Abstract
Statement of the Problem:
Pemphigus vulgaris is characterized by intraepithelial vesicles, but pathogenesis of vesicle formation in this disease has not been substantiated yet.
Purpose:
The present study investigate extrinsic apoptotic pathway in oral pemphigus vulgaris using TUNEL and important immunohistochemical markers of extrinsic pathway, TNFR1 and FasL.
Materials and Method:
In the present cross sectional study, 25 oral pemphigus vulgaris samples and 6 normal oral mucosa were analyzed for the presence of apoptosis by TUNEL and the staining of TNFR1 and FasL in basal and parabasal layers around vesicle, vesicle floor, vesicle roof and acantholytic cells. The staining expression and intensity were measured and the obtained data were analyzed by Wilcoxon and Mann-Whitney tests.
Results:
There was no or faint staining of TUNEL, FasL and TNFR1 in normal oral mucosa. In addition, there was no significant difference between the staining of TUNEL technique in different layers. The staining of TNFR1 marker was very high in all regions. FasL marker was not positive in the basal and parabasal layers around vesicle in 92% of samples but showed a varied and different staining in vesicle region. There was a significant difference between the each two markers in all layers (p <0.001).
Conclusion:
Apoptosis is probably is a preceding phenomenon to acantholysis in pemphigus vulgaris. It appears that the apoptosis occurs mostly by extrinsic pathway using proapototic mediators TNFR1 and FasL.
Keywords: Pemphigus vulgaris , Apoptosis , TUNEL , TNFR1 , FasL
Introduction
Suprabasilar vesicle in pemphigus vulgaris (PV) is created by the formation of IgG autoantibody against surface glycoproteins of epithelial cells (Desmoglein 1&3). These autoantibodies cause acantholysis.[1-3] However their exact mechanism regarding how PV-IgG antibody induces acantholysis is unknown. Apoptosis is one of the suggested mechanisms involved in pemphigus and the increased staining expression of proapoptotic factors TNFα, FasL, Fas and caspase in pemphigus has been reported by various studies.[1,4-11] Apoptosis is activated through intrinsic (mitochondrial) and extrinsic (apoptotic death receptors) pathways. Both pathways lead to activation of caspase-3. Caspase-3 induces DNA fragmentation, which can be identified by Poly ADP-Ribose Polymerase, fraction and TUNEL.[12-15]
The extrinsic apoptotic pathway is initiated by the activation of apoptotic death receptors at cell surface. The most known apoptotic receptors are TNFR1 and Fas. Fas/FasL system is one of the important mechanisms involved in the induction of apoptosis in the cells and tissues that plays a significant role in pathogenesis of PV. The cytotoxic T cells are the most important sources of FasL staining expression.[4,2-14,16-17] TNF is a potent cytokine that acts through TNFR1 and TNFR2 receptors. TNFR1 is expressed in all human tissues and is a significant signaling receptor for TNFα.[17-18]
Schmidt et al. reported that apoptosis occurs following acantholysis, but apoptosis is not a prerequisite for acantholysis.[19] Other studies have also reported apoptosis as a delayed event and have asserted that apoptosis occurs after acantholysis.20-23 On the other hand, researchers such as Pacheco-Tovar et al., Li et al., Zayed et al., Gniadecki et al., Rodrigues et al., Frusic-Zlotkin et al. and also Deyhimi&Tavakoli reported that apoptosis occurs before acantholysis and can even be the cause of acantholysis, not a secondary phenomenon.[1,3-4,7,10,24-25] Frusic-Zlotkin et al. stated that apoptotic mechanisms are inducer factors of acantholysis in PV-IgG. They observed the activated pathway of Fas and its components in the skin of PV patients around vesicles.[10] Further, Zayed et al. showed that activity of proapoptotic pathways was essential for the formation of vesicles in PV and activation of the components of apoptotic signals, including Fas, FasL, and caspase-3 could intensify vesicular response.[1] Moreover, Deyhimi&Tavakoli suggested that since TUNEL was positive in the keratinocytes around vesicle, apoptosis process occurs early in PV before acantholysis. Furthermore, they suggested that the extrinsic apoptotic pathways are probably involved in PV because of the negative immune reaction to Bax marker, a proapoptotic mitochondrial or intrinsic marker.[3] In reality, this suggestion is a predisposing factor for the present research. However Janse et al. argued that there is no evidence to support the role of apoptotic cells in acantholysis.[2]
In the present study, TUNEL technique was used to analyze the role of apoptosis in oral PV. Also, for defining the type of apoptotic pathway, the effective markers of extrinsic apoptotic pathway, namely TNF Receptor1 (TNFR1) and Fas Ligand (FasL) were used.
Materials and Method
In the present cross-sectional and descriptive-analytical study, the same 25 oral PV samples of our previous study[3] and 6 normal oral mucosa were obtained from archive of department of oral and maxillofacial pathology at school of dentistry, Isfahan University of medical sciences, Iran. All samples had histopathologic properties of PV, including acantholysis and suprabasal vesicles, which their diagnosis had been confirmed by direct immunofluorescence. The samples had also sufficient perivesicular tissue. The samples with severe inflammation, surface ulcer, and inadequate perivesicular tissue were excluded from the study. At first, sections (4-5µm) were prepared from the paraffin blocks and then deparaffinization and rehydration in five descending stages were performed on them. Next following staining procedures were done:
TUNEL (TDT-mediated dUTP-biotin nick end Labeling) technique
After washing with phosphate buffer saline (PBS), the slides were fixed with 4% paraformaldehyde and then incubated in Tween solution and after washing with PBS, incubated in Terminal Deoxynucleotidyl Transferase (TDT) end labeling solution at ambient temperature. Then, after washing with PBS, the samples were incubated with blocking buffer at room temperature, then incubated in 3% H2O2 and after washing with distilled water (DW), incubated in TUNEL (Germany, Merck, 17-141) at 37 °C. Thereafter incubation of secondary antibody (Novolink polymer RE7140-K; Novocastra (Li CA, UK) was performed in two phases: At first incubation was carried out with post primary solution and then polymer+HRP solution. Next, the secondary antibody was washed with PBS and incubated with diaminobenzydine (DAB). Afterwards, hematoxylin staining was performed and finally samples were mounted.
Natural pulmonary tissue of mouse was used as positive control and the same samples were used as negative control by eliminating the TDT.
TNFR1 Immunohistochemical staining
The samples were incubated inTris-EDTA (pH=9) in microwave for antigen retrieval. The samples were then placed in 3% H2O2 to block endogenous peroxidase activity and then were washed with PBS. Next, the Anti-TNF Receptor 1 polyclonal primary antibody (TNFR1ab19139; Abcam, USA) at 1/25 dilution was added and samples were incubated at ambient temperature. They were then incubated with Polymer Envision secondary antibody (Envision K4061; DAKO, Denmark) at room temperature. After placing the samples in DAB, opposite hematoxylin staining was performed. The samples were then mounted. Prostate adenocarcinoma was used as positive control, and the same samples were used as negative control by eliminating the primary antibody.
FasL Immunohistochemical staining
Incubation was performed inTris Ethylene Diamine Tetra acetic Acid (EDTA) and H2O2 the same as TNFR1. After washing with PBS, Anti-Fas Ligand polyclonal antibody (Anti FasL ab2440; Abcam, USA) at 1/100 dilution were added to the tissue and incubated at room temperature. Then, the samples were incubated with the secondary antibody (Biogenex QD430-XAKE; USA), including a super enhancer and SS Label solution at room temperature. Next, the samples were incubated in DAB the same as TNFR1, stained with hematoxylin and finally the mounted. Breast adenocarcinoma was used as positive control, and the same samples were used as negative control by eliminating the primary antibody.
Sample evaluation
The samples were observed by two pathologists using light microscope (Olympus BX41TF Tokyo, Japan). The cells were analyzed in basal and parabasal layers of the perivesicular mucosa, vesicle floor, vesicle roof, and intravesicular acantholytic cells in PV in addition to basal and parabasal layers of normal oral mucosa.
The staining expression level of the cells for TUNEL, FasL and TNFR1 was calculated as percent positive cells in 100 cells with 400× magnification in 10 high power fields. The results were recorded semiquantitatively and ranked as 1=1-25%, 2=26-50%, 3= 51-75% and 4= more than 75%. In addition, the staining intensity level was evaluated with 0=negative, +1=poor, +2= moderate, +3=moderate to high and +4=very high rankings.[3]
Staining Intensity Distribution (SID) index was calculated by multiplying the scores obtained for the staining expression and intensity in each sample.26-27 The obtained data analyzed by SPSS-20 software and Kruskal-Wallis, Mann-Whitney, Friedman and Wilcoxon tests. p <0.05 was considered significant.
Results
The results of SID index of TUNEL staining in basal and parabasal cells of normal oral mucosa showed significant difference with those in perivesicular tissues by Mann- Whitney test (p < 0.001), because there was not expression of TUNEL staining in normal oral tissues (Figure 1).
Figure1.
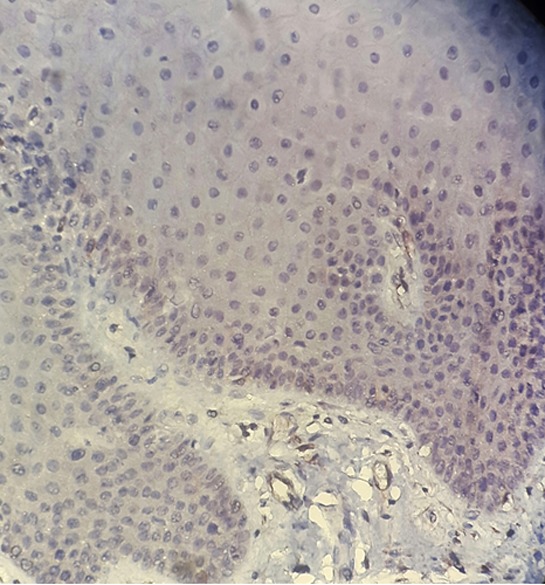
No expression of TUNEL+ cells in normal mucosa (magnification400)
The results of TUNEL staining obtained from study of staining expression in basal and parabasal cells around vesicle showed that >75% of these cells were stained in 76% and 72% of samples, respectively. The staining intensity levels in the basal and parabasal layers around vesicle were reported to be very high (+4) in 52% and 40% of samples, respectively.
The findings of TUNEL staining obtained from study of staining expression in vesicle area indicated that >75% of cells were stained in 92% of samples in the vesicle floor, 64% of samples in the vesicle roof and 72% of samples in the acantholytic cells. The staining intensity in most of these samples (approximately >65% of cases) was very high (+4) (Tables 1 and 2, Figures 2 and 3). Further, the results of Kruskal-Wallis test on SID index of TUNEL staining showed no significant difference between different layers (p = 0.381).
Table 1.
Results of the TUNEL staining expression
| Ranking of staining positivity | Number (percentage) of positive samples | ||||
|---|---|---|---|---|---|
| Perivesicular area | Vesicular area | ||||
| Basal layer | Parabasal layer | Tomb stone | Roof of vesicle | Acantholytic cells | |
| 0 | 1(4%) | 1(4%) | 0 | 0 | 0 |
| 1-25% | 3(12%) | 3(12%) | 1(4%) | 2(8%) | 2(8%) |
| 26-50% | 1(4%) | 1(4%) | 0 | 3(12%) | 2(8%) |
| 51-75% | 1(4%) | 2(8%) | 1(4%) | 4(16%) | 3(12%) |
| 75%< | 19(76%) | 18(72%) | 23(92%) | 16(64%) | 18(72%) |
| Total | 25(100%) | 25(100%) | 25(100%) | 25(100%) | 25(100%) |
Table 2.
Results of the TUNEL staining intensity
| Ranking of staining positivity | Number (percentage) of positive samples | ||||
|---|---|---|---|---|---|
| Perivesicular area | Vesicular area | ||||
| Basal layer | Parabasal layer | Tomb stone | Roof of vesicle | Acantholytic cells | |
| 0 | 1(4%) | 1(4%) | 0 | 0 | 0 |
| 1+ | 4(16%) | 5(20%) | 1(4%) | 5(20%) | 3(12%) |
| 2+ | 2(8%) | 2(8%) | 4(16%) | 1(4%) | 2(8%) |
| +3 | 5(20%) | 7(28%) | 3(12%) | 2(8%) | 4(16%) |
| +4 | 13(52%) | 10(40%) | 17(68%) | 17(68%) | 16(64%) |
| Total | 25(100%) | 25(100%) | 25(100%) | 25(100%) | 25(100%) |
Figure2.
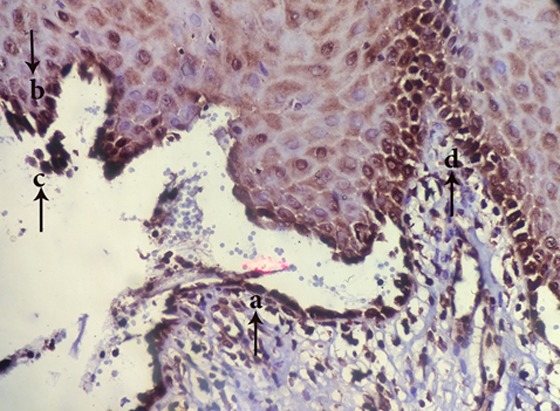
appearance of TUNEL+ cells in tombstone region (a), roof of vesicle (b), acantholytic cells(c) and Perivesicular tissue(d) (magnification400)
Figure3.
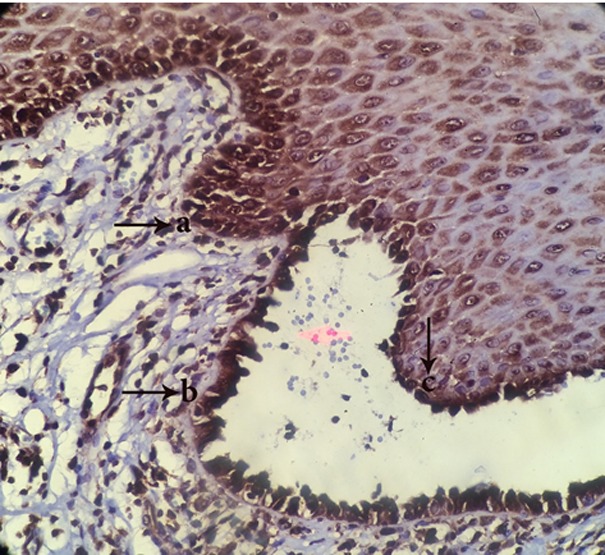
appearance of TUNEL+ cells in perivesicular tissue(a), tomb stone region(b) and roof of vesicle(c) (magnification400)
The results of SID index of TNFR1 staining in basal and parabasal cells of normal oral mucosa showed significant difference with those in perivesicular tissues by Mann-Whitney test (p <0.001), because staining expression and intensity of TNFR1 in normal oral tissues were faint with mean SID=1 (Figure 4).
Figure4.
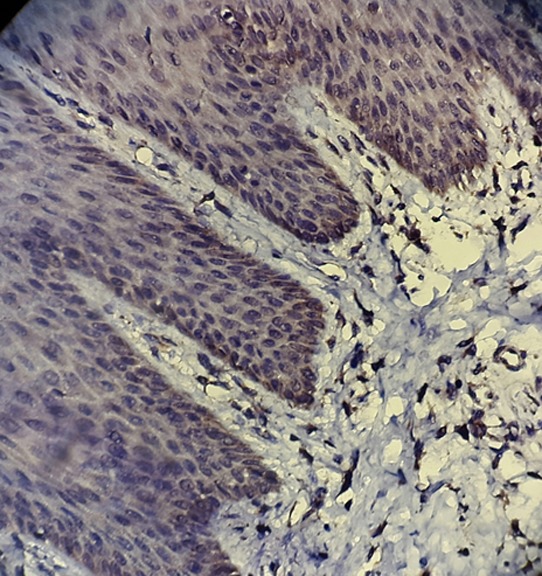
Faint staining expression and intensity of TNFR1+ cells in normal mucosa (magnification400)
Evaluation of the staining level of TNFR1 marker indicated a high expression and intensity, so that in all samples, >75% of cells were stained with very high intensity (+4) in the basal layer around vesicle, vesicle floor and acantholytic cells. This level was reported to be 92% in parabasal layer around vesicle and vesicle roof. Staining intensity was very high (+4) in 76% of samples in parabasal layer and 96% of samples in the vesicle roof ( Table 3 and Table 4, Figure 5 and Figure 6).
Table 3.
Results of theTNFR1 staining expression
| Ranking of staining positivity | Number (percentage) of positive samples | ||||
|---|---|---|---|---|---|
| Perivesicular area | Vesicular area | ||||
| Basal layer | Parabasal layer | Tomb stone | Roof of vesicle | Acantholytic cells | |
| 0 | 0 | 0 | 0 | 0 | 0 |
| 1-25% | 0 | 0 | 0 | 0 | 0 |
| 26-50% | 0 | 0 | 0 | 0 | 0 |
| 51-75% | 0 | 2(8%) | 0 | 2(8%) | 0 |
| 75%< | 25(100%) | 23(92%) | 25(100%) | 23(92%) | 25(100%) |
| Total | 25(100%) | 25(100%) | 25(100%) | 25(100%) | 25(100%) |
Table 4.
Results of the TNFR1 staining intensity
| Ranking of staining positivity | Number (percentage) of positive samples | ||||
|---|---|---|---|---|---|
| Perivesicular area | Vesicular area | ||||
| Basal layer | Parabasal layer | Tomb stone | Roof of vesicle | Acantholytic cells | |
| 0 | 0 | 0 | 0 | 0 | 0 |
| +1 | 0 | 0 | 0 | 0 | 0 |
| +2 | 0 | 1(4%) | 0 | 0 | 0 |
| +3 | 1(4%) | 5(20%) | 0 | 1(4%) | 0 |
| +4 | 24(96%) | 19(76%) | 25(100%) | 24(96%) | 25(100%) |
| Total | 25(100%) | 25(100%) | 25(100%) | 25(100%) | 25(100%) |
Figure5.
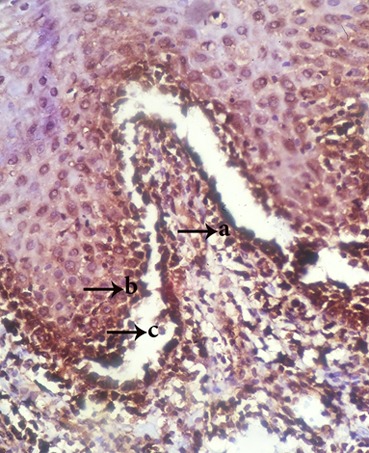
Appearance of TNFR1+ cells in tombstone region (a), roof of vesicle (b) and acantholytic cells(c) (magnification400)
Figure6.
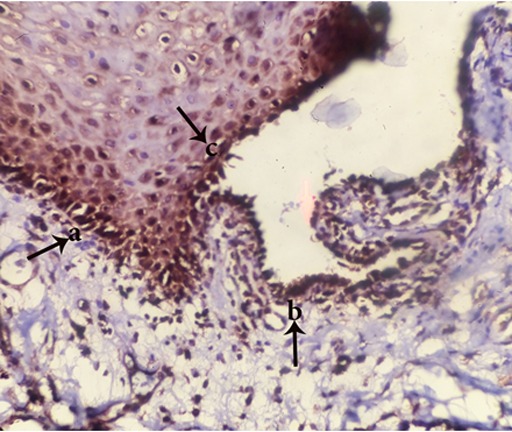
Appearance of TNFR1+ cells in perivesicular tissue (a), tomb stone region (b) and roof of vesicle(c) (magnification400)
According to the results of Mann-Whitney test on SID index of TNFR1 staining, no significant difference was reported between basal layer and vesicle floor (p= 0.317), basal layer and vesicle roof (p =0.540), basal layer and acantholytic cells (p =0.317), parabasal layer and vesicle roof (p =0.132), vesicle floor and vesicle roof (p =0.153) and also vesicle roof and acantholytic cells (p =0.153). However, a significant difference was found between basal and parabasal layers (p =0.014), parabasal layer and vesicle floor (p =0.01), parabasal layer and acantholytic cells (p =0.01) and also vesicle floor and acantholytic cells (p =0.099).
The results of SID index of FasL staining in basal and parabasal cells of normal oral mucosa did not show significant difference with those in perivesicular tissues by Mann- Whitney test (p= 0.643), because there was not expression of FasL staining in normal oral tissues, approximately similar to perivesicular tissues (Figure 7). The analysis of the staining expression rate of FasL marker in basal and parabasal layers around vesicle revealed that in 92% of all samples, staining expression and intensity of cells were zero. This marker showed a varied staining expression and intensity in vesicle area, as presented in details in the Table 5 and Table 6, Figure 8 and Figure 9.
Figure7.
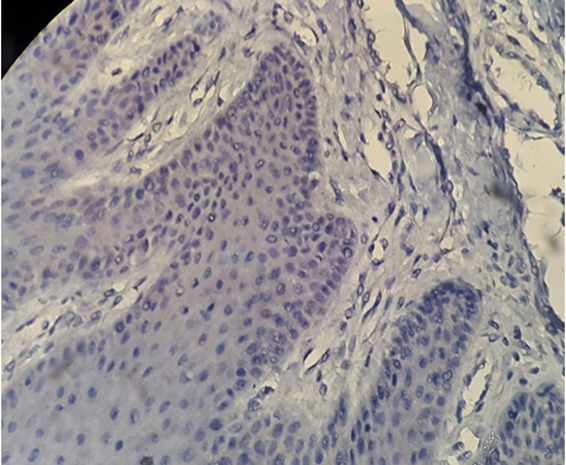
No expression of FasL+ cells in normal mucosa (magnification400)
Table 5.
Results of the FasL staining expression
| Ranking of staining positivity | Number (percentage) of positive samples | ||||
|---|---|---|---|---|---|
| Perivesicular area | Vesicular area | ||||
| Basal layer | Parabasal layer | Tomb stone | Roof of vesicle | Acantholytic cells | |
| 0 | 23(92%) | 23(92%) | 1(4%) | 4(16%) | 2(8%) |
| 1-25% | 2(8%) | 2(8%) | 6(24%) | 9(36%) | 6(24%) |
| 26-50% | 0 | 0 | 1(4%) | 8(32%) | 4(16%) |
| 51-75% | 0 | 0 | 4(16%) | 4(16%) | 4(16%) |
| 75%< | 0 | 0 | 13(52%) | 0 | 9(36%) |
| Total | 25(100%) | 25(100%) | 25(100%) | 25(100%) | 25(100%) |
Table 6.
Results of the FasL staining intensity
| Ranking of staining positivity | Number (percentage) of positive samples | ||||
|---|---|---|---|---|---|
| Perivesicular area | Vesicular area | ||||
| Basal layer | Parabasal layer | Tomb stone | Roof of vesicle | Acantholytic cells | |
| 0 | 23(92%) | 23(92%) | 1(4%) | 4(16%) | 2(8%) |
| +1 | 2(8%) | 2(8%) | 4(16%) | 6(24%) | 5(20%) |
| +2 | 0 | 0 | 7(28%) | 11(44%) | 8(32%) |
| +3 | 0 | 0 | 13(52%) | 4(16%) | 10(40%) |
| +4 | 0 | 0 | 0 | 0 | 0 |
| Total | 25(100%) | 25(100%) | 25(100%) | 25(100%) | 25(100%) |
Figure8.
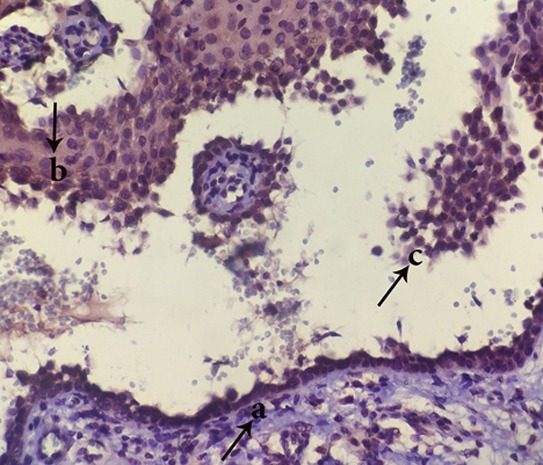
Appearance of FasL+ cells in tombstone region(a), roof of vesicle(b) and acantholytic cells(c) (magnification400)
Figure9.
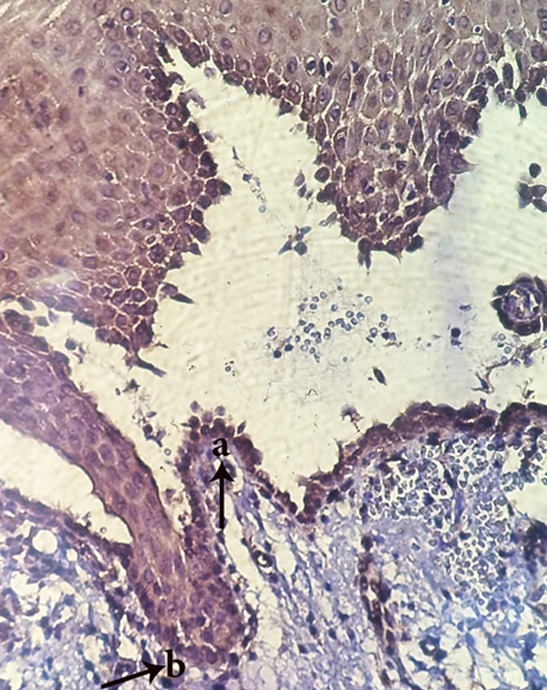
Appearance of FasL+cells in vesicle area(a) and FasL- in perivesicular tissue(b) (magnification400)
Moreover, the results of Mann-Whitney test on SID index of FasL staining showed a significant difference between basal layer and vesicle floor, basal layer and vesicle roof, basal layer and acantholytic cells, parabasal layer and vesicle floor, parabasal layer and vesicle roof, parabasal layer and acantholytic cells, vesicle floor and vesicle roof, and also vesicle roof and acantholytic cells (p ≤0.001). No significant difference was observed, however, between basal and parabasal layers (p = 0.99) and also vesicle floor and acantholytic cells (p =0.259).
In addition, Wilcoxon test on SID index was used to compare both markers in different layers and the results showed a significant difference in all cases between TNFR1 and TUNEL, TNFR1 and FasL and TUNEL and FasL in various layers (p ≤0.001).
Discussion
Considering negative immunoreactivity with Bax marker, a proapoptotic marker involving in intrinsic apoptotic pathway in our previous research in 2013[3] and based on the suggestion of same research, the present study was planned to investigate the extrinsic pathway of apoptosis in oral PV using TUNEL technique and the most important immunohistochemical markers effective in extrinsic apoptotic pathway, namely TNFR1 and FasL on the same 25 oral PV samples of previous comment.[3]
According to the results obtained from the present study and considering TUNEL negativity of normal oral mucosa, TUNEL positivity in the vesicle layer and acantholytic cells, as well as perivesicular tissues in basal and parabasal layers, it can be deduced that apoptosis is involved in the formation of vesicle or even preceding to vesicle in PV. In reality, the presence of TUNEL-positive cells around vesicle confirmed the occurrence of apoptosis in the early stages of the disease and before acantholysis.
The results of the present study are in line with those of Li et al., Zayed et al., Gniadecki et al., Pacheco- Tovar et al., Rodrigues et al., Frusic-Zlotkin et al., and also Deyhimi and Tavakoli.[1,3-4,6,10,24-25] All aforementioned authors contend that apoptosis occurs prior to acantholysis and in reality, apoptosis could be the cause of acantholysis, not a secondary phenomenon, because TUNEL-positive epidermal cells presented not only in the vesicular but also in the perivesicular epidermis of the patients with PV.
The apoptolysis hypothesis presented by Grando et al. is also indicative of concurrence of apoptosis and acantholysis, each of which is neither a prerequisite nor prior to the other one, but interdependent.[5,28]
Unlike the above studies, the findings of Schmidt et al., Waschke et al., Arredondo et al., Baroni et al. and Takahashi et al. showed that apoptosis occurs following acantholysis, and is not a prerequisite for acantholysis.[19-23] Moreover, Janse et al.[2] reported no evidence of apoptotic cells in acantholysis.
The difference between results of the present research with those of some above mentioned studies could be owing to limited number of the samples in the given studies, location of the biopsies that was cutaneous in most of the above studies, accuracy of the techniques which might not have shown low intensity rates and over fixation of the samples which their antigens might not have activated.
In the present study, TNFR1 expressed both within vesicles and around vesicles with very high intensity, which is indicative of the onset of apoptotic process before vesicle stage in PV. In normal oral mucosa, staining expression and intensity of TNFR1 were faint. These findings are indicating the overexpression of TNFR1 in apoptotic process of PV.
In the studies of Lopez-Robles et al., Felicini et al. and Rodrigues et al.,[6-7,11] inflammatory cytokine of TNFα were proposed as the factor affecting the induction of apoptotic processes. Furthermore, Chiapa-Labastida et al.[8] demonstrated the expression of TNFα within the vesicles and around vesicles. It was found out that TNFα was induced by keratinocytes in response to pemphigus autoantibodies, which consequently both stimuli induced apoptosis and then accumulation of apoptotic cells reinforced or furthered higher production of TNFα by monocytes or macrophages that in turn led to advancement of the disease. Based on the results of this study, apoptosis and acantholysis are relevant processes because antigen-antibody complex stimulates apoptotic process; however, apoptosis occurs not necessarily after acantholysis but it can simultaneously occur with acantholysis or even prior to acantholysis.[8]
Nevertheless, the expression of TUNEL around vesicle in the present study was less than the expression of TNFR1. This difference may be due to different expression rates of TNFR1 and TUNEL. In fact, TUNEL shows the morphological changes of apoptotic cells and DNA fragmentation whereas TNFR1 shows the stage before morphological changes, i.e. molecular changes of apoptosis. As it is evident, biomolecular changes always occur prior to morphological changes and are earlier, more evidently and more severe than morphological changes that take place with a time delay and consequently lower expression and intensity.
The study of the expression rate of FasL indicated a relatively well expression for this mediator in vesicle, but showed a minimum expression in the basal and parabasal layers of perivesicular region. Considering minimum expression of FasL in the basal and parabasal layers of perivesicular region, it appears that non-expression of FasL in normal oral tissues is logical. Comparison of the expression of FasL and TNFR1 markers showed that TNFR1 acts at the very early stages and before the formation of vesicle, as TNFR1 had a very high expression in the basal and parabasal layers of perivesicular region, unlike FasL that had a low expression around vesicle but a rather well expression within vesicle and acantholytic cells. Moreover, the significant difference between these markers in different layers confirms the issue that TNFR1 marker acts earlier in apoptotic cascade and even in the surrounding of vesicle. It seems that FasL acts more as an accelerator for bulla formation rather than an initiator. Nevertheless, it can be concluded that the extrinsic pathway of apoptosis is promoted through both mediators of cell death so that TNFR1 acts as an initiator of apoptotic process and FasL is activated in the following stages after TNFR1.
Another reason for the lower expression of FasL marker is that it is produced by the cells of immune system such as T cells (mainly cytotoxic T cells), natural killer cells, and macrophages that induce apoptosis by connecting to the Fas cell surface receptor.[1,4] In the present study, however, an attempt was made to select the samples with no severe inflammation in order to eliminate the confounding factor of inflammation. Therefore, the lower expression of FasL could be owing to lower inflammation of the samples, which consequently resulted in lower production of FasL. In addition, the expression of FasL and TNFR1 was observed in the inflammatory cells within connective tissue, which is a reason for the origin of these apoptotic mediators. However, since Fas receptors present in keratinocytic cells, if Fas was measured, probably higher expression and intensity of Fas compared with FasL would be reported. These results are confirmed by other studies and are in line with the findings of Puviani et al., Frusic-Zlotkin et al., Pacheco-Tovar et al. and Zayed et al.[1,4,9-10] In study of Pacheco-Tovar et al.[4] consistent with our results, Fas was increased in acantholytic cells and perivesicular keratinocytes, but FasL did not show any increase in both the control group and perivesicular keratinocytes.
Involvement of the extrinsic pathway of apoptosis in PV was reported in the studies of Orlov et al., Frusic-Zlotkin et al., and Lippens et al.[10,29-30] It was also shown that accumulation of Fas, FasL, and TNFα resulted in amplification of ability of PV-IgG, separation of cells and induction of acantholysis.[10,29-30] Furthermore, Moravvej et al.[31] reported that serum FasL level in PV patients was increased and more interestingly, serum FasL level was increased more in mucosal lesions than in cutaneous lesions, which is indicative of the role of extrinsic apoptotic pathway in the occurrence of apoptosis in mucosal lesions.
According to the results of the present study, it can be concluded that it might be feasible to mitigate the complications resulting from overconsumption of corticosteroid medications and reduce corticosteroid dose along with the use of antiapoptotic drugs such as Fas, FasL, TNFα, and TNFR1 inhibitors in patients with PV. Also based on study of Puviani et al.[9] about substantial reducing FasL by cortone, it can be concluded that these treatments are also done through the modulation of the apoptosis and so using antiapoptotic drugs such as antiFas, antiTNF and antiCaspase can reduce corticosteroid dose and consequently the complications resulting from their high dose. Li et al. and Pacheco-Tovar et al.[24,32] showed that inhibition of caspase could stop blister formation. Moreover, Ragab et al. and Frusic-Zlotkin et al.[10,33] reported that evaluation of serum TNF before treatment could be helpful as a guide for analysis of prognosis and treatment selection.
For confirmation of the obtained results from the present study, the pathophysiology of PV should be more investigated. Moreover, it is suggested to employ more advanced immunohistochemical methods such as immunogold silver staining and cell culture in order to attain results that are more accurate; however, this was not possible in the present study because of high cost and lack of availability.
Conclusion
Although there are limitations of making conclusions about the biological and pathogenic significance of molecules assessed only by their immunohistochemical expression; however , according to the results obtained by present study, it can be deduced with more certainty that:
1. Apoptosis is probably necessary for the induction of acantholysis in PV and is a preceding phenomenon to acantholysis or at least these two phenomenons are interdependent and not necessarily prerequisite to other one.
2. It appears that the apoptosis induced in PV occurs mostly, but probably not purely in extrinsic pathway.
3. Proapoptotic mediators FasL and TNFR1 are involved in the induction of apoptosis in PV.
4. Regional inflammation is involved in the induction of apoptosis.
5. Inhibition of apoptosis probably prevents acantholysis and so using TNF and FAS inhibitors can probably slow down the development of disease.
Acknowledgement
This research with institutional bioethics approval and investigative proposal number 393427 has been performed by scientific and financial support of vice-chancellery for research of Isfahan University of medical sciences. Here authors would like to thank Mr. Nasr for his excellent technical assistance in immunohistochemical and TUNEL tests.
Conflict of Interest:Authors declare that there are no conflicts of interest regarding to this study.
References
- 1.Zayed AA, Fawzy MM, El Hawary MS, Youssef ME, El-Din S, Safinaz S. Expression of Fas, Fas ligand, and caspase-3 in pemphigus vulgaris. Journal of the Egyptian Women's Dermatologic Society. 2012; 9: 144–150. [Google Scholar]
- 2.Janse IC, van der Wier G, Jonkman MF, Pas HH, Diercks GFH. No evidence of apoptotic cells in pemphigus acantholysis. J Invest Dermatol. 2014; 134: 2039–2041. doi: 10.1038/jid.2014.60. [DOI] [PubMed] [Google Scholar]
- 3.Deyhimi P, Tavakoli P. Study of apoptosis in oral pemphigus vulgaris using immunohistochemical markerBax and TUNEL technique. J Oral Pathol Med. 2013; 42: 409–414. doi: 10.1111/jop.12022. [DOI] [PubMed] [Google Scholar]
- 4.Pacheco-Tovar MG, Avalos-Díaz E, Vega-Memije E, Bollain-y-Goytia JJ, López-Robles E, Hojyo-Tomoka MT, et al. The final destiny of acantholytic cells in pemphigus is Fas mediated. J Eur Acad Dermatol Venereol. 2009; 23: 697–701. doi: 10.1111/j.1468-3083.2009.03162.x. [DOI] [PubMed] [Google Scholar]
- 5.Grando SA. Pemphigus autoimmunity: hypotheses and realities. Autoimmunity. 2012; 45: 7–35. doi: 10.3109/08916934.2011.606444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.López-Robles E, Avalos-Díaz E, Vega-Memije E, Hojyo-Tomoka T, Villalobos R, Fraire S, et al. TNFalpha and IL-6 are media-tors in the blistering process of pemphigus. Int J Dermatol. 2001; 40: 185–188. doi: 10.1046/j.1365-4362.2001.01083.x. [DOI] [PubMed] [Google Scholar]
- 7.Rodrigues DB, Pereira SA, dos Reis MA, Adad SJ, Caixeta JE, Chiba AM, et al. In situ detection of inflammatory cyto-kines and apoptosis in pemphigus foliaceuspatients. Arch Pathol Lab Med. 2009; 133: 97–100. doi: 10.5858/133.1.97. [DOI] [PubMed] [Google Scholar]
- 8.Chiapa-Labastida M, Zentella-Dehesa A, León-Dorantes G, Becker I. Pemphigus vulgaris: accumulation of apoptotic cells in dermis and epidermis possiblyrelates to pathophysiology through TNF-alpha production by phagocytes. Eur J Dermatol. 2011; 21: 874–888. doi: 10.1684/ejd.2011.1508. [DOI] [PubMed] [Google Scholar]
- 9.Puviani M, Marconi A, Cozzani E, Pincelli C. Fas ligand in pemphigus sera induces keratinocyte apoptosis through the activationof caspase-8. J Invest Dermatol. 2003; 120: 164–167. doi: 10.1046/j.1523-1747.2003.12014.x. [DOI] [PubMed] [Google Scholar]
- 10.Frusic-Zlotkin M, Pergamentz R, Michel B, David M, Mimouni D, Bregegere F, Milner Y. The interaction of pemphigus autoimmunoglobulins with epidermal cells: activation of the fas apoptotic pathway and the use of caspase activity for pathogenicity tests of pemphigus patients. Ann NY Acad Sci. 2005; 1050:371–379. doi: 10.1196/annals.1313.040. [DOI] [PubMed] [Google Scholar]
- 11.Feliciani C, Toto P, Amerio P, Pour SM, Coscione G, Shivji G, et al. In vitro and in vivo expression of interleukin-1alpha and tumor ne-crosis factor-alphamRNA in pemphigus vulgaris: interleukin-1alpha and tumor necro-sis factor-alpha are involved in acantholysis. J Invest Dermatol. 2000; 114: 71–77. doi: 10.1046/j.1523-1747.2000.00835.x. [DOI] [PubMed] [Google Scholar]
- 12.Vidya GD. Apoptosis in Health and Disease. Global Journal for Research Analysis. 2013; 2: 186–188. [Google Scholar]
- 13.Jain M, Sowmya K, Sridhara SU, Jain N, Khan S, Desai A. Apoptosis and its significance in oral diseases: An update. J Oral Dis. 2013; 2013:1–11. [Google Scholar]
- 14.Reed JC. Mechanisms of apoptosis. Am J Pathol. 2000; 157: 1415–1430. doi: 10.1016/S0002-9440(10)64779-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hashemi M, Ghavami S. Methods of Studying the Apoptosis. Journal of Rafsanjan University of Medical Sciences. 2008; 7: 71–78. [Google Scholar]
- 16.Peter ME, Krammer PH. The CD95(APO-1/Fas) DISC and beyond. Cell Death Differ. 2003; 10: 26–35. doi: 10.1038/sj.cdd.4401186. [DOI] [PubMed] [Google Scholar]
- 17.Lavrik I, Golks A, Krammer PH. Death receptor signaling. J Cell Sci. 2005; 118(Pt 2): 265–267. doi: 10.1242/jcs.01610. [DOI] [PubMed] [Google Scholar]
- 18.Schütze S, Tchikov V, Schneider-Brachert W. Regulation of TNFR1 and CD95 signalling by receptor compartmentalization. Nat Rev Mol Cell Biol. 2008; 9: 655–662. doi: 10.1038/nrm2430. [DOI] [PubMed] [Google Scholar]
- 19.Schmidt E, Gutberlet J, Siegmund D, Berg D, Wajant H, Waschke J. Apoptosis is not required for acantholysis in pemphigus vulgaris. Am J Physiol Cell Physiol. 2009; 296: C162–C172. doi: 10.1152/ajpcell.00161.2008. [DOI] [PubMed] [Google Scholar]
- 20.Waschke J, Spindler V, Bruggeman P, Zillikens D, Schmidt G, Drenckhahn D. Inhibition of Rho A activity causes pemphigus skin blistering. J Cell Biol. 2006; 175: 721–727. doi: 10.1083/jcb.200605125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arredondo J, Chernyavsky AI, Karaouni A, Grando SA. Novel mechanisms of target cell death and survival and of therapeutic action of IVIg in Pemphigus. Am J Pathol. 2005; 167: 1531–1544. doi: 10.1016/S0002-9440(10)61239-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baroni A, Buommino E, Paoletti I, Orlando M, Ruocco E, Ruocco V. Pemphigus serum and captopril induce heat shock protein 70 and inducible nitric oxide synthase overexpression, triggering apoptosis in human keratinocytes. Br J Dermatol. 2004; 150: 1070–1080. doi: 10.1111/j.1365-2133.2004.05919.x. [DOI] [PubMed] [Google Scholar]
- 23.Takahashi Y, Patel HP, Labib RS, Diaz LA, Anhalt GJ. Experimentally induced pemphigus vulgaris in neonatal BALB/c mice: a time-course study of clinical, immunologic, ultrastructural, and cytochemical changes. J Invest Dermatol. 1985; 84: 41–46. doi: 10.1111/1523-1747.ep12274679. [DOI] [PubMed] [Google Scholar]
- 24.Li N, Zhao M, Wang J, Liu Z, Diaz LA. Involvement of the apoptotic mechanism in pemphigus foliaceus autoimmune injuryof the skin. J Immunol. 2009; 182: 711–717. doi: 10.4049/jimmunol.182.1.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gniadecki R, Jemec GB, Thomsen BM, Hansen M. Relationship between keratinocyte adhesion and death: anoikis in acantholytic diseases. Arch Dermatol Res. 1998; 290: 528–532. doi: 10.1007/s004030050347. [DOI] [PubMed] [Google Scholar]
- 26.Florez-Moreno G, Henao-Ruiz M, Santa-Saenz D, Castanda-Pelaez D, Tobon-Arroyave S. Cytomorphometric and immunohistochemical comparison between central and peripheral giant cell lesions of the jaws. Oral Surg Oral Med oral Pathol oral Radiol Edod. 2008;105:625–632. doi: 10.1016/j.tripleo.2007.08.032. [DOI] [PubMed] [Google Scholar]
- 27.Deyhimi P, Hashemzadeh Z. Study of the biologic behavior of odontogenic kerato-cyst and orthokeratinaizedodontogenic cyst using TGF-alpha and P53 markers. Pathol Res Pract. 2014; 210: 201–204. doi: 10.1016/j.prp.2013.09.015. [DOI] [PubMed] [Google Scholar]
- 28.Grando SA, Bystryn JC, Chernyavsky AI, Frusić-Zlotkin M, Gniadecki R, Lotti R, et al. Apoptolysis: a novel mechanism of skin blistering in pemphigus vulgaris linking the apoptotic pathways to basal cell shrinkage and suprabasal acantholysis. Exp Dermatol. 2009; 18: 764–770. doi: 10.1111/j.1600-0625.2009.00934.x. [DOI] [PubMed] [Google Scholar]
- 29.Orlov MD, Chernyavsky AI, Arredondo J, Grando SA. Synergistic actions of pem-phigus vulgaris IgG, Fasligand and tumor necrosis factor-alpha during induction of basal cell shrinkage and acantholysis. Autoimmunity. 2006; 39: 557–562. doi: 10.1080/08916930600971729. [DOI] [PubMed] [Google Scholar]
- 30.Lippens S, Hoste E, Vandenabeele P, Agostinis P, Declercq W. Cell death in the skin. Apoptosis. 2009; 14: 549–569. doi: 10.1007/s10495-009-0324-z. [DOI] [PubMed] [Google Scholar]
- 31.Moravvej H, Yousefi M, Farrokhi B, Mosaffa N. Soluble Fas in pemphigus vul-garis. Arch Iran Med. 2011; 14: 200–201. [PubMed] [Google Scholar]
- 32.Pacheco-Tovar D, López-Luna A, Herrera-Esparza R, Avalos-Díaz E. The caspase pathway as a possible therapeutic target in experimental pemphigus. Autoimmune Dis. 2011; 2011: 563091. doi: 10.4061/2011/563091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ragab N, Abdallah M, El-Gohary E, Elewa R. Stress and serum TNF-alpha levels may predict disease outcome in patients with pemphigus: a preliminary study. Cutis. 2011; 87: 189–194. [PubMed] [Google Scholar]


