Abstract
In recent years, the role of RNA has expanded to the extent that protein-coding RNAs are now the minority with a variety of non-coding RNAs (ncRNAs) now comprising the majority of RNAs in higher organisms. A major contributor to this shift in understanding is RNA sequencing (RNA-seq), which allows a largely unconstrained method for monitoring the status of RNA from whole organisms down to a single cell. This observational power presents both challenges and new opportunities, which require specialized bioinformatics tools to extract knowledge from the data and the ability to reuse data for multiple studies. In this review, we summarize the current status of long non-coding RNA (lncRNA) research in endothelial biology. Then, we will cover computational methods for identifying, annotating, and characterizing lncRNAs in the heart, especially endothelial cells.
Keywords: bioinformatics, databases, lncRNAs, miRNAs, RNA-seq, RNA editing, RNA modifications
Introduction
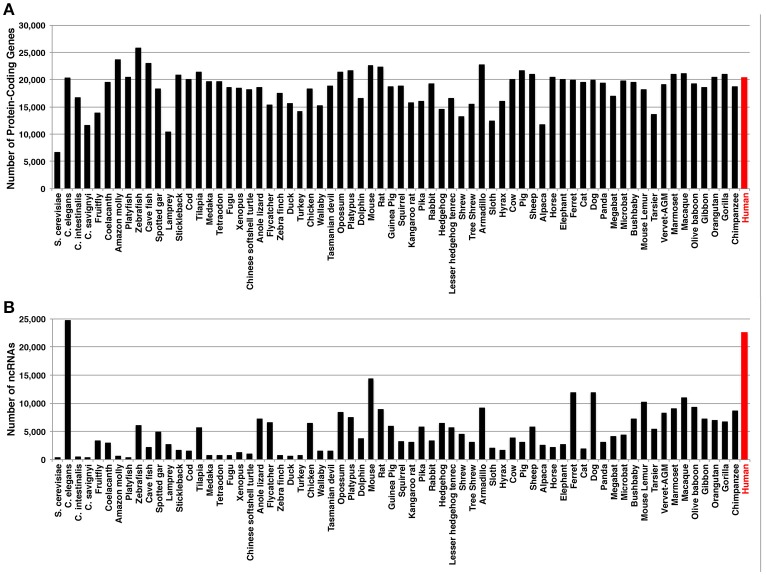
The development of next generation sequencing (NGS) and RNA sequencing (RNA-seq) has significantly improved the understanding of transcriptomes. For example, we now know that most of the human genome is transcribed (Lander et al., 2001), yet only a small percent of these RNAs code for protein (Weirick et al., 2016a). When the human genome was annotated (i.e., giving the definition to the genome by naming a particular gene and its corresponding exons), it was originally thought that the number of protein-coding genes in humans should be more than those of lower organisms (e.g., yeast, plants, fishes, amphibians) (Mercer et al., 2011; Ezkurdia et al., 2014). However, when the numbers of protein-coding genes are compared among species, the number of human genes is not more than those of lower organisms (Figure 1A). Given that humans are able to carry out more complex tasks than lower organisms, the question remains in the field: What aspect of our genome allows for the increased complexity? One school of thoughts is that proteins can be modified for various biological processes (e.g., phosphorylation of a protein for its activation). Another school suggests for the increased variety of isoforms resulting from one gene due to the alternative splicing events. In both schools, the ultimate final products are proteins as we know more about proteins than RNAs. Last school postulates that the increased number of ncRNAs (especially, lncRNAs) is at the base of the highest complexity in human, although it is highly subjective as the number of ncRNAs depends on how well the organism is studied as C. elegans has more lncRNAs than any other organisms (Figure 1B). At the moment, it is most likely that the combination of these schools of thoughts may yield important answers to the question.
Figure 1.
Numbers of (A) protein-coding genes and (B) ncRNAs, including miRNAs and lncRNAs. The information is based on the Ensembl database (Accessed on May 5, 2017).
In recent years, many review articles about lncRNAs in the heart are published (Geisler and Coller, 2013; Archer et al., 2015; Devaux et al., 2015; Iyer et al., 2015; Ounzain et al., 2015a; Philippen et al., 2015; Rizki and Boyer, 2015; Uchida and Dimmeler, 2015; Ballantyne et al., 2016; Busch et al., 2016; Lorenzen and Thum, 2016; Uchida and Bolli, 2017; Viereck and Thum, 2017; Sallam et al., 2018). Furthermore, there are large amounts of screening data available for lncRNAs expressed in the heart (Ounzain et al., 2014, 2015b,c; Kurian et al., 2015) even single-cell RNA-seq data (Chan et al., 2016; Delaughter et al., 2016; King et al., 2017; Lescroart et al., 2018; Skelly et al., 2018). However, these data sets were mostly generated for specific purposes and have largely not been analyzed for lncRNAs. Here, we will focus on endothelial cells (ECs), an important cell type in cardiovascular medicine. Furthermore, we will cover computational methods for identifying, annotating, and characterizing lncRNAs in the heart, especially ECs.
lncRNAs in endothelial cells
Vessels deliver metabolites and oxygen to the tissue and export waste products to sustain the well-being of an organism (Asahara et al., 2011). After tissue injury [e.g., myocardial infarction (MI)], ECs migrate to the site of injury to re-establish the capillary network through a process called “angiogenesis” (Jakobsson et al., 2010; Oka et al., 2014). Furthermore, ECs contribute to the multicellular communications that maintain the balance between the regeneration and dysfunctional or maladaptive healing (Cines et al., 1998; Libby, 2012; Kluge et al., 2013; Eelen et al., 2018). Although advances have been made to understand angiogenesis, the recent emergence of lncRNAs has added another layer of complexity to the genetic network of angiogenesis. To date, a number of lncRNAs are identified and characterized (Table 1) as ECs can be found throughout the human body. For example, MALAT1 regulates endothelial cell function and vessel growth via cell cycle control (Michalik et al., 2014); the histone demethylase JARID1B controls the lncRNA MANTIS, which regulates EC function and vessel growth by binding to the chromatin modifying enzyme BRG1 (Leisegang et al., 2017); and several lncRNAs bind miRNAs to function as miRNA sponges (He et al., 2015, 2017; Huang et al., 2015; Yan et al., 2015; Lu et al., 2016; Ming et al., 2016; Ma Y. et al., 2017; Sun et al., 2017; Zhang B. Y. et al., 2017; Bao et al., 2018).
Table 1.
List of lncRNAs in endothelial cells.
| LncRNA | Organism | Tissue | Cell type | Function | References |
|---|---|---|---|---|---|
| ALT1 | Human | N/A | HUVEC | Interacts with ACE2 and CUL1 to control the expression of Cyclin D1 possibly via ubiquitinatiion and degradation. | Li et al., 2017b |
| ASncmtRNA-2 | Human; mouse | Aortas of old mice | HUVEC | Might be involved in the RS establishment by participating in the cell cycle arrest in G2/M phase, possibly through the production of miR-4485 and -1973. | Bianchessi et al., 2015 |
| GAS5 | Human | Atherosclerotic plaques | HUVEC | Can be transferred from macrophages to EC in exosomes to induce apoptosis of ECs. | Chen et al., 2017 |
| GATA6-AS | Human; mouse | Cell-based Xenograft model | HUVEC | Binds the lysyl oxidase LOXL2 to impair its function as H3K4me3 deaminase. | Neumann et al., 2018 |
| H19 | Human | Brain; Glioma tissue specimens | HBMVEC | Knockdown of H19 suppressed glioma induced angiogenesis by inhibiting miR-29a, which may modulate the onset of glioma by regulating biological behaviors of glioma vascular ECs. | Jia et al., 2016 |
| H19 | Human | N/A | HUVEC | Is contained in exosomes released by CD90+ cancer cells to promote angiogenic phenotype and cell-to-cell adhesion in ECs. | Conigliaro et al., 2015 |
| HIF1A-AS2 | Human; rat | Permanent middle cerebral artery occlusion model | HUVEC | Facilitates the up-regulation of HIF-1α by sponging miR-153-3p, thereby promoting angiogenesis in hypoxia. | Li et al., 2017a |
| HOTAIR | Human | N/A | HBMVEC | Is contained in the glioma cell-derived extracellular vesicles and transmitted into ECs. | Ma X. et al., 2017 |
| HOTAIR | Human | Atherosclerotic plaques | HUVEC; HAEC | Positively regulates proliferation and migration of ECs. | Peng et al., 2017 |
| HOTTIP | Human | CAD and normal arterial tissues | HUVEC | Its overexpression induces β-catenin expression and enhances the downstream protein c-Myc expression in ECs to affect cell proliferation and migration. | Liao et al., 2018 |
| IGF2AS | Rat | Heart | mMVE | Reciprocal regulation IGF2AS and IGF2 is critical in modulating angiogenic development in myocardial tissues in type 2 diabetes. | Zhao et al., 2017 |
| LEENE | Human; mouse | Thoracic aorta and aortic arch | HUVECs; HAoEC | Serves as a guide to facilitate RNA Pol II binding to the promoter of eNOS. | Miao et al., 2018 |
| LINC00305 | Human | N/A | HUVEC | Binds miR-136 to control apoptosis. | Zhang B. Y. et al., 2017 |
| LINC00341 | Human | N/A | HUVEC | Guides EZH2 [the catalytic subunit of polycomb repressive complex 2 (PRC2)] to the promoter region of the VCAM1 gene to suppress VCAM1. | Huang et al., 2017 |
| LINC00657 | Human | N/A | HUVEC | Binds miR-590-3p to attenuate the suppression of miR-590-3p on HIF-1a, and to promote angiogenesis through VEGF, MMP-2, and MMP-9. | Bao et al., 2018 |
| lincRNA-p21 | Mouse | N/A | mouse lymphoid endothelial cell line SVEC4 | Binds miR-130b to promote cell apoptosis and induce cell cycle progression. | He et al., 2015 |
| LISPR1 | Human | N/A | HUVEC; HAoEC; HMEC | Acts as a novel regulatory unit important for S1PR1 expression and EC function. | Josipovic et al., 2018 |
| LOC100129973 | Human | N/A | HUVEC | Binds miR-4707-5p and -4767, which promote apoptosis by targeting and downregulating two apoptosis inhibitors, API5 and BCL2L12, respectively. | Lu et al., 2016 |
| MALAT1 | Human | Peripheral blood from patients diagnosed with unstable angina | HUVEC | Protects the endothelium from ox-LDL-induced endothelial dysfunction partly through competing with miR-22-3p for endogenous RNA. | Tang et al., 2015 |
| MALAT1 | Human; mouse | Mouse retinal angiogenesis model | HUVEC | Regulates EC function and vessel growth via cell cycle control. | Michalik et al., 2014 |
| MALAT1 | Human | N/A | HUVEC | Binds miR-320a, which targets the pro-proliferative gene FOXM1 for ECs. | Sun et al., 2017 |
| MALAT1 | Rat | Retina of diabetic rats | Monkey choroid, retina cell line RF/6A | Regulates EC function via p38 MAPK signaling pathway. | Liu et al., 2014 |
| MALAT1 | Human; mouse | Kidneys of diabetic mice | HUVEC | Regulates glucose-induced up-regulation of inflammatory mediators IL-6 and TNF-α through activation of SAA3. | Puthanveetil et al., 2015 |
| MANTIS | Human | Brain microvessel isolation from glioblastoma patients | HUVEC; HAoEC; HDLEC; PAEC | Regulates EC function and vessel growth by binding to the chromatin modifying enzyme BRG1. | Leisegang et al., 2017 |
| Meg3 | Mouse; monkey | Retina of diabetic mice | Monkey choroid, retina cell line RF/6A | Activates PI3k/Akt signaling. | Qiu et al., 2016 |
| Meg3 | Rat | Brain | RBMVEC | Physically interacts with p53, which binds to the promoter of Nox4 to regulate cell growth and the blood vessel growth factor expression. | Zhan et al., 2017 |
| MEG3 | Human | N/A | HUVEC | Is regulated by HIF-1α to maintain VEGFR2 expression in ECs and plays a vital role for VEGFA-mediated endothelial angiogenesis. | Ruan et al., 2018 |
| MEG3 | Human; mouse | Hind-limb ischemia in aged mice | HUVEC | Its silencing prevents aging-mediated inhibition of sprouting activity. | Boon et al., 2016 |
| MEG3 | Human; mouse | Circulating ECs from metabolic syndrome (MetS) patients | EPC | Protects ECs via decreasing miR-140-5p expression and increasing HDAC7 expression in MetS. | Liu H. Z. et al., 2016 |
| MEG3 | Human | N/A | HUVEC | Binds miR-9 to control the proliferation and angiogenesis of ECs. | He et al., 2017 |
| MIAT | Human; rat | Diabetes mellitus | HUVEC; HMVEC | Binds miR-150-5p to regulate EC function by forming a feedback loop with VEGF. | Yan et al., 2015 |
| PUNISHER | Human; zebrafish | Heart | HUVEC | Its inhibition results in severe vascular defects in zebrafish embryos and reduced cell proliferation in HUVEC. | Kurian et al., 2015 |
| PVT1 | Human | N/A | Human cerebral microvascular endothelial cell line hCMEC/D3 | Binds miR-186, which targets Atg7 and Beclin1 mRNAs. | Ma Y. et al., 2017 |
| RNCR3/LINC00599 | Human; mouse | Aortic atherosclerotic lesions | HUVEC | Forms a feedback loop with KLF2 and miR-185-5p to regulate EC function. | Shan et al., 2016 |
| SENCR | Human | N/A | HUVEC | Induces proliferation, migration, and angiogenesis. | Boulberdaa et al., 2016 |
| SIRT1 AS lncRNA | Mouse | N/A | EPC | Relieves miR-22-induced SIRT1 downregulation by competitively sponging miR-22. | Ming et al., 2016 |
| STEEL | Human | N/A | HUVEC; HMVEC | Binds the chromatin-associated enzyme PARP1 to assist its binding to the KLF2 and eNOS promoters. | Man et al., 2018 |
| TGFB2-OT1 | Human | N/A | HUVEC | Binds miR-3960, -4488, and -44459, which target CERS1, NAT8L, and LARP1, respectively, the key proteins involved in autophagy and inflammation. | Huang et al., 2015 |
| tie-1AS lncRNA | Human; mouse; Zebrafish | Zebrafish Tg(flk:EGFP) | HUVEC | Binds tie-1 mRNA and regulates tie-1 transcript levels, resulting in specific defects in endothelial cell contact junctions. | Li et al., 2010 |
| uc001pwg.1 | Human | Stenosed and nonstenotic uremic veins | HUVEC; EC derived from human-induced pluripotent stem cells | Its overexpression increases eNOS phosphorylation and NO production by affecting the expression level of nearby protein-coding gene MCAM. | Lv et al., 2017 |
Each lncRNA is listed with organism(s), tissue(s), cell type(s), and function(s) along with the corresponding reference. The abbreviations used are as follows: “coronary artery disease (CVD)”; “endothelial progenitor cells (EPCs)”; “human aortic endothelial cell (HAEC)”; “human aortic endothelial cells (HAoEC)”; “human brain microvascular endothelial cells (HBMVEC)”; “human dermal lymphatic endothelial cells (HDLEC)”; “human microvascular endothelial cells (HMEC)”; “human microvascular endothelial cells (HMVEC)”; “myocardial microvascular endothelial cells (mMVE)”; “human pulmonary artery endothelial cells (PAEC)”; “rat brain microvascular endothelial cells (RBMVEC)”; and “not applicable (N/A).”
In addition to the above lncRNAs, we recently reported the presence of an emerging class of lncRNAs called “circular RNAs (circRNAs)” in ECs (Boeckel et al., 2015). CircRNAs are byproducts of splicing events (more specifically, “backsplicing”) of mostly protein-coding genes (Jeck et al., 2013; Jeck and Sharpless, 2014; Boeckel et al., 2015), are stable and localized predominantly in the cytoplasm (Nigro et al., 1991; Cocquerelle et al., 1993). When some circRNAs are knocked down, there are phenotypes observed, which may not be observed when the parental transcripts of circRNAs are knocked down (Boeckel et al., 2015; Gerstner et al., 2016). For example, the hypoxia-regulated circRNA cZNF292, which is derived by backsplicing of ZNF292 protein-coding gene, exhibits proangiogenic activities (Boeckel et al., 2015). Some studies suggest that circRNAs function as miRNA sponges (Hansen et al., 2013; Memczak et al., 2013; Geng et al., 2016; Liu Q. et al., 2016; Zheng et al., 2016). However, recent comprehensive bioinformatics analysis (Guo et al., 2014) and our biological validation experiments (Boeckel et al., 2015; Weirick et al., 2016b) indicate that circRNAs functioning as miRNA sponges are extremely rare. Along with lncRNAs, more studies are necessary to uncover the functions of circRNAs in ECs.
RNA-seq data analysis using bioinformatics
There are two major methods of generating libraries for RNA-seq, which are based on poly-A selection and ribosomal RNA (rRNA)-depletion. Both methods are aimed at removing rRNAs, which constitute ~80% of total RNA followed by 15% transfer RNAs (tRNAs) and only 5% for all other RNAs, including protein-coding genes and lncRNAs (Lodish et al., 2000). The poly-A selection will result in the identification of protein-coding genes and lncRNAs with poly A tails (~60% of total lncRNAs; Cheng et al., 2005), while the rRNA-depletion can identify the rest of lncRNAs and circRNAs—in addition to those identified in the former method. The presence of circRNAs is detected only with the latter method as circRNAs arise from exons and/or introns that are spliced out, which are devoid of poly A tails.
Analysis of RNA-seq data usually involves a number of common computational steps to obtain the expression profiles of the RNA in a set of samples. At the start of a typical analysis pipeline, reads are trimmed to remove primers and low-quality regions of reads. Next, the reads are aligned to a genome in a “guided alignment.” In the case of the organism with no reference genome, a “de novo assembly” of the transcriptome is performed. However, de novo assembly is more error-prone and difficult to operate, thus we will simply focus on guided alignments. Traditionally, Tophat (Trapnell et al., 2012) has been the most popular aligner, but it is now being supplanted by newer programs (e.g., STAR, HISAT2), which offer greater speed and alignment accuracy (Engström et al., 2013; Conesa et al., 2016; Costa-Silva et al., 2017; Zhang C. et al., 2017).
Similar to protein-coding genes, lncRNAs undergo alternative splicing (AS) to produce isoforms (Deveson et al., 2017; White et al., 2017). The current understanding of AS is mainly based on EST-cDNA sequencing and short-read RNA-seq data. In the second-generation sequencing (e.g., Illumina-based short RNA-seq), long strands of cDNA must be broken into small segments to infer nucleotide sequences by amplification and synthesis (Metzker, 2010), which fall short of detecting intact full-length transcripts. To address this shortcoming, third-generation sequencing (also known as “long-read sequencing”) may be a solution. PacBio RS II (Pacific Biosciences, CA, U.S.A.) is the first commercialized third-generation sequencer, which utilizes a novel single molecule real-time (SMRT) technology (Schadt et al., 2010). Compared to second-generation sequencing, SMRT technology offers long read lengths (up to 92 kb), high consensus accuracy (free of systematic sequencing errors), and low degree of bias (even coverage across G+C content) (Nakano et al., 2017). When this technology is applied to any transcriptome (cDNA) sequencing (e.g., RNA-seq), it is called “Iso-Seq,” which can monitor AS (Abdel-Ghany et al., 2016). With Iso-Seq, the need for transcriptome assembly is eliminated as “one read = one transcript” with each transcript can be read from its 5′-end to poly A tail. Iso-Seq has been applied to various species and tissues (Singh et al., 2016; Cheng et al., 2017; Hoang et al., 2017a,b; Jiang et al., 2017; Jo et al., 2017; Kim et al., 2017; Kuo et al., 2017; Wang et al., 2017a,b, 2018; Xue et al., 2017; Zhang S. J. et al., 2017; Zulkapli et al., 2017; Filichkin et al., 2018) but not yet to ECs.
The largely unbiased manner in which RNA-seq captures information is another interesting aspect of the technology, which enables new findings via re-analysis of published data. For example, most of the RNA-seq studies have been focused on analyzing expression of protein-coding genes. As lncRNA are also present in the data sets, these data offer a rich resource for studying lncRNA expression patterns. We have developed a number of bioinformatics tools to exploit these resources (Gellert et al., 2013; Weirick et al., 2015, 2016b, 2017), including some specifically designed to identify lncRNAs and to associate their expressions in various tissues and cell types, including ECs (e.g., our database ANGIOGENES; Müller et al., 2016). Although ECs can be found throughout the human body, there are only few databases available that contain the expression profiles for genes expressed in ECs (e.g., Causal Biological Network database Boué et al., 2015, dbANGIO4 Savas, 2012, and PubAngioGen Li et al., 2015). Our ANGIOGENE is one of the few that contain the expression profiles of both protein-coding genes and lncRNAs in various ECs based on RNA-seq data. Furthermore, ANGIOGENES covers humans, mice, and zebrafish to allow for the screening of lncRNAs in the positional conserved regions (not necessary sequence-conserved) (Weirick et al., 2015).
There are many transcripts whose sequencing reads are present in RNA-seq data but are not annotated in the public databases, including NONCODE (Zhao et al., 2016), which is one of the hallmark databases for lncRNAs. Our previous study (Weirick et al., 2016a) shows that 77,656 novel isoforms of annotated reference transcripts and 102,848 intergenic transcripts are identified with 58,789 (75.70%) and 101,993 (99.17%) being predicted as non-coding, respectively, from 12 human tissues (Nielsen et al., 2014), while there are 181,434 annotated transcripts (87.13% out of 208,244 transcripts in Ensembl version 77) are expressed in at least one of 12 tissues analyzed. Although we could validate the presence of novel lncRNAs by RT-PCR experiments, many novel lncRNAs contain repetitive elements, such as microsatellites (Bidichandani et al., 1998) and short interspersed nuclear elements (SINE), including ALU elements (Häsler and Strub, 2006). Thus, it is highly recommended to consult the available methods to characterize lncRNAs (Li et al., 2014; Liu et al., 2017), including CAGE-seq to annotate the 5′-end of lncRNAs (Hon et al., 2017) and ribo-seq/ribosomal footprinting RNA-seq technology to understand the coding potential (Ruiz-Orera et al., 2014; Ji et al., 2015; Alvarez-Dominguez and Lodish, 2017) before proceeding to more functional experiments.
It is well-known that ECs are heterogeneous populations of cells as their activities and functions differ based on their physiological locations (Aird, 2012; Regan and Aird, 2012; Yuan et al., 2016). In order to understand such heterogeneity of ECs, it is important to perform single-cell RNA-seq (scRNA-seq) instead of bulk RNA-seq by using a piece of tissue or those in a culture dish. As the technique for scRNA-seq matures, the immediate problem is the data analysis, especially positioning each cell to a particular cell type in order to organize their molecular signatures matching to the anatomical location in which each cell was isolated from. For example, hearts contain multiple cell types (e.g., cardiomyocytes, ECs, fibroblasts, pericytes, and smooth muscle cells). In regards to ECs, their expression profiles may differ for those contained in the artery and vein. When such profiles are compared to ECs from other tissues (e.g., kidneys, lungs), there are some genes that are expressed at the similar level in all tissues while others are expressed specifically in ECs isolated from a particular tissue. In order to understand such hierarchical organization of cells, their corresponding cell types, and tissues, it is utmost importance that the ontology of each cell must be organized in relation to its corresponding cell type and tissue. To achieve this hierarchical and ontological organization, we recently introduced the usage of logic programing (Weirick et al., 2016b), which was applied to kidneys. Logic programming is a programming paradigm based on formal logic, using a set of logical sentences consisting of facts, rules, and queries (Eklund and Klawonn, 1992). For example, consider a transcript expressed in the renal cortex. The renal cortex is located within kidneys. When sequencing whole kidney under the same condition, the same transcript should be expressed. One could even descend to the level of cell types (e.g., ECs isolated from interlobular arteries, which are located within the kidney cortex). Similarly, all sequences expressed within these ECs are expressed in the kidney. Furthermore, it is well-known that high abundance sequences can overwhelm lower abundance sequences. Thus, logic programming can be useful for integrating RNA-seq data at different hierarchical levels and beyond. This can be accomplished by: (1) modeling the anatomical and experimental relationships; (2) creating rules to define various types of expression characteristics; and (3) using queries to determine expression characteristics of a given RNA. The analysis of RNA-seq data of ECs in the heart for lncRNAs, coupled with logic programming, should help to facilitate the further usage of the available RNA-seq data (e.g., single cell RNA-seq data from the heart) to test various hypotheses that were not originally intended when the data were generated. Such an approach should yield the identification of lncRNAs in a variety of conditions (e.g., expressed in atherosclerotic plaques but not in the healthy artery), which can be further validated in functional studies.
Detection of RNA editing patterns from RNA-seq data
In addition to studying lncRNAs, re-analysis of publicly-available RNA-seq data is also useful for studying RNA editing. RNA editing is a post-transcriptional modification to alter the sequence of RNA molecules (Keegan et al., 2001; Hideyama and Kwak, 2011). The full extent and reasons for RNA editing is largely unknown. However, recent studies show that the editing in exons leads to an amino acid substitutions from altered codons (Alon et al., 2015; Liscovitch-Brauer et al., 2017), whereas editing in 3′-untranslated regions (UTRs) may affect binding of RNA binding proteins (RBPs) or microRNAs (miRNAs) thereby modulating RNA stability and/or translation (Keegan et al., 2001). There are two types of RNA editing: adenosine to inosine (A-to-I) and cytidine to uridine (C-to-U). A-to-I is the most common form and occurs through RNA editing enzymes called “adenosine deaminases acting on RNA (ADARs),” which convert adenosine in double-stranded RNA into inosine (Savva et al., 2012). When reverse transcribed to complementary DNA (cDNA), an inosine is converted to guanine (“G”), which can be identified by comparison to the reference genome. A number of studies have been conducted to detect RNA editing events from RNA-seq data (Bahn et al., 2012; Park et al., 2012; Peng et al., 2012; Ramaswami et al., 2012, 2013; Solomon et al., 2013), including our recent study in ECs (Stellos et al., 2016). Because of the detection from RNA-seq data, several databases for RNA editing events have been constructed to provide evidence for the frequency of RNA editing in various conditions (Kiran and Baranov, 2010; Picardi et al., 2011, 2017; Laganà et al., 2012; Ramaswami and Li, 2014; Solomon et al., 2016; Gong et al., 2017). We recently reported that cathepsin S (CTSS), which encodes a cysteine protease associated with angiogenesis and atherosclerosis, is highly edited (Stellos et al., 2016). Such RNA editing enables the recruitment of stabilizing RBP human antigen R (HuR) to the 3′-UTR of CTSS transcript, thereby controlling CTSS mRNA stability and expression. The RNA editing enzyme ADAR1 levels and the extent of CTSS RNA editing are associated with changes in CTSS levels in patients with coronary artery diseases. Our study highlights the involvement of RNA editing in cardiovascular diseases, which has not yet been investigated (Uchida and Jones, 2018). Our finding was further supported by the recent large-scale, multi-center study analyzing RNA-seq data from the NIH Common Fund's Genotype-Tissue Expression (GTEx) program, which reported that the aorta, coronary, and tibial arteries were the most highly edited tissue type among 53 body sites from 552 individuals analyzed (Tan et al., 2017).
In humans, RNA editing occurs mostly in repetitive Alu regions (Levanon et al., 2004; Peng et al., 2012), which can be found in lncRNAs as lncRNAs can also be edited (Picardi et al., 2014; Szczesniak and Makalowska, 2016; Gong et al., 2017). Although proposed but not tested extensively, the functions of lncRNAs may depend on their conformation (e.g., 3D structures), which can be affected by their primary sequences. This folding process can be influenced by a variety of factors, including (but not limited to) RNA modifications on lncRNAs, such as RNA editing. Given that RNA editing can be readily detected from RNA-seq data, more systematic analysis of RNA editing patterns is necessary, especially targeting lncRNAs in the heart (Uchida and Jones, 2018). For this purpose, several bioinformatics tools are available to detect editing within RNA-seq data, including GIREMI (Zhang and Xiao, 2015), JACUSA (Piechotta et al., 2017), RED (Sun et al., 2016), RED-ML (Xiong et al., 2017), REDItools (Picardi and Pesole, 2013), RES-Scanner (Wang et al., 2016), and our RNAEditor (John et al., 2017).
How could we translate the concept of lncRNAs into RNA therapeutics
The one obvious usage of lncRNAs in medicine is using lncRNAs as diagnostic biomarkers as lncRNAs are more cell-type specifically expressed than protein-coding genes (Thurman et al., 2012; Gellert et al., 2013; Necsulea et al., 2014; Weirick et al., 2015). Although some progresses have been made, most of RNA-seq data analyzed so far does not consider lncRNAs due to the reasons mentioned above. Thus, without performing further RNA-seq experiments, it should be feasible to discover lncRNAs that capable of differentiating between diseased and healthy individuals by re-analyzing publicly-available RNA-seq data. For this purpose, bioinformatics tools mentioned above should be useful.
Author contributions
All authors made contributions to survey the current status of lncRNA research. All authors approved the final version of this manuscript.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The reviewer KT and handling Editor declared their shared affiliation.
Footnotes
Funding. This study was supported by the V. V. Cooke Foundation (Kentucky, U.S.A.); grant from the University of Louisville School of Medicine; an EVPRI Internal Research Grant from the Office of the Executive Vice President for Research and Innovation at the University of Louisville; University of Louisville 21st Century University Initiative on Big Data in Medicine; and the startup funding from the Mansbach Family, the Gheens Foundation and other generous supporters at the University of Louisville.
References
- Abdel-Ghany S. E., Hamilton M., Jacobi J. L., Ngam P., Devitt N., Schilkey F., et al. (2016). A survey of the sorghum transcriptome using single-molecule long reads. Nat. Commun. 7:11706. 10.1038/ncomms11706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aird W. C. (2012). Endothelial cell heterogeneity. Cold Spring Harb. Perspect. Med. 2:a006429. 10.1101/cshperspect.a006429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alon S., Garrett S. C., Levanon E. Y., Olson S., Graveley B. R., Rosenthal J. J., et al. (2015). The majority of transcripts in the squid nervous system are extensively recoded by A-to-I RNA editing. Elife 4:e05198. 10.7554/eLife.05198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Dominguez J. R., Lodish H. F. (2017). Emerging mechanisms of long noncoding RNA function during normal and malignant hematopoiesis. Blood 130, 1965–1975. 10.1182/blood-2017-06-788695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer K., Broskova Z., Bayoumi A. S., Teoh J. P., Davila A., Tang Y., et al. (2015). Long non-coding RNAs as master regulators in cardiovascular diseases. Int. J. Mol. Sci. 16, 23651–23667. 10.3390/ijms161023651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asahara T., Kawamoto A., Masuda H. (2011). Concise review: circulating endothelial progenitor cells for vascular medicine. Stem Cells 29, 1650–1655. 10.1002/stem.745 [DOI] [PubMed] [Google Scholar]
- Bahn J. H., Lee J. H., Li G., Greer C., Peng G., Xiao X. (2012). Accurate identification of A-to-I RNA editing in human by transcriptome sequencing. Genome Res. 22, 142–150. 10.1101/gr.124107.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballantyne M. D., McDonald R. A., Baker A. H. (2016). lncRNA/MicroRNA interactions in the vasculature. Clin. Pharmacol. Ther. 99, 494–501. 10.1002/cpt.355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao M. H., Li G. Y., Huang X. S., Tang L., Dong L. P., Li J. M. (2018). Long non-coding RNA LINC00657 acting as miR-590-3p sponge to facilitate low concentration oxidized low-density lipoprotein-induced angiogenesis. Mol. Pharmacol. 93, 368–375. 10.1124/mol.117.110650 [DOI] [PubMed] [Google Scholar]
- Bianchessi V., Badi I., Bertolotti M., Nigro P., D'alessandra Y., Capogrossi M. C., et al. (2015). The mitochondrial lncRNA ASncmtRNA-2 is induced in aging and replicative senescence in Endothelial Cells. J. Mol. Cell. Cardiol. 81, 62–70. 10.1016/j.yjmcc.2015.01.012 [DOI] [PubMed] [Google Scholar]
- Bidichandani S. I., Ashizawa T., Patel P. I. (1998). The GAA triplet-repeat expansion in Friedreich ataxia interferes with transcription and may be associated with an unusual DNA structure. Am. J. Hum. Genet. 62, 111–121. 10.1086/301680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeckel J. N., Jae N., Heumuller A. W., Chen W., Boon R. A., Stellos K., et al. (2015). Identification and characterization of hypoxia-regulated endothelial circular, RNA. Circ. Res. 117, 884–890. 10.1161/CIRCRESAHA.115.306319 [DOI] [PubMed] [Google Scholar]
- Boon R. A., Hofmann P., Michalik K. M., Lozano-Vidal N., Berghäuser D., Fischer A., et al. (2016). Long noncoding RNA Meg3 controls endothelial cell aging and function: implications for regenerative angiogenesis. J. Am. Coll. Cardiol. 68, 2589–2591. 10.1016/j.jacc.2016.09.949 [DOI] [PubMed] [Google Scholar]
- Boué S., Talikka M., Westra J. W., Hayes W., Di Fabio A., Park J., et al. (2015). Causal biological network database: a comprehensive platform of causal biological network models focused on the pulmonary and vascular systems. Database (Oxford) 2015:bav030. 10.1093/database/bav030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulberdaa M., Scott E., Ballantyne M., Garcia R., Descamps B., Angelini G. D., et al. (2016). A role for the long noncoding RNA SENCR in commitment and function of endothelial cells. Mol. Ther. 24, 978–990. 10.1038/mt.2016.41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch A., Eken S. M., Maegdefessel L. (2016). Prospective and therapeutic screening value of non-coding RNA as biomarkers in cardiovascular disease. Ann. Transl. Med. 4:236. 10.21037/atm.2016.06.06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan S. S., Chan H. H. W., Kyba M. (2016). Heterogeneity of Mesp1+ mesoderm revealed by single-cell RNA-seq. Biochem. Biophys. Res. Commun. 474, 469–475. 10.1016/j.bbrc.2016.04.139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Yang W., Guo Y., Chen W., Zheng P., Zeng J., et al. (2017). Exosomal lncRNA GAS5 regulates the apoptosis of macrophages and vascular endothelial cells in atherosclerosis. PLoS ONE 12:e0185406. 10.1371/journal.pone.0185406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng B., Furtado A., Henry R. J. (2017). Long-read sequencing of the coffee bean transcriptome reveals the diversity of full-length transcripts. Gigascience 6, 1–13. 10.1093/gigascience/gix086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J., Kapranov P., Drenkow J., Dike S., Brubaker S., Patel S., et al. (2005). Transcriptional maps of 10 human chromosomes at 5-nucleotide resolution. Science 308, 1149–1154. 10.1126/science.1108625 [DOI] [PubMed] [Google Scholar]
- Cines D. B., Pollak E. S., Buck C. A., Loscalzo J., Zimmerman G. A., McEver R. P., et al. (1998). Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood 91, 3527–3561. [PubMed] [Google Scholar]
- Cocquerelle C., Mascrez B., Hétuin D., Bailleul B. (1993). Mis-splicing yields circular RNA molecules. FASEB J. 7, 155–160. 10.1096/fasebj.7.1.7678559 [DOI] [PubMed] [Google Scholar]
- Conesa A., Madrigal P., Tarazona S., Gomez-Cabrero D., Cervera A., McPherson A., et al. (2016). A survey of best practices for RNA-seq data analysis. Genome Biol. 17:13 10.1186/s13059-016-0881-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conigliaro A., Costa V., Lo Dico A., Saieva L., Buccheri S., Dieli F., et al. (2015). CD90+ liver cancer cells modulate endothelial cell phenotype through the release of exosomes containing H19 lncRNA. Mol. Cancer 14:155. 10.1186/s12943-015-0426-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa-Silva J., Domingues D., Lopes F. M. (2017). RNA-Seq differential expression analysis: an extended review and a software tool. PLoS ONE 12:e0190152. 10.1371/journal.pone.0190152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaughter D. M., Bick A. G., Wakimoto H., McKean D., Gorham J. M., Kathiriya I. S., et al. (2016). Single-cell resolution of temporal gene expression during heart development. Dev. Cell 39, 480–490. 10.1016/j.devcel.2016.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaux Y., Zangrando J., Schroen B., Creemers E. E., Pedrazzini T., Chang C. P., et al. (2015). Long noncoding RNAs in cardiac development and ageing. Nat. Rev. Cardiol. 12, 415–425. 10.1038/nrcardio.2015.55 [DOI] [PubMed] [Google Scholar]
- Deveson I. W., Hardwick S. A., Mercer T. R., Mattick J. S. (2017). The dimensions, dynamics, and relevance of the mammalian noncoding transcriptome. Trends Genet. 33, 464–478. 10.1016/j.tig.2017.04.004 [DOI] [PubMed] [Google Scholar]
- Eelen G., de Zeeuw P., Treps L., Harjes U., Wong B. W., Carmeliet P. (2018). Endothelial cell metabolism. Physiol. Rev. 98, 3–58. 10.1152/physrev.00001.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund P., Klawonn F. (1992). Neural fuzzy logic programming. IEEE Trans. Neural Netw. 3, 815–818. 10.1109/72.159071 [DOI] [PubMed] [Google Scholar]
- Engström P. G., Steijger T., Sipos B., Grant G. R., Kahles A., Ratsch G., et al. (2013). Systematic evaluation of spliced alignment programs for RNA-seq data. Nat. Methods 10, 1185–1191. 10.1038/nmeth.2722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezkurdia I., Juan D., Rodriguez J. M., Frankish A., Diekhans M., Harrow J., et al. (2014). Multiple evidence strands suggest that there may be as few as 19,000 human protein-coding genes. Hum. Mol. Genet. 23, 5866–5878. 10.1093/hmg/ddu309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filichkin S. A., Hamilton M., Dharmawardhana P. D., Singh S. K., Sullivan C., Ben-Hur A., et al. (2018). Abiotic Stresses modulate landscape of poplar transcriptome via alternative splicing, differential intron retention, and isoform ratio switching. Front. Plant Sci. 9:5. 10.3389/fpls.2018.00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler S., Coller J. (2013). RNA in unexpected places: long non-coding RNA functions in diverse cellular contexts. Nat. Rev. Mol. Cell Biol. 14, 699–712. 10.1038/nrm3679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellert P., Ponomareva Y., Braun T., Uchida S. (2013). Noncoder: a web interface for exon array-based detection of long non-coding RNAs. Nucleic Acids Res. 41:e20. 10.1093/nar/gks877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng H. H., Li R., Su Y. M., Xiao J., Pan M., Cai X. X., et al. (2016). The circular RNA Cdr1as promotes myocardial infarction by mediating the regulation of miR-7a on its target genes expression. PLoS ONE 11:e0151753. 10.1371/journal.pone.0151753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerstner S., Köhler W., Heidkamp G., Purbojo A., Uchida S., Ekici A. B., et al. (2016). Specific phenotype and function of CD56-expressing innate immune cell subsets in human thymus. J. Leukoc. Biol. 100, 1297–1310. 10.1189/jlb.1A0116-038R [DOI] [PubMed] [Google Scholar]
- Gong J., Liu C., Liu W., Xiang Y., Diao L., Guo A. Y., et al. (2017). LNCediting: a database for functional effects of RNA editing in lncRNAs. Nucleic Acids Res. 45, D79–D84. 10.1093/nar/gkw835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J. U., Agarwal V., Guo H., Bartel D. P. (2014). Expanded identification and characterization of mammalian circular RNAs. Genome Biol. 15:409. 10.1186/s13059-014-0409-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen T. B., Jensen T. I., Clausen B. H., Bramsen J. B., Finsen B., Damgaard C. K., et al. (2013). Natural RNA circles function as efficient microRNA sponges. Nature 495, 384–388. 10.1038/nature11993 [DOI] [PubMed] [Google Scholar]
- Häsler J., Strub K. (2006). Alu elements as regulators of gene expression. Nucleic Acids Res. 34, 5491–5497. 10.1093/nar/gkl706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He C., Ding J. W., Li S., Wu H., Jiang Y. R., Yang W., et al. (2015). The ROLE OF LONG INTERGENIC NONCODINg RNA p21 in vascular endothelial cells. DNA Cell Biol. 34, 677–683. 10.1089/dna.2015.2966 [DOI] [PubMed] [Google Scholar]
- He C., Yang W., Yang J., Ding J., Li S., Wu H., et al. (2017). Long Noncoding RNA MEG3 negatively regulates proliferation and angiogenesis in vascular endothelial cells. DNA Cell Biol. 36, 475–481. 10.1089/dna.2017.3682 [DOI] [PubMed] [Google Scholar]
- Hideyama T., Kwak S. (2011). When does als start? ADAR2-GluA2 hypothesis for the etiology of sporadic, ALS. Front. Mol. Neurosci. 4:33. 10.3389/fnmol.2011.00033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang N. V., Furtado A., Mason P. J., Marquardt A., Kasirajan L., Thirugnanasambandam P. P., et al. (2017a). A survey of the complex transcriptome from the highly polyploid sugarcane genome using full-length isoform sequencing and de novo assembly from short read sequencing. BMC Genomics 18:395. 10.1186/s12864-017-3757-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang N. V., Furtado A., O'keeffe A. J., Botha F. C., Henry R. J. (2017b). Association of gene expression with biomass content and composition in sugarcane. PLoS ONE 12:e0183417. 10.1371/journal.pone.0183417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hon C. C., Ramilowski J. A., Harshbarger J., Bertin N., Rackham O. J., Gough J., et al. (2017). An atlas of human long non-coding RNAs with accurate 5' ends. Nature 543, 199–204. 10.1038/nature21374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S., Lu W., Ge D., Meng N., Li Y., Su L., et al. (2015). A new microRNA signal pathway regulated by long noncoding RNA TGFB2-OT1 in autophagy and inflammation of vascular endothelial cells. Autophagy 11, 2172–2183. 10.1080/15548627.2015.1106663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang T. S., Wang K. C., Quon S., Nguyen P., Chang T. Y., Chen Z., et al. (2017). LINC00341 exerts an anti-inflammatory effect on endothelial cells by repressing VCAM1. Physiol. Genomics 49, 339–345. 10.1152/physiolgenomics.00132.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer M. K., Niknafs Y. S., Malik R., Singhal U., Sahu A., Hosono Y., et al. (2015). The landscape of long noncoding RNAs in the human transcriptome. Nat. Genet. 47, 199–208. 10.1038/ng.3192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobsson L., Franco C. A., Bentley K., Collins R. T., Ponsioen B., Aspalter I. M., et al. (2010). Endothelial cells dynamically compete for the tip cell position during angiogenic sprouting. Nat. Cell Biol. 12, 943–953. 10.1038/ncb2103 [DOI] [PubMed] [Google Scholar]
- Jeck W. R., Sharpless N. E. (2014). Detecting and characterizing circular RNAs. Nat. Biotechnol. 32, 453–461. 10.1038/nbt.2890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeck W. R., Sorrentino J. A., Wang K., Slevin M. K., Burd C. E., Liu J., et al. (2013). Circular RNAs are abundant, conserved, and associated with ALU repeats. RNA 19, 141–157. 10.1261/rna.035667.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji Z., Song R., Regev A., Struhl K. (2015). Many lncRNAs, 5'UTRs, and pseudogenes are translated and some are likely to express functional proteins. Elife 4:e08890. 10.7554/eLife.08890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia P., Cai H., Liu X., Chen J., Ma J., Wang P., et al. (2016). Long non-coding RNA H19 regulates glioma angiogenesis and the biological behavior of glioma-associated endothelial cells by inhibiting microRNA-29a. Cancer Lett. 381, 359–369. 10.1016/j.canlet.2016.08.009 [DOI] [PubMed] [Google Scholar]
- Jiang X., Hall A. B., Biedler J. K., Tu Z. (2017). Single molecule RNA sequencing uncovers trans-splicing and improves annotations in Anopheles stephensi. Insect Mol. Biol. 26, 298–307. 10.1111/imb.12294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo I. H., Lee J., Hong C. E., Lee D. J., Bae W., Park S. G., et al. (2017). Isoform sequencing provides a more comprehensive view of the panax ginseng transcriptome. Genes (Basel) 8:E228. 10.3390/genes8090228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- John D., Weirick T., Dimmeler S., Uchida S. (2017). RNAEditor: easy detection of RNA editing events and the introduction of editing islands. Brief. Bioinformatics 18, 993–1001. 10.1093/bib/bbw087 [DOI] [PubMed] [Google Scholar]
- Josipovic I., Pflüger B., Fork C., Vasconez A. E., Oo J. A., Hitzel J., et al. (2018). Long noncoding RNA LISPR1 is required for S1P signaling and endothelial cell function. J. Mol. Cell. Cardiol. 116, 57–68. 10.1016/j.yjmcc.2018.01.015 [DOI] [PubMed] [Google Scholar]
- Keegan L. P., Gallo A., O'Connell M. A. (2001). The many roles of an RNA editor. Nat. Rev. Genet. 2, 869–878. 10.1038/35098584 [DOI] [PubMed] [Google Scholar]
- Kim M. A., Rhee J. S., Kim T. H., Lee J. S., Choi A. Y., Choi B. S., et al. (2017). Alternative Splicing Profile and Sex-Preferential Gene Expression in the Female and Male Pacific abalone haliotis discus hannai. Genes (Basel) 8:E99. 10.3390/genes8030099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King K. R., Aguirre A. D., Ye Y. X., Sun Y., Roh J. D., Ng R. P., Jr., et al. (2017). IRF3 and type I interferons fuel a fatal response to myocardial infarction. Nat. Med. 23, 1481–1487. 10.1038/nm.4428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiran A., Baranov P. V. (2010). DARNED: a DAtabase of RNa EDiting in humans. Bioinformatics 26, 1772–1776. 10.1093/bioinformatics/btq285 [DOI] [PubMed] [Google Scholar]
- Kluge M. A., Fetterman J. L., Vita J. A. (2013). Mitochondria and endothelial function. Circ. Res. 112, 1171–1188. 10.1161/CIRCRESAHA.111.300233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo R. I., Tseng E., Eory L., Paton I. R., Archibald A. L., Burt D. W. (2017). Normalized long read RNA sequencing in chicken reveals transcriptome complexity similar to human. BMC Genomics 18:323. 10.1186/s12864-017-3691-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurian L., Aguirre A., Sancho-Martinez I., Benner C., Hishida T., Nguyen T. B., et al. (2015). Identification of novel long noncoding RNAs underlying vertebrate cardiovascular development. Circulation 131, 1278–1290. 10.1161/CIRCULATIONAHA.114.013303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laganà A., Paone A., Veneziano D., Cascione L., Gasparini P., Carasi S., et al. (2012). miR-EdiTar: a database of predicted A-to-I edited miRNA target sites. Bioinformatics 28, 3166–3168. 10.1093/bioinformatics/bts589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lander E. S., Linton L. M., Birren B., Nusbaum C., Zody M. C., Baldwin J., et al. (2001). Initial sequencing and analysis of the human genome. Nature 409, 860–921. 10.1038/35057062 [DOI] [PubMed] [Google Scholar]
- Leisegang M. S., Fork C., Josipovic I., Richter F. M., Preussner J., Hu J., et al. (2017). Long noncoding RNA MANTIS facilitates endothelial angiogenic function. Circulation 136, 65–79. 10.1161/CIRCULATIONAHA.116.026991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lescroart F., Wang X., Lin X., Swedlund B., Gargouri S., Sànchez-Dànes A., et al. (2018). Defining the earliest step of cardiovascular lineage segregation by single-cell RNA-seq. Science 359, 1177–1181. 10.1126/science.aao4174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levanon E. Y., Eisenberg E., Yelin R., Nemzer S., Hallegger M., Shemesh R., et al. (2004). Systematic identification of abundant A-to-I editing sites in the human transcriptome. Nat. Biotechnol. 22, 1001–1005. 10.1038/nbt996 [DOI] [PubMed] [Google Scholar]
- Li A., Zhang J., Zhou Z. (2014). PLEK: a tool for predicting long non-coding RNAs and messenger RNAs based on an improved k-mer scheme. BMC Bioinformatics 15:311. 10.1186/1471-2105-15-311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K., Blum Y., Verma A., Liu Z., Pramanik K., Leigh N. R., et al. (2010). A noncoding antisense RNA in tie-1 locus regulates tie-1 function in vivo. Blood 115, 133–139. 10.1182/blood-2009-09-242180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Wang M., Mei Z., Cao W., Yang Y., Wang Y., et al. (2017a). lncRNAs HIF1A-AS2 facilitates the up-regulation of HIF-1alpha by sponging to miR-153-3p, whereby promoting angiogenesis in HUVECs in hypoxia. Biomed. Pharmacother. 96, 165–172. 10.1016/j.biopha.2017.09.113 [DOI] [PubMed] [Google Scholar]
- Li P., Liu Y., Wang H., He Y., Wang X., He Y., et al. (2015). PubAngioGen: a database and knowledge for angiogenesis and related diseases. Nucleic Acids Res. 43, D963–D967. 10.1093/nar/gku1139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Wang R., Ma J. Y., Wang M., Cui J., Wu W. B., et al. (2017b). A Human long non-coding RNA ALT1 controls the cell cycle of vascular endothelial cells via ACE2 and cyclin D1 pathway. Cell. Physiol. Biochem. 43, 1152–1167. 10.1159/000481756 [DOI] [PubMed] [Google Scholar]
- Liao B., Chen R., Lin F., Mai A., Chen J., Li H., et al. (2018). Long noncoding RNA HOTTIP promotes endothelial cell proliferation and migration via activation of the Wnt/beta-catenin pathway. J. Cell. Biochem. 119, 2797–2805. 10.1002/jcb.26448 [DOI] [PubMed] [Google Scholar]
- Libby P. (2012). Inflammation in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 32, 2045–2051. 10.1161/ATVBAHA.108.179705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liscovitch-Brauer N., Alon S., Porath H. T., Elstein B., Unger R., Ziv T., et al. (2017). Trade-off between transcriptome plasticity and genome evolution in cephalopods. Cell 169, 191–202.e111. 10.1016/j.cell.2017.03.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H. Z., Wang Q. Y., Zhang Y., Qi D. T., Li M. W., Guo W. Q., et al. (2016). Pioglitazone up-regulates long non-coding RNA MEG3 to protect endothelial progenitor cells via increasing HDAC7 expression in metabolic syndrome. Biomed. Pharmacother. 78, 101–109. 10.1016/j.biopha.2016.01.001 [DOI] [PubMed] [Google Scholar]
- Liu J. Y., Yao J., Li X. M., Song Y. C., Wang X. Q., Li Y. J., et al. (2014). Pathogenic role of lncRNA-MALAT1 in endothelial cell dysfunction in diabetes mellitus. Cell Death Dis. 5:e1506. 10.1038/cddis.2014.466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q., Zhang X., Hu X., Dai L., Fu X., Zhang J., et al. (2016). Circular RNA related to the chondrocyte ECM regulates MMP13 expression by Functioning as a MiR-136 'Sponge' in human cartilage degradation. Sci. Rep. 6:22572. 10.1038/srep22572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S. J., Horlbeck M. A., Cho S. W., Birk H. S., Malatesta M., He D., et al. (2017). CRISPRi-based genome-scale identification of functional long noncoding RNA loci in human cells. Science 355:aah7111. 10.1126/science.aah7111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodish H., Berk A., Zipursky L., Matsudaira P., Baltimore D., Darnell J. (2000). Molecular Cell Biology, 4th Edn. New York, NY: W. H. Freeman. [Google Scholar]
- Lorenzen J. M., Thum T. (2016). Long noncoding RNAs in kidney and cardiovascular diseases. Nat. Rev. Nephrol. 12, 360–373. 10.1038/nrneph.2016.51 [DOI] [PubMed] [Google Scholar]
- Lu W., Huang S. Y., Su L., Zhao B. X., Miao J. Y. (2016). Long noncoding RNA LOC100129973 suppresses apoptosis by targeting miR-4707-5p and miR-4767 in vascular endothelial cells. Sci. Rep. 6:21620. 10.1038/srep21620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv L., Qi H., Guo X., Ni Q., Yan Z., Zhang L. (2017). Long Noncoding RNA uc001pwg.1 Is downregulated in neointima in arteriovenous fistulas and mediates the function of endothelial cells derived from pluripotent stem cells. Stem Cells Int. 2017:4252974. 10.1155/2017/4252974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X., Li Z., Li T., Zhu L., Li Z., Tian N. (2017). Long non-coding RNA HOTAIR enhances angiogenesis by induction of VEGFA expression in glioma cells and transmission to endothelial cells via glioma cell derived-extracellular vesicles. Am. J. Transl. Res. 9, 5012–5021. [PMC free article] [PubMed] [Google Scholar]
- Ma Y., Wang P., Xue Y., Qu C., Zheng J., Liu X., et al. (2017). PVT1 affects growth of glioma microvascular endothelial cells by negatively regulating miR-186. Tumour Biol. 39:1010428317694326. 10.1177/1010428317694326 [DOI] [PubMed] [Google Scholar]
- Man H. S. J., Sukumar A. N., Lam G. C., Turgeon P. J., Yan M. S., Ku K. H., et al. (2018). Angiogenic patterning by STEEL, an endothelial-enriched long noncoding RNA. Proc. Natl. Acad. Sci. U.S.A. 115, 2401–2406. 10.1073/pnas.1715182115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memczak S., Jens M., Elefsinioti A., Torti F., Krueger J., Rybak A., et al. (2013). Circular RNAs are a large class of animal RNAs with regulatory potency. Nature 495, 333–338. 10.1038/nature11928 [DOI] [PubMed] [Google Scholar]
- Mercer T. R., Gerhardt D. J., Dinger M. E., Crawford J., Trapnell C., Jeddeloh J. A., et al. (2011). Targeted RNA sequencing reveals the deep complexity of the human transcriptome. Nat. Biotechnol. 30, 99–104. 10.1038/nbt.2024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzker M. L. (2010). Sequencing technologies - the next generation. Nat. Rev. Genet. 11, 31–46. 10.1038/nrg2626 [DOI] [PubMed] [Google Scholar]
- Miao Y., Ajami N. E., Huang T. S., Lin F. M., Lou C. H., Wang Y. T., et al. (2018). Enhancer-associated long non-coding RNA LEENE regulates endothelial nitric oxide synthase and endothelial function. Nat. Commun. 9:292. 10.1038/s41467-017-02113-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalik K. M., You X., Manavski Y., Doddaballapur A., Zörnig M., Braun T., et al. (2014). Long noncoding RNA MALAT1 regulates endothelial cell function and vessel growth. Circ. Res. 114, 1389–1397. 10.1161/CIRCRESAHA.114.303265 [DOI] [PubMed] [Google Scholar]
- Ming G. F., Wu K., Hu K., Chen Y., Xiao J. (2016). NAMPT regulates senescence, proliferation, and migration of endothelial progenitor cells through the SIRT1 AS lncRNA/miR-22/SIRT1 pathway. Biochem. Biophys. Res. Commun. 478, 1382–1388. 10.1016/j.bbrc.2016.08.133 [DOI] [PubMed] [Google Scholar]
- Müller R., Weirick T., John D., Militello G., Chen W., Dimmeler S., et al. (2016). ANGIOGENES: knowledge database for protein-coding and noncoding RNA genes in endothelial cells. Sci. Rep. 6:32475. 10.1038/srep32475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano K., Shiroma A., Shimoji M., Tamotsu H., Ashimine N., Ohki S., et al. (2017). Advantages of genome sequencing by long-read sequencer using SMRT technology in medical area. Hum. Cell 30, 149–161. 10.1007/s13577-017-0168-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Necsulea A., Soumillon M., Warnefors M., Liechti A., Daish T., Zeller U., et al. (2014). The evolution of lncRNA repertoires and expression patterns in tetrapods. Nature 505, 635–640. 10.1038/nature12943 [DOI] [PubMed] [Google Scholar]
- Neumann P., Jaé N., Knau A., Glaser S. F., Fouani Y., Rossbach O., et al. (2018). The lncRNA GATA6-AS epigenetically regulates endothelial gene expression via interaction with LOXL2. Nat. Commun. 9:237. 10.1038/s41467-017-02431-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen M. M., Tehler D., Vang S., Sudzina F., Hedegaard J., Nordentoft I., et al. (2014). Identification of expressed and conserved human noncoding RNAs. RNA 20, 236–251. 10.1261/rna.038927.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigro J. M., Cho K. R., Fearon E. R., Kern S. E., Ruppert J. M., Oliner J. D., et al. (1991). Scrambled exons. Cell 64, 607–613. 10.1016/0092-8674(91)90244-S [DOI] [PubMed] [Google Scholar]
- Oka T., Akazawa H., Naito A. T., Komuro I. (2014). Angiogenesis and cardiac hypertrophy: maintenance of cardiac function and causative roles in heart failure. Circ. Res. 114, 565–571. 10.1161/CIRCRESAHA.114.300507 [DOI] [PubMed] [Google Scholar]
- Ounzain S., Burdet F., Ibberson M., Pedrazzini T. (2015a). Discovery and functional characterization of cardiovascular long noncoding RNAs. J. Mol. Cell. Cardiol. 89, 17–26. 10.1016/j.yjmcc.2015.09.013 [DOI] [PubMed] [Google Scholar]
- Ounzain S., Micheletti R., Arnan C., Plaisance I., Cecchi D., Schroen B., et al. (2015b). CARMEN, a human super enhancer-associated long noncoding RNA controlling cardiac specification, differentiation and homeostasis. J. Mol. Cell. Cardiol. 89, 98–112. 10.1016/j.yjmcc.2015.09.016 [DOI] [PubMed] [Google Scholar]
- Ounzain S., Micheletti R., Beckmann T., Schroen B., Alexanian M., Pezzuto I., et al. (2015c). Genome-wide profiling of the cardiac transcriptome after myocardial infarction identifies novel heart-specific long non-coding RNAs. Eur. Heart J. 36, 353a–368a. 10.1093/eurheartj/ehu180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ounzain S., Pezzuto I., Micheletti R., Burdet F., Sheta R., Nemir M., et al. (2014). Functional importance of cardiac enhancer-associated noncoding RNAs in heart development and disease. J. Mol. Cell. Cardiol. 76, 55–70. 10.1016/j.yjmcc.2014.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park E., Williams B., Wold B. J., Mortazavi A. (2012). RNA editing in the human ENCODE RNA-seq data. Genome Res. 22, 1626–1633. 10.1101/gr.134957.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Y., Meng K., Jiang L., Zhong Y., Yang Y., Lan Y., et al. (2017). Thymic stromal lymphopoietin-induced HOTAIR activation promotes endothelial cell proliferation and migration in atherosclerosis. Biosci. Rep. 37:BSR20170351. 10.1042/BSR20170351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Z., Cheng Y., Tan B. C., Kang L., Tian Z., Zhu Y., et al. (2012). Comprehensive analysis of RNA-Seq data reveals extensive RNA editing in a human transcriptome. Nat. Biotechnol. 30, 253–260. 10.1038/nbt.2122 [DOI] [PubMed] [Google Scholar]
- Philippen L. E., Dirkx E., da Costa-Martins P. A., De Windt L. J. (2015). Non-coding RNA in control of gene regulatory programs in cardiac development and disease. J. Mol. Cell. Cardiol. 89, 51–58. 10.1016/j.yjmcc.2015.03.014 [DOI] [PubMed] [Google Scholar]
- Picardi E., D'erchia A. M., Gallo A., Montalvo A., Pesole G. (2014). Uncovering RNA editing sites in long non-coding RNAs. Front. Bioeng. Biotechnol. 2:64. 10.3389/fbioe.2014.00064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picardi E., D'erchia A. M., Lo Giudice C., Pesole G. (2017). REDIportal: a comprehensive database of A-to-I RNA editing events in humans. Nucleic Acids Res. 45, D750–D757. 10.1093/nar/gkw767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picardi E., Pesole G. (2013). REDItools: high-throughput RNA editing detection made easy. Bioinformatics 29, 1813–1814. 10.1093/bioinformatics/btt287 [DOI] [PubMed] [Google Scholar]
- Picardi E., Regina T. M., Verbitskiy D., Brennicke A., Quagliariello C. (2011). REDIdb: an upgraded bioinformatics resource for organellar RNA editing sites. Mitochondrion 11, 360–365. 10.1016/j.mito.2010.10.005 [DOI] [PubMed] [Google Scholar]
- Piechotta M., Wyler E., Ohler U., Landthaler M., Dieterich C. (2017). JACUSA: site-specific identification of RNA editing events from replicate sequencing data. BMC Bioinformatics 18:7. 10.1186/s12859-016-1432-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puthanveetil P., Chen S., Feng B., Gautam A., Chakrabarti S. (2015). Long non-coding RNA MALAT1 regulates hyperglycaemia induced inflammatory process in the endothelial cells. J. Cell. Mol. Med. 19, 1418–1425. 10.1111/jcmm.12576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu G. Z., Tian W., Fu H. T., Li C. P., Liu B. (2016). Long noncoding RNA-MEG3 is involved in diabetes mellitus-related microvascular dysfunction. Biochem. Biophys. Res. Commun. 471, 135–141. 10.1016/j.bbrc.2016.01.164 [DOI] [PubMed] [Google Scholar]
- Ramaswami G., Li J. B. (2014). RADAR: a rigorously annotated database of A-to-I RNA editing. Nucleic Acids Res. 42, D109–D113. 10.1093/nar/gkt996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaswami G., Lin W., Piskol R., Tan M. H., Davis C., Li J. B. (2012). Accurate identification of human Alu and non-Alu RNA editing sites. Nat. Methods 9, 579–581. 10.1038/nmeth.1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaswami G., Zhang R., Piskol R., Keegan L. P., Deng P., O'connell M. A., et al. (2013). Identifying RNA editing sites using RNA sequencing data alone. Nat. Methods 10, 128–132. 10.1038/nmeth.2330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regan E. R., Aird W. C. (2012). Dynamical systems approach to endothelial heterogeneity. Circ. Res. 111, 110–130. 10.1161/CIRCRESAHA.111.261701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizki G., Boyer L. A. (2015). Lncing epigenetic control of transcription to cardiovascular development and disease. Circ. Res. 117, 192–206. 10.1161/CIRCRESAHA.117.304156 [DOI] [PubMed] [Google Scholar]
- Ruan W., Zhao F., Zhao S., Zhang L., Shi L., Pang T. (2018). Knockdown of long noncoding RNA MEG3 impairs VEGF-stimulated endothelial sprouting angiogenesis via modulating VEGFR2 expression in human umbilical vein endothelial cells. Gene 649, 32–39. 10.1016/j.gene.2018.01.072 [DOI] [PubMed] [Google Scholar]
- Ruiz-Orera J., Messeguer X., Subirana J. A., Alba M. M. (2014). Long non-coding RNAs as a source of new peptides. Elife 3:e03523. 10.7554/eLife.03523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallam T., Sandhu J., Tontonoz P. (2018). Long noncoding RNA discovery in cardiovascular disease: decoding form to function. Circ. Res. 122, 155–166. 10.1161/CIRCRESAHA.117.311802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savas S. (2012). A curated database of genetic markers from the angiogenesis/VEGF pathway and their relation to clinical outcome in human cancers. Acta Oncol. 51, 243–246. 10.3109/0284186X.2011.636758 [DOI] [PubMed] [Google Scholar]
- Savva Y. A., Rieder L. E., Reenan R. A. (2012). The ADAR protein family. Genome Biol. 13:252. 10.1186/gb-2012-13-12-252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schadt E. E., Turner S., Kasarskis A. (2010). A window into third-generation sequencing. Hum. Mol. Genet. 19, R227–R240. 10.1093/hmg/ddq416 [DOI] [PubMed] [Google Scholar]
- Shan K., Jiang Q., Wang X. Q., Wang Y. N., Yang H., Yao M. D., et al. (2016). Role of long non-coding RNA-RNCR3 in atherosclerosis-related vascular dysfunction. Cell Death Dis. 7:e2248. 10.1038/cddis.2016.145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh N., Sahu D. K., Chowdhry R., Mishra A., Goel M. M., Faheem M., et al. (2016). IsoSeq analysis and functional annotation of the infratentorial ependymoma tumor tissue on PacBio RSII platform. Meta Gene 7, 70–75. 10.1016/j.mgene.2015.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skelly D. A., Squiers G. T., McLellan M. A., Bolisetty M. T., Robson P., Rosenthal N. A., et al. (2018). Single-cell transcriptional profiling reveals cellular diversity and intercommunication in the mouse heart. Cell Rep. 22, 600–610. 10.1016/j.celrep.2017.12.072 [DOI] [PubMed] [Google Scholar]
- Solomon O., Eyal E., Amariglio N., Unger R., Rechavi G. (2016). e23D: database and visualization of A-to-I RNA editing sites mapped to 3D protein structures. Bioinformatics 32, 2213–2215. 10.1093/bioinformatics/btw204 [DOI] [PubMed] [Google Scholar]
- Solomon O., Oren S., Safran M., Deshet-Unger N., Akiva P., Jacob-Hirsch J., et al. (2013). Global regulation of alternative splicing by adenosine deaminase acting on RNA (ADAR). RNA 19, 591–604. 10.1261/rna.038042.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stellos K., Gatsiou A., Stamatelopoulos K., Perisic Matic L., John D., Lunella F. F., et al. (2016). Adenosine-to-inosine RNA editing controls cathepsin S expression in atherosclerosis by enabling HuR-mediated post-transcriptional regulation. Nat. Med. 22, 1140–1150. 10.1038/nm.4172 [DOI] [PubMed] [Google Scholar]
- Sun J. Y., Zhao Z. W., Li W. M., Yang G., Jing P. Y., Li P., et al. (2017). Knockdown of MALAT1 expression inhibits HUVEC proliferation by upregulation of miR-320a and downregulation of FOXM1 expression. Oncotarget 8, 61499–61509. 10.18632/oncotarget.18507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., Li X., Wu D., Pan Q., Ji Y., Ren H., et al. (2016). RED: a Java-MySQL software for identifying and visualizing RNA editing sites using rule-based and statistical filters. PLoS ONE 11:e0150465. 10.1371/journal.pone.0150465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szcześniak M. W., Makaĺowska I. (2016). lncRNA-RNA interactions across the human transcriptome. PLoS ONE 11:e0150353. 10.1371/journal.pone.0150353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan M. H., Li Q., Shanmugam R., Piskol R., Kohler J., Young A. N., et al. (2017). Dynamic landscape and regulation of RNA editing in mammals. Nature 550, 249–254. 10.1038/nature24041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y., Jin X., Xiang Y., Chen Y., Shen C. X., Zhang Y. C., et al. (2015). The lncRNA MALAT1 protects the endothelium against ox-LDL-induced dysfunction via upregulating the expression of the miR-22-3p target genes CXCR2 and AKT. FEBS Lett. 589, 3189–3196. 10.1016/j.febslet.2015.08.046 [DOI] [PubMed] [Google Scholar]
- Thurman R. E., Rynes E., Humbert R., Vierstra J., Maurano M. T., Haugen E., et al. (2012). The accessible chromatin landscape of the human genome. Nature 489, 75–82. 10.1038/nature11232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapnell C., Roberts A., Goff L., Pertea G., Kim D., Kelley D. R., et al. (2012). Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat. Protoc. 7, 562–578. 10.1038/nprot.2012.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida S., Bolli R. (2017). Short and long noncoding RNAs regulate the epigenetic status of cells. Antioxid. Redox Signal. [Epub ahead of print]. 10.1089/ars.2017.7262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida S., Dimmeler S. (2015). Long noncoding RNAs in cardiovascular diseases. Circ. Res. 116, 737–750. 10.1161/CIRCRESAHA.116.302521 [DOI] [PubMed] [Google Scholar]
- Uchida S., Jones S. P. (2018). RNA Editing: Unexplored Opportunities in the Cardiovascular System. Circ. Res. 122, 399–401. 10.1161/CIRCRESAHA.117.312512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viereck J., Thum T. (2017). Long Noncoding RNAs in pathological cardiac remodeling. Circ. Res. 120, 262–264. 10.1161/CIRCRESAHA.116.310174 [DOI] [PubMed] [Google Scholar]
- Wang J., Li Z., Lei M., Fu Y., Zhao J., Ao M., et al. (2017a). Integrated DNA methylome and transcriptome analysis reveals the ethylene-induced flowering pathway genes in pineapple. Sci. Rep. 7:17167. 10.1038/s41598-017-17460-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Yao L., Li B., Meng Y., Ma X., Wang H. (2017b). Single-molecule long-read transcriptome dataset of halophyte halogeton glomeratus. Front. Genet. 8:197. 10.3389/fgene.2017.00197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., Wang P., Liang F., Ye Z., Li J., Shen C., et al. (2018). A global survey of alternative splicing in allopolyploid cotton: landscape, complexity and regulation. New Phytol. 217, 163–178. 10.1111/nph.14762 [DOI] [PubMed] [Google Scholar]
- Wang Z., Lian J., Li Q., Zhang P., Zhou Y., Zhan X., et al. (2016). RES-Scanner: a software package for genome-wide identification of RNA-editing sites. Gigascience 5:37. 10.1186/s13742-016-0143-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weirick T., John D., Dimmeler S., Uchida S. (2015). C-It-Loci: a knowledge database for tissue-enriched loci. Bioinformatics 31, 3537–3543. 10.1093/bioinformatics/btv410 [DOI] [PubMed] [Google Scholar]
- Weirick T., John D., Uchida S. (2017). Resolving the problem of multiple accessions of the same transcript deposited across various public databases. Brief. Bioinformatics 18, 226–235. 10.1093/bib/bbw017 [DOI] [PubMed] [Google Scholar]
- Weirick T., Militello G., Müller R., John D., Dimmeler S., Uchida S. (2016a). The identification and characterization of novel transcripts from RNA-seq data. Brief. Bioinformatics 17, 678–685. 10.1093/bib/bbv067 [DOI] [PubMed] [Google Scholar]
- Weirick T., Militello G., Ponomareva Y., John D., Döring C., Dimmeler S., et al. (2016b). Logic programming to infer complex RNA expression patterns from RNA-seq data. Brief. Bioinform. 19, 199–209. 10.1093/bib/bbw117 [DOI] [PubMed] [Google Scholar]
- White E. J., Matsangos A. E., Wilson G. M. (2017). AUF1 regulation of coding and noncoding RNA. Wiley Interdiscip. Rev. RNA 8:e1393. 10.1002/wrna.1393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong H., Liu D., Li Q., Lei M., Xu L., Wu L., et al. (2017). RED-ML: a novel, effective RNA editing detection method based on machine learning. Gigascience 6, 1–8. 10.1093/gigascience/gix012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue X. Y., Majerciak V., Uberoi A., Kim B. H., Gotte D., Chen X., et al. (2017). The full transcription map of mouse papillomavirus type 1 (MmuPV1) in mouse wart tissues. PLoS Pathog. 13:e1006715. 10.1371/journal.ppat.1006715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan B., Yao J., Liu J. Y., Li X. M., Wang X. Q., Li Y. J., et al. (2015). lncRNA-MIAT regulates microvascular dysfunction by functioning as a competing endogenous RNA. Circ. Res. 116, 1143–1156. 10.1161/CIRCRESAHA.116.305510 [DOI] [PubMed] [Google Scholar]
- Yuan L., Chan G. C., Beeler D., Janes L., Spokes K. C., Dharaneeswaran H., et al. (2016). A role of stochastic phenotype switching in generating mosaic endothelial cell heterogeneity. Nat. Commun. 7:10160. 10.1038/ncomms10160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan R., Xu K., Pan J., Xu Q., Xu S., Shen J. (2017). Long noncoding RNA MEG3 mediated angiogenesis after cerebral infarction through regulating p53/NOX4 axis. Biochem. Biophys. Res. Commun. 490, 700–706. 10.1016/j.bbrc.2017.06.104 [DOI] [PubMed] [Google Scholar]
- Zhang B. Y., Jin Z., Zhao Z. (2017). Long intergenic noncoding RNA 00305 sponges miR-136 to regulate the hypoxia induced apoptosis of vascular endothelial cells. Biomed. Pharmacother. 94, 238–243. 10.1016/j.biopha.2017.07.099 [DOI] [PubMed] [Google Scholar]
- Zhang C., Zhang B., Lin L. L., Zhao S. (2017). Evaluation and comparison of computational tools for RNA-seq isoform quantification. BMC Genomics 18:583. 10.1186/s12864-017-4002-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Xiao X. (2015). Genome sequence-independent identification of RNA editing sites. Nat. Methods 12, 347–350. 10.1038/nmeth.3314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S. J., Wang C., Yan S., Fu A., Luan X., Li Y., et al. (2017). Isoform evolution in primates through independent combination of alternative RNA processing events. Mol. Biol. Evol. 34, 2453–2468. 10.1093/molbev/msx212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Li H., Fang S., Kang Y., Wu W., Hao Y., et al. (2016). NONCODE 2016: an informative and valuable data source of long non-coding RNAs. Nucleic Acids Res. 44, D203–D208. 10.1093/nar/gkv1252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z., Liu B., Li B., Song C., Diao H., Guo Z., et al. (2017). Inhibition of long noncoding RNA IGF2AS promotes angiogenesis in type 2 diabetes. Biomed. Pharmacother. 92, 445–450. 10.1016/j.biopha.2017.05.039 [DOI] [PubMed] [Google Scholar]
- Zheng Q., Bao C., Guo W., Li S., Chen J., Chen B., et al. (2016). Circular RNA profiling reveals an abundant circHIPK3 that regulates cell growth by sponging multiple miRNAs. Nat. Commun. 7:11215. 10.1038/ncomms11215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zulkapli M. M., Rosli M. A. F., Salleh F. I. M., Mohd Noor N., Aizat W. M., Goh H.H. (2017). Iso-Seq analysis of Nepenthes ampullaria, Nepenthes rafflesiana and Nepenthes x hookeriana for hybridisation study in pitcher plants. Genom Data 12, 130–131. 10.1016/j.gdata.2017.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]