Abstract
Concomitantly with the increase in the prevalences of overweight/obesity, nonalcoholic fatty liver disease (NAFLD) has worldwide become the main cause of chronic liver disease in both adults and children. Patients with fatty liver display features of metabolic syndrome (MetS), like insulin resistance (IR), glucose intolerance, hypertension and dyslipidemia. Recently, epidemiological studies have linked obesity, MetS, and NAFLD to decreased bone mineral density and osteoporosis, highlighting an intricate interplay among bone, adipose tissue, and liver. Osteoprotegerin (OPG), an important symbol of the receptor activator of nuclear factor-B ligand/receptor activator of nuclear factor kappa B/OPG system activation, typically considered for its role in bone metabolism, may also play critical roles in the initiation and perpetuation of obesity-related comorbidities. Clinical data have indicated that OPG concentrations are associated with hypertension, left ventricular hypertrophy, vascular calcification, endothelial dysfunction, and severity of liver damage in chronic hepatitis C. Nonetheless, the relationship between circulating OPG and IR as a key feature of MetS as well as between OPG and NAFLD remains uncertain. Thus, the aims of the present review are to provide the existent knowledge on these associations and to discuss briefly the underlying mechanisms linking OPG and NAFLD.
Keywords: Nonalcoholic fatty liver disease, Insulin resistance, Metabolic syndrome, Osteoprotegerin, Receptor activator of nuclear factor kappa B, Receptor activator of nuclear factor kappa B ligand
Core tip: Recently, epidemiological studies have linked obesity, metabolic syndrome, and nonalcoholic fatty liver disease (NAFLD) to decreased bone mineral density and osteoporosis, highlighting an intricate interplay among bone, adipose tissue, and liver. Osteoprotegerin (OPG), an important symbol of the receptor activator of nuclear factor-B ligand/receptor activator of nuclear factor kappa B/OPG axis activation, has recently been suggested to have critical roles in the initiation and perpetuation of obesity-related comorbidities including NAFLD. The available studies have reported either positive or negative associations between OPG and NAFLD. Thus, more research is needed to clarify its role in this liver disease.
INTRODUCTION
Concomitantly with the increase in the prevalences of overweight/obesity, nonalcoholic fatty liver disease (NAFLD) has worldwide become the main cause of chronic liver disease in both adults and children[1,2]. NAFLD implies accumulation of lipids within hepatocytes, with a spectrum ranging from simple steatosis to steatohepatitis (NASH), progressive to cirrhosis[3-5]. Although patients with NAFLD have a high risk of mortality from liver complications, the primary cause of mortality in such patients is cardiovascular disease (CVD)[6]. Indeed, NAFLD may be considered in adults as well as in children a multisystem disease affecting several extra-hepatic organs and involving a range of extra-hepatic chronic diseases, in particular type 2 diabetes, CVD, and chronic renal disease[7-11]. These diseases have the same underlying pathophysiological features associated with metabolic syndrome (MetS), including insulin resistance (IR), chronic systemic inflammation and hyperlipidemia. Recently, epidemiological studies have linked obesity, MetS, and NAFLD to decreased bone mineral density (BMD) and osteoporosis, highlighting an intricate interplay among bone, adipose tissue, and liver[12-14]. With regard to this, the association between NAFLD and decreased BMD has been also reported in the pediatric obese population[15,16].
Osteoprotegerin (OPG), an important symbol of the receptor activator of nuclear factor-B ligand (RANKL)/receptor activator of nuclear factor kappa B (RANK)/OPG axis activation, has recently been highlighted as an important factor of the biochemical mechanisms underlying the association between MetS and CVD[17-20]. Tumor necrosis factor (TNF) superfamily molecules, namely, RANKL, its receptor (RANK), and its soluble (decoy) receptor, OPG, mediate interactions (RANKL-OPG axis) that exert multiple actions on bone metabolism, endocrine functions, and the immune system[21-23]. The RANKL-OPG axis is typically considered for its role in bone metabolism, but proinflammatory cytokines [e.g., interleukin (IL)-1b, IL-6, and TNF-α] that are regulated by the RANKL-OPG axis in mediating bone resorption in osteoporosis, may also play critical roles in the initiation and perpetuation of obesity-related comorbidities[21-23]. There is arising evidence that RANKL/RANK/OPG system participate in the pathogenesis of atherosclerosis and CVD by expanding the detrimental actions of inflammation and multiple risk factors including dyslipidemia, endothelial dysfunction, type 2 diabetes, and high blood pressure[20].
Clinical data have displayed that circulating OPG concentrations are associated with hypertension and left ventricular hypertrophy in the general population, with vascular calcification and altered endothelial function in subjects with and without diabetes, and with severity of liver damage in patients with chronic hepatitis C[22-25]. Moreover, epidemiological studies have shown that OPG concentrations may predict morbidity and mortality from CVD[26]. Nonetheless, still the association of circulating OPG with IR as a key feature of MetS as well as of OPG with NAFLD remains uncertain. Thus, the aims of the present review are to provide the existent knowledge on these associations and to discuss briefly the possible underlying mechanisms linking OPG and NAFLD. We searched in MEDLINE and EMBASE databases utilizing the words “OPG”, “RANKL”, “RANK”, “IR”, “MetS”, and “NAFLD” individually and in combination to recruit all published articles from 1990 to 2018.
OPG/RANK/RANKL SYSTEM
OPG, first recognized in 1997, is a cytokine belonging to the superfamily of TNF receptor[27-30]. It has been termed OPG for its protective role in bone. The OPG gene discovered and cloned in 1998 is a single -copy gene localized on chromosome 8 (8q24) consisting of five exons over 29 kilobases[31]. Fom a biochemical aspect, OPG is a glycoprotein with a primary structure of 401 aminoacids and a molecular weight of 60 kilodaltons. OPG has seven structural domains, which actuate its biological functions in specific manners[32]. The amino terminal domains one to four, containing plenty of cysteine, impart osteclastogenesis inhibitory characteristics. Domains five and six at the carboxy terminal end include apoptosis-mediating death domain homologous regions. Domain seven encloses a heparin-binding region along with a free cysteine residue required for disulfide bond formation and dimerization. In fact, further to its monomeric structure, OPG may be completed at the cys-400 residue in the heparin binding domain to constitute a disulphide-linked dimer[32]. Before being secreted as monomeric and dimeric forms, the twenty-one aminoacid’s signal peptide of OPG is split from the N-terminal achieving a 380 aminoacid’s mature OPG protein. Therefore, as long as the OPG monomer is biologically active, OPG homodimer molecule is more active and its production is necessary to generate complete biological activity in vitro and in vivo. This is because the homodimer form possesses higher affinity for the RANKL ectodomain than the OPG monomer. RANKL and TNF-related apoptosis-inducing ligand (TRAIL) bind to OPG with similar affinities[32].
OPG is highly expressed in various organs and tissues including osteoblasts, lungs, cardiac tissue, renal tissue, hepatic tissue, spleen, thymus, prostate, ovary, small intestine, thyroid, lymphnodes, trachea, adrenal gland, testis, and bone marrow, endothelial cells and vascular smooth cells, while it is encountered at very low levels in brain, placenta, and skeletal muscle[23,26,33]. OPG has also been discovered by means of immunohistochemistry in atherosclerotic plaques of aortas and coronary arteries. Furthermore, OPG expression has recently been demonstrated in human adipose tissue[34].
RANK, an additional member of the TNF receptor superfamily, is expressed on the surface of hematopoietic precursor cells and mediates signaling that activates osteoclastogenesis[35]. Its ligand RANKL is typically expressed on osteoblast/stromal cell surfaces. RANKL is also encountered in stimulated T-lymphocytes, lymph nodes, thymus, mammary gland, lungs, spleen and bone marrow. It is a transmembrane protein, however, in the blood is also present a soluble form (sRANKL). sRANKL seems to derive from cleavage of membrane RANKL or to be produced by T-lymphocytes. Membrane-bound RANKL or sRANKL binds to RANK through interaction with specific molecules such as TNF receptor-associated factor (TRAF) proteins. The most important role of TRAFs in RANK-RANKL signaling is the stimulation of NF-kBs as well as mitogen-activated protein kinases and interferon-regulatory molecules. TRAF proteins may also take part to chronic inflammatory state and infection[36].
ROLE OF OPG/RANK/RANKL SYSTEM IN BONE AND OTHER TISSUES
The wide variety of cells and tissues in major organ systems such as the skeletal, vascular, and immune systems as well as other systems producing OPG, RANKL, and RANK support their role in the function of these organs (Figure 1). Typically, the OPG/RANK/RANKL axis regulates remodeling of bone as well as differentiation and activation of osteoclasts, and thus, the crucial equilibrium between formation and resorption of bone. RANKL binds to RANK on osteoprogenitor cells and controls osteoclastogenesis and bone resorption. OPG acting as a soluble decoy receptor, negatively regulates this interaction and competes with RANK, preventing RANKL-RANK interactions.
Figure 1.
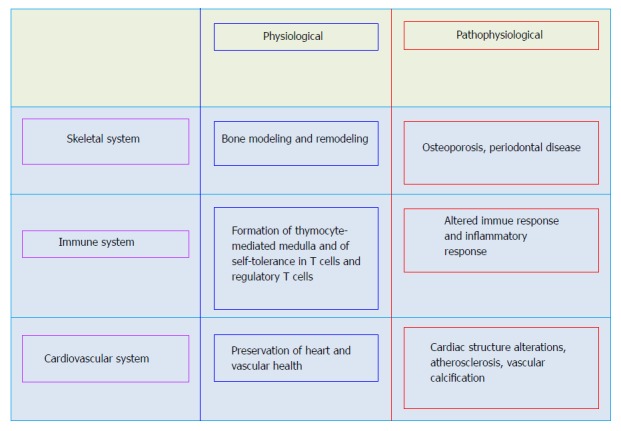
Role of osteoprotegerin/ receptor activator of nuclear factor-B ligand/receptor activator of nuclear factor kappa B axis in physiological and pathophysiological conditions.
While OPG is expressed in the vessels of healthy mice, RANK and RANKL are not detected in the arteries of healthy adult mice. In contrast, RANKL and RANK have been discovered in the calcified arteries of OPG-/- mice and RANK expression occurred simultaneously with the appearance of multinuclear osteoclast-like cells[37]. These findings suggest that vascular OPG protects against RANK/RANKL induced osteoclast formation. In humans, RANKL and RANK are often undetected in the non-diseased vessel, while OPG is expressed in normal arteries. However, early as well as advanced human atherosclerotic lesions of carotid arteries and abdominal aortas manifest both RANKL and OPG immunoreactivity and mRNA expression[38-40].
Immune cells express OPG, RANKL, and RANK and these are believed to regulate inflammatory and immune responses[41-43]. Binding of RANKL to RANK augments dentritic cells’ survival, enhances the immunostimulatory capacity of dentritic cells, and modulates activated T-cells. In particular, RANKL/RANK signaling in the immune system controls the development of thymocyte-mediated medulla, and the development of self-tolerance in T cells as well as the number of regulatory T cells (Treg). RANKL also regulates the production of proinflammatory cytokines in macrophages[41]. An important function of OPG in the immune system is related to the cytotoxic ligand TRAIL, a potent activator of apoptosis. Binding of OPG to TRAIL inhibits cell apoptosis[44].
Yet, OPG, RANKL, and RANK have been demonstrated to be expressed in normal brain of rodents. Notably, in normal brain, RANKL/RANK signaling has been related to fever and body temperature control. The stimulation of RANKL/RANK signaling obtained by the deletion of OPG or the administration of RANKL has been demonstrated to prevent the exacerbation of infart volume as well as cerebral edema through the inhibition of the production of pro-inflammatory cytokines[45].
The multiple actions of OPG/RANKL/RANK axis, including modulation of cell survival, mineralization and inflammation suggest a potential role as mediator of metabolic complications including insulin resistance, type 2 diabetes, MetS and NAFLD.
CLINICAL STUDIES
Insulin resistance
NAFLD is strictly associated with IR, which is also a main determinant in the pathogenesis of type 2 diabetes and MetS. Even if investigators agree that IR is determined by alterations in intracellular insulin signaling, various causes have been suggested to explain by what means such insulin signaling alterations originate in NAFLD. Inflammation, activation of endoplasmic reticulum stress pathways, and deposit of lipids in hepatocytes have all been proposed to determine IR in NAFLD[46,47].
Numerous studies have reported on the association between OPG and IR with contrastant results[48-62]. In a cohort of 106 subjects with obesity, including eighteen with type 2 diabetes, Gannage-Yared et al[48] demonstrated a positive relationship between OPG and IR as evaluated by the homeostasis model assessment for IR (HOMA-IR). In a cross-sectional study, Yaturu et al[49] demonstrated that OPG was significantly associated with insulin levels and IR as well as with C-reactive protein (CRP) and TNF-α in patients affected by type 2 diabetes, most likely reflecting the proinflammatory state in this population. Pepene et al[50] reported a positive association of OPG with HOMA-IR in a cohort of women with polycystic ovary syndrome. Akinci et al[51] found that women with a history of gestational diabetes mellitus developing MetS showed increased OPG values compared to women who did not fulfill MetS criteria. Yet, these authors showed that OPG concentrations were associated with markers of IR, with carotid intima-media thickness (IMT) and with subclinical inflammation. Suliburska et al[52] found that HOMA-IR values and OPG values were significantly increased in obese adolescents than in the control group. A significant positive correlation between OPG and IR was found. In a large population of individuals with normal glucose tolerance (n = 599), with impaired glucose tolerance (n = 730) and with newly diagnosed diabetes (n = 327), respectively, Niu et al[53] demonstrated that elevated circulating OPG levels were independently related to impaired glucose regulation and a higher risk of microalbuminuria. Bilgir et al[54] found that circulating OPG and sRANKL values were significantly increased in prediabetic patients than in control individuals. There was a positive relationship between sRANKL and OPG. Yet, sRANKL was positively associated with body mass index (BMI), HOMA-IR, and inflammatory markers such as high-sensitivity CRP. Duan et al[55] demonstrated that circulating OPG concentrations were increased in Chinese postmenopausal women with diabetes and prediabetes. Moreover, serum OPG levels showed significant correlation with IR. Mashavi et al[56] showed that OPG values were significantly increased in postmenopausal women affected by osteoporosis and impaired glucose metabolism (including impaired glucose tolerance and type 2 diabetes) than women with normal glucose tolerance. OPG concentrations were independently associated with IR as evaluated by HOMA-IR. Recently, Daniele et al[57] found that high OPG concentrations were correlated with increased endogenous glucose production (primarily reflecting liver glucose production) and hepatic IR in individuals with impaired glucose regulation, supporting the possibility that OPG could have a role in glucose homeostasis derangement that usually precede overt type 2 diabetes.
There have also been some studies demonstrating a negative relationship between OPG and IR, though they were predominantly based on healthy populations. In a healthy population (exhibiting normal glucose tolerance and exercise stress tests, thus excluding hyperglycemia and ischemic heart disease, respectively), Ashley et al[58] found that OPG correlated inversely with HOMA-IR, and suggested that high IR in healthy subjects is associated with low levels of circulating OPG. In a subsequent study, these authors showed that OPG was higher in patients with abnormal glucose tolerance compared to normoglycemic healthy subjects[59]. Nonethless, OPG did not correlate with the severity of IR as evaluated by HOMA-IR either on univariate or multiple linear regression, suggesting that OPG elevation in these individuals may be due to other factors. In agreement with these findings, Ugur-Altun and colleagues[60,61] in two separate studies - the former involving obese patients without diabetes vs lean healthy subjects, the latter healthy young women - found a negative relationship between OPG and IR. Ayina Ayina et al[62] demonstrated that HOMA-IR was inversely associated with OPG values in women with obesity, meaning that elevated OPG concentrations may be expression of high insulin sensitivity.
The heterogeneity of the results of the studies on the association between OPG and IR might reflect differences in the population included in terms of gender, age, ethnic background, and, importantly, in terms of metabolic-associated diseases. Indeed, a positive relationship has been found in studies that involved individuals with high levels of IR, such as those affected by type 2 diabetes and a previous history of gestational diabetes, while a negative relationship in those that involved healthy subjects. It should be acknowledged that elevated circulating OPG has emerged as a strong, independent predictor of CVD[63,64]. In particular, plasma OPG is considered a marker of vascular calcifications[65], a feature often seen in patients with impaired glucose homeostasis[66] and recently shown to involve insulin actions[67]. OPG concentrations in patients affected by obesity and type 2 diabetes may thus reflect the presence of CVD.
Metabolic syndrome
Scant and contrastant literature is available on the association between OPG and MetS. Initial studies found no correlation between OPG and MetS[68,69]. In particular, in a cohort of elderly Lebanese men, Gannage-Yared et al[68] found no significant difference in OPG concentrations between individuals with and without MetS. Similar findings were reported by Nabipour et al[69] in a population-based sample of postmenopausal women. In subsequent studies, however, an association between OPG and MetS has been reported. In individuals with peripheral artery disease, circulating concentrations of OPG were raised in obese patients with MetS[70]. Akinci et al[51] found that women with a history of gestational diabetes mellitus developing the MetS showed increased OPG levels than women who did not fulfill the MetS criteria. These findings were previously reported by the same authors in a sample of 128 women with previous gestational diabetes and 67 age-matched controls. OPG values were associated with obesity, IR, and carotid IMT[71].
Recently, Pérez de Ciriza et al[72] demonstrated that patients with MetS had significantly elevated OPG concentrations than those without the syndrome. Of note, OPG values significantly and positively correlated with the number of cardiovascular risk factors. In addition, OPG expression in adipose tissue was endorsed, and MetS patients expressed elevated OPG mRNA values compared to those without. Bernardi et al[73] demonstrated that circulating OPG was higher in patients with MetS compared to controls. In high-fat diet fed C57BL6 mice, they also found that OPG was elevated, and that OPG administration promoted systemic and adipose tissue proinflammatory changes resembling those observed in HDF fed mice. Finally, in patients with type 2 diabetes, Tavintharan et al[74] found OPG to be a significant predictor of MetS also after adjustment for age, sex, ethnic origin, glucose levels, and microvascular complications.
The variation of the results of the studies on the association between MetS and OPG may be in part explained by differences in the population included in terms of gender, age and associated diseases, and importantly, in diagnostic criteria utilized.
NAFLD
There are few studies on the relationship between OPG and NAFLD, with either positive or negative associations having being described[75-80] (Table 1). In a cross-sectional study, Yilmaz et al[75] first reported that OPG levels were significantly decreased in patients with definite and borderline NASH than in subjects with simple liver steatosis. The authors also found a negative relationship between OPG and HOMA-IR, and between OPG and serum transaminases values. Thus, low OPG concentration in subjects affected by NAFLD may reflect the effects of IR, as well as the occurrence of severe liver necroinflammation. Yang et al[76] tested the accuracy of non-invasive biological markers for identification of NASH, including OPG, in 179 patients with biopsy-proven NAFLD (training group) and 91 age- and sex-matched healthy controls. Further 63 subjects with NAFLD were separately included as validation group. Serum levels of OPG decreased progressively from controls to patients with NAFLD but without NASH, and reached the lowest levels in patients with NASH. Sensitivity and specificity of OPG for assessing NASH were 81.30% and 74.60%, respectively. In a case-control study involving 746 patients affected by type 2 diabetes (of whom 367 with ultrasound-diagnosed NAFLD), Niu et al[77] demonstrated that the OPG concentrations were significantly decreased in patients with NAFLD compared to patients without liver involvement. The subjects in the lowest OPG quartile were at higher risk for NAFLD. Finally, Erol et al[78] evaluated the association of OPG concentrations with obesity, IR, and NAFLD in children and adolescents. OPG concentrations in the youth with obesity were significantly decreased than in controls. Among obese youths, those with high fasting insulin and high HOMA-IR values displayed significantly lower OPG values. Patients with hepatic steatosis had lower OPG concentrations than those without liver involvement, although they did not reach statistical significance. In contrast, Ayaz et al[79] demostrated that patients with NAFLD diagnosed via ultrasonography had OPG levels significantly higher compared to controls. Monseu et al[80] determined the association between OPG and visceral adipose tissue and liver fat content as measured by magnetic resonance imaging, as well as other markers of the MetS in dysmetabolic adults. OPG levels were positively correlated with visceral fat liver and liver fat content, as well as liver markers such as alanine aminotransferase and HOMA-IR index.
Table 1.
Studies assessing the association between osteoprotegerin and nonalcoholic fatty liver disease
| Ref. | Study design | Population | Findings |
| Yilmaz et al[75], 2010 | Cross-sectional study | 56 adult patients with histological-proven definite NASH; 26 with borderline NASH; 17 with simple fatty liver; and 58 healthy controls without evidence of liver disease (normal results on liver function tests and normal liver ultrasound). | OPG levels were significantly decreased in patients with definite NASH and borderline NASH than in controls. No significant differences were found between patients with simple fatty liver and controls. |
| Ayaz et al[79], 2014 | Case-control study | 60 adult patients with ultrasound-proven NAFLD and 30 control subjects. | OPG levels were significantly increased in patients with NAFLD compared to control subjects. |
| Yang et al[76], 2015 | Cross-sectional study | 179 patients with biopsy-proven NAFLD (training group) and 91 age- and gender-matched healthy subjects. 63 other NAFLD patients were separately collected as validation group. | Serum levels of OPG decreased in a stepwise fashion in controls, non-NASH NAFLD patients and NASH patients. |
| Monseu et al[80], 2016 | Cross-sectional study | 314 adult subjects with at least one criterion for metabolic syndrome. | OPG levels were positively associated with both liver markers (such as alanine aminotransferase, gamma-glutamyl transferase and ferritin levels) and increased liver fat content as assessed by magnetic resonance imaging. |
| Niu et al[77], 2016 | Case-control study | 746 adult patients with type 2 diabetes, of whom 367 with ultrasound-proven NAFLD. | OPG levels were significantly decreased in patients with NAFLD compared to those without NAFLD. |
| Participants in the lowest OPG quartile had a significantly increased risk for NAFLD (OR = 3.49, 95%CI: 1.86-6.94). | |||
| Erol et al[78], 2016 | Cross-sectional study | 107 children with obesity of whom 62 had ultrasound-proven NAFLD and 37 control subjects. | OPG levels in the obese group were significantly lower than in controls. Among obese youths, those with high fasting insulin and high HOMA-IR values had significantly lower OPG levels. Patients with hepatic steatosis had lower OPG concentrations than those without liver involvement, although they did not reach statistical significance. |
NASH: Non-alcoholic steatohepatitis; OPG: Osteoprotegerin; NAFLD: Nonalcoholic fatty liver disease; HOMA-IR: Homeostasis model assessment for insulin resistance.
Some points must be considered when interpreting the results of the few aforementioned studies. First, half of them have included a small sample size. Second, the clinical heterogeneity of patients’ population enrolled in the studies. Third, methodologic heterogeneity in defining the reference standard. In fact, liver disease was differently evaluated, with the majority of the studies utilizing ultrasonography that is known to be unable to assess severity of liver disease such as NASH.
BIOLOGICAL ROLE OF OPG IN NAFLD
OPG acting like a decoy receptor for TRAIL and RANKL neutralizes their biological actions. Of note, TRAIL is a relevant inductor of apoptosis in hepatocyte cells[81]. Because enhanced hepatocyte apoptosis has a key role in the progression of liver disease, that is from simple steatosis to NASH[82], it is tempting to suppose that the decrease in circulating concentrations of OPG in NAFLD, observed in the majority of the studies, might be responsible for alterations in the mechanisms protecting against hepatocyte apoptosis. Notably, accumulation of OPG is closely related to reduced apoptosis in several cell types[81,83]. These findings may imply that OPG exert a common defensive effect on the pathophysiologic derangements responsible for NAFLD through at least two different mechanisms: The first mechanism involves IR, while the second is based on protection of hepatocytes from cell death by apoptosis. Nonethless, the exact mechanisms responsible for the decrease of OPG in subjects with NAFLD and NASH need additional studies.
ANIMAL DATA
The development of transgenic technologies in mice has led to advances in knowledge of the role of OPG/RANKL/RANK system in bone metabolism and cardiometabolic functions. Concerning cardiometabolic disorders, Hao et al[84] showed that OPG-/- mice exhibited a significant increase in systolic blood pressure since early stages of life, and that this rise was in parallel with the osteoporotic change in these mice. OPG-/- mice also presented a higher heart weight/body weight ratio than age-matched wild-type mice, indicating that OPG plays an important role in the preservation of cardiac structure. Kiechl et al[85] developed hepatocyte-specific RANK knockout (RANKLKO) mice and compared them with wild-type mice. While RANKWT mice experienced insulin resistance after 4 wk of a high-fat diet (NFD), RANKLKO mice did not. A very recent study demonstrated that mice lacking β-catenin in osteoblasts exhibit during the postnatal period reduced bone mass, increased glucose level, reduced insulin production, reduced fat accumulation and increased energy expenditure. OPG overexpression normalized not only the reduced bone mass but also the reduced fat accumulation and increased energy expenditure[86].
CONCLUSION
Contention still exists on the exact role of OPG/RANKL/RANK system in IR and NAFLD. The available studies have reported either positive or negative associations between OPG and IR as well as between OPG and NAFLD. As previously outlined, possible explanations of the discordant results may be related to differences in the study population in terms of gender, age, ethnic background, and, importantly, in terms of cardiometabolic-associated diseases. Interestingly, OPG seems to have a dichotomous role in humans, as suggested in CVD. In healthy subjects, the proatherogenic and antiatherogenic effects are being held in a fine balance, while in the presence of persistent risk factors the proatherogenic pathway becomes predominant. Moreover, there are differences between human and animal studies. Observational studies in human subjects show that circulating OPG concentrations are associated positively with severity and progression of coronary artery disease, atherosclerosis, and vascular calcification whereas animal studies support a protective role for OPG[87]. Future studies are necessary to clarify the role of OPG in NAFLD.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Conflict-of-interest statement: Authors have no conflicts of interest to disclose.
Peer-review started: March 30, 2018
First decision: April 27, 2018
Article in press: May 11, 2018
P- Reviewer: Liu HK S- Editor: Wang XJ L- Editor: A E- Editor: Huang Y
Contributor Information
Lucia Pacifico, Policlinico Umberto I Hospital, Sapienza University of Rome, Rome 00161, Italy. lucia.pacifico@uniroma1.it.
Gian Marco Andreoli, Policlinico Umberto I Hospital, Sapienza University of Rome, Rome 00161, Italy.
Miriam D’Avanzo, Policlinico Umberto I Hospital, Sapienza University of Rome, Rome 00161, Italy.
Delia De Mitri, Policlinico Umberto I Hospital, Sapienza University of Rome, Rome 00161, Italy.
Pasquale Pierimarchi, Institute of Translational Pharmacology, National Research Council, Rome 00083, Italy.
References
- 1.Nogrady B. Childhood obesity: A growing concern. Nature. 2017:551. doi: 10.1038/d41586-017-05868-y. [DOI] [PubMed] [Google Scholar]
- 2.Hales CM, Fryar CD, Carroll MD, Freedman DS, Ogden CL. Trends in Obesity and Severe Obesity Prevalence in US Youth and Adults by Sex and Age, 2007-2008 to 2015-2016. JAMA. 2018;319:1723–1725. doi: 10.1001/jama.2018.3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221–1231. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 4.Ekstedt M, Hagström H, Nasr P, Fredrikson M, Stål P, Kechagias S, Hultcrantz R. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology. 2015;61:1547–1554. doi: 10.1002/hep.27368. [DOI] [PubMed] [Google Scholar]
- 5.Hagström H, Tynelius P, Rasmussen F. High BMI in late adolescence predicts future severe liver disease and hepatocellular carcinoma: a national, population-based cohort study in 1.2 million men. Gut. 2017 doi: 10.1136/gutjnl-2016-313622. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 6.Targher G, Day CP, Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N Engl J Med. 2010;363:1341–1350. doi: 10.1056/NEJMra0912063. [DOI] [PubMed] [Google Scholar]
- 7.Byrne CD, Targher G. NAFLD: a multisystem disease. J Hepatol. 2015;62:S47–S64. doi: 10.1016/j.jhep.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 8.Musso G, Gambino R, Tabibian JH, Ekstedt M, Kechagias S, Hamaguchi M, Hultcrantz R, Hagström H, Yoon SK, Charatcharoenwitthaya P, et al. Association of non-alcoholic fatty liver disease with chronic kidney disease: a systematic review and meta-analysis. PLoS Med. 2014;11:e1001680. doi: 10.1371/journal.pmed.1001680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anstee QM, Targher G, Day CP. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat Rev Gastroenterol Hepatol. 2013;10:330–344. doi: 10.1038/nrgastro.2013.41. [DOI] [PubMed] [Google Scholar]
- 10.Pacifico L, Chiesa C, Anania C, De Merulis A, Osborn JF, Romaggioli S, Gaudio E. Nonalcoholic fatty liver disease and the heart in children and adolescents. World J Gastroenterol. 2014;20:9055–9071. doi: 10.3748/wjg.v20.i27.9055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pacifico L, Bonci E, Andreoli GM, Di Martino M, Gallozzi A, De Luca E, Chiesa C. The Impact of Nonalcoholic Fatty Liver Disease on Renal Function in Children with Overweight/Obesity. Int J Mol Sci. 2016;17 doi: 10.3390/ijms17081218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Poggiogalle E, Donini LM, Lenzi A, Chiesa C, Pacifico L. Non-alcoholic fatty liver disease connections with fat-free tissues: A focus on bone and skeletal muscle. World J Gastroenterol. 2017;23:1747–1757. doi: 10.3748/wjg.v23.i10.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yilmaz Y. Review article: non-alcoholic fatty liver disease and osteoporosis--clinical and molecular crosstalk. Aliment Pharmacol Ther. 2012;36:345–352. doi: 10.1111/j.1365-2036.2012.05196.x. [DOI] [PubMed] [Google Scholar]
- 14.Targher G, Lonardo A, Rossini M. Nonalcoholic fatty liver disease and decreased bone mineral density: is there a link? J Endocrinol Invest. 2015;38:817–825. doi: 10.1007/s40618-015-0315-6. [DOI] [PubMed] [Google Scholar]
- 15.Pacifico L, Bezzi M, Lombardo CV, Romaggioli S, Ferraro F, Bascetta S, Chiesa C. Adipokines and C-reactive protein in relation to bone mineralization in pediatric nonalcoholic fatty liver disease. World J Gastroenterol. 2013;19:4007–4014. doi: 10.3748/wjg.v19.i25.4007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pardee PE, Dunn W, Schwimmer JB. Non-alcoholic fatty liver disease is associated with low bone mineral density in obese children. Aliment Pharmacol Ther. 2012;35:248–254. doi: 10.1111/j.1365-2036.2011.04924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baud’huin M, Lamoureux F, Duplomb L, Rédini F, Heymann D. RANKL, RANK, osteoprotegerin: key partners of osteoimmunology and vascular diseases. Cell Mol Life Sci. 2007;64:2334–2350. doi: 10.1007/s00018-007-7104-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leibbrandt A, Penninger JM. RANK/RANKL: regulators of immune responses and bone physiology. Ann N Y Acad Sci. 2008;1143:123–150. doi: 10.1196/annals.1443.016. [DOI] [PubMed] [Google Scholar]
- 19.Buso G, Faggin E, Pauletto P, Rattazzi M. Osteoprotegerin in cardiovascular disease: ally or enemy? Curr Pharm Des. 2014;20:5862–5869. doi: 10.2174/1381612820666140212195711. [DOI] [PubMed] [Google Scholar]
- 20.Montagnana M, Lippi G, Danese E, Guidi GC. The role of osteoprotegerin in cardiovascular disease. Ann Med. 2013;45:254–264. doi: 10.3109/07853890.2012.727019. [DOI] [PubMed] [Google Scholar]
- 21.Kartsogiannis V, Zhou H, Horwood NJ, Thomas RJ, Hards DK, Quinn JM, Niforas P, Ng KW, Martin TJ, Gillespie MT. Localization of RANKL (receptor activator of NF kappa B ligand) mRNA and protein in skeletal and extraskeletal tissues. Bone. 1999;25:525–534. doi: 10.1016/s8756-3282(99)00214-8. [DOI] [PubMed] [Google Scholar]
- 22.Rochette L, Meloux A, Rigal E, Zeller M, Cottin Y, Vergely C. The role of osteoprotegerin in the crosstalk between vessels and bone: Its potential utility as a marker of cardiometabolic diseases. Pharmacol Ther. 2018;182:115–132. doi: 10.1016/j.pharmthera.2017.08.015. [DOI] [PubMed] [Google Scholar]
- 23.Pérez de Ciriza C, Lawrie A, Varo N. Osteoprotegerin in Cardiometabolic Disorders. Int J Endocrinol. 2015;2015:564934. doi: 10.1155/2015/564934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Omland T, Drazner MH, Ueland T, Abedin M, Murphy SA, Aukrust P, de Lemos JA. Plasma osteoprotegerin levels in the general population: relation to indices of left ventricular structure and function. Hypertension. 2007;49:1392–1398. doi: 10.1161/HYPERTENSIONAHA.107.087742. [DOI] [PubMed] [Google Scholar]
- 25.Noheria A, Mosley TH Jr, Kullo IJ. Association of serum osteoprotegerin with left ventricular mass in African American adults with hypertension. Am J Hypertens. 2010;23:767–774. doi: 10.1038/ajh.2010.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Venuraju SM, Yerramasu A, Corder R, Lahiri A. Osteoprotegerin as a predictor of coronary artery disease and cardiovascular mortality and morbidity. J Am Coll Cardiol. 2010;55:2049–2061. doi: 10.1016/j.jacc.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 27.Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Lüthy R, Nguyen HQ, Wooden S, Bennett L, Boone T, et al. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell. 1997;89:309–319. doi: 10.1016/s0092-8674(00)80209-3. [DOI] [PubMed] [Google Scholar]
- 28.Tsuda E, Goto M, Mochizuki S, Yano K, Kobayashi F, Morinaga T, Higashio K. Isolation of a novel cytokine from human fibroblasts that specifically inhibits osteoclastogenesis. Biochem Biophys Res Commun. 1997;234:137–142. doi: 10.1006/bbrc.1997.6603. [DOI] [PubMed] [Google Scholar]
- 29.Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Goto M, Mochizuki SI, Tsuda E, Morinaga T, Udagawa N, et al. A novel molecular mechanism modulating osteoclast differentiation and function. Bone. 1999;25:109–113. doi: 10.1016/s8756-3282(99)00121-0. [DOI] [PubMed] [Google Scholar]
- 30.Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S, Tomoyasu A, Yano K, Goto M, Murakami A, et al. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci USA. 1998;95:3597–3602. doi: 10.1073/pnas.95.7.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hilton MJ, Gutiérrez L, Zhang L, Moreno PA, Reddy M, Brown N, Tan Y, Hill A, Wells DE. An integrated physical map of 8q22-q24: use in positional cloning and deletion analysis of Langer-Giedion syndrome. Genomics. 2001;71:192–199. doi: 10.1006/geno.2000.6438. [DOI] [PubMed] [Google Scholar]
- 32.Yamaguchi K, Kinosaki M, Goto M, Kobayashi F, Tsuda E, Morinaga T, Higashio K. Characterization of structural domains of human osteoclastogenesis inhibitory factor. J Biol Chem. 1998;273:5117–5123. doi: 10.1074/jbc.273.9.5117. [DOI] [PubMed] [Google Scholar]
- 33.Boyce BF, Xing L. Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch Biochem Biophys. 2008;473:139–146. doi: 10.1016/j.abb.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.An JJ, Han DH, Kim DM, Kim SH, Rhee Y, Lee EJ, Lim SK. Expression and regulation of osteoprotegerin in adipose tissue. Yonsei Med J. 2007;48:765–772. doi: 10.3349/ymj.2007.48.5.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165–176. doi: 10.1016/s0092-8674(00)81569-x. [DOI] [PubMed] [Google Scholar]
- 36.Xie P. TRAF molecules in cell signaling and in human diseases. J Mol Signal. 2013;8:7. doi: 10.1186/1750-2187-8-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Min H, Morony S, Sarosi I, Dunstan CR, Capparelli C, Scully S, Van G, Kaufman S, Kostenuik PJ, Lacey DL, et al. Osteoprotegerin reverses osteoporosis by inhibiting endosteal osteoclasts and prevents vascular calcification by blocking a process resembling osteoclastogenesis. J Exp Med. 2000;192:463–474. doi: 10.1084/jem.192.4.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Collin-Osdoby P. Regulation of vascular calcification by osteoclast regulatory factors RANKL and osteoprotegerin. Circ Res. 2004;95:1046–1057. doi: 10.1161/01.RES.0000149165.99974.12. [DOI] [PubMed] [Google Scholar]
- 39.Golledge J, McCann M, Mangan S, Lam A, Karan M. Osteoprotegerin and osteopontin are expressed at high concentrations within symptomatic carotid atherosclerosis. Stroke. 2004;35:1636–1641. doi: 10.1161/01.STR.0000129790.00318.a3. [DOI] [PubMed] [Google Scholar]
- 40.Schoppet M, Al-Fakhri N, Franke FE, Katz N, Barth PJ, Maisch B, Preissner KT, Hofbauer LC. Localization of osteoprotegerin, tumor necrosis factor-related apoptosis-inducing ligand, and receptor activator of nuclear factor-kappaB ligand in Mönckeberg’s sclerosis and atherosclerosis. J Clin Endocrinol Metab. 2004;89:4104–4112. doi: 10.1210/jc.2003-031432. [DOI] [PubMed] [Google Scholar]
- 41.Maruyama K, Takada Y, Ray N, Kishimoto Y, Penninger JM, Yasuda H, Matsuo K. Receptor activator of NF-kappa B ligand and osteoprotegerin regulate proinflammatory cytokine production in mice. J Immunol. 2006;177:3799–3805. doi: 10.4049/jimmunol.177.6.3799. [DOI] [PubMed] [Google Scholar]
- 42.Ohigashi I, Nitta T, Lkhagvasuren E, Yasuda H, Takahama Y. Effects of RANKL on the thymic medulla. Eur J Immunol. 2011;41:1822–1827. doi: 10.1002/eji.201141480. [DOI] [PubMed] [Google Scholar]
- 43.Loser K, Mehling A, Loeser S, Apelt J, Kuhn A, Grabbe S, Schwarz T, Penninger JM, Beissert S. Epidermal RANKL controls regulatory T-cell numbers via activation of dendritic cells. Nat Med. 2006;12:1372–1379. doi: 10.1038/nm1518. [DOI] [PubMed] [Google Scholar]
- 44.Emery JG, McDonnell P, Burke MB, Deen KC, Lyn S, Silverman C, Dul E, Appelbaum ER, Eichman C, DiPrinzio R, et al. Osteoprotegerin is a receptor for the cytotoxic ligand TRAIL. J Biol Chem. 1998;273:14363–14367. doi: 10.1074/jbc.273.23.14363. [DOI] [PubMed] [Google Scholar]
- 45.Shimamura M, Nakagami H, Osako MK, Kurinami H, Koriyama H, Zhengda P, Tomioka H, Tenma A, Wakayama K, Morishita R. OPG/RANKL/RANK axis is a critical inflammatory signaling system in ischemic brain in mice. Proc Natl Acad Sci USA. 2014;111:8191–8196. doi: 10.1073/pnas.1400544111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kumashiro N, Erion DM, Zhang D, Kahn M, Beddow SA, Chu X, Still CD, Gerhard GS, Han X, Dziura J, et al. Cellular mechanism of insulin resistance in nonalcoholic fatty liver disease. Proc Natl Acad Sci USA. 2011;108:16381–16385. doi: 10.1073/pnas.1113359108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stefan N, Kantartzis K, Häring HU. Causes and metabolic consequences of Fatty liver. Endocr Rev. 2008;29:939–960. doi: 10.1210/er.2008-0009. [DOI] [PubMed] [Google Scholar]
- 48.Gannagé-Yared MH, Yaghi C, Habre B, Khalife S, Noun R, Germanos-Haddad M, Trak-Smayra V. Osteoprotegerin in relation to body weight, lipid parameters insulin sensitivity, adipocytokines, and C-reactive protein in obese and non-obese young individuals: results from both cross-sectional and interventional study. Eur J Endocrinol. 2008;158:353–359. doi: 10.1530/EJE-07-0797. [DOI] [PubMed] [Google Scholar]
- 49.Yaturu S, Rains J, Jain SK. Relationship of elevated osteoprotegerin with insulin resistance, CRP, and TNF-alpha levels in men with type 2 diabetes. Cytokine. 2008;44:168–171. doi: 10.1016/j.cyto.2008.07.471. [DOI] [PubMed] [Google Scholar]
- 50.Pepene CE, Ilie IR, Marian I, Duncea I. Circulating osteoprotegerin and soluble receptor activator of nuclear factor κB ligand in polycystic ovary syndrome: relationships to insulin resistance and endothelial dysfunction. Eur J Endocrinol. 2011;164:61–68. doi: 10.1530/EJE-10-0720. [DOI] [PubMed] [Google Scholar]
- 51.Akinci B, Celtik A, Yuksel F, Genc S, Yener S, Secil M, Ozcan MA, Yesil S. Increased osteoprotegerin levels in women with previous gestational diabetes developing metabolic syndrome. Diabetes Res Clin Pract. 2011;91:26–31. doi: 10.1016/j.diabres.2010.09.028. [DOI] [PubMed] [Google Scholar]
- 52.Suliburska J, Bogdanski P, Gajewska E, Kalmus G, Sobieska M, Samborski W. The association of insulin resistance with serum osteoprotegerin in obese adolescents. J Physiol Biochem. 2013;69:847–853. doi: 10.1007/s13105-013-0261-8. [DOI] [PubMed] [Google Scholar]
- 53.Niu Y, Yang Z, Li X, Zhang W, Lu S, Zhang H, Chen X, Zhu L, Xing Y, Ning G, et al. Association of osteoprotegerin with impaired glucose regulation and microalbuminuria: the REACTION study. BMC Endocr Disord. 2015;15:75. doi: 10.1186/s12902-015-0067-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bilgir O, Yavuz M, Bilgir F, Akan OY, Bayindir AG, Calan M, Bozkaya G, Yuksel A. Relationship between insulin resistance, hs-CRP, and body fat and serum osteoprotegerin/RANKL in prediabetic patients. Minerva Endocrinol. 2018;43:19–26. doi: 10.23736/S0391-1977.17.02544-5. [DOI] [PubMed] [Google Scholar]
- 55.Duan P, Yang M, Wei M, Liu J, Tu P. Serum Osteoprotegerin Is a Potential Biomarker of Insulin Resistance in Chinese Postmenopausal Women with Prediabetes and Type 2 Diabetes. Int J Endocrinol. 2017;2017:8724869. doi: 10.1155/2017/8724869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mashavi M, Menaged M, Shargorodsky M. Circulating osteoprotegerin in postmenopausal osteoporotic women: marker of impaired glucose regulation or impaired bone metabolism. Menopause. 2017;24:1264–1268. doi: 10.1097/GME.0000000000000914. [DOI] [PubMed] [Google Scholar]
- 57.Daniele G, Winnier D, Mari A, Bruder J, Fourcaudot M, Pengou Z, Hansis-Diarte A, Jenkinson C, Tripathy D, Folli F. The potential role of the osteopontin-osteocalcin-osteoprotegerin triad in the pathogenesis of prediabetes in humans. Acta Diabetol. 2018;55:139–148. doi: 10.1007/s00592-017-1065-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ashley DT, O’Sullivan EP, Davenport C, Devlin N, Crowley RK, McCaffrey N, Moyna NM, Smith D, O’Gorman DJ. Similar to adiponectin, serum levels of osteoprotegerin are associated with obesity in healthy subjects. Metabolism. 2011;60:994–1000. doi: 10.1016/j.metabol.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 59.O’Sullivan EP, Ashley DT, Davenport C, Penugonda L, Kelleher G, Devlin N, Crowley R, O’Shea P, Agha A, Thompson CJ, et al. A comparison of osteoprotegerin with adiponectin and high-sensitivity C-reactive protein (hsCRP) as a marker for insulin resistance. Metabolism. 2013;62:34–38. doi: 10.1016/j.metabol.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 60.Ugur-Altun B, Altun A. Circulating leptin and osteoprotegerin levels affect insulin resistance in healthy premenopausal obese women. Arch Med Res. 2007;38:891–896. doi: 10.1016/j.arcmed.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 61.Ugur-Altun B, Altun A, Gerenli M, Tugrul A. The relationship between insulin resistance assessed by HOMA-IR and serum osteoprotegerin levels in obesity. Diabetes Res Clin Pract. 2005;68:217–222. doi: 10.1016/j.diabres.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 62.Ayina Ayina CN, Sobngwi E, Essouma M, Noubiap JJ, Boudou P, Etoundi Ngoa LS, Gautier JF. Osteoprotegerin in relation to insulin resistance and blood lipids in sub-Saharan African women with and without abdominal obesity. Diabetol Metab Syndr. 2015;7:47. doi: 10.1186/s13098-015-0042-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jorsal A, Tarnow L, Flyvbjerg A, Parving HH, Rossing P, Rasmussen LM. Plasma osteoprotegerin levels predict cardiovascular and all-cause mortality and deterioration of kidney function in type 1 diabetic patients with nephropathy. Diabetologia. 2008;51:2100–2107. doi: 10.1007/s00125-008-1123-8. [DOI] [PubMed] [Google Scholar]
- 64.Clancy P, Oliver L, Jayalath R, Buttner P, Golledge J. Assessment of a serum assay for quantification of abdominal aortic calcification. Arterioscler Thromb Vasc Biol. 2006;26:2574–2576. doi: 10.1161/01.ATV.0000242799.81434.7d. [DOI] [PubMed] [Google Scholar]
- 65.Lehto S, Niskanen L, Suhonen M, Rönnemaa T, Laakso M. Medial artery calcification. A neglected harbinger of cardiovascular complications in non-insulin-dependent diabetes mellitus. Arterioscler Thromb Vasc Biol. 1996;16:978–983. doi: 10.1161/01.atv.16.8.978. [DOI] [PubMed] [Google Scholar]
- 66.Olesen P, Nguyen K, Wogensen L, Ledet T, Rasmussen LM. Calcification of human vascular smooth muscle cells: associations with osteoprotegerin expression and acceleration by high-dose insulin. Am J Physiol Heart Circ Physiol. 2007;292:H1058–H1064. doi: 10.1152/ajpheart.00047.2006. [DOI] [PubMed] [Google Scholar]
- 67.Wang CC, Sorribas V, Sharma G, Levi M, Draznin B. Insulin attenuates vascular smooth muscle calcification but increases vascular smooth muscle cell phosphate transport. Atherosclerosis. 2007;195:e65–e75. doi: 10.1016/j.atherosclerosis.2007.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gannagé-Yared MH, Fares F, Semaan M, Khalife S, Jambart S. Circulating osteoprotegerin is correlated with lipid profile, insulin sensitivity, adiponectin and sex steroids in an ageing male population. Clin Endocrinol (Oxf) 2006;64:652–658. doi: 10.1111/j.1365-2265.2006.02522.x. [DOI] [PubMed] [Google Scholar]
- 69.Nabipour I, Kalantarhormozi M, Larijani B, Assadi M, Sanjdideh Z. Osteoprotegerin in relation to type 2 diabetes mellitus and the metabolic syndrome in postmenopausal women. Metabolism. 2010;59:742–747. doi: 10.1016/j.metabol.2009.09.019. [DOI] [PubMed] [Google Scholar]
- 70.Golledge J, Leicht AS, Crowther RG, Glanville S, Clancy P, Sangla KS, Spinks WL, Quigley F. Determinants of endothelial function in a cohort of patients with peripheral artery disease. Cardiology. 2008;111:51–56. doi: 10.1159/000113428. [DOI] [PubMed] [Google Scholar]
- 71.Akinci B, Demir T, Celtik A, Baris M, Yener S, Ozcan MA, Yuksel F, Secil M, Yesil S. Serum osteoprotegerin is associated with carotid intima media thickness in women with previous gestational diabetes. Diabetes Res Clin Pract. 2008;82:172–178. doi: 10.1016/j.diabres.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 72.Pérez de Ciriza C, Moreno M, Restituto P, Bastarrika G, Simón I, Colina I, Varo N. Circulating osteoprotegerin is increased in the metabolic syndrome and associates with subclinical atherosclerosis and coronary arterial calcification. Clin Biochem. 2014;47:272–278. doi: 10.1016/j.clinbiochem.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 73.Bernardi S, Fabris B, Thomas M, Toffoli B, Tikellis C, Candido R, Catena C, Mulatero P, Barbone F, Radillo O, et al. Osteoprotegerin increases in metabolic syndrome and promotes adipose tissue proinflammatory changes. Mol Cell Endocrinol. 2014;394:13–20. doi: 10.1016/j.mce.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 74.Tavintharan S, Pek LT, Liu JJ, Ng XW, Yeoh LY, Su Chi L, Chee Fang S. Osteoprotegerin is independently associated with metabolic syndrome and microvascular complications in type 2 diabetes mellitus. Diab Vasc Dis Res. 2014;11:359–362. doi: 10.1177/1479164114539712. [DOI] [PubMed] [Google Scholar]
- 75.Yilmaz Y, Yonal O, Kurt R, Oral AY, Eren F, Ozdogan O, Ari F, Celikel CA, Korkmaz S, Ulukaya E, et al. Serum levels of osteoprotegerin in the spectrum of nonalcoholic fatty liver disease. Scand J Clin Lab Invest. 2010;70:541–546. doi: 10.3109/00365513.2010.524933. [DOI] [PubMed] [Google Scholar]
- 76.Yang M, Xu D, Liu Y, Guo X, Li W, Guo C, Zhang H, Gao Y, Mao Y, Zhao J. Combined Serum Biomarkers in Non-Invasive Diagnosis of Non-Alcoholic Steatohepatitis. PLoS One. 2015;10:e0131664. doi: 10.1371/journal.pone.0131664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Niu Y, Zhang W, Yang Z, Li X, Fang W, Zhang H, Wang S, Zhou H, Fan J, Qin L, et al. Plasma osteoprotegerin levels are inversely associated with nonalcoholic fatty liver disease in patients with type 2 diabetes: A case-control study in China. Metabolism. 2016;65:475–481. doi: 10.1016/j.metabol.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 78.Erol M, Bostan Gayret O, Tekin Nacaroglu H, Yigit O, Zengi O, Salih Akkurt M, Tasdemir M. Association of Osteoprotegerin with Obesity, Insulin Resistance and Non-Alcoholic Fatty Liver Disease in Children. Iran Red Crescent Med J. 2016;18:e41873. doi: 10.5812/ircmj.41873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ayaz T, Kirbas A, Durakoglugil T, Durakoglugil ME, Sahin SB, Sahin OZ, Kirvar A, Tasci F. The relation between carotid intima media thickness and serum osteoprotegerin levels in nonalcoholic fatty liver disease. Metab Syndr Relat Disord. 2014;12:283–289. doi: 10.1089/met.2013.0151. [DOI] [PubMed] [Google Scholar]
- 80.Monseu M, Dubois S, Boursier J, Aubé C, Gagnadoux F, Lefthériotis G, Ducluzeau PH. Osteoprotegerin levels are associated with liver fat and liver markers in dysmetabolic adults. Diabetes Metab. 2016;42:364–367. doi: 10.1016/j.diabet.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 81.Reid P, Holen I. Pathophysiological roles of osteoprotegerin (OPG) Eur J Cell Biol. 2009;88:1–17. doi: 10.1016/j.ejcb.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 82.Feldstein AE, Gores GJ. Apoptosis in alcoholic and nonalcoholic steatohepatitis. Front Biosci. 2005;10:3093–3099. doi: 10.2741/1765. [DOI] [PubMed] [Google Scholar]
- 83.Chamoux E, Houde N, L’Eriger K, Roux S. Osteoprotegerin decreases human osteoclast apoptosis by inhibiting the TRAIL pathway. J Cell Physiol. 2008;216:536–542. doi: 10.1002/jcp.21430. [DOI] [PubMed] [Google Scholar]
- 84.Hao Y, Tsuruda T, Sekita-Hatakeyama Y, Kurogi S, Kubo K, Sakamoto S, Nakamura M, Udagawa N, Sekimoto T, Hatakeyama K, et al. Cardiac hypertrophy is exacerbated in aged mice lacking the osteoprotegerin gene. Cardiovasc Res. 2016;110:62–72. doi: 10.1093/cvr/cvw025. [DOI] [PubMed] [Google Scholar]
- 85.Kiechl S, Wittmann J, Giaccari A, Knoflach M, Willeit P, Bozec A, Moschen AR, Muscogiuri G, Sorice GP, Kireva T, et al. Blockade of receptor activator of nuclear factor-κB (RANKL) signaling improves hepatic insulin resistance and prevents development of diabetes mellitus. Nat Med. 2013;19:358–363. doi: 10.1038/nm.3084. [DOI] [PubMed] [Google Scholar]
- 86.Yao Q, Yu C, Zhang X, Zhang K, Guo J, Song L. Wnt/β-catenin signaling in osteoblasts regulates global energy metabolism. Bone. 2017;97:175–183. doi: 10.1016/j.bone.2017.01.028. [DOI] [PubMed] [Google Scholar]
- 87.Morony S, Tintut Y, Zhang Z, Cattley RC, Van G, Dwyer D, Stolina M, Kostenuik PJ, Demer LL. Osteoprotegerin inhibits vascular calcification without affecting atherosclerosis in ldlr(-/-) mice. Circulation. 2008;117:411–420. doi: 10.1161/CIRCULATIONAHA.107.707380. [DOI] [PMC free article] [PubMed] [Google Scholar]


