Abstract
MicroRNA (miR)-423-5p is a potential target for the diagnosis and therapy of heart failure and cancer. The present study aimed to investigate the expression and role of miR-423-5p in ovarian cancer. miR-423-5p expression in ovarian tissues and plasma collected from ovarian cancer patients and healthy volunteers was analyzed by polymerase chain reaction analysis. In addition, a cell proliferation assay, clonogenic assay and Matrigel-based assay were performed to evaluate the role of miR-423-5p in ovarian cancer cells. The results demonstrated that miR-423-5p was downregulated in ovarian cancer tissues and plasma from ovarian cancer patients, compared with healthy individuals. Of note, miR-423-5p expression in ovarian tissues and plasma was demonstrated to be inversely correlated with ovarian cancer progression. Transfection with miR-423-5p efficiently increased miR-423-5p expression in A2780-s and A2780-cp cells, which had low miR-423-5p expression. Ectopic overexpression of miR-423-5p reduced cell proliferation, colony formation and invasion of ovarian cancer cells. In conclusion, the present study indicated that miR-423-5p may serve as a diagnostic indicator and functions as a tumor suppressor in ovarian cancer.
Keywords: ovarian cancer, miR-423-5p, diagnosis, progression
Introduction
Ovarian cancer is the second most prevalent gynecological cancer type and the fifth most common cause of cancer-associated mortality in women, of which epithelial ovarian carcinoma is the most common pathological type, accounting for 85–90% of ovarian cancer cases (1–3). Consistent with the poor prognosis due to the extensive metastasis of ovarian cancer cells into the peritoneal cavity, the majority of ovarian cancer patients experience a relapse within 2 years (2,4). Despite the development of several approaches for targeted therapies for ovarian cancer, the prognosis of patients with advanced disease has not improved much in the last 2 decades (1,5,6). This may be due to chemoresistance and difficulties in early detection, as most patients are not diagnosed with ovarian cancer until reaching the advanced stages (stage III or IV) (7,8). Therefore, it is urgently required to identify novel therapeutic and diagnostic targets to help improve the prognosis of ovarian cancer patients.
MicroRNAs (miRNAs) are small RNA molecules of ~22 nucleotides in length that mediate the post-transcriptional regulation of gene expression by binding mainly to the 3′-end of mRNA transcripts to induce translational repression and/or mRNA degradation (9,10). miRNAs have numerous critical roles in the regulation of the proliferation, differentiation, apoptosis, invasion and metastasis of tumor cells (11–13). Emerging evidence suggests a role of miRNAs in cancer, with potential use as novel disease-associated biomarkers (14–16). Changes in tissue and circulating levels of miRNA have been described in esophageal cancer (17) and lung cancer (13). Accordingly, the development of miRNAs as diagnostic biomarkers and therapeutic targets for cancer is feasible, and has prospective clinical applications.
miR-423-5p was identified as a circulating biomarker for heart failure (18) and inducer of apoptosis in cardiomyocytes by targeting β-linked N-acetylglucosamine (O-GlcNAc) transferase (19). In tumors, miR-423-5p has been demonstrated to contribute to the development of malignant phenotypes and temozolomide resistance in glioblastoma (20), and increase autophagy in hepatocellular carcinoma cells (21). Furthermore, plasma miR-423-5p levels have promising potential to serve as a novel biomarker for colorectal cancer detection, particularly at its early stage (22). miR-423-5p was observed to respond to Sorafenib therapy for hepatocellular carcinoma, since 75% of patients with increased plasma miR423-5p levels achieved partial remission or stable disease after 6 months from the beginning of therapy (21). These results demonstrated the potential role of miR-423-5p in the diagnosis and therapy of cancer. However, the expression and role of miR-423-5p in ovarian cancer has remained to be determined, which was therefore the aim of the present study.
Materials and methods
Clinical samples
The subjects of the present study were 40 ovarian cancer patients treated at Sichuan Provincial People's Hospital (Chengdu, China) and the Second People's Hospital of Neijiang City (Neijiang, China) from January 2016 to May 2017. All patients were diagnosed with epithelial ovarian cancer and complete clinical data for these patients were available (age range, 52 to 75 years old; mean age, 61.2; 15 patients were diagnosed with metastasis; Table I). The control group consisted of 20 patients that had been diagnosed with ovarian endometriosis (age range 51 to 64 years old; mean age, 58.6). Human ovarian cancer tissues and normal ovarian endometriosis tissues were obtained with signed written informed consent under a general waiver from the Academic Medical Center institutional review board for the proper secondary use of human material (Sichuan, China). Fasting peripheral blood (5 ml) was drawn from each patient and placed in anti-coagulative tubes at room temperature for 30 min, followed by centrifugation at 4,000 × g for 5 min at 4°C. The plasma supernatant was collected and stored at −80°C until use. All of the experiments described were approved by the ethics committee of Sichuan Provincial People's Hospital (Chengdu, China).
Table I.
Clinicopathological characteristics of patients with ovarian cancer.
| Characteristic | N (%) |
|---|---|
| Age (years) | |
| ≤60 | 23 (57.5) |
| >60 | 17 (42.5) |
| TNM stage (%) | |
| I | 8 (20) |
| II–III | 19 (47.5) |
| IV | 13 (32.5) |
| Metastasis | |
| Yes | 15 (37.5) |
| No | 25 (62.5) |
TNM, tumor-nodes-metastasis.
Cell culture and transfection
The human ovarian cancer cell lines A2780s, A2780cp, SKOV3, CAOV3 and PA-1 were obtained from the American Type Culture Collection (Manassas, VA, USA), and the normal ovarian epithelial cell line HOEC and was obtained from Jennio Biotech (Guangzhou, China). HOEC cells were and passaged for <10 passages in the laboratory. The cells were cultured in Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) containing 10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.) at 37°C in a humidified atmosphere containing 5% CO2. The molecular agomir miR-423-5p expression system (cat. no. miR40004748-1-2) and negative control (miR-NC; cat. no. miR04201-1-10) were purchased from Guangzhou RiboBio Co., Ltd. (Guangzhou, China). miR-423-5p and miR-NC were transfected into cells using miRNA transfection agent (riboFECT CP; cat. no. C10511; RiboBio Co., Ltd., Guangzhou, China) following the manufacturer's protocol.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR) analysis
Total RNA was extracted from ovarian (cancer) tissues and cell using TRIzol® reagent (Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol. The miRNeasy Serum/Plasma kit (cat. no. 217184; Qiagen, Hilden, Germany) was used to extract miRNA from plasma. qPCR for miR-423-5p was performed using miRNA primers obtained from Guangzhou RiboBio Co., Ltd. The sequences were designed with the Bulge-Loop™ primer set, but not specified due to the rules of the company. RT was performed on the isolated total RNA using a Reverse Transcription kit (cat. no. RR047A; Takara Bio, Inc., Otsu, Japan) and qPCR was performed using a Real Time PCR kit (cat. no. RR430A; Takara Bio, Inc.). RT was performed using 1 µg total RNA in 2 µl water and the reaction conditions were 65°C for 5 min, 30°C for 10 min, 42°C for 10–30 min and 2°C for 3 min. The qPCR conditions were as follows: Denaturation at 94°C for 2 min, amplification for 30 cycles at 94°C for 0.5 min, annealing at 58°C for 0.5 min and extension at 72°C for 1 min, followed by a terminal elongation step at 72°C for 10 min. The qPCR analysis was performed on a Bio-Rad CFX96 thermal cycler (Bio-Rad Laboratories, Inc., Hercules, CA, USA). mRNA expression was quantified using the 2−ΔΔCq method (23). RT and qPCR Experiments were performed three times. U6 was used as the internal control.
Cell viability assay
At 48 h post miR-423-5p and miR-NC transfection, the transfected cells were collected and counted. Cells were seeded in 96-well plates at 1,000 cells/well in 0.1 ml DMEM with 10% FBS. Following 0, 24, 48 and 72 h of incubation, a Cell Counting Kit-8 (CCK-8; Dojindo, Shanghai, China) was used to determine cell viability. Subsequent to 3 h of incubation with CCK-8 reagent, the absorbance of each well was measured at 450 nm using a micro-plate reader (Thermo Fisher Scientific, Inc.). Four independent experiments were performed.
Colony formation assay
At 48 h post miR-423-5p and miR-NC transfection, the transfected cells were collected and counted. Cells were seeded on 6-well plates at 1,000 cells/well in 2.0 ml DMEM with 10% FBS. The cells were cultured for ~14 days and then fixed with 4% paraformaldehyde for 15 min, followed by staining with crystal violet (Beyotime Institute of Biotechnology, Haimen, China) at room temperature for 15 min. The number of colonies (>50 cells) in each well was counted and analyzed. Three independent experiments were performed.
Matrigel®-based invasion assay
The Matrigel®-based invasion assay was performed as described in a previous study (24). miR-423-5p and miR-NC transfected cells (2,000 cells) in serum-free medium (0.1 ml) were seeded in the upper chamber of a 24-well Transwell insert (pore size, 8 µm; BD Biosciences, Bedford, MA, USA) with the filter pre-coated with 50 µl Matrigel® (BD Biosciences) diluted at 1:5 in DMEM medium. The lower chambers were filled with 500 µl DMEM containing 10% FBS as a chemoattractant. After 48 h of incubation, the cells that had not invaded through the pores were carefully wiped off with a wet cotton swab and the inserts were fixed with 4% paraformaldehyde for 15 min at room temperature, followed by staining with crystal violet (Beyotime Institute of Biotechnology) for 15 min at room temperature. The number of invaded cells in each millicell was counted and analyzed. Three independent experiments were performed.
Statistical analysis
Values are expressed as the mean ± standard deviation. For statistical comparison of quantitative data between two groups, Students' t-test was performed. If multiple groups were present, one-way analysis of variance followed by Dunnett's multiple comparisons test was used. All statistical analyses were performed using SPSS 20.0 statistical software (SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
miR-423-5p is downregulated in ovarian cancer
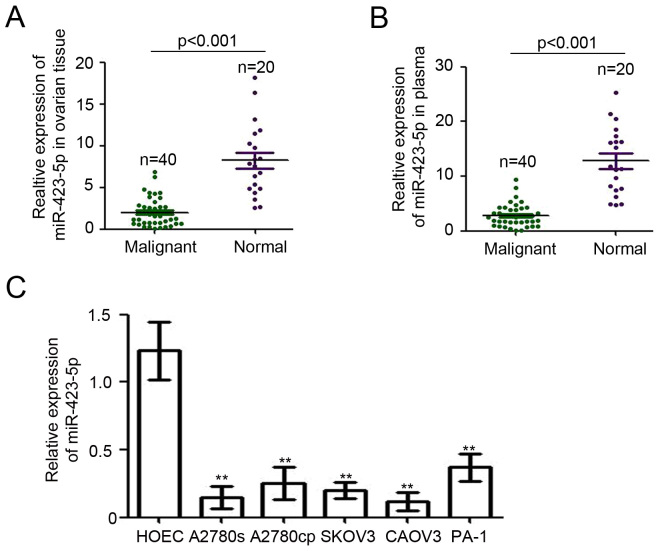
To determine the expression of miR-423-5p in patients with ovarian cancer, RT-qPCR was performed. Epithelial ovarian tissues of 40 ovarian cancer patients with different tumor grades (Table I) and 20 adjacent noncancerous (endometriosis) ovarian tissues were collected for this analysis. Compared with that in ovarian endometriosis tissues, miR-423-5p expression relative to U6 was significantly reduced in ovarian cancer tissues (8.24±0.97 vs. 2.0±0.27; Fig. 1A). Furthermore, miR-423-5p levels in plasma were also determined. As presented in Fig. 1B, the levels of miR-423-5p in the plasma from ovarian cancer patients were determined to be lower than those in patients with ovarian endometriosis (2.80±0.32 vs. 12.79±1.36). Next, the expression of miR-423-5p was examined in ovarian cancer cell lines. The normal ovarian epithelial cell line HOEC was used as a normal control. The results indicated that miR-423-5p expression was downregulated in all ovarian cancer cell lines compared with that in the normal ovarian epithelial cell line (Fig. 1C). Collectively, these results demonstrated the downregulation of miR-423-5p in the tumor tissues and plasma of ovarian cancer patients as well as in ovarian cancer cell lines.
Figure 1.
miR-423-5p is downregulated in ovarian cancer. miR-423-5p expression was determined in (A) ovarian tumor tissues of 40 ovarian cancer patients and 20 ovarian endometriosis tissues, (B) plasma samples of 40 ovarian cancer patients and 20 plasma samples of healthy controls and in (C) the normal ovarian epithelial cell line HOEC and the ovarian cancer cell lines A2780s, A2780cp, SKOV3, CAOV3 and PA-1. Reverse transcription-quantitative polymerase chain reaction was employed and U6 was used as a loading control. **P<0.01 vs. HOEC. miR, microRNA.
miR-423-5p is inversely associated with ovarian cancer progression
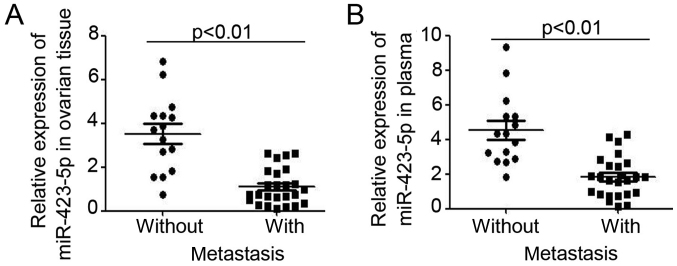
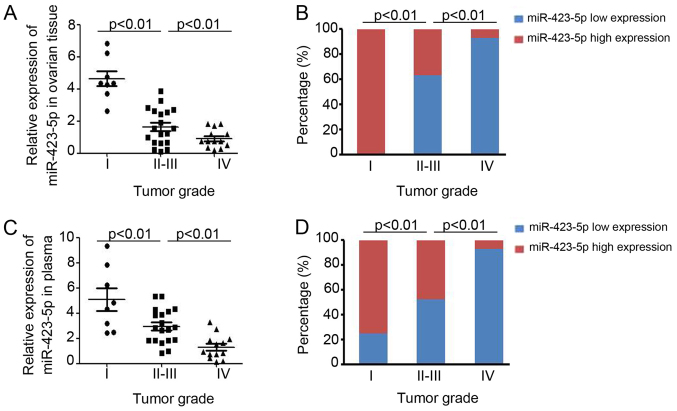
Next, the relative expression of miR-423-5p was analyzed in ovarian cancer patients stratified into two groups depending on the absence or presence of metastasis. As presented in Fig. 2A, lower miR-423-5p expression was demonstrated in ovarian cancer tissues from patients with metastasis. Furthermore, analysis of miR-423-5p levels in plasma also indicated lower levels in ovarian cancer patients with metastasis (Fig. 2B). miR-423-5p expression in ovarian tissues and plasma was then compared for ovarian cancer patients at different stages, which were stratified using International Federation of Gynecology and Obstetrics staging (25) (Fig. 3). In ovarian cancer patients with a higher tumor stage, lower expression of miR-423-5p was demonstrated in ovarian cancer tissues (stage I vs. stage II–III vs. stage IV: 4.64±0.47 vs. 1.64±0.26 vs. 0.92±0.16; Fig. 3A) and in plasma (stage I vs. stage II–III vs. stage IV: 5.08±0.89 vs. 2.13±0.75 vs. 1.29±0.27; Fig. 3C). Further analysis also demonstrated that miR-423-5p expression is inversely correlated with the tumor grade (Fig. 3B and D). Collectively, these results proved the capacity of miR-423-5p levels in ovarian cancer tissues and plasma to indicate ovarian cancer progression.
Figure 2.
miR-423-5p is downregulated in ovarian cancer with metastasis. The ovarian cancer patients were divided into two groups: Patients with metastasis or without metastasis. The expression of miR-423-5p in (A) Primary ovarian tumor tissues and in (B) plasma of the two groups was analyzed. miR, microRNA.
Figure 3.
miR-423-5p is inversely associated with ovarian cancer progression. The patients were divided into groups according to their tumor stages (I, II–III and IV) determined based on the clinical diagnostic criteria. (A) Expression of miR-423-5p in ovarian tissues and (B) percentage of patients with high and low expression of mir-423-5p in ovarian tissues of different tumor stages. (C) Expression of miR-423-5p in plasma and (D) percentage of patients with high and low expression of mir-423-5p in plasma of patients with different tumor stages. miR, microRNA.
Overexpression of miR-423-5p inhibits ovarian cancer cell proliferation
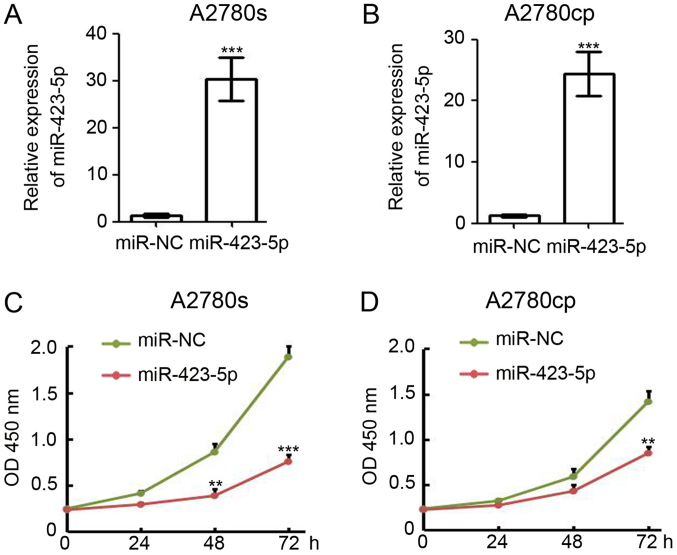
Based on the results obtained with the clinical samples in the present study, miR-423-5p is a potential tumor suppressor in ovarian cancer. Thus, a gain-of-function approach was employed to investigate the function of miR-423-5p in A2780s and A2780cp cells. The cells were transfected with miR-423-5p and miR-NC (negative control) and collected for total RNA extraction at 48 h post-transfection. RT-qPCR analysis indicated that miR-423-5p was efficiently expressed in miR-423-5p-transfected A2780s cells (miR-423-5p/miR-NC expression ratio, 30.3±2.7; Fig. 4A) and A2780cp cells (miR-423-5p/miR-NC expression ratio, 24.4±2.1; Fig. 4B). To determine whether miR-423-5p has any effect on cell proliferation in vitro, 1,000 miR-423-5p and miR-NC transfected cells were seeded into the wells of a 96-well plate for the CCK-8 assay. The results indicated that ectopic expression of miR-423-5p significantly reduced the proliferation of A2780s cells (Fig. 4C). miR-423-5p expression also markedly reduced A2780cp cell proliferation (Fig. 4D). Collectively, these results suggest that miR-423-5p suppresses ovarian cancer cell proliferation.
Figure 4.
Overexpression of miR-423-5p inhibits ovarian cancer proliferation. (A and B) At 48 h post miR-NC and miR-423-5p transfection, reverse transcription-quantitative polymerase chain reaction was employed to detect miR-423-5p expression in (A) A2780s and (B) A2780cp cells. U6 was used as a loading control. Experiments were performed in triplicate. (C and D) A Cell Counting Kit-8 assay was used to determine the viability of (C) A2780s and (D) A2780cp cells at 0, 24, 48 and 72 h post miR-NC and miR-423-5p transfection. **P<0.01, ***P<0.001 vs. miR-NC group. miR, microRNA; miR-NC, scrambled control miR; OD, optical density.
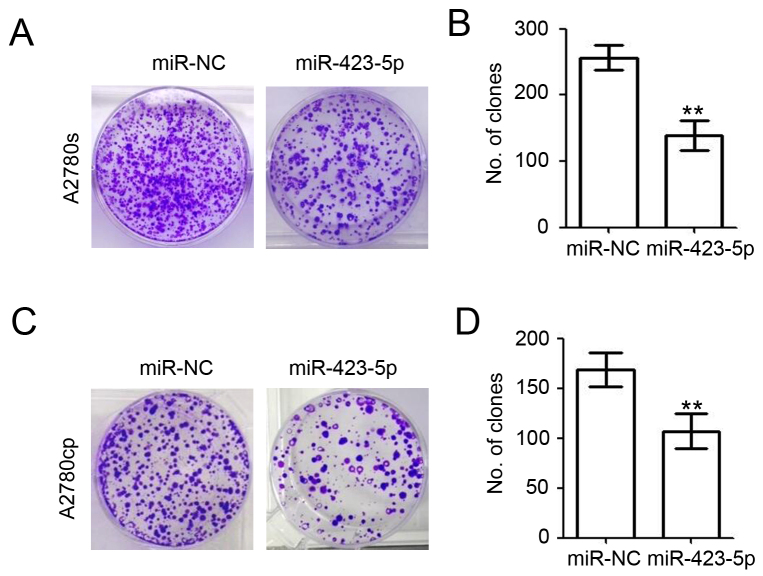
Overexpression of miR-423-5p impairs colony formation of ovarian cancer cells
A colony formation assay was performed to further investigate the roles of miR-423-5p in ovarian cancer (Fig. 5). At 48 h post miR-NC and miR-423-5p transfection, 1,000 A2780s and A2780cp cells were seeded into the wells of a 6-well plate containing 2 ml DMEM with 10% FBS. Ten days later, crystal violet was used to stain the colonies of A2780s (Fig. 5A) and A2780cp cells (Fig. 5C). The results also demonstrated the inhibitory role of miR-423-5p regarding the colony formation ability of A2780s cells (miR-423-5p vs. miR-NC: 132.0±5.7 vs. 187.3±10.3; decreased by 29.5%; Fig. 5B). Overexpression of miR-423-5p also impaired the colony formation ability of A2780cp cells (miR-423-5p vs. miR-NC: 157.3±7.8 vs. 256.7±10.9; decreased by 38.7%; Fig. 5D). These results demonstrated the inhibitory role of miR-423-5p regarding the colony formation of ovarian cancer cells.
Figure 5.
Overexpression of miR-423-5p impairs colony formation of ovarian cancer cells. At 48 h post miR-NC and miR-423-5p transfection, A2780s and A2780cp cells were seeded in a 6-well plate at 1,000 cells/well in complete medium. Ten days later, crystal violet was used to stain the colonies of (A and B) A2780s and (C and D) A2780cp cells was counted and quantified. Experiments were performed in triplicate. **P<0.01 vs. miR-NC group. miR, microRNA; miR-NC, scrambled control miR.
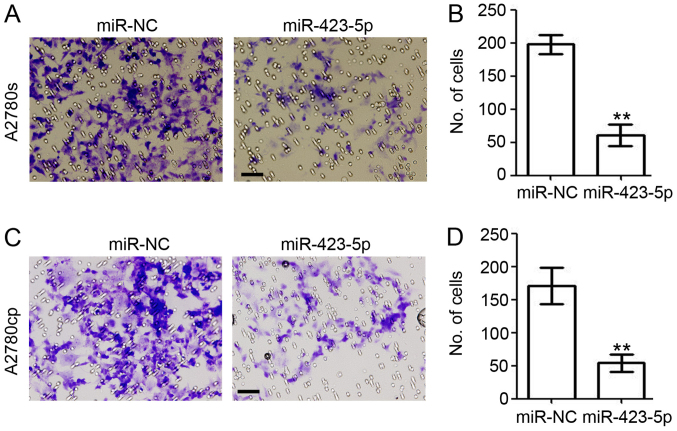
Overexpression of miR-423-5p reduces the invasion ability of ovarian cancer
Next, the present study investigated the role of miR-423-5p in cell invasion by performing a Matrigel®-based invasion assay (Fig. 6). In the miR-423-5p-transfected A2780s cells, the amount of invaded cells was reduced by 70.4% (miR-423-5p vs. miR-NC: 60.7±9.2 vs. 198.0±8.2; Fig. 6A and B). In A2780cp cells, ectopic expression of miR-423-5p also significantly reduced cell invasion (miR-423-5p vs. miR-NC: 54.0±7.4 vs. 171.0±15.9; Fig. 6C and D). Collectively, the present results further demonstrated the role of miR-423-5p in inhibiting the invasion of ovarian cancer cells.
Figure 6.
Overexpression of miR-423-5p reduces the invasion ability of ovarian cancer. At 48 h post miR-NC and miR-423-5p transfection, A2780s and A2780cp cells were collected and subjected to a Matrigel-based invasion assay. The number of invaded (A and B) A2780s and (C and D) A2780cp cells per field of view were counted and analyzed. Scale bar=100 µm. Experiments were performed in triplicate. **P<0.01 vs. miR-NC group. miR, microRNA; miR-NC, scrambled control miR.
Discussion
Early detection has long been key to the successful treatment of multiple life-threatening diseases, including ovarian cancer. It has been reported that miR-141 and miR-200a/b/c are the most significantly overexpressed miRs, whereas miR-199, miR140, miR-145 and miR-125b are significantly downregulated in ovarian cancer (26). Decreased miR-145 expression was detected in serum of patients with malignant and benign ovarian tumors compared with that in healthy controls, and miR-145 may potentially serve as a biomarker for the detection of ovarian cancer (27). The present study was the first to report that miRNA-423-5p is downregulated in ovarian tissues and plasma from ovarian cancer patients. miR-423-5p expression in ovarian tissue and plasma was identified to be significantly associated with metastasis and tumor progression of ovarian cancer. Furthermore, miR-423-5p was indicated to function as a tumor suppressor in ovarian cancer by inhibiting cell proliferation, colony formation and invasion. Taken together, the present results suggest that miRNA-423-5p functions as a tumor suppressor in ovarian cancer and may potentially be utilized as a negative diagnostic indicator.
Aberrant expression of miRNA-423-5p has been reported in several cancer types. The plasma levels of miR-423-5p were decreased in patients with colon cancer, but increased in patients with inflammatory bowel disease (22). The sensitivity of miR-423-5p in detecting colon cancer at the early stage was determined as 88.89% and the plasma concentration of miR-423-5p was increased in patients with clinical improvement after the surgery (22). Secretory miR-423-5p was upregulated in vitro and in vivo by sorafenib treatment and its increase was correlated with the response to therapy in hepatocarcinoma (21). Furthermore, in pancreatic cancer, miR-423-5p was either downregulated or upregulated with a significant inter-individual variation (28). However, miR-423-5p was demonstrated to be overexpressed in glioma tissues and corresponding glioma stem cells (29). In the present study, the ovarian tissue and plasma miRNA-423-5p levels in ovarian cancer patients were detected by RT-qPCR, and patients with ovarian endometriosis served as control subjects. The results indicated that the average miRNA-423-5p expression was markedly lower in the ovarian tumor tissues and plasma of ovarian cancer patients compared with that in the samples of ovarian endometriosis patients. Subgroup analysis revealed that the expression levels of miRNA-423-5p were lower in the ovarian cancer tissue and plasma of patients with a higher tumor stage or those with tumor metastasis. The heterogeneity of miR-423-5p expression may be caused by the heterogeneity of the tumor tissues. Collectively, these results demonstrated that miR-423-5p is a potential diagnostic biomarker and an indicator of tumor progression in ovarian cancer. In the future, in order to analyze the receiver operator characteristic curves for ovarian cancer diagnosis by plasma miRNA-423-5p, more clinical samples should be collected for the determination of miR-423-5p levels. Furthermore, the survival data of ovarian cancer patients should be collected for exploring the correlation between miR-423-5p expression and the clinical outcome for ovarian cancer patients.
Previous studies have demonstrated that in different tumor types, miR-423-5p may have the opposite role to that in ovarian cancer. In gastric cancer, miRNA-423-5p was reported to participate in proliferation/invasion-associated processes via negatively regulating the expression of trefoil factor 1, which is a tumor suppressor gene, in the stomach (30). Furthermore, miR-423-5p expression enhanced glioma cell proliferation, angiogenesis and invasion via targeting inhibitor of growth family member 4 and activating important signaling molecules, including AKT and extracellular signal-regulated kinase 1/2 (31). In another study, miR-423-5p knockdown notably enhanced the inhibitory effect of apigenin on the proliferation of glioma stem cells and the promotion of their apoptosis through the mitochondrial pathway (29). However, hepatocellular cancer cells transfected with miR-423-5p exhibited an increase in the S-phase population of the cell cycle, paralleled by a similar-size increase in autophagic cells (21). miR-423-5p was indicated to be an inducer of apoptosis in cardiomyocytes by targeting O-GlcNAc transferase (19). The present results suggested that miR-423-5p functions as a tumor suppressor in ovarian cancer according to its inhibitory role on cell proliferation, colony formation and cell invasion. However, the direct targets of miR-423-5p in ovarian cancer remain to be elucidated and further in-depth research is required.
In conclusion, miR-423-5p in the tumor tissues and plasma of ovarian cancer patients was indicated to be inversely associated with metastasis and tumor progression. Therefore, miRNA-423-5p may serve as an important molecular marker for the diagnosis of ovarian cancer and an indicator of its progression. In the future, the correlation between miR-423-5p expression and the clinical outcome of ovarian cancer patients should be further explored.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
Data and materials supporting the findings of this study are available within the article.
Authors' contributions
XT, XZ and YH were involved in acquisition of the data. SC and FL were involved in the analysis and interpretation of the data. XZ, GY and NY were involved in the collection of human tissues. NY was involved in the conception and design of the present study.
Ethics approval and consent to participate
Human ovarian cancer tissues and ovarian tissues from patients with ovarian endometriosis were obtained with written and signed informed consent under a general waiver for the proper secondary use of human material by the institutional review board of the Academic Medical Center (Chengdu, China) and were obtained from Sichuan Provincial People's Hospital and The Second People's Hospital of Neijiang City (Neijiang, China).
Consent for publication
Not applicable.
Competing interests
All authors declare that there are no competing interests.
References
- 1.Chang SJ, Bristow RE, Chi DS, Cliby WA. Role of aggressive surgical cytoreduction in advanced ovarian cancer. J Gynecol Oncol. 2015;26:336–342. doi: 10.3802/jgo.2015.26.4.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kujawa KA, Lisowska KM. Ovarian cancer-from biology to clinic. Postepy Hig Med Dosw (Online) 2015;69:1275–1290. doi: 10.5604/17322693.1184451. [DOI] [PubMed] [Google Scholar]
- 3.Dinkelspiel HE, Champer M, Hou J, Tergas A, Burke WM, Huang Y, Neugut AI, Ananth CV, Hershman DL, Wright JD. Long-term mortality among women with epithelial ovarian cancer. Gynecol Oncol. 2015;138:421–428. doi: 10.1016/j.ygyno.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Devi Uma K, Purushotham N, Jayashree N. Management of ovarian cancer in younger women. Rev Recent Clin Trials. 2015;10:263–269. doi: 10.2174/1574887110666150923112047. [DOI] [PubMed] [Google Scholar]
- 5.Gasparri ML, Attar R, Palaia I, Perniola G, Marchetti C, Di Donato V, Farooqi AA, Papadia A, Panici PB. Tumor infiltrating lymphocytes in ovarian cancer. Asian Pac J Cancer Prev. 2015;16:3635–3638. doi: 10.7314/APJCP.2015.16.9.3635. [DOI] [PubMed] [Google Scholar]
- 6.Grisham RN, Hyman DM, Iyer G. Targeted therapies for treatment of recurrent ovarian cancer. Clin Adv Hematol Oncol. 2014;12:158–162. [PubMed] [Google Scholar]
- 7.Au KK, Josahkian JA, Francis JA, Squire JA, Koti M. Current state of biomarkers in ovarian cancer prognosis. Future Oncol. 2015;11:3187–3195. doi: 10.2217/fon.15.251. [DOI] [PubMed] [Google Scholar]
- 8.Dong X, Men X, Zhang W, Lei P. Advances in tumor markers of ovarian cancer for early diagnosis. Indian J Cancer. 2014;51(Suppl 3):e72–e76. doi: 10.4103/0019-509X.154049. [DOI] [PubMed] [Google Scholar]
- 9.Slezak-Prochazka I, Durmus S, Kroesen BJ, van den Berg A. MicroRNAs, macrocontrol: Regulation of miRNA processing. RNA. 2010;16:1087–1095. doi: 10.1261/rna.1804410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu J, Wang S, Zhang W, Qiu J, Shan Y, Yang D, Shen B. Screening key microRNAs for castration-resistant prostate cancer based on miRNA/mRNA functional synergistic network. Oncotarget. 2015;6:43819–43830. doi: 10.18632/oncotarget.6102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun X, Zhang J. Dysfunctional miRNA-mediated regulation in chromophobe renal cell carcinoma. PLoS One. 2016;11:e0156324. doi: 10.1371/journal.pone.0156324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sathyapalan T, David R, Gooderham NJ, Atkin SL. Increased expression of circulating miRNA-93 in women with polycystic ovary syndrome may represent a novel, non-invasive biomarker for diagnosis. Sci Rep. 2015;5:16890. doi: 10.1038/srep16890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen Y, Min L, Ren C, Xu X, Yang J, Sun X, Wang T, Wang F, Sun C, Zhang X. miRNA-148a serves as a prognostic factor and suppresses migration and invasion through Wnt1 in non-small cell lung cancer. PLoS One. 2017;12:e0171751. doi: 10.1371/journal.pone.0171751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zedan AH. Heterogeneity of miRNA expression in localized prostate cancer with clinicopathological correlations. PLoS One. 2017;12:e0179113. doi: 10.1371/journal.pone.0179113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gautam A, Kumar R, Dimitrov G, Hoke A, Hammamieh R, Jett M. Identification of extracellular miRNA in archived serum samples by next-generation sequencing from RNA extracted using multiple methods. Mol Biol Rep. 2016;43:1165–1178. doi: 10.1007/s11033-016-4043-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakamura K, Sawada K, Yoshimura A, Kinose Y, Nakatsuka E, Kimura T. Clinical relevance of circulating cell-free microRNAs in ovarian cancer. Mol cancer. 2016;15:48. doi: 10.1186/s12943-016-0536-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li SP, Su HX, Zhao D, Guan QL. Plasma miRNA-506 as a prognostic biomarker for esophageal squamous cell carcinoma. Med Sci Monitor. 2016;22:2195–2201. doi: 10.12659/MSM.899377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tijsen AJ, Creemers EE, Moerland PD, de Windt LJ, van der Wal AC, Kok WE, Pinto YM. MiR423-5p as a circulating biomarker for heart failure. Circ Res. 2010;106:1035–1039. doi: 10.1161/CIRCRESAHA.110.218297. [DOI] [PubMed] [Google Scholar]
- 19.Luo P, He T, Jiang R, Li G. MicroRNA-423-5p targets O-GlcNAc transferase to induce apoptosis in cardiomyocytes. Mol Med Rep. 2015;12:1163–1168. doi: 10.3892/mmr.2015.3491. [DOI] [PubMed] [Google Scholar]
- 20.Li S, Zeng A, Hu Q, Yan W, Liu Y, You Y. miR-423-5p contributes to a malignant phenotype and temozolomide chemoresistance in glioblastomas. Neuro Oncol. 2017;19:55–65. doi: 10.1093/neuonc/now129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stiuso P, Potenza N, Lombardi A, Ferrandino I, Monaco A, Zappavigna S, Vanacore D, Mosca N, Castiello F, Porto S, et al. MicroRNA-423-5p promotes autophagy in cancer cells and is increased in serum from hepatocarcinoma patients treated with sorafenib. Mol Ther Nucleic Acids. 2015;4:e233. doi: 10.1038/mtna.2015.8. [DOI] [PubMed] [Google Scholar]
- 22.Fang Z, Tang J, Bai Y, Lin H, You H, Jin H, Lin L, You P, Li J, Dai Z, et al. Plasma levels of microRNA-24, microRNA-320a and microRNA-423-5p are potential biomarkers for colorectal carcinoma. J Exp Clin Cancer Res. 2015;34:86. doi: 10.1186/s13046-015-0198-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 24.Dai L, Cui X, Zhang X, Cheng L, Liu Y, Yang Y, Fan P, Wang Q, Lin Y, Zhang J, et al. SARI inhibits angiogenesis and tumour growth of human colon cancer through directly targeting ceruloplasmin. Nat Commun. 2016;7:11996. doi: 10.1038/ncomms11996. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 25.Falcetta FS, Lawrie TA, Medeiros LR, da Rosa MI, Edelweiss MI, Stein AT, Zelmanowicz A, Moraes AB, Zanini RR, Rosa DD. Laparoscopy vs. laparotomy for FIGO stage I ovarian cancer. Cochrane Database Syst Rev. 2016;10:CD005344. doi: 10.1002/14651858.CD005344.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iorio MV, Visone R, Di Leva G, Donati V, Petrocca F, Casalini P, Taccioli C, Volinia S, Liu CG, Alder H, et al. MicroRNA signatures in human ovarian cancer. Cancer Res. 2007;67:8699–8707. doi: 10.1158/0008-5472.CAN-07-1936. [DOI] [PubMed] [Google Scholar]
- 27.Liang H, Jiang Z, Xie G, Lu Y. Serum microRNA-145 as a novel biomarker in human ovarian cancer. Tumour Biol. 2015;36:5305–5313. doi: 10.1007/s13277-015-3191-y. [DOI] [PubMed] [Google Scholar]
- 28.Ali S, Saleh H, Sethi S, Sarkar FH, Philip PA. MicroRNA profiling of diagnostic needle aspirates from patients with pancreatic cancer. Br J Cancer. 2012;107:1354–1360. doi: 10.1038/bjc.2012.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wan Y, Fei X, Wang Z, Jiang D, Chen H, Wang M, Zhou S. miR-423-5p knockdown enhances the sensitivity of glioma stem cells to apigenin through the mitochondrial pathway. Tumour Biol. 2017;39:1010428317695526. doi: 10.1177/1010428317695526. [DOI] [PubMed] [Google Scholar]
- 30.Liu J, Wang X, Yang X, Liu Y, Shi Y, Ren J, Guleng B. miRNA423-5p regulates cell proliferation and invasion by targeting trefoil factor 1 in gastric cancer cells. Cancer Lett. 2014;347:98–104. doi: 10.1016/j.canlet.2014.01.024. [DOI] [PubMed] [Google Scholar]
- 31.Li S, Zeng A, Hu Q, Yan W, Liu Y, You Y. miR-423-5p contributes to a malignant phenotype and temozolomide chemoresistance in glioblastomas. Neuro Oncol. 2017;19:55–65. doi: 10.1093/neuonc/now129. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data and materials supporting the findings of this study are available within the article.