard C, Ailal F, Bobby Gaspar H, Al-Herz W, et al. The 2017 IUIS phenotypic classification for primary immunodeficiencies. J Clin Immunol (2018) 38(1):129–43. 10.1007/s10875-017-0465-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lucas M, Lee M, Lortan J, Lopez-Granados E, Misbah S, Chapel H. Infection outcomes in patients with common variable immunodeficiency disorders: relationship to immunoglobulin therapy over 22 years. J Allergy Clin Immunol (2010) 125(6):1354–1360e4. 10.1016/j.jaci.2010.02.040 [DOI] [PubMed] [Google Scholar]
- 3.Durandy A, Kracker S, Fischer A. Primary antibody deficiencies. Nat Rev Immunol (2013) 13(7):519–33. 10.1038/nri3466 [DOI] [PubMed] [Google Scholar]
- 4.Baumgart KW, Britton WJ, Kemp A, French M, Roberton D. The spectrum of primary immunodeficiency disorders in Australia. J Allergy Clin Immunol (1997) 100(3):415–23. 10.1016/S0091-6749(97)70257-4 [DOI] [PubMed] [Google Scholar]
- 5.Kirkpatrick P, Riminton S. Primary immunodeficiency diseases in Australia and New Zealand. J Clin Immunol (2007) 27(5):517–24. 10.1007/s10875-007-9105-z [DOI] [PubMed] [Google Scholar]
- 6.Bruton OC. Agammaglobulinaemia. Pediatrics (1952) 9(6):722–8. [PubMed] [Google Scholar]
- 7.Minegishi Y, Rohrer J, Coustan-Smith E, Lederman HM, Pappu R, Campana D, et al. An essential role for BLNK in human B cell development. Science (1999) 286(5446):1954–7. 10.1126/science.286.5446.1954 [DOI] [PubMed] [Google Scholar]
- 8.Minegishi Y, Coustan-Smith E, Rapalus L, Ersoy F, Campana D, Conley ME. Mutations in Ig-alpha (CD79a) result in a complete block in B-cell development. J Clin Invest (1999) 104(8):1115–21. 10.1172/JCI7696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yel L, Minegishi Y, Coustan-Smith E, Buckley RH, Trubel H, Pachman LM, et al. Mutations in the mu heavy-chain gene in patients with agammaglobulinemia. N Engl J Med (1996) 335(20):1486–93. 10.1056/NEJM199611143352003 [DOI] [PubMed] [Google Scholar]
- 10.Minegishi Y, Coustan-Smith E, Wang YH, Cooper MD, Campana D, Conley ME. Mutations in the human lambda5/14.1 gene result in B cell deficiency and agammaglobulinemia. J Exp Med (1998) 187(1):71–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gathmann B, Grimbacher B, Beauté J, Dudoit Y, Mahlaoui N, Fischer A, et al. The European internet-based patient and research database for primary immunodeficiencies: results 2006-2008. Clin Exp Immunol (2009) 157(Suppl 1):3–11. 10.1111/j.1365-2249.2009.03954.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Selenius JS, Martelius T, Pikkarainen S, Siitonen S, Mattila E, Pietikäinen R, et al. Unexpectedly high prevalence of common variable immunodeficiency in Finland. Front Immunol (2017) 8:1190. 10.3389/fimmu.2017.01190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Conley ME, Casanova JL. Discovery of single-gene inborn errors of immunity by next generation sequencing. Curr Opin Immunol (2014) 30:17–23. 10.1016/j.coi.2014.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maffucci P, Filion CA, Boisson B, Itan Y, Shang L, Casanova JL, et al. Genetic diagnosis using whole exome sequencing in common variable immunodeficiency. Front Immunol (2016) 7:220. 10.3389/fimmu.2016.00220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bogaert DJ, Dullaers M, Lambrecht BN, Vermaelen KY, De Baere E, Haerynck F. Genes associated with common variable immunodeficiency: one diagnosis to rule them all? J Med Genet (2016) 53(9):575–90. 10.1136/jmedgenet-2015-103690 [DOI] [PubMed] [Google Scholar]
- 16.European Society for Immunodeficiencies. New Clinical Diagnosis Criteria for the ESID Registry. Geneva: (2017). Available from: https://esid.org/Working-Parties/Registry/Diagnosis-criteria [Google Scholar]
- 17.Bonilla FA, Barlan I, Chapel H, Costa-Carvalho BT, Cunningham-Rundles C, de la Morena MT, et al. International Consensus Document (ICON): common variable immunodeficiency disorders. J Allergy Clin Immunol Pract (2016) 4(1):38–59. 10.1016/j.jaip.2015.07.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ameratunga R, Brewerton M, Slade C, Jordan A, Gillis D, Steele R, et al. Comparison of diagnostic criteria for common variable immunodeficiency disorder. Front Immunol (2014) 5:415. 10.3389/fimmu.2014.00415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Conley ME, Notarangelo LD, Etzioni A. Diagnostic criteria for primary immunodeficiencies. representing PAGID (Pan-American Group for Immunodeficiency) and ESID (European Society for Immunodeficiencies). Clin Immunol (1999) 93(3):190–7. 10.1006/clim.1999.4799 [DOI] [PubMed] [Google Scholar]
- 20.Gabrielsen AE, Good RA. Thymoma. Ann Intern Med (1966) 65(3):607–11. 10.7326/0003-4819-65-3-607 [DOI] [PubMed] [Google Scholar]
- 21.Malphettes M, Gérard L, Galicier L, Boutboul D, Asli B, Szalat R, et al. Good syndrome: an adult-onset immunodeficiency remarkable for its high incidence of invasive infections and autoimmune complications. Clin Infect Dis (2015) 61(2):e13–9. 10.1093/cid/civ269 [DOI] [PubMed] [Google Scholar]
- 22.Picard C, Bobby Gaspar H, Al-Herz W, Bousfiha A, Casanova JL, Chatila T, et al. International union of immunological societies: 2017 primary immunodeficiency diseases committee report on inborn errors of immunity. J Clin Immunol (2018) 38(1):96–128. 10.1007/s10875-017-0464-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salzer U, Bacchelli C, Buckridge S, Pan-Hammarström Q, Jennings S, Lougaris V, et al. Relevance of biallelic versus monoallelic TNFRSF13B mutations in distinguishing disease-causing from risk-increasing TNFRSF13B variants in antibody deficiency syndromes. Blood (2009) 113(9):1967–76. 10.1182/blood-2008-02-141937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boisson B, Wang YD, Bosompem A, Ma CS, Lim A, Kochetkov T, et al. A recurrent dominant negative E47 mutation causes agammaglobulinemia and BCR(-) B cells. J Clin Invest (2013) 123(11):4781–5. 10.1172/JCI71927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chapel H, Lucas M, Lee M, Bjorkander J, Webster D, Grimbacher B, et al. Common variable immunodeficiency disorders: division into distinct clinical phenotypes. Blood (2008) 112(2):277–86. 10.1182/blood-2007-11-124545 [DOI] [PubMed] [Google Scholar]
- 26.Resnick ES, Moshier EL, Godbold JH, Cunningham-Rundles C. Morbidity and mortality in common variable immune deficiency over 4 decades. Blood (2012) 119(7):1650–7. 10.1182/blood-2011-09-377945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gathmann B, Mahlaoui N, CEREDIH. Gérard L, Oksenhendler E, Warnatz K, et al. Clinical picture and treatment of 2212 patients with common variable immunodeficiency. J Allergy Clin Immunol (2014) 134(1):116–26. 10.1016/j.jaci.2013.12.1077 [DOI] [PubMed] [Google Scholar]
- 28.Graziano V, Pecoraro A, Mormile I, Quaremba G, Genovese A, Buccelli C, et al. Delay in diagnosis affects the clinical outcome in a cohort of CVID patients with marked reduction of IgA serum levels. Clin Immunol (2017) 180:1–4. 10.1016/j.clim.2017.03.011 [DOI] [PubMed] [Google Scholar]
- 29.Westh L, Mogensen TH, Dalgaard LS, Bernth Jensen JM, Katzenstein T, Hansen AE, et al. Identification and characterization of a nationwide Danish adult common variable immunodeficiency cohort. Scand J Immunol (2017) 85(6):450–61. 10.1111/sji.12551 [DOI] [PubMed] [Google Scholar]
- 30.Ameratunga R, Koopmans W, Woon ST, Leung E, Lehnert K, Slade CA, et al. Epistatic interactions between mutations of TACI (TNFRSF13B) and TCF3 result in a severe primary immunodeficiency disorder and systemic lupus erythematosus. Clin Transl Immunology (2017) 6(10):e159. 10.1038/cti.2017.41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Schouwenburg PA, Davenport EE, Kienzler AK, Marwah I, Wright B, Lucas M, et al. Application of whole genome and RNA sequencing to investigate the genomic landscape of common variable immunodeficiency disorders. Clin Immunol (2015) 160(2):301–14. 10.1016/j.clim.2015.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kienzler AK, Hargreaves CE, Patel SY. The role of genomics in common variable immunodeficiency disorders. Clin Exp Immunol (2017) 188(3):326–32. 10.1111/cei.12947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kanegane H, Tsukada S, Iwata T, Futatani T, Nomura K, Yamamoto J, et al. Detection of bruton’s tyrosine kinase mutations in hypogammaglobulinaemic males registered as common variable immunodeficiency (CVID) in the Japanese immunodeficiency registry. Clin Exp Immunol (2000) 120(3):512–7. 10.1046/j.1365-2249.2000.01244.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Farmer JR, Ong MS, Barmettler S, Yonker LM, Fuleihan R, Sullivan KE, et al. Common variable immunodeficiency non-infectious disease endotypes redefined using unbiased network clustering in large electronic datasets. Front Immunol (2017) 8:1740. 10.3389/fimmu.2017.01740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barton JC, Bertoli LF, Barton JC. Comparisons of CVID and IgGSD: referring physicians, autoimmune conditions, pneumovax reactivity, immunoglobulin levels, blood lymphocyte subsets, and HLA-A and -B typing in 432 adult index patients. J Immunol Res (2014) 2014:542706. 10.1155/2014/542706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Driessen GJ, Dalm VA, van Hagen PM, Grashoff HA, Hartwig NG, van Rossum AM, et al. Common variable immunodeficiency and idiopathic primary hypogammaglobulinemia: two different conditions within the same disease spectrum. Haematologica (2013) 98(10):1617–23. 10.3324/haematol.2013.085076 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.