Abstract
Statement of the Problem:
One major goal of tissue engineering and regenerative medicine is to find an appropriate source of mesenchymal stem cells (MSCs) with higher differentiation ability.
Purpose:
In this experimental study, the osteogenic and chondrogenic differentiation ability of buccal fat pad derived MSCs (BFP-MSCs) with gingival derived cells (GDCs) were compared.
Materials and Method:
BFP-MSCs and GDCs were cultured enzymatically and expanded. The expanded cells were analyzed for membrane-associated markers, using flow cytometry. Then the ability of these cells to differentiate into osteocyte and chondrocyte was assessed morphologically and by mRNA expression of collagen I (COLL), BGLA and bone morphogenetic protein 2 (BMP2) using qRT-PCR.
Results:
Flow cytometry analysis showed that both BFP-MSCs and GDCs expressed the characteristic stem cell markers such as CD73, CD44, and CD90, whereas they did not express hematopoietic markers. Mineralized calcium deposition was observed apparently in BFP-MSCs cultured in osteogenic medium but GDCs showed fewer mineralized nodules. The mRNA expression levels of BGLA and BMP2 showed 7×105 and 733-fold more mRNA expression in BFP-MSCs treated with differentiation media compared to the control group. In chondrogenic differentiation, BFP-MSCs transformed from a spindle to a cuboidal shape while GDCs showed only a slight transformation. In addition, mRNA expression of COLL showed 282-fold higher expression in BFP-MSCs in comparison to the control group. Such significant difference in mRNA expression of BGLA, BMP2, and COLL was not observed in GDCs compared to their corresponding controls.
Conclusion:
Based on the present results, BFP yields a greater proportion of stem cells compared to gingiva. Therefore, this tissue can be introduced as an easily available source for the treatment of periodontal defects and other maxillofacial injuries.
Keywords: Mesenchymal stem cell , Buccal fat pad , Gingiva , Chondrocyte , Osteocyte
Introduction
Mesenchymal stem cells (MSCs) are a heterogeneous population of non-hematopoietic stem cells with a high capacity for self-renewal and regeneration. These cells are capable of differentiating into various lineages including osteogenic, adipogenic, and chondrogenic lineages in the presence of a series of stimulations.[1] Several studies have reported the successful treatment of bone and cartilage defects, vascular ischemia, and coronary artery disease upon local administration of MSCs to the sites of injury.[2-3]
MSCs are present in many adult tissues, such as synovium, muscle, adipose tissue, and bone marrow.[4] Adipose (fat) tissue represents an abundant and accessible source of MSCs, and deriving of these cells is accompanied with minimal patient discomfort. Therefore, adipose tissue may be a practical and appealing source of donor tissue for clinical applications.[5]
Buccal fat pad (BFP), one of the encapsulated fat masses in the cheek, is located between the buccinator muscle and several superficial muscles including masseter, zygomaticus major, and zygomaticus minor on both sides of the face.[6-7] BFP is a new and easily accessible source of MSCs and BFP derived MSCs (BFP-MSCs) have virtually the same characteristics as adipose derived stem cell (ASCs). It means that they have the ability of giving rise to various cell lineages and therefore could be suitable for clinical uses such as periodontal defect treatment.[8]
Gingiva as the soft tissue surrounding the teeth has unique structure that contributes to the resistance against shear stress or friction.2 Previous studies have demonstrated that gingival tissue possess progenitors or adult stem cells with similar properties to MSCs such as pluripotency, self-renewal ability and immunomodulatory properties and play a crucial role in the repair and regeneration of periodontal tissues. [9-11]
In this study, for the first time the osteogenic and chondrogenic capabilities of BFP-MSCs and gingival derived cells (GDCs) have been compared. Results of this study may contribute to obtain a more accessible source of stem cells for periodontal, oral implant and maxillo-facial surgeries.
Materials and Method
Subjects
In this experimental study, BFP was obtained from five healthy individuals undergoing elective orthognathic surgery at Rajaee Hospital, Shiraz University of Medical Sciences, Shiraz, Iran. The age range of the individuals undergoing oral surgery was 19-28 years and no history of diabetes or other systemic complications were reported for them.
Human gingival samples were collected from five patients undergoing crown-lengthening surgery, with age range of 35-45 years, and with no history of periodontal or systemic diseases. The gingival tissues were obtained as part of routine periodontal surgery at the School of Dentistry, Shiraz University of Medical Sciences, Shiraz, Iran. Informed consent was obtained from all the patients. The informed consent and experimental protocols in this study were reviewed and approved by the Ethics Committee of Shiraz University of Medical Sciences, Shiraz, Iran.
Both BFP and gingival tissue samples were maintained and transferred to Stem Cell and Cancer Laboratory of Shiraz Institute for Cancer Research (ICR), Shiraz University of Medical Sciences, in a media containing 50U/mL penicillin, streptomycin, amphotericin B and DMEM culture medium (Gibco, USA) containing 10% fetal bovine serum (FBS, Gibco, USA).
Isolation and culture of BFP-MSCs and GDCs
Samples were washed several times with sterile phosphate-buffered saline (PBS), chopped into small pieces, and then digested using 0.2 % collagenase type I. The resulted soup was centrifuged and the pellet was carefully put on Ficoll (Biosera, UK) and centrifuged again. The second white layer was washed with PBS and finally re-suspended in DMEM containing 10% fetal bovine serum and 1% penicillin/streptomycin (Biosera, UK) and maintained in a humidified incubator at 37˚C and 5% CO2. The medium was changed every 4 days. After 3-4 days, individual cell colonies were visible upon microscopic examination. The cells were analyzed by flow cytometry and differentiation protocols in passage 3.
Cell staining, data acquisition and flow cytometry analysis
Expression of mesenchymal markers on extracted cells was analyzed by flow cytometry. BFP-MSCs and GDCs were detached by trypsin-EDTA (Biosera, UK), re-suspended in PBS at a density of 1×106 cells/mL. The selected cells were then incubated with PE-conjugated anti-CD166, anti-CD105, anti-CD44 antibodies, FITC-conjugated anti-CD34, anti-CD45, anti-CD14, and APC-conjugated anti-CD90, and anti-CD73, as well as dye/isotype matched antibodies (all from BD Biosciences, USA) in dark environment for 30 minutes. Afterwards unbound antibodies were washed out, and each cell sample was assessed on a FACSCalibur flow cytometer (BD Bioscience, USA) .The data was analyzed by FlowJo software package. Positive cells were counted and compared with the signals of the corresponding antibody isotype controls.
Differentiation of human BFP-MSCs and GDCs to chondrocyte and osteocyte
The potential of BFP-MSCs and GDCs to differentiate into chondrogenic and osteogenic lineages was examined using the following procedures. 1×105 cells were cultured in each well of 4-well tissue culture plate for differentiation. When the cultures were 60-80% confluent, an appropriate differentiation kit (STEMPRO Differentiation Kit; GIBCO, USA) was used for differentiating the cells regarding the manufacturer’s instructions. On days 7, 14 and 21 post treatment, cells were stained with Alizarin red (Merck, Germany) and Safranin (Merck, Germany) for evaluating osteogenic and chondrogenic differentiation, respectively. For the control group, 1×105 cells were cultured with common media (DMEM and 10% FBS), without differentiation agents, and the same procedure of staining and RNA isolation was carried out.
RNA isolation and reverse transcription
When the culture flasks were 80-90% confluent, in passage 3, cells were cultured in three separated plates and the total RNA was extracted on 7, 14 and 21 days post culture by RNX Plus solution (Cinnagene, Iran). During RNA extraction, DNase I (GIBCO, USA) was added to avoid DNA contamination.
Then cDNA was synthesized from 5µg of the total RNA, using the ReverAid first strand cDNA synthesis Kit Fermentas, Lithuania) according to recommended instructions.
Quantitative Real-Time PCR (qRT-PCR)
The expression and quantity of BGLA, bone morphogenetic protein 2 (BMP2) (as osteogenic markers) and collagen I (COLL) (as a chondrogenic marker) gene transcripts were determined using an ABI thermal cycler. Briefly approximately 2µl cDNA was amplified in a final volume of 20µl including 10µl of SYBR Green I PCR Master Mix (Fermentas, Lithuania), 0.3µl of each forward and reverse primers and 7.4µl DEPC treated water. 18s rRNA housekeeping gene expression was used to normalize the level of target gene expression.
Thermal cycling for all the genes was set up through a denaturation step at 95°C for 10 minutes, followed by 50 cycles (denaturation at 95°C for 15 s, annealing and extension at 60ºC for 60s). Table 1 shows the forward and reverse primers for 18s rRNA, BGLA, BMP2 and COLL genes. Primers were designed using Primer-Blast online software (<http://www.ncbi.nlm.nih. go/tools/primer-blast>).
Table 1.
The sequences of primers used in qRT-PCR method
| Forward primer | Reverse primer | |
|---|---|---|
| COLL | 5'-TGCCCCATCTGCCCAACTGA -3' | 5'-TGCAGGTCCCTGAGGCCC-3' |
| BGLA | 5'-GAGCCCTCACACTCCTCGC-3′ | 5'-CAGCCAACTCGTCACAGTCC-3' |
| BMP2 | 5'-GAGGCAAAGAAAAGGAACGGAC-3' | 5'-GCAGCAACGCTAGAAGACAG-3' |
| 18sRNA | 5'-GTTGATTAAGTCCCTGCCCT-3' | 5'-TCCGAGGGCCTCACTAAACC-3' |
Statistical Analysis
The relative amounts of BGLA, BMP2, and COLL transcripts were determined from ΔCt and 2−ΔΔCt formulas. Relative expressions were plotted and evaluated by means of Prism 5 software (San Diego, CA, USA).
Results
Flow cytometry analysis
The BFP-MSCs and GDCs were harvested in passage 3 and identified by a FACSCalibur flow cytometer. Flow cytometric analysis of both BFP-MSCs and GDCs is shown in Figure 1 and Table 2. Comparison of forward (F-) and side (S-) scatter (SC) dot plots demonstrated that both cells were similar in granularity and size.
Figure1.
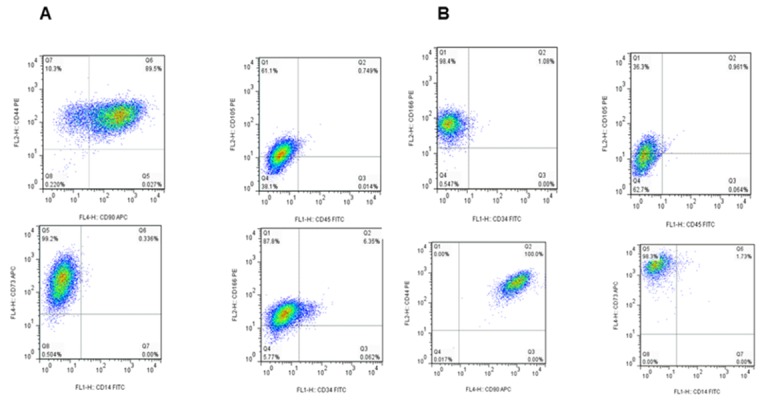
Flow cytometry analysis of mesenchymal specific markers on BFP-MSCs (A) and GDCs (B) obtained from BFP and gingival specimens, respectively. Both cells isolated were positive for the expression of CD90, CD73, and CD44 but negative for the expression of CD14, CD45, and CD34
Table 2.
Mesenchymal, lymphocyte or leukocyte antigens and hematopoietic markers on BFP-MSCs and GDCs obtained from related tissues.
| MSCs type | Cell surface markers(mean ±SD) | |||||||
|---|---|---|---|---|---|---|---|---|
| CD166 | CD105 | CD44 | CD90 | CD73 | CD14 | CD34 | CD45 | |
| BFP- MSCs | 94.4±2.2 | 85±4.7 | 99.7±0.7 | 93.2±6.3 | 97.2±1.8 | 0 | 4.7±3 | 0 |
| GDCs | 87.4±18.2 | 39.5±15.2 | 99.8±0.3 | 99.8±0.3 | 97.4±3 | 0 | 1 | o.9 |
MSCs: Mesenchymal stem cells, BFP-MSCs: Buccal fat pad mesenchymal stem cells, GDCs: Gingival derived cells
Both BFP-MSCs and GDCs expressed the characteristic stem cell markers such as CD73, CD166, CD44, CD90 and CD105, whereas they did not express or rarely expressed lymphocyte or leukocyte antigens and hematopoietic markers such as CD14, CD45, and CD34 (Table 2 and Figure 1). As shown in Table 2, the expression of all markers had similar mean percentage in both cell types except for CD105 which had 2-fold higher expression in BFP-MSCs compared to GDCs (85±4.7% vs 39.5±15.2%).
Osteogenic and chondrogenic differentiation
Morphological changes
Under a phase contrast microscope, mineralized calcium deposition was observed in BFP-MSCs cultured in osteogenic medium (OM) on day7 until 21, which strongly increased on day 21(Figure 2b). GDCs cultured in OM showed some mineralized nodule formation on day 21, but they were fewer than those observed in differentiated BFP-MSCs (Figure 2e). There was no mineralized nodule formation in both cell types (BFP-MSCs and GDCs) cultured in normal media, without differentiation agents, as the control group at all time periods (Figure 2a and 2d). In chondrogenic differentiation, cells transformed from a spindle to a cuboidal shape in BFP-MSCs while GDCs showed only a slight transformation (Figure 2c and 2f). In control group, no transformation was observed.
Figure2.
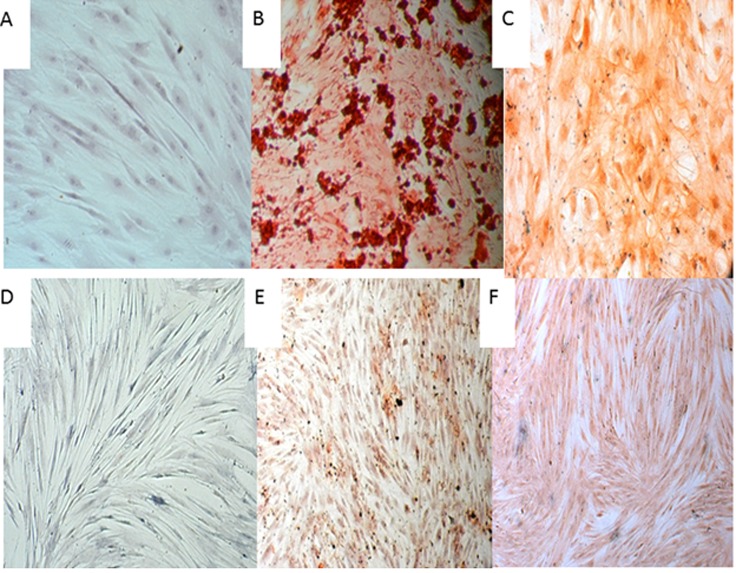
Morphology of BFP-MSCs and GDCs after osteocyte and chondrocyte differentiation (day 21). a-c: adipose derived cells: a: control, b: osteogenic differentiation, c: Chondrogenic differentiation. d-f: gingival- derived mesenchymal stem cells. d: Control, e: osteocyte differentiation, f: Chondrogenic differentiation
Expressions of BGLA and BMP2 as osteogenic and COLL as chondrogenic markers
Total RNA was isolated from both cell types during differentiation on days 7, 14 and 21 and subsequently mRNA level of BGLA, BMP2 and COLL were evaluated using qRT-PCR.
As depicted in Figure 3, BGLA and BMP2 gene transcripts showed a higher expression in BFP-MSCs on 14 and 21 days post culture compared to GDCs. Accordingly, BGLA mRNA had approximately 7×105-fold more expression at day 21 compared to the control group after culturing with osteogenic media. In addition, BMP2 showed 733-fold more mRNA expression in BFP-MSCs treated with differentiation media compared to control. No obvious changes were observed in mRNA expression of these two genes after treating GDCs with osteogenic media.
Figure3.
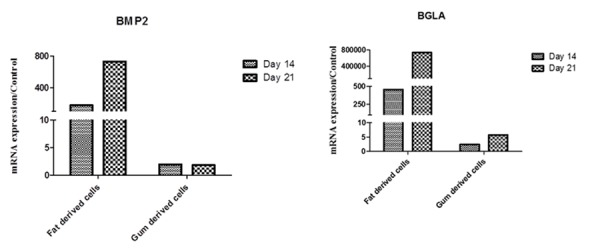
Evaluation of BGLA and BMP2 genes expression in BFP-MSCs and GDCs differentiated to osteocytes after 14 and 21 days post treatment of the cells. The expression and quantity of BGLA and BMP2 gene transcripts was determined by qRT-PCR
Similarly, differentiation to chondrocytes showed a higher ability of BFP-MSCs for transformation compared to GDCs. COLL mRNA showed a 282-fold higher expression at day 21 in BFP-MSCs but not in GDCs compared to control (Figure 4).
Figure4.
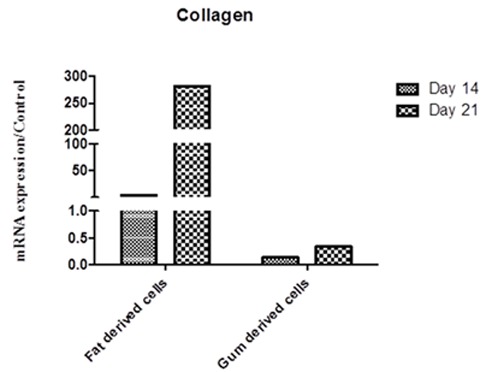
Expression of COLL gene transcript 14 and 21 days after treatment of BFP-MSCs and GDCs with chondrogenic differentiation media. The expression and quantity of COLL gene transcript was determined using qRT-PCR
Discussion
MSCs are present in various organs and are known as attractive resources in regenerative medicine because of their outstanding characteristics including extensive expansion and differentiation into various cell types. In engineering medicine, the availability of stem cell source, simple isolation and the ability to expand the cells into large numbers is desirable.[12]
BFP and gingiva are two unique oral reservoirs for MSCs that usually yield to a high number of progenitor cells compared to other sources such as bone marrow.[13] Adipose tissue holds great promise in regenerative medicine; it is accessible in sufficient quantities as waste material, and it contains more progenitor cells giving rise to diverse cell populations in comparison to bone marrow.[6-7] The common anatomical areas for obtaining fat tissue are abdomen, breast, knee, and cheek. BFP is a deep fat pad located in cheek, which is usually discarded during plastic surgery but because of having a high number of stem cells, could be a suitable candidate in facial reconstruction procedures.[6-7] As well as BFP, gingival tissue harbors a population of cells that have similar features compared to stem cells. Gingiva is the most applicable stem cell source compared to the other dental origins.[11,14] Gingival tissue can be simply obtained as a byproduct from the clinically resected gingival tissues during periodontal surgeries. The availability and ease of isolation of stem cells from gingiva renders it as a candidate for the source of progenitor cells in the regenerative treatment of periodontal defects.[11,14] Zhang et al.[11] isolated a population of progenitor/stromal cells from gingival tissues that showed stem cell characteristics.
To find a better source of MSCs for future therapies of periodontal defects, we obtained and cultured cells from two intra oral sources including adipose and gingival tissues, and then compared their stemness features and differentiation ability to osteocyte and chondrocyte. Subsequently differentiation was confirmed by detection of COLL, BGLA and BMP2 mRNA expressions.
Based on the results, BFP-MSCs and GDCs could easily grow and expand from small specimens of both tissues. Surface staining of mesenchymal specific markers indicated that features of both cell types were similar to other known MSC sources. Moreover, both types of cells did not express or rarely expressed non-mesenchymal markers such as CD14, CD45, and CD34. The expression of all the investigated mesenchymal markers were similar between these two populations except for the expression of CD105 (Endoglin) which is the TGF-β receptor15 and the difference in its expression was expected, because it may have been modified based on the tissue source.
It is reported that despite the lack of a specific cell-surface marker for adult MSCs of distinct tissue origins, these cells express a panel of mesenchymal cell markers such as CD73, CD90, CD105, and CD44 but are negative for endothelial and hematopoietic markers such as CD31, CD34, and CD45.16 Similar to our study, a recent study has revealed that BFP-MSCs expressed stem cell markers such as CD73, CD90, and CD105, whereas they did not express lymphocyte or leukocyte antigens and hematopoietic markers such as CD14, CD31, and CD34.[17] Similarly, it has been shown that GDCs consistently express CD29, CD44, CD73, and CD90 and are negative for CD34 and CD45, but are positive for CD105, CD146, and Stro-1 in variable population subsets.[2]
Moreover, we investigated the differentiation ability of BFP-MSCs and GDCs to osteocyte and chondrocyte. The findings demonstrated that both cell types could be induced to give rise to these lineages.
Our pervious study on isolated ASCs from breast tissue showed that these cells could represent hepatogenic and chondrogenic differentiation markers on the certain differentiating conditions.[18] In addition, a pervious study indicated that adipose tissue isolated from BFP contained MSCs with stemness properties that were able to differentiate towards the adipogenic and osteogenic lineages.[17] Like BFP-MSCs, it has been reported that gingiva-derived MSCs can also differentiate to osteocytes, adipocytes, and chondrocytes when they are properly induced.[2] Our results also indicated that cellular transformation towards chondrocyte and osteocyte and the expression of corresponding genes including COLL, BGLA, and BMP2 genes were considerably higher in BFP-MSCs than GDCs.
GDCs cultured in osteogenic media showed some mineralized nodule formations, but fewer than those observed within the culture of BFP-MSCs. Those nodules were associated with slight transformation.
The findings of the present study imply that BFP can be a better alternative source of stem cells than gingival tissue. ASCs from BFP are easily reachable in abundant quantities with a minimal invasive harvesting method and allow a high number of ASCs to be derived. Moreover, the chance of contamination during surgical removal of gingiva increases due to the presence of oral normal flora. Another important concern in culture of gingival tissue is the growth of fibroblasts, because most of the stromal cells in gingival connective tissue are fibroblasts. Fibroblasts and stem cells share common properties including a spindle shape, plastic adherence and overlapping cell surface antigens but there are no specific markers to discriminate fibroblast from stem cells, however the differentiation ability of stem cells is not identified for fibroblasts.[19] Fibroblasts derived from different tissue origins can exhibit multilineage differentiation potentials and immunomodulatory functions. Nevertheless, because of the lack of definite markers and the unidentified in vivo identity of MSCs, the exact relationship between MSCs and fibroblasts remains elusive.[2]
Tomasello et al.[20] showed that the chronic inflammatory condition that exists in periodontitis does not negatively influence the number or the stem cell marker profile of GDCs. Actually they confirmed that overexpression of proinflammatory cytokines permit a higher osteogenic differentiation potential of extracted stem cells.[20] From a clinical point of view, these findings convince us for using inflamed periodontal tissue for future researches and in tissue engineering applications.
Conclusion
Based on the present findings, adipose tissue, particularly BFP, can be introduced as a simply accessible source for extraction of stem cells. This tissue yields greater proportion of stem cells compared to other sources such as gingiva. Considering these features, BFP may be an outstanding cell source for clinical use and regenerative treatments in maxillofacial bony and periodontal defects.
Acknowledgement
The authors would like to thank all the participants in the study. This work was supported by a grant from Shiraz University of Medical Sciences [Grant No. 6265] and Shiraz Institute for Cancer Research [ICR-100-504]. This research was done as a requirement for the Dentistry thesis defended by Dr. Hamid Ghaderi.
Conflict of Interest:The authors declare that they have no conflicts of interest concerning this article.
References
- 1.Deng J, Petersen BE, Steindler DA, Jorgensen ML, Laywell ED. Mesenchymal stem cells spontaneously express neural proteins in culture and are neurogenic after transplantation. Stem Cells. 2006; 24: 1054–1064. doi: 10.1634/stemcells.2005-0370. [DOI] [PubMed] [Google Scholar]
- 2.Zhang QZ, Nguyen AL, Yu WH, Le AD. Human oral mucosa and gingiva: a unique reservoir for mesenchymal stem cells. J Dent Res. 2012; 91: 1011–1018. doi: 10.1177/0022034512461016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Docheva D, Popov C, Mutschler W, Schieker M. Human mesenchymal stem cells in contact with their environment: surfacecharacteristics and the integrin system. J Cell Mol Med. 2007;11: 21–38. doi: 10.1111/j.1582-4934.2007.00001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Noël D, Djouad F, Jorgense C. Regenerative medicine through mesenchymal stem cells for bone and cartilage repair. Curr Opin Investig Drugs. 2002;3: 1000–1004. [PubMed] [Google Scholar]
- 5.Cowan CM, Shi YY, Aalami OO, Chou YF, Mari C, Thomas R, et al. Adipose-derived adult stromal cells heal critical-size mouse calvarial defects. Nat Biotechnol. 2004;22: 560–567. doi: 10.1038/nbt958. [DOI] [PubMed] [Google Scholar]
- 6.Zhang HM, Yan YP, Qi KM, Wang JQ, Liu ZF. Anatomical structure of the buccal fat pad and its clinical adaptations. Plast Reconstr Surg. 2002;109: 2509–2518. doi: 10.1097/00006534-200206000-00052. [DOI] [PubMed] [Google Scholar]
- 7.Gassner HG, Rafii A, Young A, Murakami C, Moe KS, Larrabee WF Jr. Surgical anatomy of the face: implications for modern face-lift techniques. Arch Facial Plast Surg. 2008;10: 9–19. doi: 10.1001/archfacial.2007.16. [DOI] [PubMed] [Google Scholar]
- 8.Farré-Guasch E, Martí-Pagè C, Hernádez-Alfaro F, Klein-Nulend J, Casals N. Buccal fat pad, an oral access source of human adipose stem cells with potential for osteochondral tissue engineering: an in vitro study. Tissue Eng Part C Methods. 2010;16: 1083–1094. doi: 10.1089/ten.TEC.2009.0487. [DOI] [PubMed] [Google Scholar]
- 9.Tomar GB, Srivastava RK, Gupta N, Barhanpurkar AP, Pote ST, Jhaveri HM, et al. Human gingiva-derived mesenchymal stem cells are superior to bone marrow-derived mesenchymal stem cells for cell therapy in regenerative medicine. Biochem Biophys Res Commun. 2010;393: 377–383. doi: 10.1016/j.bbrc.2010.01.126. [DOI] [PubMed] [Google Scholar]
- 10.Mitrano TI, Grob MS, Carrión F, Nova-Lamperti E, Luz PA, Fierro FS, et al. Culture and characterization of mesenchymal stem cells from human gingival tissue. J Periodontol. 2010;81: 917–925. doi: 10.1902/jop.2010.090566. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Q, Shi S, Liu Y, Uyanne J, Shi Y, Shi S, et al. Mesenchymal stem cells derived from human gingiva are capable of immunomodulatory functions and ameliorate inflammation-related tissue destructionin experimental colitis. J Immunol. 2009;183: 7787–7798. doi: 10.4049/jimmunol.0902318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen FM, Jin Y. Periodontal tissue engineering and regeneration: current approaches and expandingopportunities. Tissue Eng Part B Rev. 2010;16: 219–255. doi: 10.1089/ten.TEB.2009.0562. [DOI] [PubMed] [Google Scholar]
- 13.Bunnell BA, Flaat M, Gagliardi C, Patel B, Ripoll C. Adipose-derived stem cells: isolation, expansion and differentiation. Methods. 2008;45: 115–120. doi: 10.1016/j.ymeth.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Häkkinen L, Uitto VJ, Larjava H. Cell biology of gingival wound healing. Periodontol 2000. 2000;24: 127–152. [PubMed] [Google Scholar]
- 15.Valluru M, Staton CA, Reed MW, Brown NJ. Transforming Growth Factor-β and Endoglin Signaling Orchestrate Wound Healing. Front Physiol. 2011;2: 89. doi: 10.3389/fphys.2011.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fournier BP, Ferre FC, Couty L, Lataillade JJ, Gourven M, Naveau A, et al. Multipotent progenitor cells in gingival connective tissue. Tissue Eng Part A. 2010;16: 2891–2899. doi: 10.1089/ten.TEA.2009.0796. [DOI] [PubMed] [Google Scholar]
- 17.Broccaioli E, Niada S, Rasperini G, Ferreira LM, Arrigoni E, Yenagi V, et al. Mesenchymal Stem Cells from Bichat's Fat Pad: In Vitro Comparison with Adipose-Derived Stem Cells from Subcutaneous Tissue. Biores Open Access. 2013;2: 107–117. doi: 10.1089/biores.2012.0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Razmkhah M, Jaberipour M, Erfani N, Habibagahi M, Talei AR, Ghaderi A. Adipose derived stem cells (ASCs) isolated from breast cancer tissue express IL-4, IL-10 and TGF-β1 and upregulate expression of regulatory molecules on T cells: do they protect breast cancer cells from the immune response? Cell Immunol. 2011;266: 116–122. doi: 10.1016/j.cellimm.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 19.Alt E, Yan Y, Gehmert S, Song YH, Altman A, Gehmert S, et al. Fibroblasts share mesenchymal phenotypes with stem cells, but lack their differentiation and colony-forming potential. Biol Cell. 2011;103: 197–208. doi: 10.1042/BC20100117. [DOI] [PubMed] [Google Scholar]
- 20.Tomasello L, Mauceri R, Coppola A, Pitrone M, Pizzo G, Campisi G, et al. Mesenchymal stem cells derived from inflamed dental pulpal and gingival tissue: a potential application for bone formation. Stem Cell Res Ther. 2017;8: 179. doi: 10.1186/s13287-017-0633-z. [DOI] [PMC free article] [PubMed] [Google Scholar]


