Abstract
The objective of this study was to investigate the effect of equine chorionic gonadotropin (eCG) on ovarian follicles at three stages of development (emergence, dominance and early static phases) during the first follicular wave (FFW) in Holstein heifers. Heifers (n=20) were randomly assigned into four experimental groups (n=5 in each group). Heifers received eCG (500 IU; Folligon®; Intervet, Holland; i.m) a) on the day of follicle emergence (day of ovulation; group 1), b) on the dominant phase (dominant follicle (DF): the first day in which follicle was observed at ≥10 mm; group 2, and c) on the early static phase (group 3) of the FFW. Control group heifers did not receive any treatment. Daily ultrasonography was conducted to monitor ovarian structure throughout estrous cycle. All treatment group heifers, regardless of the stage of follicle development, displayed follicle growth after eCG injection. Administration of eCG, in group 1, hastened DF detection and induced co-dominant follicles; whereas, in groups 2 and 3, it delayed DF regression, and increased cycle length compared to control. In all treatment group heifers, DF was present 84 h after eCG injection. Maximum diameter of corpus luteum was larger in eCG treated groups compared to control (P<0.05). In conclusion, depending on the time of eCG administration throughout the FFW (emergence, dominant and early static phases), co-dominancy, maintenance of DF, enhancement of follicle and corpus luteum growth and increase in estrous cycle length could be observed in Holstein heifers.
Key Words: Dominant phase, eCG, Emergence, Holstein heifers, Static phase
Introduction
Follicle growth in cattle occurs in a wave like pattern (Ginther et al., 1989a ▶). Predominantly, two (Ginther et al., 1989a ▶; Knopf et al., 1989 ▶; Ahmad et al., 1997 ▶; Burke et al., 2000 ▶) and three (Savio et al., 1988 ▶; Sirois and Fortune, 1988 ▶; Ahmad et al., 1997 ▶; Burke et al., 2000 ▶) follicular waves were observed throughout estrous cycle. Each follicle wave starts with the recruitment of a cohort of follicles followed by the selection of growing follicle as dominant follicle and regression of subordinate follicles (Savio et al., 1988 ▶; Ginther et al., 1989b ▶; Adams et al., 1992 ▶; Ginther et al., 2000 ▶). Then dominant follicle remained at statistic phase followed by its regression in the presence of corpus luteum (Ginther et al., 1989b ▶).
Several hormonal interventions could affect the fate of the ovarian follicle within follicular wave. The ovarian follicle could be luteinized or ovulated following GnRH administration (Macmillan and Thatcher, 1991 ▶; Moghaddam et al., 2001 ▶; Souza et al., 2009; Niasari-Naslaji et al., 2012 ▶) or regressed subsequent to the administration of regressing agents such as estradiol and progesterone (Bo et al., 1995 ▶; Niasari-Naslaji et al., 2001 ▶). Within follicular wave, a cohort of ovarian follicles could continue to grow in the presence of follicle stimulating hormone (FSH) throughout superovulation (Bevers et al., 1989 ▶; Dieleman et al., 1989 ▶) or a single follicle might become persistent in the presence of high Luteinizing hormone (LH) pulse frequency induced by low progestogen concentrations (Savio et al., 1993 ▶; Niasari-Naslaji et al., 2001 ▶, Niasari-Naslaji et al., 2012 ▶).
Equine chorionic gonadotropin (eCG) is a glycoprotein molecule, with FSH and LH like activities in non-equid species (Gonzalez-Menico et al., 1978 ▶; Newcomb et al., 1979 ▶; Murphy et al., 1991 ▶; Murphy et al., 2012 ▶) with the half-life of 45.6 h in cattle (Schams et al., 1978 ▶). The main objectives of using eCG in cattle could be summarized into three categories: a) supporting the growth of follicles in superovulation programs (Monniaux et al., 1983 ▶; Goulding et al., 1996), b ▶) enhancing the growth and ovulation of the existing follicle near the end of estrous synchronization programs (Garcia-Ispierto et al., 2012 ▶; Núnez-Olivera et al., 2014 ▶; Pessoa et al., 2016 ▶), following parturition (Rostami et al., 2011 ▶; Voigani et al., 2013 ▶) and for resynchronization of non-pregnant cows (Bartolome et al., 2012) and c ▶) increasing the diameter of CL and its progesterone production (Souza et al., 2006; O’Hara et al., 2016 ▶). We have hypothesized that the administration of eCG at different stages of follicle development could change the fate of the follicle within follicular wave. This, in turn, will enable us to manage the variation of response following eCG administration. The aim of the present study was to investigate the effect of eCG administration at emergence, dominance and early static phases of the first follicular wave (FFW) in Holstein heifers.
Materials and Methods
Location and animals
This study was carried out, between December and January 2015, at the experimental dairy herd of the Veterinary Research Institute, Faculty of Veterinary Medicine, University of Tehran (latitude: 35°39'8'N; longitude: 51°26'38'E; altitude: 1029 m). Healthy cyclic Holstein heifers (n=20; 14 months of age; 350 ± 20 kg LW) were selected for this study. Heifers were housed in an open shed barn and received total mixed ration according to NRC recommendation (NRC, 2001).
Experimental design
Heifers were synchronized using two doses of PGF2α analogue (Cloprostenol; Estroplan®, Parnell, Canada) 14 days apart. Following ovulation (day 0 of experiment), heifers were randomly divided into 4 experimental groups (n=5 in each group) and received eCG (500 IU; Folligon®; Intervet, Holland; i.m) at different stages of follicular development. Heifers received eCG during the FFW: at the emergence of follicular wave (group 1), at the detection of morphologically dominant follicle (≥10 mm; group 2) and at the second day of static phase (group 3). Control group heifers did not receive any treatment. Ovarian structures (follicle and CL) were monitored daily using ultrasonography for the completion of one estrous cycle.
Ultrasonography
Daily ovarian ultrasound examinations were conducted, throughout the experiment, using a real time ultrasound scanner (Emperor, V-9; China) equipped with 7.5 MHz linear array trans-rectal transducer. During each examination, the diameter of follicles (≥4 mm in diameter) and CL were recorded using the internal electronic calipers. The horizontal and vertical diameters of ovarian structures were recorded and the average diameters were used for further analysis. New follicle wave emergence was defined as the day in which the dominant follicle was, retrospectively, identified at a diameter of 4-5 mm (Ginther et al., 1989a ▶; Knopf et al., 1989 ▶). Morphologically dominant follicle refers to the presence of at least one follicle of ≥10 mm in diameter, originated from new follicular wave. Initiation of static phase was defined when the detected dominant follicle remained at similar diameter for at least two days followed by follicle regression. The day of ovulation was confirmed by the disappearance of the largest follicle followed by the subsequent formation of corpus luteum.
Statistical analysis
Data were analyzed using GLM procedure including Tukey statement in the model, if assumptions of parametric tests were in place. Otherwise, Kruskal-Wallis procedure was used to analyze the data. Follicle growth rate was estimated using Proc Rsreg, including Lackfit in the model. In case the lack of fit was not significant, and the linear relationship between time and follicle size was significant, linear regression was used to estimate follicle growth rate. Data were presented as mean±SE of the mean.
Results
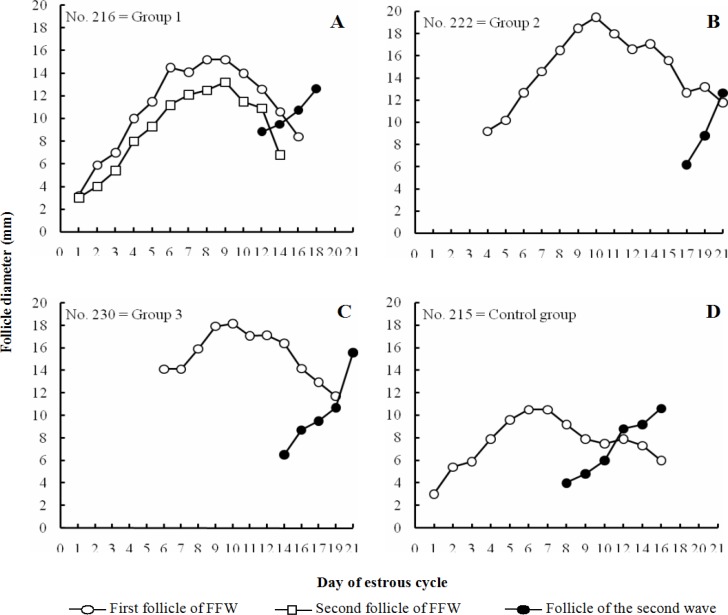
All treatment group heifers that received eCG, at the emergence of follicular wave (group 1), at the detection of dominant follicle (group 2) and at the early static phase (group 3), have shown follicle growth following eCG administration (Figs. 1A-C). Administration of eCG, concurrent with the emergence of follicular wave, did not affect the length of estrous cycle (treatment: 21.5 ± 0.65 days; control: 20.4 ± 0.24 days; Table 1; P>0.05). However, eCG treatment increased the cycle length when it was administrated at the detection of morphologically dominant follicle (22.8 ± 0.49 days) and at the early static phase (22.8 ± 0.73 days) compared to control (Fig. 1D; 20.4 ± 0.24 days; Table 1; P<0.05). All experimental groups displayed two follicular waves throughout estrous cycle.
Fig. 1.
Follicle growth pattern throughout estrous cycle in a representative heifer belonged to the group of Holstein heifers that received eCG (500 IU) throughout the FFW: on the day of follicle emergence (group 1; n=5; A), at dominant phase (group 2; n=5; B) and at early static phase (group 3; n=5; C), or remained untreated as control (n=5; D
Table 1.
Follicular dynamics and corpus luteum formation in a group of Holstein heifers that received eCG (500 IU, IM) throughout the FFW: on the day of follicle emergence (group 1; n=5), at the dominant phase (group 2; n=5) and at the early static phase (group 3; n=5) compared to control (n=5). Data were presented as mean±SEM
| Parameters | Control | Group 1 | Group 2 | Group 3 |
|---|---|---|---|---|
| Estrus cycle | ||||
| Length | 20.4 ± 0.24a | 21.5 ± 0.65ab | 22.8 ± 0.49b | 22.8 ± 0.73b |
| Number of follicular wave | 2 | 2 | 2 | 2 |
| Follicle growth rate (mm/day) | ||||
| First wave follicle growth rate | 1.2 ± 0.1a | 1.6 ± 0.15a | 1.6 ± 0.13a | 1.3 ± 0.23a |
| Second wave follicle growth rate | 1.1 ± 0.08ab | 0.8 ± 0.13a | 1.4 ± 0.11b | 1.1 ± 0.07ab |
| Follicle characteristics | ||||
| Day of DF detection | 4.8 ± 0.37a | 3.2 ± 0.25b | ||
| Maximum diameter of the first wave DF | 12.3 ± 0.49a | 13.7 ± 0.73a | 18.3 ± 0.47b | 17.3 ± 0.65b |
| The day in which the first wave DF reached at maximum diameter | 6.4 ± 0.4a | 6.2 ± 0.48a | 9.8 ± 0.86b | 9.7 ± 0.85b |
| Day of the second follicle wave emergence | 7.6 ± 0.24a | 9a | 13 ± 0.89b | 13 ± 0.63b |
| Day of follicle regression | 9.2 ± 0.37a | 9.2 ± 0.25a | 12.4 ± 0.75b | 13.7 ± 1.31b |
| Corpus luteum characteristics | ||||
| Maximum CL diameter | 21.2 ± 0.81a | 25.3 ± 1.03b | 24.3 ± 1.19b | 25.5 ± 0.94b |
| The day in which CL reached at maximum diameter | 6.8 ± 0.37a | 8.2 ± 0.25b | 9.2 ± 0.25b | 9.2 ± 0.37b |
Values within rows with different superscripts differ (P<0.05)
Administration of eCG on the day of follicle emergence advanced the detection of morphologically dominant follicle (treatment: 3.2 ± 0.25 days vs control: 4.8 ± 0.37 days; P<0.05; Table 1) and induced the growth of two dominant follicles (co-dominance) compared to control group in which single follicle grew and became dominant (Fig. 1A). The follicle growth rate was similar among control (1.2 ± 0.1 mm/day), group 1 (1.6 ± 0.15 mm/day), group 2 (1.6 ± 0.13 mm/day) and group 3 (1.3 ± 0.23 mm/day) heifers (Table 1; P>0.05). Dominant follicle reached at maximum diameter earlier in control (day: 6.4 ± 0.4) and group 1 (day: 6.2 ± 0.48) compared to group 2 (day: 9.8 ± 0.86) and group 3 (day: 9.7 ± 0.85; P<0.05; Table 1). The maximum diameter of the first wave dominant follicle was smaller in control (12.3 ± 0.49 mm) and group 1 (13.7 ± 0.73 mm) than in group 2 (18.3 ± 0.47 mm) and group 3 (17.3 ± 0.65 mm; P<0.05; Table 1).
The day of the first wave dominant follicle regression was delayed following administration of eCG on dominant (group 2: 12.4 ± 0.75) and early static (group 3: 13.7 ± 1.31) phases compared to the heifers that received eCG on the day of follicle emergence (group 1: 9.2 ± 0.25) and control group (9.2 ± 0.37; P<0.05; Table 1). The emergence of the second follicular wave was delayed in group 2 (13 ± 0.89) and 3 (13 ± 0.63) heifers compared to control (7.6 ± 0.24) and group 1 (9) heifers (P<0.05; Table 1).
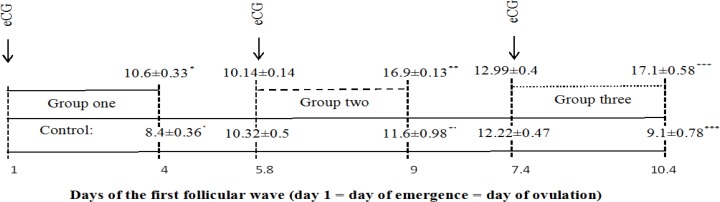
Almost 84 h after eCG administration, the diameter of ovarian follicle was significantly different between treatment groups and control counterparts (control: 8.4 ± 0.36 mm vs group 1: 10.6 ± 0.33 mm, P<0.05; control: 11.6 ± 0.98 mm vs group 2: 16.9 ± 0.13 mm, P<0.01; control: 9.1 ± 0.78 mm vs group 3: 17.1 ± 0.58 mm, P<0.001; Fig. 2).
Fig. 2.
Experimental design and the diameter of ovarian follicle (mean±SEM) for the group of Holstein heifers that received eCG (500 IU): on the day of follicle emergence (group 1; n=5; * P<0.05), at dominant phase (group 2; n=5; ** P<0.01) and at early static phase (group 3; n=5; *** P<0.01), or remained untreated as control (n=5)
The maximum diameter of corpus luteum was significantly larger in treated groups (group 1: 25.3 ± 1.03 mm, group 2: 24.3 ± 1.19 mm, group 3: 25.5 ± 0.94 mm) compared to control (21.2 ± 0.081; P<0.05; Table 1; Fig. 3). The day in which corpus luteum reached to the maximum diameter was delayed in treated groups compared with control (P<0.05; Table 1).
Fig. 3.
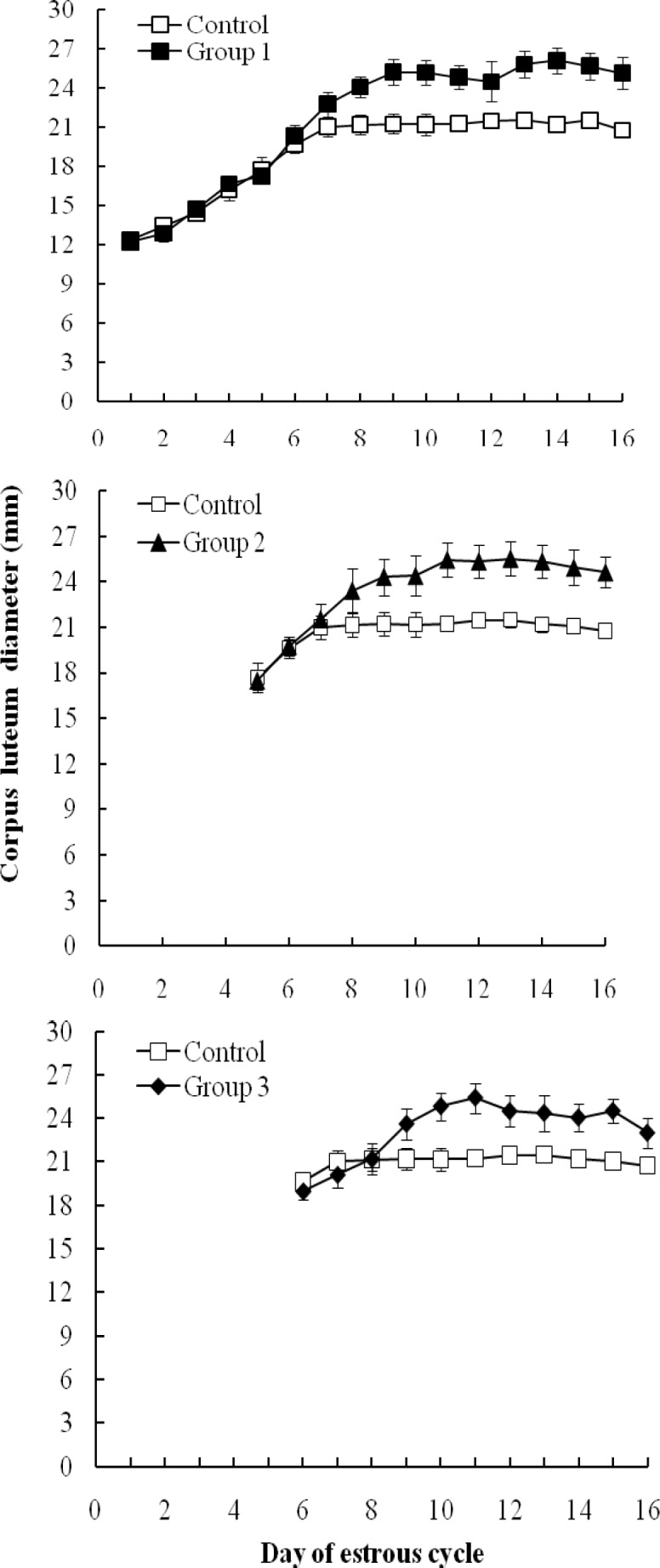
Corpus luteum diameter (mm) throughout estrous cycle in the group of Holstein heifers that received eCG (500 IU) throughout the FFW: on the day of follicle emergence (group 1; n=5(, at dominant phase (group 2; n=5( and at early static phase (group 3; n=5( compared to control (n=5
Discussion
The objective of the present study was to evaluate the effect of eCG, prescribed at different times within the FFW, on follicle dynamics and corpus luteum formation in Holstein heifers. In the present study, regardless of the stage of follicle development, eCG did not affect the number of follicular wave, but induced follicle growth. The enhancing effect of eCG on follicle growth was evident throughout superovulation (Monniaux et al., 1983 ▶; Goulding et al., 1996 ▶) and estrous synchronization (Sa Filho et al., 2010 ▶; Garcia-Ispierto et al., 2012 ▶; Pessoa et al., 2016 ▶) programs. The FSH and LH like effects of eCG is implemented through its attachment to FSH and LH receptors in theca and granulosa cells (Gonzalez-Menico et al., 1978 ▶; Soumano et al., 1998 ▶; Murphy, 2012 ▶).
In the present study, injection of eCG, concurrent with the emergence of the FFW supported the simultaneous growth of two follicles. This is consistent with the result of the previous study in which eCG administration prior to the follicle selection (day 3 after ovulation) could enhance multiple follicle development in a dose dependent manner (O’Hara et al., 2016 ▶). Accordingly, 250 and 500 IU eCG may not be able to induce co-dominancy; whereas, 750 and 1000 IU of eCG could induce twin follicle growth (O’Hara et al., 2016 ▶). The result of the present study indicated that 500 IU eCG, administered at the day of follicle emergence, is sufficient to induce co-dominancy. Therefore, the amount and the time of eCG administration are important factors for co-dominancy of ovarian follicles in cattle.
Administration of eCG at dominant (group 2) and early static (group 3) phases of follicular wave delayed follicle regression and emergence of the second follicle wave subsequent with an increase in the length of estrus cycle (group 2: 22.8 ± 0.49; group 3: 22.8 ± 0.73 days). This is in agreement with the previous study in which high doses of eCG (750-1000 IU) on day 3 of the estrous cycle could delay the emergence of the second follicular wave and increase estrous cycle length (O’Hara et al., 2016 ▶). In contrast, eCG administration concurrent with the emergence of the FFW, did not affect estrous cycle length (21.5 ± 0.65 days) and was similar to control (20.4 ± 0.24 days). The estrous cycle length of the latter two groups was similar to the mean length of estrous cycle in heifers with two dominant follicles (20.5 ± 1.3 days; Savio et al., 1988 ▶). Several factors could affect the length of estrous cycle such as number of follicular waves (Ginther et al., 1989a ▶) and life span of the CL (Ginther, 1970 ▶; Sirois and Fortune, 1990 ▶; Wiltbank et al., 2002 ▶; Niasari-Naslaji et al., 2012 ▶; O’Hara et al., 2016 ▶). Heifers with three follicular waves have longer estrous cycle compared to those with two waves (Ginther et al., 1989a ▶; Taylor and Rajamahendran, 1991 ▶; Adams, 1999 ▶). Heifers at the first estrus after reaching puberty (Gonzalez-Padilla et al., 1975 ▶) and in dairy cows at the first ovulation following parturition (Odde et al., 1980 ▶) are more likely to have short estrous cycle due to the lack of progesterone priming (Inskeep et al., 1988 ▶). It is possible to induce short estrous cycle in cattle, by injecting prostaglandin F2α analogues (Taponen et al., 2002 ▶). In the present study, injection of eCG, at dominant or early static phases, increased cycle length. Similar phenomena could occur when progesterone concentrations are maintained between 1 and 2 ng/ml, resulting in the prolongation of follicular dominance and increase in cycle length (Sirois and Fortune, 1990 ▶).
The growth rate of follicle within the FFW was recorded as 1.2 ± 0.1, 1.6 ± 0.15, 1.6 ± 0.13 and 1.3 ± 0.23 in control, groups 1, 2 and 3, respectively (P>0.05). These follicle growth rates in Holstein heifers are within the range reported in the previous studies (Ginther et al., 1989b ▶; Sirois and Fortune, 1990 ▶).
The maximum diameter of dominant follicle (DF) was greater and the day in which DF was detected was delayed when eCG was administered at dominant (18.3 ± 0.47 mm; 9.8 ± 0.86 days) and early static (17.3 ± 0.65 mm; 9.7 ± 0.85) phases compared to eCG administration at follicle emergence (13.7 ± 0.73; 6.2 ± 0.48 days) and control (12.3 ± 0.49 mm; 6.4 ± 0.4 days; P<0.05). The maximum diameter of the first wave ovarian follicle in Holstein heifers was reported to be 13.6 ± 0.8 mm (Sirois and Fortune, 1988 ▶) and 10.78 ± 0.55 mm (Niasari-Naslaji et al., 2012 ▶), which is similar to the diameter of follicle in group 1 and control but is less than those in group 2 and 3 in the present study. In many studies, the day in which DF reached at the maximum diameter was around day 6 and 7 (Ginther et al., 1989b ▶; Sirois and Fortune, 1990 ▶), which is similar to control and group 1 but is less than in groups 2 and 3.
In this study, eCG administration on the day of follicle emergence hastened early detection of DF (day: 3.2 ± 0.25) compared with control (day: 4.8 ± 0.37). This clearly indicates that the effect of eCG on follicle dynamics depends on the stage of follicle development within follicular wave. Equine chorionic gonadotropin could maintain the growth of the DF for a longer time even in the presence of functional CL; whereas, it is well documented that the growth rate and maximum diameter of ovarian follicle is suppressed via the progesterone negative feedback (Sirois et al., 1988 ▶, 1990).
Present results showed that maximum CL diameter in treatment groups was significantly larger than that in control group. In addition, in all treatment groups the day in which maximum diameter of CL appeared was later than control. Conclusively, administration of eCG at different stages of the FFW could participate in further luteal development. This finding is in accordance with the previous study in which the administration of eCG on day 3 resulted in dose-dependent rise in CL diameter and progesterone production (O’Hara et al., 2016 ▶). Equine chorionic gonadotropin might exert the direct effect on theca and granulosa cells (Pulley et al., 2013 ▶) and/or gene expression regulation of prolactin receptors, leading to CL volume expansion and progesterone synthesis (Fatima et al., 2012 ▶; Rigoglio et al., 2013 ▶).
In conclusion, administration of eCG on various stages of the follicle development within the FFW could result in different outcomes including: co-dominancy, when it is administered on the day of follicle emergence, persistence of follicle growth, delayed follicle regression and increased cycle length, when it is administered at dominant and early static phases. Moreover, eCG could increase CL diameter, when it is administered at any stage of follicle development. Furthermore, morphologically dominant follicle could be present 84 h following eCG administration. Accordingly, further research is underway in our research group to incorporate eCG throughout pre-synchronization programs in cattle.
Acknowledgements
The authors express their sincere appreciation for the financial support of the Deputy for Research of University of Tehran and the kind assistance of the staff of the dairy farm within the Institute of Veterinary Research, at the Faculty of Veterinary Medicine, University of Tehran.
References
- Adams, GP. Comparative patterns of follicular development and selection in ruminants. J. Reprod. Fertil. 1999;54:17–32. [PubMed] [Google Scholar]
- Adams, GP, Matteri, RL, Kastelic, JP, Ko, JC, Ginther, OJ. Association between surges of follicle-stimulating hormone and the emergence of follicular waves in heifers. J. Reprod. Fertil. 1992;94:177–188. doi: 10.1530/jrf.0.0940177. [DOI] [PubMed] [Google Scholar]
- Ahmad, N, Townsend, EC, Dailey, RA, Inskeep, EK. Relationship of hormonal patterns and fertility to ocurrence of two or three waves of ovarian follicles, before and after breeding, in beef cows and heifers. Anim. Reprod. Sci. 1997;49:13–28. doi: 10.1016/s0378-4320(97)00057-2. [DOI] [PubMed] [Google Scholar]
- Bartolome, JA, Perez Wallace, S, de la Sota, RL, Thatcher, WW. The effect of administering equine chorionic gonadotropin (eCG) and human chorionic gonadotropin (hCG) post artificial insemination on fertility of lactating dairy cows. Theriogenology. 2012;78:1110–1116. doi: 10.1016/j.theriogenology.2012.05.006. [DOI] [PubMed] [Google Scholar]
- Bevers, MM, Dieleman, SJ, van Tol, HTM, Blankenstein, DM, van den Broek, J. Changes in pulsatile secretion patterns of LH, FSH, progesterone, androstenedione and oestradiol in cows after superovulation with PMSG. J. Reprod. Fertil. 1989;87:745–754. doi: 10.1530/jrf.0.0870745. [DOI] [PubMed] [Google Scholar]
- Bo, GA, Adams, GP, Caccia, M, Martinez, M, Pierson, RA, Mapletoft, RJ. Ovarian follicular wave emergence after treatment with progesterone and estradiol in cattle. Anim. Reprod. Sci. 1995;39:193–204. [Google Scholar]
- Burke, CR, Day, ML, Bunt, CR, Macmillan, KL. Use of a small dose of estradiol benzoate during diestrus to synchronize development of the ovulatory follicle in cattle. J. Anim. Sci. 2000;78:145–151. doi: 10.2527/2000.781145x. [DOI] [PubMed] [Google Scholar]
- Dieleman, SJ, Bevers, MM, Wurth, YA, Gielen, JT, Willemse, AH. Improved embryo yield and condition of donor ovaries in cows after PMSG superovulation with monoclonal anti-PMSG administered shortly after the preovulatory LH peak. Theriogenology. 1989;31:473–487. doi: 10.1016/0093-691x(89)90552-9. [DOI] [PubMed] [Google Scholar]
- Fátima, LA, Baruselli, PS, Gimenes, LU, Binelli, M, Rennó, FP, Murphy, BD, Papa, PC. Global gene expression in the bovine corpusluteum is altered after stimulatory and superovulatory treatments. Reprod. Fertil. Dev. 2012;25:998–1011. doi: 10.1071/RD12155. [DOI] [PubMed] [Google Scholar]
- Garcia-Ispierto, I, López-Helguera, I, Martino, A, López-Gatius, F. Reproductive performance of anoestrous high-producing dairy cows improved by adding equine chorionic gonadotrophin to a progesterone-based oestrous synchronizing protocol. Reprod. Dom. Anim. 2012;47:752–758. doi: 10.1111/j.1439-0531.2011.01954.x. [DOI] [PubMed] [Google Scholar]
- Ginther, OJ. Effect of progesterone on length of oestrous cycle in cattle. Am. J. Vet. Res. 1970;31:493–496. [PubMed] [Google Scholar]
- Ginther, OJ, Bergfelt, DR, Kulick, LJ, Kot, K. Selection of the dominant follicle in cattle: role of estradiol. Biol. Reprod. 2000;63:383–389. doi: 10.1095/biolreprod63.2.383. [DOI] [PubMed] [Google Scholar]
- Ginther, OJ, Kastelic, JP, Knopf, L. Composition and characteristics of follicular waves during the bovine estrous cycle. Anim. Reprod. Sci. 1989b;20:187–200. [Google Scholar]
- Ginther, OJ, Knopf, L, Kastelic, JP. Temporal associations among ovarian events in cattle during oestrous cycle with two and three follicular waves. J. Reprod. Fertil. 1989a;41:154–247. doi: 10.1530/jrf.0.0870223. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Menico, F, Manns, J, Murphy, BD. FSH and LH activity of PMSG from mares at different stages of gestation. Anim. Reprod. Sci. 1978;1:137–144. [Google Scholar]
- Gonzalez-Padilla, E, Niswender, GD, Wiltbank, JN. Puberty in beef heifers II Effect of injections of progesterone and estradiol-17β on serum LH, FSH and ovarian activity. J. Anim. Sci. 1975;40:1105–1109. doi: 10.2527/jas1975.4061105x. [DOI] [PubMed] [Google Scholar]
- Goulding, D, Williams, DH, Rochei, JF, Boland, MP. Factors affecting superovulations in heifers treated with PMSG. Theriogenology. 1996;45:765–773. doi: 10.1016/0093-691x(96)00006-4. [DOI] [PubMed] [Google Scholar]
- Inskeep, EK, Braden, TD, Lewis, PE, Garcia-Winder, M, Niswender, GD. Receptors for luteinizing hormone and follicle-stimulating hormone in largest follicles of postpartum beef cows. Biol. Reprod. 1988;38:587–591. doi: 10.1095/biolreprod38.3.587. [DOI] [PubMed] [Google Scholar]
- Knopf, L, Kastelic, JP, Scallenberger, E, Ginther, OJ. Ovarian follicular dynamics in heifers: test of two-wave hypothesis by ultrasonography monitoring individual follicles. Dom. Anim. Endocrinol. 1989;6:111–119. doi: 10.1016/0739-7240(89)90040-4. [DOI] [PubMed] [Google Scholar]
- Macmillan, KL, Thatcher, WW. Effect of an agonist of gonadotropin-releasing hormone on ovarian follicle in cattle. Biol. Reprod. 1991;45:883–889. doi: 10.1095/biolreprod45.6.883. [DOI] [PubMed] [Google Scholar]
- Moghaddam, AA, Niasari-Naslaji, A, Bolorchi, M. Effect of steroid and GnRH, given on the day of estrus, on ovarian follicle characteristics in Holstein heifers. J. Fac. Vet. Med. 2001;56:45–52. [Google Scholar]
- Monniaux, D, Chupin, D, Saumande, J. Superovulatory responses of cattle. Theriogenology. 1983;19:55–81. [Google Scholar]
- Murphy, BD. Equine chorionic gonadotropin: an enigmatic but essential tool. Anim. Reprod. 2012;9:223–230. [Google Scholar]
- Murphy, BD, Martinuk, SD. Equine chorionic gonadotropin. Endocrinol. Rev. 1991;12:27–44. doi: 10.1210/edrv-12-1-27. [DOI] [PubMed] [Google Scholar]
- Newcomb, R, Christie, WB, Rowson, LEA, Walters, DE, Bousfield, WED. Influence of dose, repeated treatment and batch of hormone on ovarian response in heifers treated with PMSG. J. Reprod. Fertil. 1979;56:113–118. doi: 10.1530/jrf.0.0560113. [DOI] [PubMed] [Google Scholar]
- Niasari-Naslaji, A, Eslami, M, Nazem, Y. Ovulatory response of different GnRH analogues and subsequent corpus luteum lifespan in the presence of norgestomet in Holstein heifers. Iran. J. Vet. Res. 2012;13:36–41. [Google Scholar]
- Niasari-Naslaji, A, Hosseini, SM, Sarhaddi, F, Bolourchi, M, Birjandi, MR. Steriod priming shortnes prostaglandin-based estrus synchronization program from 14 to 7 days in cattle. Theriogenology. 2001;56:735–743. doi: 10.1016/s0093-691x(01)00603-3. [DOI] [PubMed] [Google Scholar]
- Núnez-Olivera, R, de Castroa, T, García-Pintos, C, Bó, G, Piaggio, J, Menchaca, A. Ovulatory response and luteal function after eCG administration at the end of a progesterone and estradiol based treatment in postpartum anestrous beef cattle. Anim. Reprod. Sci. 2014;146:111–116. doi: 10.1016/j.anireprosci.2014.02.017. [DOI] [PubMed] [Google Scholar]
- Odde, KG, Wards, HS, Kiracofe, GH, McKee, RJ, Kittok, RJ. Short estrous cycles and associated serum progesterone levels in beef cows. Theriogenology. 1980;14:105–112. doi: 10.1016/0093-691x(80)90097-7. [DOI] [PubMed] [Google Scholar]
- O’Hara, L, Forde, N, Duffy, P, Randi, F, Kelly, AK, Vaenza, A, Rodriguez, P, Lonergan, P. Effect of combined exogenous progesterone with luteotrophic support via equine chorionic gonadotrophin (eCG) on corpus luteum development, circulating progesterone concentration and embryo development in cattle. Reprod, Fertil. Dev. 2016;28:269–277. doi: 10.1071/RD14019. [DOI] [PubMed] [Google Scholar]
- Pessoa, GA, Martini, AP, Carloto, GW, Rodrigues, MCC, Claro Júnior, I, Baruselli, PS, Brauner, CC, Rubin, MIB, Corrêa, MN, Leivas, FG, Sá Filho, MF. Different doses of equine chorionic gonadotropin on ovarian follicular growth and pregnancy rate of suckled Bos taurus beef cows subjected to timed artificial insemination protocol. Theriogenology. 2016;85:792–799. doi: 10.1016/j.theriogenology.2015.09.057. [DOI] [PubMed] [Google Scholar]
- Pulley, SL, Wallace, LD, Mellieon, HIJr, Stevenson, JS. Ovarian characteristics, serum concentrations of progesterone and estradiol, and fertility in lactating dairy cows in response to equine chorionic gonadotropin. Theriogenology. 2013;79:127–134. doi: 10.1016/j.theriogenology.2012.09.017. [DOI] [PubMed] [Google Scholar]
- Rigoglio, NN, Fátima, LA, Hanassaka, JY, Pinto, GL, Machado, ASD, Gimenes, LU, Baruselli, PS, Rennó, FP, Moura, CEB, Watanabe, IS, Papa, PC. Equine chorionic gonadotropin alters luteal cell mor-phologic features related to progesterone synthesis. Theriogenology. 2013;79:673–679. doi: 10.1016/j.theriogenology.2012.11.023. [DOI] [PubMed] [Google Scholar]
- Rostami, B, Niasari-Naslaji, A, Vojgani, M, Nikjou, D, Amanlou, HM, Gerami, A. Effect of eCG on early resumption of ovarian activity in postpartum dairy cows. Anim. Reprod. Sci. 2011;128:100–106. doi: 10.1016/j.anireprosci.2011.09.006. [DOI] [PubMed] [Google Scholar]
- Sá Filho, MF, Ayres, H, Ferreira, RM, Marques, MO, Reis, EL, Silva, RC, Rodrigues, CA, Madureira, EH, Bó, GA, Baruselli, PS. Equine chorionic gonadotropin and gonadotropin-releasing hormone enhance fertility in a norgestomet-based, timed artificial insemination protocol in suckled Nelore (Bos indicus) cows. Theriogenology. 2010;73:651–658. doi: 10.1016/j.theriogenology.2009.11.004. [DOI] [PubMed] [Google Scholar]
- Savio, JD, Keenan, L, Boland, MP, Roche, JF. Pattern of growth of dominant follicles during the oestrous cycle of heifers. J. Reprod. Fertil. 1988;83:663–671. doi: 10.1530/jrf.0.0830663. [DOI] [PubMed] [Google Scholar]
- Savio, JD, Thatcher, WW, Badinga, L, de la Sota, RL, Wolfeson, D. Regulation of dominant follicle turnover during the estrous cycle in cows. J. Reprod. Fertil. 1993;97:197–203. doi: 10.1530/jrf.0.0970197. [DOI] [PubMed] [Google Scholar]
- Schams, D, Mentzer, C, Schallenberger, E, Hahn, J, Hahn, R. Some studies of pregnant mare serum gonadotropin and on endocrine responses after application for superovulation in cattle. In: Sreenan J, editor. Control of reproduction in the cow. 8th Edn. Martinus Nijhoff: The Hague; 1978. pp. 122–143. [Google Scholar]
- Sirois, J, Fortune, JE. Ovarian follicular dynamics during the estrous cycle in heifers monitored by real-time ultrasonography. Biol. Reprod. 1998;39:308–317. doi: 10.1095/biolreprod39.2.308. [DOI] [PubMed] [Google Scholar]
- Sirois, J, Fortune, JE. Lengthening the bovine estrous cycle with low levels of exogenous progesterone: a model for studying ovarian follicular dominance. Endocrinology. 1990;127:916–925. doi: 10.1210/endo-127-2-916. [DOI] [PubMed] [Google Scholar]
- Soumano, K, Lussier, JG, Price, CA. Levels of messenger RNA encoding ovarian receptors for FSH and LH in cattle during superovulation with equine chorionic gonadotropin versus FSH. J. Endocrinol. 1998;156:373–378. doi: 10.1677/joe.0.1560373. [DOI] [PubMed] [Google Scholar]
- Souza, AH, Cunha, AP, Silva, EPB, Gümen, A, Ayres, H, Guenther, JN, Wiltbank, MC. Comparison of gonadorelin products in lactating dairy cows: efficacy based on induction of ovulation of an accessory follicle and circulating luteinizing hormone profiles. Theriogenology. 2009;72:271–279. doi: 10.1016/j.theriogenology.2009.02.016. [DOI] [PubMed] [Google Scholar]
- Souza, AH, Wosniacki, AM, Torres-Junior, JRS, Martins, CM, Ayres, H, Baruselli, PS. Factors that affect the corpus luteum volume during estrous cycle in high producer Holstein cows. Acta Sci. Vet. 2006;34:368 . (abst) [Google Scholar]
- Taponen, J, Kulcsár, M, Katila, T, Kátai, L, Huszenicza, G, Rodriguez-Martinez, H. Short estrous cycles and estrous signs after premature ovulations induced with cloprostenol and gonadotropin-releasing hormone in cyclic dairy cows. Theriogenology. 2002;58:1291–1302. doi: 10.1016/s0093-691x(02)00957-3. [DOI] [PubMed] [Google Scholar]
- Taylor, C, Rajamahendran, R. Folicular dynamics, corpus luteum growth and regression in lactating dairy cattle. Can. J. Anim. Sci. 1991;7l:61–68. doi: 10.3168/jds.s0022-0302(91)78151-4. [DOI] [PubMed] [Google Scholar]
- Vojgani, M, Akbarinejad, V, Niasari-Naslaji, A. Administration of eCG on day 6 postpartum could enhance reproductive performance of Holstein dairy cows. Anim. Reprod. Sci. 2013;138:159–162. doi: 10.1016/j.anireprosci.2013.03.003. [DOI] [PubMed] [Google Scholar]
- Wiltbank, MC, Gumen, A, Sartori, R. Physiological classification of anovulatory conditions in cattle. Theriogenology. 2002;57:21–52. doi: 10.1016/s0093-691x(01)00656-2. [DOI] [PubMed] [Google Scholar]