Abstract
The effects of three selective oral inhibitors, fluvoxamine (FLU), ketoconazole (KET), and verapamil (VER), on the pharmacokinetics (PK) of florfenicol (FFC) were investigated in chickens. The chickens were administered orally with saline solution (SAL), FLU (60 mg/kg), KET (25 mg/kg), or VER (9 mg/kg) for 7 consecutive days. Florfenicol was given to the chickens at a single dose of 30 mg/kg orally. Blood samples were collected from each chicken at 0 to 12 h post-administration of FFC. The plasma concentration of FFC was analyzed by high-performance liquid chromatography (HPLC). The AUC of FFC increased and the CLs of FFC decreased with oral co-administration of KET in chickens, and the Cmax of FFC increased with VER. While the AUC, the CLs and the Cmax of FFC were all invariable with FLU. These data suggested that CYP 3A played a key role in the PK of FFC in chickens, however, P-glycoprotein (P-gp) and CYP 1A did not. The results imply that the adverse drug-drug interaction may occur in the use of FFC if the co-administrated drugs are the substrates, inducers or inhibitors of CYP 3A or/and P-gp.
Key Words: CYP 1A, CYP 3A, Florfenicol, P-glycoprotein, pharmacokinetics
Introduction
Florfenicol (FFC) (2,2-dichloro-N-[(1R,2S)-3-fluoro-1-hydroxy-1-(4-methylsulfonylphenyl) propan-2-yl] acetamide), a synthetic broad-spectrum antibiotic, offers the advantages of low toxicity and a broad antimicrobial spectrum (Shin et al., 2005 ▶; Wei et al., 2016 ▶). Therefore, FFC has been widely used in husbandry industry to cure and prevent infections for sensitive bacteria (Soback et al., 1995 ▶; Atef et al., 2001 ▶; Verner-Jeffreys et al., 2017 ▶). In poultry farms, FFC is used for the treatment of gastrointestinal and respiratory tract infections (Shen et al., 2003 ▶). Although the pharmacokinetics (PK) of FFC has been extensively studied in chickens (Afifi and AboEl-Sooud, 1997 ▶; Anadón et al., 2008 ▶; Poźniak et al., 2017 ▶), the primary enzymes and transporters that are involved in the PK of FFC remain unclear.
Pharmacokinetics is a branch of pharmacology dedicated to analyzing the fate of substances administered to living organisms. It describes how the body affects a specific xenobiotic/chemical after exposure through the mechanisms of absorption and distribution, as well as the metabolic changes of the substance in the body (e.g. by metabolic enzymes such as cytochrome P450 (CYP 450) or glucuronosyl-transferase enzymes), and the routes of metabolites excretion of the drug (Azizi et al., 2013 ▶). Cytochrome P450 is a super-family of enzymes and mainly responsible for catalyzing the metabolism of many drugs. The CYP 1A subfamily mainly participates in the metabolism of aromatic compounds, while the CYP 3A subfamily metabolizes most of the therapeutic drugs (Tsuji et al., 2007 ▶; Zhou, 2008 ▶; He and Feng, 2015 ▶). P-glycoprotein (P-gp) is a class of important transporters which are expressed in the intestine along with CYP 3A and forms a barrier of the transmembrane transportation of drugs (Pal and Mitra, 2006 ▶; Lee et al., 2017 ▶). P-glycoprotein and⁄or CYP 3A play an important role in the disposition of FFC in rabbits (Liu et al., 2011 ▶). Florfenicol could be useful in controlling common bacterial infections in different avian species, but no data are available for the primary enzymes that are involved in the FFC metabolism in chickens. It has been documented that the disposition kinetics of FFC represent obvious differences between different species and should not be extrapolated for rabbits to chickens without pharmacokinetic data (Ismail and El-Kattan, 2009 ▶). Chicken accounts for a large share of human food consumption and FFC is extensively metabolized in chickens, and its main metabolite, florfenicol amine (FFA) may be a risk to human health (Filazi et al., 2014 ▶). Therefore, understanding the roles of CYP 1A, CYP 3A and P-gp in the PK of FFC is important to explore the PK profile, avoid the possible adverse drug-drug interactions and prevent the harm caused by FFA residues in the edible tissues (Wang et al., 2013 ▶).
Fluvoxamine (FLU), a selective serotonin reuptake inhibitor, is regarded as a potent CYP1A2 inhibitor (Yasui-Furukori et al., 2004 ▶). Ketoconazole (KET), an imidazole anti-fungal drug, is an inhibitor of CYP 3A. Ketoconazole has been shown to inhibit testosterone hydroxylation in kidney in bobwhite quail (Cortright et al., 2006 ▶; Davidson Peiris and Wusirika, 2017 ▶). Verapamil (VER) is a calcium channel blocker and is commonly used to control hypertension, chest pain and arrhythmia (Ledwitch et al., 2016 ▶). Verapamil is also a potent and selective inhibitor of P-gp inhibitor which can increase the concentrations and the AUC in serum of many drugs (Zhang et al., 2017 ▶). To aid in determining the role of CYP 3A, CYP1A2 and P-gp in FFC metabolism in chickens, inhibition studies were conducted using KET, FLU and VER on the PK of FFC to investigate the roles of CYP 450 and P-gp in this paper.
Materials and Methods
Chemicals
Florfenicol (purity 99.6%) was provided by Biok Biology Co., Ltd. (Hangzhou, Zhejiang, China), and was dissolved in polyethylene glycol-300 (Tokyo Kasei Kogyo Co., Ltd., Tokyo, Japan) to a concentration of 50 g/L for the animal experiments immediately prepared before oral administration. Chloramphenicol analytical standards (purity 99%) were purchased from Schering-Plough Corporation (Shanghai, China) and Merck Corporation (Darmstadt, Germany). Maleic acid FLU tablets were purchased from Netherland Solvay Pharmaceuticals, Inc. (Chendu China). Ketoconazole tablets and VER hydrochloride tablets were purchased from Xi’an-Janssen Pharmaceutical Co., Ltd. (Xi’an, China). Acetonitrile was purchased from Merck Corporation (Darmstadt, Germany) and was of high-performance liquid chromatography (HPLC) grade. All other reagents that were used for extraction and analysis were analytical reagent grade or better, and were commercially available.
Chickens
One-day-old male AA broiler chickens provided by Hewei Agricultural Development Share Co., Ltd. (Xuancheng, Anhui, China) were used in this study. The animals were fed with a balanced ration of feed (antibacterial-free) and water ad libitum before starting the experiments. This investigation (animal study protocol No. 201207003) was approved by the Institutional Animal Care and Use Committee of College of Animal Science and Technology, Henan University of Science and Technology. According to the recommendations of the NRC (1994) ▶, the temperature, humidity, and light were controlled under suitable conditions.
Experimental design
Twenty-eight day old chickens (0.96 ± 0.24 kg, male) were divided into four groups (6 chickens per group). The chickens in the first group were given the volume of saline solution (SAL) as the other groups via oral administration once a day during the study. Those in the second, third, and fourth groups were administered FLU (60 mg/kg), KET (25 mg/kg), and VER (9 mg/kg) by oral gavage, respectively, for 7 consecutive days. At the end of day 7 of administrating, all chickens were given a single dose of 30 mg/kg of FFC by oral gavage administration at 30 min post final administration.
Firstly, the brachial wing vein of chickens was obtuse separated. Just before and at 0.08, 0.17, 0.25, 0.5, 1, 2, 3, 4, 6, 8, 10, 12 h post-FFC administration, 1 ml blood samples were taken from the left brachial wing vein of each chicken and were collected in heparinized tube. Plasma was separated from sample after centrifugation (3500 rpm for 10 min) at room temperature and stored at -20C until analysis.
Analytical method
Apparatus and chromatographic conditions
The quantitative analysis of FFC was performed with a HPLC (Agilent 1200 series; Agilent Technologies Co., Ltd., Palo Alto, Santa Clara, CA, USA). The HPLC system was equipped with a G1311A quaternary pump, a G1328B MAN injector, a G1314B ultraviolet wavelength detector set at 224 nm, a G1316A column temperature box set at 40°C, and an instrument 1 online/offline analysis software. HPLC analysis was carried out on a 5-µm Eclipse XDB-C18 column (250 4.6 mm). The mobile phase solution consisted of 50 mmol/L aqueous KH2PO4–acetonitrile (77:23 [vol/vol]). The elution flow rate was 0.9 ml/min.
Sample preparation
The frozen plasma samples were defrosted at room temperature and centrifuged for 5 min at 3500 rpm before analysis. A 200 µL supernatant of plasma was accurately collected and transferred to a new polyethylene centrifuge tube. Immediately, 5 µL of the internal standard solution (1 mg/ml chloramphenicol) and 600 µL ethyl acetate were added to the tube successively. After vortexing for 5 min, the tube was centrifuged at 12,000 rpm for 10 min. The upper layer was transferred to another centrifuge tube, and the underlying layer was re-extracted once with 600 µL ethyl acetate. The two extracts were pooled together and dried under a stream of nitrogen at 45°C, then dissolved in 200 µL of the mobile phase and centrifuged for 10 min at 12,000 rpm. Finally, 15 µL of the supernatant was injected into the HPLC system for analysis.
Quantification
Stock standard solutions (4000 μg/ml) of FFC standards were prepared by accurately dissolving 40 mg of the compounds into 10 ml of acetonitrile. The working standard solutions were prepared through serial dilution of the stock solutions with mobile phase solution to 400, 200, 80, 40, 20, 8, 4, 2, 1, 0.4, and 0.2 µg/ml. 1 mg/ml chloramphenicol solution was used as an internal standard. Both the stock and working standard solutions were stable for 1 month at -20°C in the dark.
180 µL blank plasma samples were supplemented with 20 µL working standard solutions of FFC and 5 µL internal standard to prepare plasma standard samples (containing FFC 0.02-40 µg/ml correspondingly). The plasma standard samples were analyzed according to the sample preparation items as mentioned above. Calibration curve was constructed by plotting peak area ratios (FFC to chloramphenicol) versus the corresponding FFC concentrations. The FFC con-centrations of the unknown samples were calculated from the calibration curve.
Method validation
Specificity
The specificity was evaluated by comparing blank individual plasma samples from chickens, blank plasma samples spiked with the FFC and internal standard, and plasma samples after oral administration of FFC.
Linearity
Calibration curves were prepared and assayed in duplicate in three consecutive days to assess linearity. Linearity was evaluated by calculating the FFC/internal standard peak area ratio versus FFC concentration using a weighted least-squares linear regression (weighting factor, 1/x2). The lowest to the highest concentration of FFC was the linearity range of the calibration curve.
Limits of detection and limits of quantification
The limits of detection (LODs) and limits of quantification (LOQs) were estimated from the signal of the FFC peak in spiked samples. They were defined as the concentrations that resulted in a detectable peak area 3 and 6 times the noise level, respectively.
Precision and accuracy
The intra-day accuracy and precision were determined by analyzing five replicates of the three level samples-spiked with high, middle, and low concentrations of FFC (the final concentrations were 8, 1, and 0.125 μg/ml, respectively) on the same day. The inter-day accuracy and precision were measured by analyzing the respective spiked samples on five consecutive days. The intra- and inter-day precisions were defined by the relative standard deviation (RSD), respectively. The accuracy expressed as the approximate extent of the detected FFC concentrations to the true concentrations, which was calculated by comparing the detected concentration of drug in spiked plasma samples with the true concentrations
Extraction recovery
The extraction recovery of FFC from plasma were determined by comparison of the peak areas of the analytes and internal standard in plasma experienced the complete preparation procedure with those spiked into the prepared blank plasma.
Pharmacokinetic data analysis
Pharmacokinetic analysis of plasma concentration-time data of FFC was carried out using a practical pharmacokinetic 3p87 program (Wang et al., 2013 ▶). A pharmacokinetic compartment model was selected by application of Akaike’s information criterion (AIC method) and weighting factor (Yamaoka et al., 1978 ▶; Suo et al., 2007 ▶; Wang et al., 2013 ▶).
Lag time corresponds to the finite time taken for FFC to appear in systemic circulation following oral administration:
V/F: The apparent distribution volume
AUC: The area under the plasma concentration-time curves calculated by means of the trapezoidal rule
Cmax and Tmax: The peak plasma drug concentration and the time required to attain peak concentration, respectively
The apparent clearance (CL/F) was determined as:
CL/F = Dose/AUC
Following oral administration, the plasma concentration-time data of FFC for each broiler was fitted in one-compartment open models according to the following equations:
Ct = Error! × (e–Ke·t – e–Ka·t)
In the above formula,
Ct: The plasma drug concentration at time
t: Ka means the absorption rate constants
e: The base of natural logarithm
Ke: Elimination rate constants
F: The absolute percentage bioavailability
X0 and V: The dose of administration and the volume of distribution
The absorption half-life (t1/2Kα) is given by the expression:
t1/2Kα = Error!
The elimination half-life (t1/2β) is given by the expression:
t1/2β = Error!
Statistical analysis
The results were presented as mean ± standard error (mean±SE). The data were statistically analyzed using the one-way ANOVA with SPSS 16.0 software (SPSS Inc., Chicago, IL, USA). P-values of <0.05 were considered to be statistically significant.
Results
A specific and sensitive HPLC method was developed for quantitation of FFC in chicken plasma. Using this analytical method to analyze blank samples, spiked samples, and samples from broilers administered FFC, the retention time of FFC and the internal standard was free of interference. The LOD and LOQ of FFC was 0.02 and 0.04 µg/ml in HPLC method, respectively. The calibration curve prepared from the chicken plasma spiked with known amounts of FFC was linear between 0.02 and 40 µg/ml. The correlation coefficient (r2) of the calibration curve was >0.9999. As shown in Table 1, analytical accuracy, intra-day precision, inter-day precision, and extraction recovery data of FFC (n=5) all met the requirements at three levels (0.125, 1, and 8 µg/ml) in chicken plasma. The assay was suitable to study the pharmacokinetic characteristics following oral dose of FFC extract in chickens.
Table 1.
Accuracy, precision, and extraction recovery data of the method
| Spiked concentration (µg/ml) | Detected concentration (µg/ml) | Accuracy (%) | Intra-day |
Inter-day |
Extraction recovery (%) |
|---|---|---|---|---|---|
| RSD (%) | RSD (%) | ||||
| 0.125 | 0.129 ± 0.011 | 103.2 ± 8.8 | 4.9 | 3.5 | 90.8 ± 7.7 |
| 1 | 1.054 ± 0.023 | 105.4 ± 2.3 | 4.1 | 4.6 | 103.5 ± 2.3 |
| 8 | 8.389 ± 0.595 | 104.9 ± 7.4 | 3.2 | 2.4 | 105.2 ± 7.5 |
Values are presented as mean±SE (n=5)
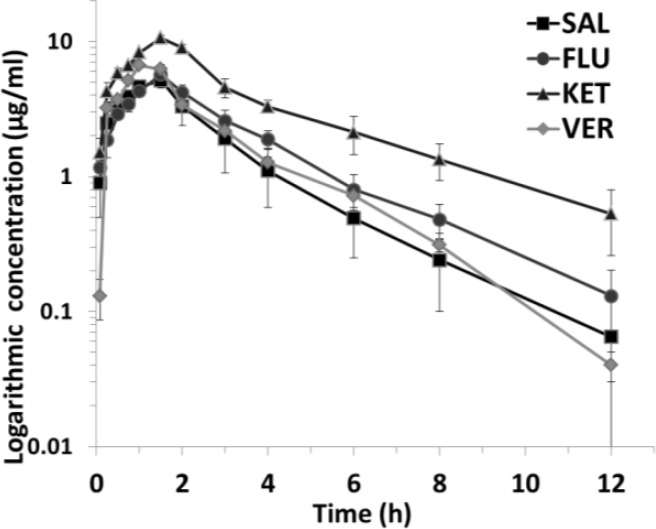
No significant adverse effects were observed in broilers when FFC was co-administered orally with SAL, FLU, KET or VER. The pharmacokinetic profile of FFC is illustrated in Fig. 1. The concentration of FFC is best described by a one-compartment open model. As shown in Table 2, the AUC and CLs of FFC in FFC + SAL group were 13.8 ± 2.18 μg/ml·h and 2.28 ± 0.34 L/kg/h, respectively. No significant difference of the AUC and CLs of FFC was found when CYP 1A was inhibited by FLU (P>0.05). However, compared with the FFC + SAL group, the inhibition of CYP 3A by KET significantly increased the AUC to about 3 times, and decreases the CLs of FFC (P<0.01, AUC = 35.04 ± 2.11 μg/ml·h, P<0.01, CLs = 0.86 ± 0.06 L/kg/h). In addition, the Cmax of FFC in the FFC + VER group was significantly increased, but the AUC was not changed relative to a control group.
Fig. 1.
Mean plasma concentration of FFC (µg/ml) following oral administration in broilers. FFC (30 mg/kg b.wt.) was co-administered orally with SAL, FLU (60 mg/kg), KET (25 mg/kg) or VER (9 mg/kg) (mean±SE, n=6)
Table 2.
Pharmacokinetic parameters of FFC following-oral administration in broiler chickens a
| Parameter | Unit | FFC + SAL | FFC + FLU | FFC + KET | FFC + VER |
|---|---|---|---|---|---|
| A | µg/ml | 15.17 ± 0.70 | 16.21 ± 1.84 | 25.73 ± 2.23** | 16.52 ± 0.31 |
| t1/2ka | h | 0.51 ± 0.03 | 0.66 ± 0.04* | 0.59 ± 0.02 | 0.42 ± 0.003 |
| t1/2ke | h | 1.14 ± 0.14 | 1.41 ± 0.02 | 1.55 ± 0.12* | 1.09 ± 0.02 |
| Tmax | h | 1.07 ± 0.09 | 1.35 ± 0.05** | 1.32 ± 0.03* | 0.94 ± 0.001 |
| Cmax | µg/ml | 4.33 ± 0.33 | 4.44 ± 0.26 | 8.66 ± 0.18** | 5.55 ± 0.22** |
| AUC | mg·h/L | 13.8 ± 2.18 | 17.62 ± 1.51 | 35.04 ± 2.11** | 15.96 ± 0.80 |
| CL/Fs | L/kg·h | 2.28 ± 0.34 | 1.73 ± 0.14 | 0.86 ± 0.06** | 1.89 ± 0.10 |
| V/F | L/kg | 3.63 ± 0.19 | 3.51 ± 0.25 | 1.91 ± 0.03** | 2.97 ± 0.10* |
Note: FFC (30 mg/kg b.wt.) was co-administered orally with SAL, FLU (60 mg/kg), KET (25 mg/kg) or VER (9 mg/kg) (mean±SE, n=6) (mean±SE, n=6).
P<0.05 and
P<0.01 compared with the FFC + SAL group
Discussion
Florfenicol is used alone or with other drugs to cure and prevent bacteria infections in veterinary clinical practice (Ghoddusi et al., 2015 ▶; Razmyar and Zamani, 2016 ▶). When the drug combination is used, the PK drug-drug interactions may occur. In addition to chemical factors, physiological and biochemical factors such as metabolism enzymes, transporting proteins also may result in these interactions. Liu et al. (2011) ▶ reported that an obvious increase of T1⁄2Ke was found in the FFC + VER group, but not in the FFC + FLU group or FFC + KET group. The CL/F in the FFC + VER group or the FFC + KET group, but not in the FFC + FLU group, was remarkably slower than that of FFC alone group in rabbits. P-glycoprotein and/or CYP 3A have been found to affect the disposition of FFC in rabbits (Liu et al., 2011 ▶). It is a little different in rabbits metabolism, the present study showed that when CYP 3A was inhibited by KET, the AUC of FFC significantly increased and the CLs of FFC significantly decreased. However, when CYP 1A was inhibited by FLU, neither the AUC nor the CLs of FFC was changed. In general, VER should increase the Cmax in serum and the AUC of many drugs. Our results showed the Cmax of FFC was increased, but the AUC was not changed when P-gp was inhibited by VER. It suggested that CYP 3A play a key role in the PK of FFC in broiler chickens, the P-gp should be involved in the process and CYP 1A may not be important. The result implied that the adverse drug-drug interaction may occur in the use of FFC if the co-administered drugs are the substrates, inducers or inhibitors of CYP 3A or/and P-gp.
In human and animal, CYP 3A is very abundant in the liver and the intestine, and metabolizes severaldrugs including some synthetic steroids and most macrolide antibiotics (Lee et al., 2017 ▶). In addition, P-gp is expressed in the intestine along with CYP 3A and form a transport barrier to drug absorption (Pal and Mitra, 2006 ▶). If the drug was a substrate of P-gp or P-gp was inhibited, the absorption of the drug would be increased and results in an increase of the drug concentration in the blood. However, the activity of CYP 3A was not decreased and a greater amount of drug was eliminated from the body which may not result in the AUC of the drug increasing. This may explain why the Cmax of FFC was increased while the AUC of FFC was not changed when co-administered with VER. On the other hand, when CYP 3A was inhibited by some co-administered drugs, the metabolism of FFC was decreased, and the drug was accumulated in the animal body which resulted in augmentations of the Cmax and the AUC of FFC. These may further cause a risk of toxicity to animals that are exposed to FFC and the prolonged withdrawal time of the edible tissues. Conversely, if CYP 3A was induced, the Cmax and the AUC of FFC decreased which may lead to a failure of therapy.
Now, our study showed that FLU, KET and VER are not the specific inhibitors in the pharmacokinetics of FFC in chickens. They are the potent and selective inhibitors of CYP enzymes or/and P-gp (Yasui-Furokori et al., 2004 ▶; Zhou, 2008 ▶; Athukuri ans Neerati, 2017 ▶). Therefore, some other factors in the PK of FFC in chicken cannot be ruled out, and more and further study should be performed.
In conclusion, CYP 3A may play a key role in the metabolism of FFC and P-gp is likely involved in the absorption of FFC in chickens. To avoid the adverse drug-drug interactions, more attention should be paid when the substrates, inducers or inhibitors of CYP 3A and/or P-gp are co-administrated with FFC in poultry farms.
Acknowledgements
We gratefully acknowledge the support by the National Key Technology R&D Program (2015BAD11B01) and the Special Fund for Agro-Scientific Research in the Public Interest (201303038) of China.
Conflict of interest
The authors declare that they have no competing interests.
References
- Afifi, NA, AboEl-Sooud, K. Tissue concentration and pharmacokinetics of florfenicol in broiler chickens. Br. Poult. Sci. 1997;38:425–428. doi: 10.1080/00071669708418013. [DOI] [PubMed] [Google Scholar]
- Anadón, A, Martínez, MA, Martínez, M, Ríos, A, Caballero, V, Ares, I, Martínez-Larrañaga, MR. Plasma and tissue depletion of florfenicol and florfenicol-amine in chickens. J. Agr. Food Chem. 2008;56:11049–11056. doi: 10.1021/jf802138y. [DOI] [PubMed] [Google Scholar]
- Atef, M, El-genda, YI, Amer, AMM, El-Aty, AMA. Disposition kinetics of florfenicol in goats by using two analytical methods. J. Vet. Med. A Physiol. Pathol. Clin. Med. 2001;48:129–136. doi: 10.1046/j.1439-0442.2001.00339.x. [DOI] [PubMed] [Google Scholar]
- Athukuri, BL, Neerati, P. Enhanced oral bio-availability of domperidone with piperine in male Wistar rats: involvement of CYP3A1 and P-gp inhibition. J. Pharm. Pharm. Sci. 2017;20:28–37. doi: 10.18433/J3MK72. [DOI] [PubMed] [Google Scholar]
- Azizi, J, Ismail, S, Mansor, SM. Mitragyna speciosa Korth leaves extracts induced the CYP450 catalyzed aminopyrine-N-demethylase (APND) and UDP-glucuronosyl transferase (UGT) activities in male Sprague-Dawley rat livers. Drug Metabol. Drug Interact. 2013;28:95–105. doi: 10.1515/dmdi-2012-0039. [DOI] [PubMed] [Google Scholar]
- Cortright, KA, Craigmill, AL. Cytochrome P450-dependent metabolism of midazolam in hepatic microsomes from chickens, turkeys, pheasant and bobwhite quail. J. Vet. Pharmacol. Ther. 2006;29:469–476. doi: 10.1111/j.1365-2885.2006.00793.x. [DOI] [PubMed] [Google Scholar]
- Davidson Peiris, E, Wusirika, R. A case report of compound heterozygous CYP24A1 mutations leading to nephrolithiasis successfully treated with ketoconazole. Case Rep. Nephrol. Dial. 2017;7:167–171. doi: 10.1159/000485243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filazi, A, Sireli, UT, Yurdakok, B, Aydin, FG, Kucukosmanoglu, AG. Depletion of florfenicol and florfenicol amine residues in chicken eggs. Br. Poult. Sci. 2014;55:460–465. doi: 10.1080/00071668.2014.935701. [DOI] [PubMed] [Google Scholar]
- Ghoddusi, A, Nayeri Fasaei, B, Karimi, V, Ashrafi Tamai, I, Moulana, Z, Zahraei Salehi, T. Molecular identification of Salmonella infantis isolated from backyard chickens and detection of their resistance genesby PCR. Iran. J. Vet. Res. 2015;16:293–297. [PMC free article] [PubMed] [Google Scholar]
- He, X, Feng, S. Role of metabolic enzymes P450 (CYP) on activating procarcinogen and their poly-morphisms on the risk of cancers. Curr. Drug Metab. 2015;16:850–863. doi: 10.2174/138920021610151210164501. [DOI] [PubMed] [Google Scholar]
- Ismail, M, El-Kattan, YA. Comparative pharma-cokinetics of florfenicol in the chicken, pigeon and quail. Br. Poult. Sci. 2009;50:144–149. doi: 10.1080/00071660802613286. [DOI] [PubMed] [Google Scholar]
- Ledwitch, KV, Barnes, RW, Roberts, AG. Unravelling the complex drug-drug interactions of the cardiovascular drugs, verapamil and digoxin, with P-glycoprotein. Biosci. Rep. 2016;36:e00309. doi: 10.1042/BSR20150317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, J, Kim, AH, Yi, S, Lee, S, Yoon, SH, Yu, KS, Jang, IJ, Cho, JY. Distribution of exogenous and endogenous CYP3A markers and related factors in healthy males and females. AAPS J. 2017 doi: 10.1208/s12248-017-0090-8. doi: 10.1208/s12248-017-0090-8. [DOI] [PubMed] [Google Scholar]
- Liu, N, Guo, M, Mo, F, Sun, YH, Yuan, Z, Cao, LH, Jiang, SX. Involvement of P-glycoprotein and cytochrome P450 3A in the metabolism of florfenicol of rabbits. J. Vet. Pharmacol. Therap. 2011;35:202–205. doi: 10.1111/j.1365-2885.2011.01310.x. [DOI] [PubMed] [Google Scholar]
- NRC. Nutrient requirements of poultry. 9th Rev. Edn. Washington, D.C: Natl. Acad. Press; 1994. pp. 19–34. [Google Scholar]
- Pal, D, Mitra, AK. MDR- and CYP3A4-mediated drug-drug interactions. J. Neuroimmune Pharmacol. 2006;1:323–339. doi: 10.1007/s11481-006-9034-2. [DOI] [PubMed] [Google Scholar]
- Poźniak, B, Pawłowski, P, Pasławska, U, Grabowski, T, Suszko, A, Lis, M, Świtała, M. The influence of rapid growth in broilers on florfenicol pharmacokinetics-allometric modelling of the pharmacokinetic and haemo-dynamic parameters. Br. Poult. Sci. 2017;58:184–191. doi: 10.1080/00071668.2016.1261994. [DOI] [PubMed] [Google Scholar]
- Razmyar, J, Zamani, AH. An outbreak of yolk sac infection and dead-in-shell mortality in common canary (Serinus canaria) caused by Klebsiella pneumoniae. Iran. J. Vet. Res. 2016;17:141–143. [PMC free article] [PubMed] [Google Scholar]
- Shen, J, Hu, D, Wu, X, Coats, JR. Bioavailability and pharmacokinetics of florfenicol in broiler chickens. Vet. Pharmacol. Ther. 2003;26:337–341. doi: 10.1046/j.1365-2885.2003.00495.x. [DOI] [PubMed] [Google Scholar]
- Shin, SJ, Kang, SG, Nabin, R, Kang, ML, Yoo, HS. Evaluation of the antimicrobial activity of florfenicol against bacteria isolated from bovine and porcine respiratory disease. Vet. Microbiol. 2005;106:73–77. doi: 10.1016/j.vetmic.2004.11.015. [DOI] [PubMed] [Google Scholar]
- Soback, S, Paape, MJ, Filep, R, Varma, KJ. Florfenicol pharmacokinetics in lactating cows after intravenous, intramuscular and intramammary administra-tion. J. Vet. Pharmacol. Ther. 1995;18:413–417. doi: 10.1111/j.1365-2885.1995.tb00618.x. [DOI] [PubMed] [Google Scholar]
- Suo, XB, Zhang, H, Wang, YQ. HPLC determination of andrographolide in rat whole blood: study on the pharmacokinetics of andrographolide incorporated in liposomes and tablets. Biomed. Chromatogr. 2007;21:730–734. doi: 10.1002/bmc.812. [DOI] [PubMed] [Google Scholar]
- Tsuji, PA, Walle, T. Benzo[a]pyrene-induced cytochrome P450 1A and DNA binding in cultured trout hepatocytes-inhibition by plant polyphenols. Chem. Biol. Interact. 2007;169:25–31. doi: 10.1016/j.cbi.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verner-Jeffreys, DW, Brazier, T, Perez, RY, Ryder, D, Card, RM, Welch, TJ, Hoare, R, Ngo, T, McLaren, N, Ellis, R, Bartie, KL, Feist, SW, Rowe, WMP, Adams, A, Thompson, KD. Detection of the florfenicol resistance gene floR in Chryseobacterium isolates from rainbow trout Exception to the general rule? FEMS Microbiol. Ecol. 2017;93(4) doi: 10.1093/femsec/fix015. doi: 10.1093/femsec/fix015. [DOI] [PubMed] [Google Scholar]
- Wang, GY, Tu, P, Chen, X, Guo, YG, Jiang, SX. Effect of three polyether ionophores on pharmacokinetics of florfenicol in male broilers. J. Vet. Pharmacol. Ther. 2013;36:494–501. doi: 10.1111/jvp.12020. [DOI] [PubMed] [Google Scholar]
- Wei, CF, Shien, JH, Chang, SK, Chou, CC. Florfenicol as a modulator enhancing antimicrobial activity: example using combination with Thiamphenicol against Pasteurella multocida. Front Microbiol. 2016;7:389. doi: 10.3389/fmicb.2016.00389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaoka, K, Nakagawa, T, Uno, T. Application of Akaike’s information criterion (AIC) in the evaluation of linear pharmacokinetic equations. J. Food Biochem. 1978;100:609–618. doi: 10.1007/BF01117450. [DOI] [PubMed] [Google Scholar]
- Yang, YC, Zhang, WG, Tang, ZM, Liu, CX, Sun, RY, Yu, ZL. 3P87 practical pharmacokinetics program. Information of the CPA. 1988;5:67. [Google Scholar]
- Yasui-Furukori, N, Takahata, T, Nakagami, T, Yoshiya, G, Inoue, Y, Kaneko, S, Tateishi, T. Different inhibitory effect of fluvoxamine on omeprazole metabolism between CYP2C19 genotypes. Br. J. Clin. Pharmacol. 2004;57:487–494. doi: 10.1111/j.1365-2125.2004.02047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y, Wang, C, Liu, Z, Meng, Q, Huo, X, Liu, Q, Sun, P, Yang, X, Sun, H, Ma, X, Liu, K. P-gp is involved in the intestinal absorption and biliary excretion of afatinib in vitro and in rats. Pharmacol. Rep. 2017;70:243–250. doi: 10.1016/j.pharep.2017.10.005. [DOI] [PubMed] [Google Scholar]
- Zhou, SF. Drugs behave as substrates, inhibitors and inducers of human cytochrome P450 3A4. Curr. Drug Metab. 2008;9:310–322. doi: 10.2174/138920008784220664. [DOI] [PubMed] [Google Scholar]