Abstract
Objective(s):
Trans-chalcone as the parent member of the chalcone series reduces circulating levels of insulin and glucose. However, the cellular mechanism of these effects is poorly understood. Sirtuin 1 (SIRT1) as a direct target of miR-34a controls homeostasis of glucose, and also improves insulin sensitivity. Therefore, the present study for the first time investigated the influence of trans-chalcone on the miR-34a/SIRT1 pathway as a possible mechanism for its hypoglycemic and hypoinsulinemic effects.
Materials and Methods:
In this study, thirty male rats were randomly divided into three groups (n=10): solvent control (NS), oral administration of trans-chalcone for 2 (N2T) and 6 weeks (N6T) groups. Then, hepatic levels of miR-34a and SIRT1 were measured through the qRT-PCR method.
Results:
Trans-chalcone reduced food intake, body weight gain, and serum glucose as well as insulin levels. Also, this chalcone inhibited hepatic miR-34a expression and significantly elevated SIRT1 mRNA level.
Conclusion:
Trans-chalcone as an insulin-sensitizing chalcone partly acts through the miR-34a/SIRT1 pathway.
Keywords: Liver, MiR-34a, Rat, SIRT1, Trans-chalcone
Introduction
Chalcones are the main class of natural compounds which are plentiful in eatable plants. These compounds have been subject of attention because of their wide biological activities such as anti-diabetic, antioxidant, anti-inflammatory, and hepatoprotective effects (1-5). Trans-chalcone with a simple structure is a flavonoids precursor and a parent of chalcone series (3, 6-8). It has been shown that trans-chalcone reduces the body weights and circulating levels of glucose and insulin in healthy and diabetic rats. Therefore, it is possible that trans-chalcone may have an insulin-like influence in these animals (9). Hyperinsulinemia and hyperglycemia are characteristics of insulin resistance, which is the main feature of metabolic syndrome (10, 11).
Micro-ribonucleic acids (miRNAs) are small noncoding RNAs that negatively control the expression of their target genes in post-transcriptional level. Certain miRNAs regulate insulin signalling and affect insulin resistance (12). One of the best-studied miRNAs which has important roles in glucose homeostasis and insulin production is miR-34a (12). This miRNA directly targets and inhibits expression of Sirtuin 1 (SIRT1) as a NAD-dependent protein deacetylase with anti-aging properties. It has been shown that SIRT1 protects mammals against metabolic disease (13-16). The main physiologic effects of this deacetylase are regulation of metabolic pathways in the metabolic tissues (liver, adipose tissue, and pancreas) and reaction to oxidative stress (14, 15, 17). In this case, SIRT1 regulates insulin secretion, lipid metabolism and homeostasis of glucose, and also improves insulin sensitivity (10, 14, 16).
The liver has a key role in the insulin-dependent regulation of glucose homeostasis. In the insulin resistance conditions, low hepatic response to the insulin hormone leads to hyperglycemia (18). Some previous studies have suggested that flavonoids exert their positive effects on health and metabolic diseases by regulation of hepatic miRNAs (19-25). Therefore, hypoglycemic and hypoinsulinemic effects of trans-chalcone may be partly due to its effect on the miR-34a/SIRT1 pathway. This study for the first time investigated effects of trans-chalcone on miR-34a and SIRT1 in male rats.
Materials and Methods
Animals
Male Wistar rats (200–250 g) were obtained and kept in a room under standard conditions (22 °C, 12 hr light:12 hr dark cycle). Animals had free access to water and normal chow. Animal studies were directed according to the Regulations for the Use and Care of Laboratory Animals (1996, published by National Academy Press, 2101 Constitution Ave. NW, Washington, DC 20055, USA) and Animal Care Review Committee of Tabriz University of Medical Sciences (IR.TBZMED.REC.1395.582).
Experimental protocol
After the acclimatization period (1 week), thirty male rats were randomly divided into three groups (n=10): NS (10% Tween 80 as trans-chalcone solvent (26), for 6 weeks by daily oral gavage), N2T (trans-chalcone 20 mg/kg (3, 9) in 5th and 6th weeks by daily oral gavage), and N6T (trans-chalcone 20 mg/kg for 6 weeks by daily oral gavage) (Figure 1). At the beginning and the end of the treatment period, the body weights and the food intakes were measured in all groups.
Figure 1.
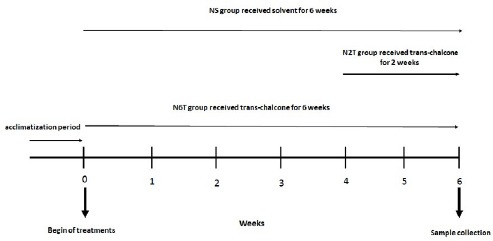
Study design and experimental groups
After 8 hr fasting, rats were deeply anesthetized by intraperitoneal injection of a combination of ketamine (60 mg/kg) and xylazine (10 mg/kg) (27). Then, the blood samples were obtained from inferior vena cava for biochemical measurements and the liver samples were immediately excised for gene expression analysis. Biochemical measurements (serum insulin and glucose levels) were done by ELISA kit (Rat insulin ELISA; Mercodia, Uppsala, Sweden) and a commercial kit (Pars Azmoon, Tehran, Iran), respectively. Also, gene expressions (miR-34a and SIRT1 mRNA) were assessed by real-time PCR.
Real-time quantitative PCR
To determine the expression levels of hepatic miR-34a and SIRT1, total RNA including miRNA and mRNA was extracted from liver samples with the miR-amp kit (Parsgenome Co, Iran) and RNX-Plus solution kit (Fermentase, Cinagen Co Iran). Complementary DNA (cDNA) synthesis from mRNA samples, was done using Thermo Fisher Scientific Revert Aid Reverse Transcriptase in accordance with manufacturer’s instructions. In brief, total RNA (3 μg) was reversed to cDNA using 1 µl of M-MuLV RevertAid Reverse Transcriptase (200 U/µl), 1 μl of random hexamer primers (100 μM), 2 µl of dNTPs (10 mM), and 0.5 µl of Thermo Fisher Scientific RiboLock RNase-inhibitor (40 U/µl), incubated for 10 min at 25 °C, then 60 min at 42 °C in a final volume of 20 µl. The reaction was ended by heating at 70 °C for 5 min. Moreover, synthesis of cDNA from miRNA samples was performed using the miR-amp kit (Parsgenome Co Iran). Real-time PCR was done by means of SYBR Green PCR Master Mix (Fermentase, Cinagen Co Iran) on a real-time PCR machine (Rotor-Gene 3000) (28). Housekeeping (reference) genes for SIRT1 mRNA and miR-34a were β-actin and miR-191, respectively. Relative quantitative expressions of these genes were determined through the 2−ΔΔCt method as described previously (27). Sequences of miR-34a and SIRT1, as well as β-actin and miR-191 primers, are listed in Table 1.
Table 1.
Primers set list for mRNAs and miRNAs
| Gene | Primers Sequencea | Gene bank Accession number | Product length (bp) |
|---|---|---|---|
| SIRT1 | F: AGG GAACCTCTGCCTCATCTAC R: GGCATACTCGCCACCTAA |
XM_008772947.2 | 100 |
| β-actin | F: TACAGCTTCACCACCACAGC R: AT GCC ACA GGA TTC CAT ACC |
NM_031144.3 | 190 |
| Gene name | Accession number | Target sequenceb | |
| rno-miR-34a-5p | MIMAT0000815 | UGGCAGUGUCUUAGCUGGUUGU | |
| rno-miR-191-5p | MIMAT0000866 | CAACGGAAUCCCAAAAGCAGCUG |
Sequences were derived from NCBI (www.ncbi.nlm.nih.gov)
Sequence was derived from miRBase (www.mirbase.org)
Statistical analysis
All data are expressed as mean±SEM. The SPSS 16.0 software (SPSS Inc., IL, U.S.A.) was used for analysis of data. Because of the normal distribution of data, which was confirmed by Shapiro-Wilk analysis, the differences between groups were assessed by one-way analysis of variance (ANOVA) followed by Tukey’s Post hoc test and P<0.05 was considered statistically significant.
Results
The effect of trans-chalcone on food intake and body weight gain
Analysis of the food intake data revealed that in the initiation of the study, this variable was not significantly different between different groups (Data are not shown), but trans-chalcone significantly reduced (P<0.05) food consumption after 2 and 6 weeks of treatment (Figure 2) compared with the NS group. Consistent with reduction of food intake, trans-chalcone significantly decreased (P<0.001) body weight gain in the N6T group compared with NS (Figure 3). However, there was no significant change in body weight gain of N2T compared with NS group.
Figure 2.
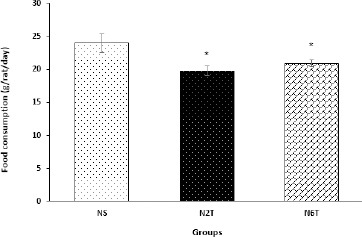
Last day food consumption in the study groups. Food consumption was significantly reduced in N2T and N6T groups compared with the NS group (NS: solvent control, N2T: trans-chalcone for 2 weeks, N6T: trans-chalcone for 6 weeks). Data were expressed as mean±SEM. * P<0.05 versus NS
Figure 3.
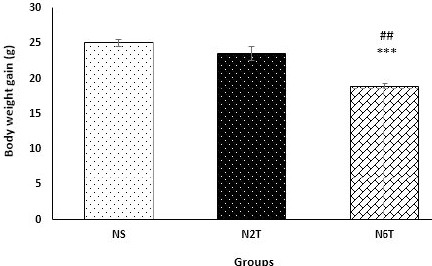
Body weight gain in the study groups. Body weight gain was significantly reduced in the N6T group compared with the NS group. There was a significant difference between body weight gain in N2T and N6T groups (NS: solvent control, N2T: trans-chalcone for 2 weeks, N6T: trans-chalcone for 6 weeks). Data were expressed as mean±SEM. *** P<0.001 versus NS, ## P<0.01 versus N2T
The effect of trans-chalcone on serum glucose and insulin levels
As shown in Figure 4, trans-chalcone significantly diminished (P<0.001) serum glucose levels after 2 and 6 weeks of oral administration. Also, trans-chalcone significantly reduced (P<0.001) serum insulin levels in N2T and N6T groups compared with the NS group (Figure 5).
Figure 4.
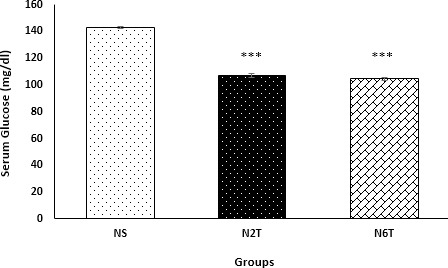
Serum glucose levels in the study groups. Serum levels of glucose were significantly reduced in N2T and N6T groups compared with the NS group (NS: solvent control, N2T: trans-chalcone for 2 weeks, N6T: trans-chalcone for 6 weeks). Data were expressed as mean±SEM. *** P<0.001 versus NS
Figure 5.
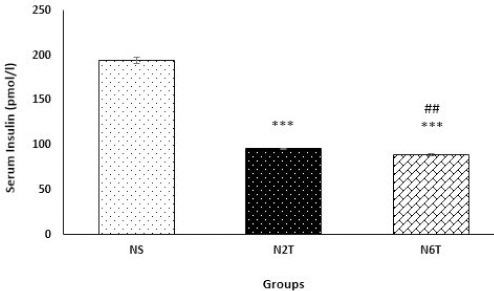
Serum insulin levels in the study groups. Serum levels of insulin hormone were significantly reduced in N2T and N6T groups compared with the NS group. There was a significant difference between serum insulin levels in N2T and N6T groups (NS: solvent control, N2T: trans-chalcone for 2 weeks, N6T: trans-chalcone for 6 weeks). Data were expressed as mean ± SEM. *** P<0.001 versus NS, ## P<0.01 versus N2T
The effect of trans-chalcone on miR-34a/SIRT1 pathway
For examining the hypothesis that trans-chalcone exerts its insulin-sensitizing effect through the miR-34a/SIRT1 pathway, this study measured the trans-chalcone induced changes in the expression of miR-34a and SIRT1 mRNA. As expected, trans-chalcone significantly decreased (P<0.001) hepatic miR-34a in N2T (by 39%) and N6T (by 56%) groups compared with the NS group (Figure 6). Also as shown in Figure 7, chalcone upregulated mRNA levels of SIRT1 in N2T and N6T groups, which was significant (P<0.05) in the N2T group compared with NS.
Figure 6.
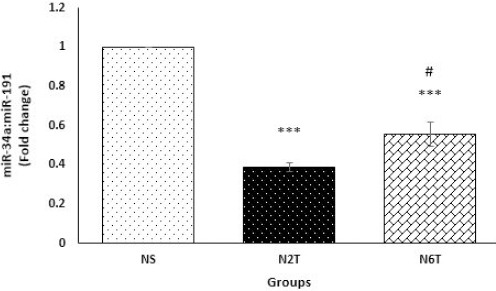
Hepatic expression levels of miR-34a in the study groups. Hepatic levels of miR-34a were significantly reduced in N2T and N6T groups compared with the NS group. There was a significant difference between hepatic levels of miR-34a in N2T and N6T groups (NS: solvent control, N2T: trans-chalcone for 2 weeks, N6T: trans-chalcone for 6 weeks). Data were expressed as mean±SEM. *** P<0.001 versus NS, # P<0.05 versus N2T
Figure 7.
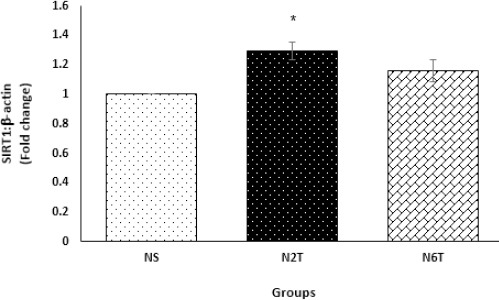
Hepatic expression levels of SIRT1 mRNA in the study groups. Hepatic level of SIRT1 was significantly elevated in the N2T group compared with the NS group (NS: solvent control, N2T: trans-chalcone for 2 weeks, N6T: trans-chalcone for 6 weeks). Data were expressed as mean±SEM. * P<0.05 versus NS
Discussion
The present study evaluated the effects of oral administration of trans-chalcone on insulin sensitivity and the miR-34a/SIRT1 pathway as its possible mechanism in healthy rats. As described in the results section, treatment with trans-chalcone decreased food intake, body weight gain, and serum insulin as well as glucose levels. Consistent with our finding, some previous studies showed that flavonoids and trans-chalcone improved glucose homeostasis and insulin sensitivity (9, 29-31). In this regard, Knekt et al. (30) showed that some flavonoids (quercetin and myricetin) diminished type 2 diabetes occurrence in human subjects. Another study suggested that there is an association between consumption of anthocyanins and low risk of type 2 diabetes in adult persons (29). Also, it has been shown by two previous works that intake of flavonoid-enriched chocolate reduced insulin resistance (32, 33). Furthermore, Karkhaneh et al. (31) demonstrated that trans-chalcone diminished body weight and insulin resistance in cholesterol-fed mice. A similar study reported body weight, food intake, insulin, and glucose-lowering effects of this flavonoids precursor in healthy and type 1 diabetic rats (9). Taken together, beneficial effects of trans-chalcone on insulin sensitivity have been shown by present and previous studies.
As mentioned in the introduction, direct regulation of SIRT1 by miR-34a and its important role in insulin sensitivity have been documented (13, 14, 34). This study investigated the effect of trans-chalcone on this pathway as a possible mechanism for its insulin-sensitizing action. In this regard, main findings of this study showed the inhibitory influence of trans-chalcone on hepatic miR-34a in parallel with elevation of hepatic SIRT1 expression. Consistent with the present study, previous works reported that miR-34a exerted pro-diabetic and pro-obesity functions through repression of its target, SIRT1 (13, 35). A study (16) suggested that miR-34a overexpression reduced SIRT1 expression and elevated free fatty acid induced apoptosis in rat hepatocytes. Also, Choi et al. (13) revealed that overexpression of this miRNA in the lean mice caused reduction of SIRT1 activity and obesity-associated outcomes such as glucose intolerance and hepatic fat deposition. Moreover, they suggested that elevation of miR-34a diminished hepatic SIRT1 activity in obesity. On the contrary, silencing of miR-34a alleviated steatosis and glucose intolerance in diet-induced obese mice (13). In this regard, another study indicated that miR-34a was dysregulated during metabolic syndrome (obesity and diabetes) (36). Therefore, miR-34a is a possible therapeutic target for steatosis and type 2 diabetes treatment (13).
In agreement with the present study, it has been shown that flavonoids could increase SIRT1 expression (37, 38). Quercetin as a natural flavonoid enhanced expression of SIRT1 in mice (37). Also, troxerutin (a flavonoid with hepatoprotective effect) elevated hepatic SIRT1 protein expression in 2,2′,4,4′-tetrabromodiphenyl ether-induced liver injury (38). However, 3,2’,3’,4’-tetrahydroxychalcone (a synthesized chalcone) inhibited the SIRT1 activity and could be considered an anti-cancer agent (39). This disagreement may be due to the different structures of 3,2’,3’,4’-tetrahydroxychalcone and trans-chalcone. To sum up, the present study suggested a new mechanism for hypoglycemic and hypoinsulinemic effects of trans-chalcone. Here, we showed that this simple chalcone inhibited miR-34a, which led to elevation of SIRT1 expression as its target gene.
Conclusion
This study proposed that trans-chalcone exerts its insulin-sensitizing effect through the miR-34a/SIRT1 pathway. Also, appetite lowering and anti-obesity effects of this chalcone may be other mechanisms of its insulin-sensitizing action. However, these effects were observed in normal healthy state and the influence of trans-chalcone on the miR-34a/SIRT1 pathway in pathologic conditions remains to be investigated.
Acknowledgment
This study was supported by a grant from Drug Applied Research Center of Tabriz University of Medical Sciences, Tabriz, Iran. Our data were derived from the thesis of Ms Elham Karimi-Sales for a PhD degree in physiology (thesis serial no. : 94/5-7/13).
Conflicts of Intrest
The authors declare that there are no conflicts of interest.
References
- 1.Matos MJ, Vazquez-Rodriguez S, Uriarte E, Santana L. Potential pharmacological uses of chalcones: a patent review (from June 2011 –2014) Expert Opin Ther Pat. 2015;25:351–366. doi: 10.1517/13543776.2014.995627. [DOI] [PubMed] [Google Scholar]
- 2.Orlikova B, Tasdemir D, Golais F, Dicato M, Diederich M. Dietary chalcones with chemopreventive and chemotherapeutic potential. Genes Nutr. 2011;6:125–147. doi: 10.1007/s12263-011-0210-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singh H, Sidhu S, Chopra K, Khan M. Hepatoprotective effect of trans-chalcone on experimentally induced hepatic injury in rats: inhibition of hepatic inflammation and fibrosis. Can J Physiol Pharma. 2016;94:879–887. doi: 10.1139/cjpp-2016-0071. [DOI] [PubMed] [Google Scholar]
- 4.Jalalvand F, Amoli MM, Yaghmaei P, Kimiagar M, Ebrahim-Habibi A. Acarbose versus trans-chalcone: comparing the effect of two glycosidase inhibitors on obese mice. Arch Endocrinol Metab. 2015;59:202–209. doi: 10.1590/2359-3997000000038. [DOI] [PubMed] [Google Scholar]
- 5.Batovska DI, Todorova IT. Trends in utilization of the pharmacological potential of chalcones. Curr Clin Pharmacol. 2010;5:1–29. doi: 10.2174/157488410790410579. [DOI] [PubMed] [Google Scholar]
- 6.Aksöz BE, Ertan R. Chemical and Structural Properties of Chalcones I. FABAD J Pharm Sci. 2011;36:223–242. [Google Scholar]
- 7.Rahman MA. Chalcone: A valuable insight into the recent advances and potential pharmacological activities. Chem Sci J. 2011;2011:CSJ–29. [Google Scholar]
- 8.Yaghmaei P, Kheirbakhsh R, Dezfulian M, Haeri-Rohani A, Larijani B, Ebrahim-Habibi A. Indole and trans-chalcone attenuate amyloid βplaque accumulation in male Wistar rat in vivo effectiveness of two anti-amyloid scaffolds. Arch Ital Biol. 2013;151:106–113. doi: 10.4449/aib.v151i3.1479. [DOI] [PubMed] [Google Scholar]
- 9.Najafian M, Ebrahim-Habibi A, Yaghmaei P, Parivar K, Larijani B. Core structure of flavonoids precursor as an antihyperglycemic and antihyperlipidemic agent: an in vivo study in rats. Acta Biochim Pol. 2010;57:553–560. [PubMed] [Google Scholar]
- 10.Liang F, Kume S, Koya D. SIRT1 and insulin resistance. Nat Rev Endocrinol. 2009;5:367–373. doi: 10.1038/nrendo.2009.101. [DOI] [PubMed] [Google Scholar]
- 11.Chen YR, Fang SR, Fu YC, Zhou XH, Xu MY, Xu WC. Calorie restriction on insulin resistance and expression of SIRT1 and SIRT4 in rats. Biochem Cell Biol. 2010;88:715–722. doi: 10.1139/O10-010. [DOI] [PubMed] [Google Scholar]
- 12.Chakraborty C, Doss CG, Bandyopadhyay S, Agoramoorthy G. Influence of miRNA in insulin signaling pathway and insulin resistance: micro-molecules with a major role in type-2 diabetes. Wiley Interdiscip Rev RNA. 2014;5:697–712. doi: 10.1002/wrna.1240. [DOI] [PubMed] [Google Scholar]
- 13.Choi SE, Fu T, Seok S, Kim DH, Yu E, Lee KW, et al. Elevated microRNA-34a in obesity reduces NAD+levels and SIRT1 activity by directly targeting NAMPT. Aging Cell. 2013;12:1062–1072. doi: 10.1111/acel.12135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun C, Zhang F, Ge X, Yan T, Chen X, Shi X, et al. SIRT1 improves insulin sensitivity under insulin-resistant conditions by repressing PTP1B. Cell Metab. 2007;6:307–319. doi: 10.1016/j.cmet.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 15.Guarente L. Sirtuins, aging, and metabolism. Cold Spring Harb Symp Quant Biol. 2011;76:81–90. doi: 10.1101/sqb.2011.76.010629. [DOI] [PubMed] [Google Scholar]
- 16.Castro RE, Ferreira DM, Afonso MB, Borralho PM, Machado MV, Cortez-Pinto H, et al. MiR-34a/SIRT1/p53 is suppressed by ursodeoxycholic acid in the rat liver and activated by disease severity in human non-alcoholic fatty liver disease. J Hepatol. 2013;58:119–125. doi: 10.1016/j.jhep.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 17.Guarente L. Sirtuins as potential targets for metabolic syndrome. Nature. 2006;444:868–874. doi: 10.1038/nature05486. [DOI] [PubMed] [Google Scholar]
- 18.Klover PJ, Mooney RA. Hepatocytes: critical for glucose homeostasis. Int J Biochem Cell B. 2004;36:753–758. doi: 10.1016/j.biocel.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 19.Yang YM, Seo SY, Kim TH, Kim SG. Decrease of microRNA-122 causes hepatic insulin resistance by inducing protein tyrosine phosphatase 1B, which is reversed by licorice flavonoid. Hepatology. 2012;56:2209–2220. doi: 10.1002/hep.25912. [DOI] [PubMed] [Google Scholar]
- 20.Baselga-Escudero L, Arola-Arnal A, Pascual-Serrano A, Ribas-Latre A, Casanova E, Salvado MJ, et al. Chronic administration of proanthocyanidins or docosahexaenoic acid reverses the increase of miR-33a and miR-122 in dyslipidemic obese rats. PLoS One. 2013;8:e69817. doi: 10.1371/journal.pone.0069817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baselga-Escudero L, Bladé C, Ribas-Latre A, Casanova E, Salvadó MJ, Arola L, et al. Grape seed proanthocyanidins repress the hepatic lipid regulators miR-33 and miR-122 in rats. Mol Nutr Food Res. 2012;56:1636–1646. doi: 10.1002/mnfr.201200237. [DOI] [PubMed] [Google Scholar]
- 22.Baselga-Escudero L, Pascual-Serrano A, Ribas-Latre A, Casanova E, Salvadó MJ, Arola L, et al. Long-term supplementation with a low dose of proanthocyanidins normalized liver miR-33a and miR-122 levels in high-fat diet–induced obese rats. Nutr Res. 2015;35:337–345. doi: 10.1016/j.nutres.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 23.Baselga-Escudero L, Blade C, Ribas-Latre A, Casanova E, Suarez M, Torres JL, et al. Resveratrol and EGCG bind directly and distinctively to miR-33a and miR-122 and modulate divergently their levels in hepatic cells. Nucleic Acids Res. 2014;42:882–892. doi: 10.1093/nar/gkt1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Joven J, Espinel E, Rull A, Aragonès G, Rodríguez-Gallego E, Camps J, et al. Plant-derived polyphenols regulate expression of miRNA paralogs miR-103/107 and miR-122 and prevent diet-induced fatty liver disease in hyperlipidemic mice. BBA-Gen Subjects. 2012;1820:894–899. doi: 10.1016/j.bbagen.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 25.Baselga-Escudero L, Blade C, Ribas-Latre A, Casanova E, Salvadó M-J, Arola L, et al. Chronic supplementation of proanthocyanidins reduces postprandial lipemia and liver miR-33a and miR-122 levels in a dose-dependent manner in healthy rats. J Nutr Biochem. 2014;25:151–156. doi: 10.1016/j.jnutbio.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 26.Okunrobo LO, Usifoh CO, Uwaya JO. Anti-inflammatory and gastroprotective properties of some chalcones. Acta Pol Pharm. 2006;63:195–199. [PubMed] [Google Scholar]
- 27.Yousefzadeh N, Jeddi S, Alipour MR. Effect of Fetal Hypothyroidism on Cardiac Myosin Heavy Chain Expression in Male Rats. Arq Bras Cardiol. 2016;107:147–153. doi: 10.5935/abc.20160099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Habibi P, Alihemmati AR, Nasirzadeh MR, Yousefi H, Habibi MR, Ahmadiasl N. Involvement of microRNA-133 and -29 in cardiac disturbances in diabetic ovariectomized rats. Iran J Basic Med Sci. 2016;19:1177–1185. [PMC free article] [PubMed] [Google Scholar]
- 29.Wedick NM, Pan A, Cassidy A, Rimm EB, Sampson L, Rosner B, et al. Dietary flavonoid intakes and risk of type 2 diabetes in US men and women. Am J Clin Nutr. 2012;95:925–933. doi: 10.3945/ajcn.111.028894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Knekt P, Kumpulainen J, Jarvinen R, Rissanen H, Heliovaara M, Reunanen A, et al. Flavonoid intake and risk of chronic diseases. Am J Clin Nutr. 2002;76:560–568. doi: 10.1093/ajcn/76.3.560. [DOI] [PubMed] [Google Scholar]
- 31.Karkhaneh L, Yaghmaei P, Parivar K, Sadeghizadeh M, Ebrahim-Habibi A. Effect of trans-chalcone on atheroma plaque formation, liver fibrosis and adiponectin gene expression in cholesterol-fed NMRI mice. Pharmacol Rep. 2016;68:720–727. doi: 10.1016/j.pharep.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 32.Shrime MG, Bauer SR, McDonald AC, Chowdhury NH, Coltart CE, Ding EL. Flavonoid-rich cocoa consumption affects multiple cardiovascular risk factors in a meta-analysis of short-term studies. J Nutr. 2011;141:1982–1988. doi: 10.3945/jn.111.145482. [DOI] [PubMed] [Google Scholar]
- 33.Curtis PJ, Sampson M, Potter J, Dhatariya K, Kroon PA, Cassidy A. Chronic ingestion of flavan-3-ols and isoflavones improves insulin sensitivity and lipoprotein status and attenuates estimated 10-year CVD risk in medicated postmenopausal women with type 2 diabetes: a 1-year, double-blind, randomized, controlled trial. Diabetes Care. 2012;35:226–232. doi: 10.2337/dc11-1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ding J, Li M, Wan X, Jin X, Chen S, Yu C, et al. Effect of miR-34a in regulating steatosis by targeting PPARαexpression in nonalcoholic fatty liver disease. Sci Rep. 2015;5:13729. doi: 10.1038/srep13729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rokavec M, Li H, Jiang L, Hermeking H. The p53/miR-34 axis in development and disease. J Mol Cell Biol. 2014;6:214–230. doi: 10.1093/jmcb/mju003. [DOI] [PubMed] [Google Scholar]
- 36.Price NL, Ramírez CM, Fernández-Hernando C. Relevance of microRNA in metabolic diseases. Crit Rev Cl Lab Sci. 2014;51:305–320. doi: 10.3109/10408363.2014.937522. [DOI] [PubMed] [Google Scholar]
- 37.Davis JM, Murphy EA, Carmichael MD, Davis B. Quercetin increases brain and muscle mitochondrial biogenesis and exercise tolerance. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1071–1077. doi: 10.1152/ajpregu.90925.2008. [DOI] [PubMed] [Google Scholar]
- 38.Zhang ZF, Zhang YQ, Fan SH, Zhuang J, Zheng YL, Lu J, et al. Troxerutin protects against 2,2',4,4'-tetrabromodiphenyl ether (BDE-47)-induced liver inflammation by attenuating oxidative stress-mediated NAD(+)-depletion. J Hazard Mater. 2015;283:98–109. doi: 10.1016/j.jhazmat.2014.09.012. [DOI] [PubMed] [Google Scholar]
- 39.Kahyo T, Ichikawa S, Hatanaka T, Yamada MK, Setou M. A novel chalcone polyphenol inhibits the deacetylase activity of SIRT1 and cell growth in HEK293T cells. J Pharmacol Sci. 2008;108:364–371. doi: 10.1254/jphs.08203fp. [DOI] [PubMed] [Google Scholar]


