Abstract
Objective(s):
The aim of this study was to prepare fraction and determine the biological activities of the polyphenol-enriched fraction of Berberis integerrima Bunge fruits.
Materials and Methods:
In this assay fraction was extracted by column chromatography, using Amberlite column as the stationary phase. Phenol and flavonoids in the extract and fraction were analyzed by high performance liquid chromatography (HPLC). DNA protection ability, antioxidant and xanthine oxidase inhibition capacities of this fraction were also examined.
Results:
Phenol and flavonoid content measurement and HPLC analyses of this fraction confirmed that phenol and flavonoids were increased in fraction in comparison to extract (before using Amberlite column). In antioxidant measurement assay, the trolox equivalent values were 1.05± 0.04 and 0.8±0.11 in oxygen radical absorbance capacity (ORAC) and the EC50 values for cellular antioxidant activity were 55.51±0.21 and 95.67±0.13 µg/ml for quercetin and the fraction, respectively. The xanthine oxidase inhibition percentages were 97.6±0.003 and 90.2 6±0.003 in 100 µg/ml concentration of fraction and vitamin C respectively. Comet assay analysis showed that this fraction protects human lymphocytes against H2O2-induced DNA damages at 12.5 to 100 µg/ml concentrations.
Conclusion:
This study suggests that Amberlite column as the stationary phase help to improve phenolic compound in separating fractions. The results showed that B. integerrima fruits are rich in phenolic compounds and they are potent antioxidants with protective effects on oxidative damages. They might be used as functional ingredients in food and supplements.
Keywords: Antioxidant activity, Berberis integerrima Bunge, Comet assay, DNA protection, Xanthine oxidase inhibitor
Introduction
Reactive oxygen species (ROS) are cellular metabolic products, categorized as free radicals (superoxide anion radicals (O2•−)) and non-free radical species (hydrogen peroxide (H2O2)). Oxidative stress is as a result of the disparity between ROS production and the cellular defense mechanism. The presence of extra ROS in the cells may eventually cause damage to the biomacromolecule. Protein, lipid and nucleic acid can be the target of these radicals (1, 2). The footprints of these types of damage were found in the etiology of cancers, as well as chronic and neurodegenerative diseases (3). Consequently, enzymatic and non-enzymatic pathways attempted to reduce the incidence of these oxidative stress-associated diseases (2). For instance, the enzymatic mechanisms involved catalase, peroxidase (1) and non-enzymatic pathways involved albumin, myoglobin and ferritin (4). Medicinal plants are still great sources of novel pharmacological studies. Their secondary metabolites are responsible for these therapeutic effects. Many evidences confirmed the protective effects of some plants or their isolated compounds against the carcinogenesis and mutagenesis of ROS (5). Among the various natural products, phenolic compounds are known as the potent antioxidant and protective on DNA damage (1).
Xanthine oxidase (XO) is responsible for the last two steps in purine metabolism by converting hypoxanthine to xanthine, then xanthine to uric acid and H2O2 (6). XO activity procedure leads to ROS products. Gout, hyperuricemia, hepatitis, carcinogenesis, and aging are involved in this enzyme activity. Consequently, XO inhibitors are considered in different pathological conditions in relation to the formation of uric acid and superoxide anion radical. Besides classic XO inhibitors with different side effects, several natural products have been suggested to reduce uric acid in gout, such as caffeic acid, rutin, and chestnut honey (7).
Berberis integerrima Bunge belongs to the Berberidaceae family with various secondary metabolites and therapeutic effects (8). This medicinal plant is used in the treatment of different diseases such as bleeding, swollen gums teeth, sore throat, fever, malaria, hepatitis, inflammation and diarrhea (9).
In the present investigation, polyphenol rich fraction was isolated from B. integerrima fruits by using Amberlite as the stationary phase and it was evaluated for its antioxidant activity, XO inhibition and protective effects against DNA damage induced by H2O2 on isolated human lymphocytes.
Materials and Methods
Extraction
Fruits of Berberis integerrima were collected from Kohmar (Fars Province, Iran) during August and September 2105. The voucher numbers for fruits was P.M. 396. They were deposited at Museum of Medicinal Plants, Shiraz University of Medical Sciences, Shiraz, Iran.
The fruits were freeze-dried using freeze dryer (Zirbus, Germany) and ground into powder. The powder (100 g) was macerated with ethanol (70%, 1 L) in a dark place, followed by filtration. The extract was concentrated using a rotary evaporator (Heidolph, Germany) under vacuum, followed by speed vacuum to get 60 g gummy material. The crude extract (30 g) was suspended in trifluoroacetic acid (TFA) (Samchun, Korea) (0.3%), was filtered and poured into the decanter, where it was properly shaken with ethyl acetate (Samchun, Korea). This extraction procedure was repeated three times and the aqueous phase was collected in each repeat. The aqueous phase was loaded on Amberlite column XAD-7 (2.5 × 45 cm) (Sigma-Aldrich, St Louis, MO, USA) as the stationary phase. This phase was rinsed with distilled water containing TFA, respectively. At the last time, the stationary phase was rinsed with methanol containing TFA, and then the extract was collected and concentrated using a rotary evaporator. Yield of extraction fractionation was 20%.
Analysis and purification by high performance liquid chromatography (HPLC)
To obtain a highly purified fraction, stock solution of (1 mg/ml), B. integerrima in methanol was prepared. The current solution was analyzed by HPLC (LC-10 VP, Shimadzu, Japan) using analytical column (C18, 4.6×250 mm, 5 μm, Erouspher, Kanuer). The mobile phase contained Solvent A: 0.1% TFA in water and Solvent B: methanol. The elution gradiant systems were as follows: Solvent A (0 min: 100%; 15 min: 80%; 25 min: 60%, 40 min: 0%,). The flow rate was 1 ml/min, the injection volume was 20 μl, and detection wavelength was 342 nm. Through the use of a preparative column, isolation was done using the preparative Kanuer system (Model 1800) using preparative column (C18, 20 × 250 mm, 7 μm, Erouspher, Kanuer). The mobile phase was also used for the analysis, while flow rate and injection volume were 13.5 ml/min and 2000 μl, respectively.
Phenol content analysis
The total phenolic constituents of the extracts were evaluated using Folin-Ciocalteu’s phenol reagent and gallic acid (Sigma-Aldrich, Co LLC, USA) as standard (10). For the calibration curve, different concentrations of gallic acid were mixed with Folin-Ciocalteu reagent and sodium carbonate (7.5 g/l). The mixture was kept at 20 °C for 30 min and the absorption was read at 765 nm. Plant extract was mixed with the same reagents as described previously. All samples were performed in triplicate.
Total content of phenolic compounds in plant express as gallic acid equivalents (GAE) and were calculated by this formula:
C= c. V/m
C: total content of phenolic compounds
c: the concentration of gallic acid established from the calibration curve (mg/ml)
v: the volume of extract (ml)
m: the weight of plant extract (g)
Determination of flavonoid content
The total flavonoid contents of B. integerrima extract were measured using the previously described colorimetric method (11). The sample solution was mixed with distilled water and then with NaNO2 (% 0.15) solution. NaOH solution (4%) was added to the mixture after 6 min. Then, water was added to get the final volume to 5 ml; the mixture was mixed and incubated for another 15 min. Absorbance of the reaction was measured at 510 nm against reagent blank. Quercetin (Sigma-Aldrich, Co LLC, USA) was used as the standard for total flavonoid content measurement. The results were expressed as milligram of quercetin equivalent per 1 g of the extract. Data was recorded as mean ± standard deviation (SD) for three replicates.
Antioxidant analysis
Oxygen radical absorbance capacity assay (ORAC)
ORAC assay was done using Polar star omega device (BMG LABTECH GmbH, Germany) according to the method defined by Hossain et al (12). The final assay mixture contained AAPH (Sigma-Aldrich, Co LLC, USA) (240 Mm), fluorescein (10 Nm) (Sigma-Aldrich, Co. LLC, USA) and 25 μl of the sample (25-400 µg/ml) or phosphate buffer as the blank. The quercetin was used as standard (0.6-3.6 µg/ml). The fluorescence of this mixture was recorded every 90 sec per cycle using fluorescent filters. Different concentrations of Trolox (Sigma-Aldrich, Co. LLC, USA) (3-50 µg/ml) were used to give a standard curve to compare the ORAC values of various samples. The data were analyzed using data analysis software, MARS, linked with Omega reader control software. The difference between the “area under the fluorescence decay curve” (AUC) of blank and each sample were expressed as Trolox equivalents (TE).
Antioxidant assay for cellular antioxidant activity (CAA)
HepG2 cells at a concentration of 6×104 cells in 100 µl growth media per well were incubated for 24 hr. After 24 hr seeding, the growth medium was removed and cells were washed gently 3 times with PBS. Triplicate wells were treated with DCFH-DA (Sigma-Aldrich, Co LLC, USA) probe solution (50 µl) and with either quercetin standards or (0.4-2 µg/ml) samples (3-250 µg/ml) dissolved in the treatment medium. The microplate was incubated for 60 min at 37 °C. Then the liquid was removed and cells were washed 3 times using PBS and then the last phase was washed and the liquid was removed and discarded. Lastly, AAPH (free radical initiator solution) was added to all wells and the plate was read on the Polar star omega (13).
Xanthin oxidase (XO) assay
The effects of the extract on XO activity were evaluated using xanthine oxidase Activity Assay Kit (Sigma-Aldrich, Co LLC, USA) according to protocol. According to this protocol, the xanthin oxidase activity of the extract and vitamin C at 100 µg/ml concentration was measured.
XO inhibitory activity was evaluated by the following formula,
% enzyme inhibition = (1 – b/a) × 100
where “a” is the activity of the enzyme without plant extract and “b” is the activity of XO with extract (14).
Comet assay
Isolation of human lymphocytes
Informed consent was taken from all volunteers and all procedures performed in this study were conducted in line with Declaration of Helsinki (15).
Peripheral blood was taken from 10 healthy volunteers (25 to 30 years) with no history of smoking or chronic use of medication. Samples were collected into cell preparation tubes containing 10% (EDTA) in PBS as anticoagulant agent. Blood (5 ml) was diluted 1:1 with PBS and the suspension was placed over the lymphocyte separation medium carefully in a tube and then centrifuged for 20 min at 2000 rpm, and gradient-separated lymphocytes were recovered, diluted with PBS and centrifuged at 1500 rpm for 10 min again. The pellets were resuspended in PBS, and then cells were counted in a Neobauer chamber. The cell concentration was adjusted to 5000 cells/ml. Trypan blue dye exclusion technique was used to check cell viability (16).
In vitro comet assay experiments with human lymphocytes
The alkaline comet assay was carried out according to the guidelines of Singh et al. (17) for comet assay with some modifications. The cells were treated with different concentrations of polyphenol fraction and H2O2 (50 µM) simultaneously, for 20 min in the dark at 4 °C to prevent repair of the induced oxidative DNA damage (18). Positive control cells were treated with H2O2 in PBS.
The cells were harvested and centrifuged at 3000 rpm for 10 min and then washed with PBS. The cell pellets were mixed with 100 μl of 0.75% (w/v) low melting point agarose, then spread on a fully frosted microscopic slide precoated with 1% normal melting agarose, covered with a cover slip and kept for 10 min at 4 °C.
After removing the cover slips, the slides were immersed in freshly prepared cold lysing solution [2.5 M NaCl, 100 mM Na2EDTA, 10 mM Tris, 1% (v/v) triton X-100, 10% DMSO, pH 10.0] at 4 °C for at least 2 hr. The slides were presoaked in freshly prepared alkaline electrophoresis buffer for 30 min. Electrophoresis was carried out for 45 min at 4 °C. All procedural steps were performed under yellow light conditions to minimize additional DNA damage.
Then slides were placed vertically in a neutralizing tank and washed with neutralizing solution (0.4 m tris HCl buffer, pH 7.5). Finally, the slides were stained with propidium iodide (Sigma-Aldrich, Co LLC, USA) (20 µg/ml) dispensed directly onto the slides and covered with a coverslip. The slides were studied by a fluorescent microscope (Olympus-BX61). All experiments were performed at least three times. For each slide, 50 selected cells were analyzed with Casplab software.
Statistical analysis
For statistical analyses of the results, data was ana-
lyzed using one-way analysis of variance (ANOVA), followed by Tukey’s post tests done by SPSS (version 20). Values were expressed as mean±standard error of mean (SEM) of triplicate experiments. Statistical significance was determined at P<0.05.
Results
Quantification of phenolic compounds and HPLC analysis
The results of the total phenolic and total flavonoids content were shown in Table 1. The total phenolic and total flavonoids content of the extract increased significantly after being loaded on the column. Possibly, Amberlite column (XAD-7) which used as stationary phase in the extraction process, separate nonpolar compound from extract and increase phenolic and flavonoids concentrations. It can be seen also in the HPLC chromatogram in Figure 1. More polar compounds such as polysaccharides with retention time less than 30 min, were separated by XAD-7.
Table 1.
Total phenolic and total flavonoids content of extract of Berberis integerrima before (extract) and after (phenolic rich fraction) loading on column
| Sample | Total phenolic (mg GAE/g extract) | Total flavonoids (mg QE/g extract) |
|---|---|---|
| Extract | 130.52±0.42 | 52.35±0.52 |
| Phenolic rich fraction | 396.44±0.4 | 138.71±0.28 |
Figure 1.
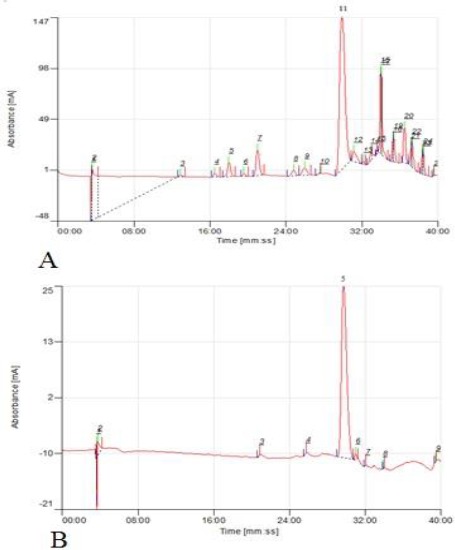
Typical chromatograms of HPLC of extract (A) and phenolic rich fraction of Berberis integerrima Bunge fruits (B)
Antioxidant assay
In ORAC assay, AAPH decompose to peroxyl radicals which are capable of reacting with a fluorescent probe (19). This assay shows the ability of the sample to participate in hydrogen atom transfer (HAT) reactions; since the peroxyl radical (ROO·) was generated hydrogen atom of the antioxidant transferred to these radicals (20). The Trolox equivalents value for the fraction and quercetin in ORAC assay were 0.8±0.11 and 1.05±0.04 respectively.
CAA is the assay to determine intracellular oxidative stress and predict antioxidant activity of the sample in cellular conditions. The results of this method depend on antioxidants properties such as cellular uptake, distribution and metabolism (19). In this assay, the fraction was able to prevent oxidation of 2’,7’-dichlorofluorescin (DCFH) to DCF by peroxyl radicals in HepG2 cells. The reduction of DCF fluorescent in the cells exhibits the antioxidant capacity (21).
In the present study, EC50 values for the inhibition of DCFH oxidation are 55.51±0.21 and 95.67±0.13 µg/ml for quercetin and the fraction, respectively.
Xanthin oxidase activity
It is known that XO is a source of cellular ROS and oxidative stress (22). Several researches have revealed the significant role of XO in the pathology of several diseases such as vascular injuries, as well as inflammatory and chronic heart failure (23). So, during the past decades, screening for the novel XO inhibitors especially natural derivate has been considered (23, 24). Polyphenol compounds are known as the potent XO inhibitors (25). The result of XO inhibition percentage of B. integerrima fraction is 97.6±0.003% and this value is 90.2 6± 0.003% for vitamin C.
Comet assay
The capacity of the samples to protect cells against damage induced by H2O2 shows the cellular antioxidant power (26). In the presence of transition metal ions (especially Fe2+) in Fenton reaction, H2O2 generates hydroxyl radicals which are more reactive and prompt reactive radicals (27). Therefore, in protective assay studies, cells were exposed to H2O2 and an antioxidant sample simultaneously, for a short time and DNA damage were measured using comet assay. The lower frequency of DNA breaks shows more antioxidant activities (26). Over the past decades, the Comet assay has been developed as a standard, simple, and reliable method for examining the DNA damage caused by ROS (26, 28).
In the present study, different levels of DNA damage induced by H2O2 were measured using the Comet assay. The results showed that 50 and 100 µM of H2O2 caused DNA damage (Figure 2). For the B. integrimma fraction, a range of concentrations (100- 800 µg/ml) did not reveal any genotoxicity and could be considered as non-genotoxic concentrations (Figure 3).
Figure 2.
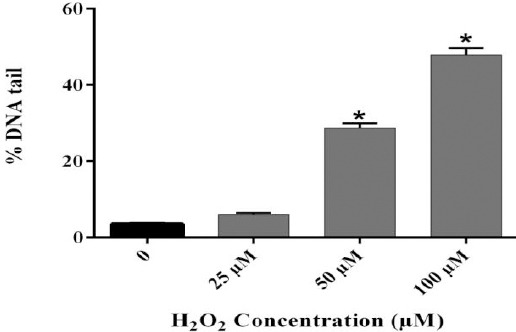
DNA damage of human lymphocytes treated with H2O2. Human lymphocytes were incubated for 15 min at 4 °C with different concentrations of H2O2. Results are mean±SEM (n=6 slides × 50 lymphocytes). * P<0.05
Figure 3.
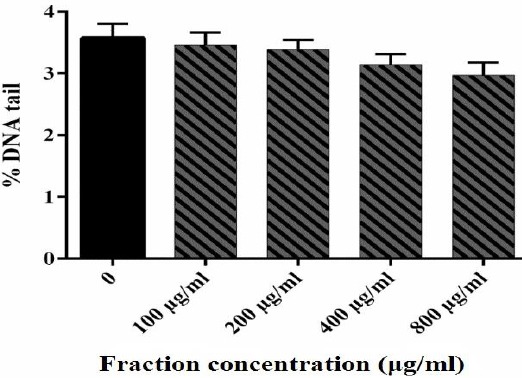
DNA damage of human lymphocytes treated with B. integerrima extract. Human lymphocytes were incubated for 15 min at 4 °C with different concentrations of fraction. Results are mean±SEM (n = 6 slides × 50 lymphocytes)
As shown in Figure 4, DNA damage induced by H2O2 decreased by 12.5, 25, 50, and 100 μg/ml of the fraction significantly and the percentage of DNA tail reduced from 30% to less than 10%. Although, H2O2 induced serious DNA damage, but B. integrimma fraction protects lymphocyte against DNA damage effectively.
Figure 4.
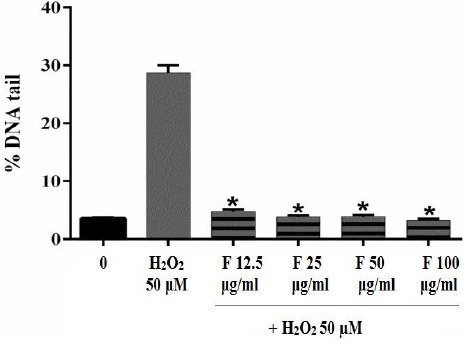
Effect of B. integerrima extract on lymphocyte DNA damaged induced by 50 μM H2O2. Human lymphocytes were incubated for 15 min at 4 °C with a combination of 50 μM H2O2 with different concentrations of B. integerrima fraction (F12.5-F100 μg/ml). Results are mean±SEM (n=6 slides×50 lymphocytes) * P<0.05
Discussion
The isolated compounds from different species of Berberis include Berberine, Oxyacanthine, Bermamine, Palmatine, Jateorrhizinem, Columbamine and Berberubine (29-30). These different secondary metabolites suggest the application of Berberis species in different medicinal conditions as well as folk and traditional medicine (30). More evidences confirmed the biological effects of these secondary metabolites and proposed them as preventive and treatment factors. Occasionally, among these compounds, phenolic compounds are known as extremely important radical scavengers and potent antioxidants (31).
In this assay, Amberlite XAD-7 was used as stationary phase in extraction process. These columns were able to interact with nonpolar and moderate polar phenolic compounds with intensive hydrogen bonds between hydroxyl groups of these compounds and the ester groups on the column surfaces (32). The total phenolic and flavonoids content of extract increase significantly after extract loaded on column. It seems that Amberlite phase separate non phenolic (polar) compound such as polysaccharides and protein from extract and increase the concentration of phenolic and flavonoids content. The results of HPLC chromatogram showed more concentrated phenolic compound in fraction in comparison to extract.
Although there are few reported data on the total phenolic content of Berberis sp., the total amount of phenol obtained in this study was more than the total phenolic content value of B. integerrima leaves (33) Berberis crataegina fruits (34) and B. integerrima fruits (35).
This is supported by antioxidant activity measured using ORAC and CAA assays and high amount of phenol and flavonoid compound in this fraction, since previous evidence showed the correlation between antioxidant activity and phenolic compounds in herbs (36).
In this assay, B. integerrima fraction inhibits XO while there is no previous report of XO inhibitory activity of this plant. B. integerrima fruits showed rich sources of poly phenol and anthocyanin. It should be noted that there are no previously published studies on the chemical constituents and physicochemical characteristics of B. integerrima fruits. However, anthocyanin can be considered as a XO inhibitor as reported by Bräunlich et al (37).
In comet assay analysis, DNA damage induced by H2O2 decreased by 12.5-100 μg/ml of the fraction significantly and the percentage of DNA tail reduced. Although, H2O2 induced serious DNA damage, but B. integrimma fraction protects lymphocyte against DNA damage effectively. This is supported by antioxidant activity measured using ORAC and CAA assays and high amount of phenol and flavonoid compound in this fraction. As shown, the antioxidant activities of phenolic compounds are due to their structures (38-40). Three parts of their structure play important role in radical scavenging effects. Phenolic hydroxyl groups were able to donate a hydrogen atom or an electron to free radical, hydrocarbon backbone delocalizes an unpaired electron (39) and dihydroxy groups were able to conjugate to transition metals such as Cu+ or Fe2+ to inhibit free radical formation by these metals in Fenton reaction (39). Consequently, the antioxidant activity of phenolic compounds was performed by different mechanisms: scavenging radical species, donating hydrogen atoms or electron, enzyme inhibition, chelating metal cations and up regulating or boosting cellular antioxidants (38-40).
The different reports about the medical application of B. integerrima such as anti-inflammatory, anticancer, hepatoprotective, hypoglycemic and hypolipidemic activities can be attributed to the radical scavenging and protective effects of this plant against ROS (33).
Conclusion
This study recommends that using Amberlite column as the stationary phase enrich phenolic compound in fraction. Since B. integerrima is a rich source of phenolic and flavonoid content, it can be an antioxidant, xanthin oxidase inhibitor and antigenotoxic. It might be a protective agent against the DNA damage caused by chemicals. Though, additional investigation should be carried out in order to determine the mechanisms of its chemopreventive activity.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgment
This study was financially supported by Shiraz University of Medical Sciences, Shiraz, Iran (Grant number (94-01-70-11231).
References
- 1.Skała E, Sitarek P, Różalski M, Krajewska U, Szemraj J, Wysokińska H, et al. Antioxidant and DNA repair stimulating effect of extracts from transformed and normal roots of Rhaponticum carthamoides against induced oxidative stress and DNA damage in cho cells. Oxid Med Cell Longev. 2016;2016 doi: 10.1155/2016/5753139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kang JS, Kim DJ, Kim GY, Cha HJ, Kim S, Kim HS, et al. Ethanol extract of Prunus mume fruit attenuates hydrogen peroxide-induced oxidative stress and apoptosis involving nrf2/ho-1 activation in c2c12 myoblasts. Rev Bras Farmacogn. 2016;26:184–190. [Google Scholar]
- 3.Ehtesham-Gharaee M, Eshaghi A, Shojaee S, Asili J, Emami S.A, Behravan J, et al. Protective effects of Scutellaria lindbergii root extract against oxidative-induced cell and DNA damage in mouse fibroblast-like cells. Drug Chem Toxicol2015; 38:293–299. doi: 10.3109/01480545.2014.954047. [DOI] [PubMed] [Google Scholar]
- 4.Arora M, Mahat R.K, Kumar S, Tyagi S, Batra J. Oxidative stress and its relation to glycemic control in patients of type 2 diabetes mellitus. Int J Med Sci Public Health. 2016;5:1173–1177. [Google Scholar]
- 5.Sghaier M.B, Ismail M.B, Bouhlel I, Ghedira K, Chekir-Ghedira L. Leaf extracts from Teucrium ramosissimum protect against DNA damage in human lymphoblast cell k562 and enhance antioxidant, antigenotoxic and antiproliferative activity. Environ Toxicol Pharmacol. 2016;44:44–52. doi: 10.1016/j.etap.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 6.Mahdi-Pour B, Jothy S.L, Latha L.Y, Chen Y, Sasidharan S. Antioxidant activity of methanol extracts of different parts of Lantana camara. Asian Pac J Trop Biomed. 2012;2:960–965. doi: 10.1016/S2221-1691(13)60007-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sahin H. Honey as an apitherapic product: Its inhibitory effect on urease and xanthine oxidase. J Enzyme Inhib Med Chem. 2016;31:490–494. doi: 10.3109/14756366.2015.1039532. [DOI] [PubMed] [Google Scholar]
- 8.Sabahi Z, Khoshnood-Mansoorkhani M J, Rahmani Namadi S, Moein M. Antidiabetic and synergistic effects of anthocyanin fraction from Berberis integerrima fruit on streptozotocin-induced diabetic rats model. Tips. 2016;2(1):43–50. [Google Scholar]
- 9.Ashraf H, Heidari R, Nejati V. Antihyperglycemic and antihyperlipidemic effects of fruit aqueous extract of Berberis integerrima Bge in streptozotocin-induced diabetic rats. IJPR. 2014;13:1313–1318. [PMC free article] [PubMed] [Google Scholar]
- 10.Moein S, Moein M. Relationship between antioxidant properties and phenolics in Zhumeria majdae. J Med Plant Res. 2010;4:517–521. [Google Scholar]
- 11.Moein M, Moein S, Ahmadizadeh S. Radical scavenging and reducing power of Salvia mirzayanii subfractions. Molecules. 2008;13:2804–2813. doi: 10.3390/molecules13112804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hossain M, Barry-Ryan C, Martin-Diana A.B, Brunton N. Effect of drying method on the antioxidant capacity of six lamiaceae herbs. Food Chem. 2010;123:85–91. [Google Scholar]
- 13.Olsen E, Hansen E, Isaksson J, Isaksson J, Andersen J. Cellular antioxidant effect of four bromophenols from the red algae Vertebrata lanosa. Mar Drugs. 2013;11:2769–2784. doi: 10.3390/md11082769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berboucha M, Ayouni K, Atmani D, Benboubetra M. Kinetic study on the inhibition of xanthine oxidase by extracts from two selected Algerian plants traditionally used for the treatment of inflammatory diseases. J Med Food. 2010;13:896–904. doi: 10.1089/jmf.2009.0164. [DOI] [PubMed] [Google Scholar]
- 15.Carlson RV, Boyd KM, Webb D. The Revision of the Declaration of Helsinki: past, present and future. Brit J Clin Pharmacol. 2004;57:695–713. doi: 10.1111/j.1365-2125.2004.02103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singh N.P, McCoy M.T, Tice R.R, Schneider E.L. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res. 1988;175:184–191. doi: 10.1016/0014-4827(88)90265-0. [DOI] [PubMed] [Google Scholar]
- 17.Collins A.R, Duthie S.J, Dobson V.L. Direct enzymic detection of endogenous oxidative base damage in human lymphocyte DNA. Carcinoge. 1993;14:1733–1735. doi: 10.1093/carcin/14.9.1733. [DOI] [PubMed] [Google Scholar]
- 18.Mellado-Ortega E, Zabalgogeazcoa I, de Aldana BRV, Arellano JB. Solutions to decrease a systematic error related to aaph addition in the fluorescence-based orac assay. Anal Biochem. 2017;519:27–29. doi: 10.1016/j.ab.2016.12.009. [DOI] [PubMed] [Google Scholar]
- 19.Park JH, Lee M, Park E. Antioxidant activity of orange flesh and peel extracted with various solvents. Prev Nutr Food Sci. 2014;19:291–298. doi: 10.3746/pnf.2014.19.4.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang X-D, Yi X, Zhang T, Jun-Jiang L. Assessing plant antioxidants by cellular antioxidant activity assay based on microfluidic cell chip with arrayed microchannels. Chin J Anal Chem. 2016;44:604–609. [Google Scholar]
- 21.Mashino T, Takigawa Y, Saito N, Wong L Q, Mochizuki M. Antioxidant activity and xanthine oxidase inhibition activity of reductic acid: ascorbic acid analogue. Bioorg Med Chem Lett. 2000;10:2783–2785. doi: 10.1016/s0960-894x(00)00570-9. [DOI] [PubMed] [Google Scholar]
- 22.Vitale RM, Antenucci L, Gavagnin M, Raimo G, Amodeo P. Structure-activity relationships of fraxamoside as an unusual xanthine oxidase inhibitor. J Enzyme Inhib Med Chem. 2017;32:345–354. doi: 10.1080/14756366.2016.1252758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berboucha M, Ayouni K, Atmani D, Atmani D, Benboubetra M. Kinetic study on the inhibition of xanthine oxidase by extracts from two selected algerian plants traditionally used or the treatment of inflammatory diseases. J Med Food. 2010;13:896–904. doi: 10.1089/jmf.2009.0164. [DOI] [PubMed] [Google Scholar]
- 24.Kostić DA, Dimitrijević DS, Stojanović GS, Palić IR, Đorđević AS, Ickovski J D. Xanthine oxidase: isolation, assays of activity, and inhibition. J Chem. 2015;2015:1–8. [Google Scholar]
- 25.Azqueta A, Collins A. Polyphenols and DNA damage: A mixed blessing. Nutr. 2016;3:8. doi: 10.3390/nu8120785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Winterbourn C.C. Reconciling the chemistry and biology of reactive oxygen species. Nat Chem Biol. 2008;4:278–286. doi: 10.1038/nchembio.85. [DOI] [PubMed] [Google Scholar]
- 27.Ostling O, Johanson K.J. Microelectrophoretic study of radiation-induced DNA damages in individual mammalian cells. Biochem Biophys Res Commun. 1984;123:291–298. doi: 10.1016/0006-291x(84)90411-x. [DOI] [PubMed] [Google Scholar]
- 28.Ashraf H, Heidari R, Nejati V, Ilkhanipoor M. Effects of aqueous extract of Berberis integerrima root on some physiological parameters in streptozotocin-induced diabetic rats. IJPR. 2013;12:425–434. [PMC free article] [PubMed] [Google Scholar]
- 29.Mokhber-Dezfuli N, Saeidnia S, Gohari A.R, Kurepaz-Mahmoodabadi M. Phytochemistry and pharmacology of berberis species. Pharmacogn Rev. 2014;8:8–15. doi: 10.4103/0973-7847.125517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kich D.M, Bitencourt S, Caye B, Faleiro D, Alves C, Silva J, et al. Lymphocyte genotoxicity and protective effect of Calyptranthes tricona (myrtaceae) against H2O2-induced cell death in MCF-7 cells. Mol Cell Biochem. 2017;424:35–43. doi: 10.1007/s11010-016-2840-9. [DOI] [PubMed] [Google Scholar]
- 31.da Costa Lopes A.M, Brenner M, Falé P, Roseiro L.B, Bogel-Łukasik R. Extraction and purification of phenolic compounds from lignocellulosic biomass assisted by ionic liquid, polymeric resins, and supercritical CO2. ACS SustainChem Engin. 2016;4:3357–3367. [Google Scholar]
- 32.Rezaeian S, Pourianfar H.R, Janpoor J. Antioxidant properties of several medicinal plants growing wild in northeastern iran. Asian J Plant Sci Res. 2015;5:63–68. [Google Scholar]
- 33.Charehsaz M, Sipahi H, Celep E, Üstündağ A, Ülker Ö.C, Duydu Y, et al. The fruit extract of berberis crataegina dc: Exerts potent antioxidant activity and protects DNA integrity. DARU. 2015;23:24. doi: 10.1186/s40199-015-0108-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Farideh S, Poorakbar L. The survey of antioxidant properties of phenolic compounds in fresh and dry hybrid barberry fruits (Berberis integerrima×vulgaris) Cumhuriyet Sci J. 2015;36:1609–1617. [Google Scholar]
- 35.Senevirathne M, Kim S-H, Jeon Y-J. Protective effect of enzymatic hydrolysates from highbush blueberry (Vaccinium corymbosum l.) against hydrogen peroxide-induced oxidative damage in chinese hamster lung fibroblast cell line. Nutr Res Pract. 2010;4:183–190. doi: 10.4162/nrp.2010.4.3.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bräunlich M, Slimestad R, Wangensteen H, Brede C, Malterud K E, Barsett H. Extracts, anthocyanins and procyanidins from Aronia melanocarpa as radical scavengers and enzyme inhibitors. Nutr. 2013;5:663–678. doi: 10.3390/nu5030663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Balasundram N, Sundram K, Samman S. Phenolic compounds in plants and agr-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food chem. 2006;99:191–203. [Google Scholar]
- 38.Dai J, Mumper R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules. 2010;15:7313–7352. doi: 10.3390/molecules15107313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fredotović Ž, Šprung M, Soldo B, Ljubenkov I, Budić-Leto I, Bilušić T, et al. Chemical Composition and Biological Activity of Allium cepa L and Allium ×cornutum (Clementi ex Visiani 1842) Methanolic Extracts. Molecules. 2017;22:448. doi: 10.3390/molecules22030448. [DOI] [PMC free article] [PubMed] [Google Scholar]


