Abstract
Background
Interest in the use of extremely low-frequency (ELF) electromagnetic field (EMF) for the treatment of pain and inflammation is increasing due to the ability of this promising therapy to compete with pharmaceuticals without the adverse effects caused by drugs. However, there continues to be concerns regarding cytotoxic and genotoxic effects that may occur as a result of exposure to EMF.
Objective
To investigate this concern, we tested the effect of our known therapeutic 5 Hz, 0.4 milliTesla (mT) EMF on a human mesenchymal stromal cell (hMSC) line to determine whether ELF-EMF exposure would cause cytotoxic or genotoxic effects.
Methods
Treated samples along with controls were exposed to 5 Hz, 0.4 mT ELF-EMF for 20 min/day, 3×/week for 2 weeks and then assayed for cell viability, proliferation rates, and chromosome breaks.
Results
Cytogenetic analysis of the viability and proliferation rates along with analysis of morphological genome stability showed no cytotoxicity, and no chromosome breaks per karyotype analysis—therefore no genotoxicity.
Conclusion
Exposure to an ELF-EMF of 5 Hz, 0.4 mT for 20 min/day, 3×/week for 2 weeks does not cause cytotoxic or genotoxic effects in hMSCs.
Keywords: chromosome breaks, cytotoxicity, extremely low frequency electromagnetic field, genotoxicity, mesenchymal stromal cells/pericytes
Introduction
Extremely low frequency electromagnetic fields (ELF-EMF) penetrate through the skin into the body’s deep tissue to affect cell function.1–3 Studies report ELF-EMF to be effective in the treatment of pain and inflammation4–7 and also tissue regeneration and wound healing.8,9 Evidence shows that mechanisms of action include Ca2+ ion flux, and expression/activation of Ca2+ ion binding proteins such as calmodulin, increase the cytosolic Ca2+ concentration to affect signaling pathways targeting tissues such as bone, cartilage, and nerve for pain regulation and tissue regeneration.10,11 For many years, it was thought that EMF exposure would only cause harmful effects in the body, but it is now understood that the amount of energy (field strength), frequency of the field, and length of time of exposure are the parameters that determine whether EMF is harmful or beneficial.12
Mesenchymal stromal cells (MSCs)/pericytes are a heterogeneous, tissue-specific population of cells, located in perivascular areas throughout the body that, depending on the organ and on the pathology, assume tissue-specific roles to respond/mitigate cellular events.13 Therefore, any treatment using ELF-EMF would certainly reach, and impact these cells, making them an ideal subject in which to test cytotoxicity and genotoxicity. Here, we investigated whether our known therapeutic ELF-EMF of 5 Hz, 0.4 mT field4,5 could be cytotoxic/genotoxic to MSCs/pericytes upon exposure for 20 min/day, 3×/week for 2 weeks. We found that MSCs’ viability, proliferation rates, and morphological genome stability were not affected or altered by ELF-EMF, when used at the tested field strength and frequency.
Materials and Methods
Cell Culture
Human bone mononuclear cells (BMNCs) were obtained from AllCells (Alameda, CA). BMNCs were enriched for the Stro-1+ fraction using a Stro-1 antibody (R&D Systems, Minneapolis, MN) and magnetic bead cell sorting (Miltenyi Biotec, Inc., Auburn, CA). Stro-1+ cells were expanded in vitro at 37℃ in 5% CO2 humidified air, in MSC-GM (growing media). At confluence, cells were detached with 0.25% trypsin (Invitrogen Corp., Carlsbad, CA), and trypsin was neutralized with media containing 10% fetal bovine serum (Thermo Fisher Scientific, Grand Island, NY). Characterization by flow cytometry, and by functional studies, demonstrated that these cells displayed markers characteristic of bone marrow-derived mesenchymal cells/pericytes, including CD105, CD146, CXCL12, CD90, CD44, and CD29, and that they were able to undergo trilineage differentiation into adipocytes, cartilage, and bone.14 Cells were cultured in T-75 flasks using 36 mL of media per flask, incubated at 37℃, with 5% CO2, and grown to 100% confluency before being exposed to the ELF-EMF.
ELF-EMF Exposure
Flasks containing confluent cells were removed from incubators and the media changed. Flasks with cells were placed in 37℃ water bath and exposed to a 5 Hz, 0.4 mT uniform ELF-EMF generated by a Helmholtz coil (Figure 1) for 20 min, 3×/week (Monday, Wednesday, and Friday) for 2 weeks. Flasks were placed in center of ELF-EMF field to assure uniform cell exposure.
Figure 1.
ELF-EMF Emitting Device Setup. EMF Emitting Device Is an 11″ Diameter Helmholtz Coil Equipped With a Warm Bath Heated With Ceramic Heating Element to Maintain 37℃ Constant Temperature. Oscilloscope Is Used to Measure Frequency. Gauss Meter Is Used to Measure Field Strength.
Both treated and control cells originated from the same batch of isolated cells. Controls were subject to the same media change, but they were placed in the EMF device with field turned off (sham).
Cell Viability (Live/Dead) Assay
Cells were washed prior to assay using 1 mL of Dulbecco’s phosphate-buffered saline. In both sham and treated cells, imaging was performed using MSCs (1 × 106 cells/mL) that were transferred to coverslips (Mat-Tek, No. 1.5 cover glass) embedded with 35 mm circular plates in the cover glass. To determine viability, a live/dead stain calcein assay kit (Invitrogen Molecular Probes, Eugene, OR) was used according to manufacturer’s instructions. This kit provides a 2-color fluorescence cell viability system that uses 2 probes to measure recognized parameters of cell viability—intracellular esterase activity (generating green fluorescence to determine live cell activity) and plasma membrane integrity using ethidium homodimer-1 (EthD-1 generating red fluorescence to determine dead cells). Cells were counted, and all images were quantitatively assessed based on live/dead cell percentages using CellSens Standard software installed on Leica DMI 4000B inverted microscope.
Proliferation Rate Assay
To determine cell proliferation rates, PrestoBlue Cell Proliferation Assay (Invitrogen, Eugene, OR) was used, according to manufacturer’s instructions, to measure rates of proliferation during the time of exposure to ELF-EMF. PrestoBlue uses a permeable resazurin-based solution that functions as an indicator of cell proliferation. The reagent is modified by the reduction environment of the viable cell and turns red in color, becoming highly fluorescent. This color change can be detected using a plate reader that measures optical rates of excitation and emission. For this assay, a volume of 90 µL of cells (1 × 106/mL) were plated in 16 wells of 2 separate 96-well plates (1 plate treated with ELF-EMF exposed cells, and the other with control cells). Next, they were incubated at 37℃, with 5% CO2, for 24 h (considered day 0) before the first time point was taken. This gave the cells time to adhere to the wells. The second time point was taken at 7 days, and the third time point was taken at 14 days.
At each time point, assay was performed by transferring 90 µL of cell media from each well of the 96-well cell plates into corresponding separate 96-well plates (treatment and CTRL) for testing. Fresh media was then added to the cells and replaced in the incubator. The cell media used for testing was treated with 200 µL of PrestoBlue (10% solution) mixed into each well and then incubated for 20 min. Proliferation rate measurements were taken using a Spectramax M5 plate reader (Molecular Devices LLC, Sunnyvale, CA) for fluorescence reading (excitation 535 nm and emission 615).
Genotoxicity Analysis
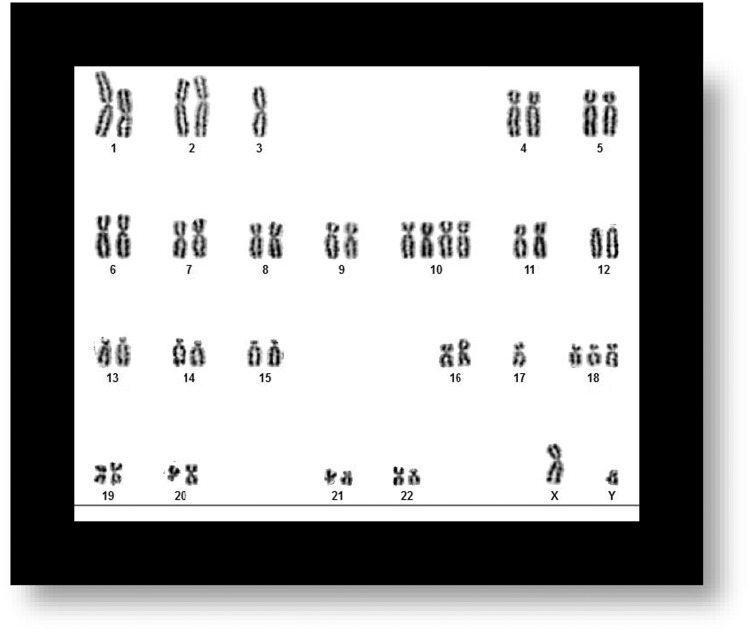
To determine karyotype stability, we first determined that MSC karyotype before treatment was stable (Figure 2). Next, we stained a total of 12 cell samples on slides (treatment with EMF (n = 6), and control cells CTRL/sham (n = 6), to determine whether our ELF-EMF treatment causes genotoxic effects. Slides were stained for 1 min 40 s with a working solution of 1 mL Giemsa stain prepared from a commercially available stock solution (R66 solution, Sci Supply group, Collingwood, Ontario, Canada), mixed with 50 mL Gurrs buffer (GibCo Life technologies, Thermo Fisher Scientific, Waltham, MA), and then washed 2 × 1 min in Gurrs buffer. One sample each (control and treatment) was stained with Giemsa (mixed with trypsin) to show banding of chromosomes in order to determine the cell sample karyotype.
Figure 2.
Untreated Samples of MSCs. Sample Shows Stable Karyotype in MSC Sample Before Being Exposed to EMF.
Statistical Methods
Experimental results are presented as ± the standard error of the mean and were analyzed with Prism (GraphPad Software, Inc., La Jolla, CA). Student’s paired t test was used to determine significance of difference between means, with P < .05 considered statistically significant for all tests.
Results
Impact of ELF-EMF-5-0.4 on Cell Viability
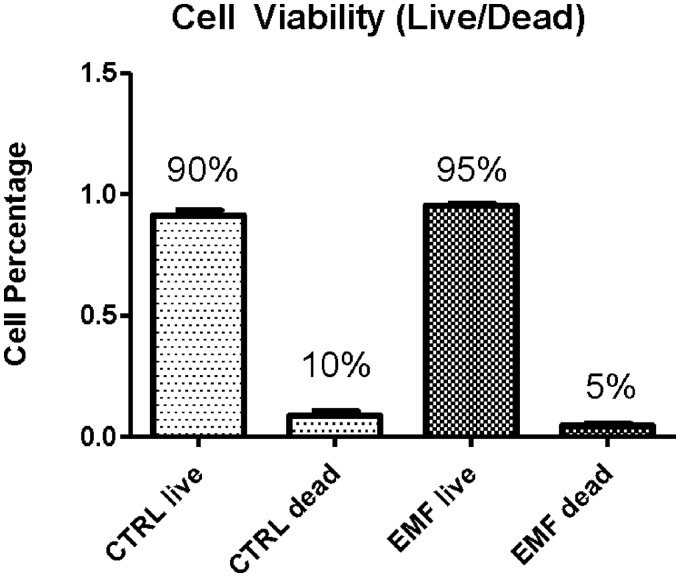
In order to test whether ELF-EMF, when administered at the known therapeutic dosimetry of 5 Hz, 0.4 mT (ELF-EMF-5-0.4), could have cytotoxic/genotoxic effects, we began by investigating whether ELF-EMF-5-0.4 had an impact on cell viability. Experimental groups included ELF-EMF-5-0.4 exposed (n = 3) versus control (CTRL/sham, n = 3) groups, in which cells were placed in Helmholtz Coil with field turned off, as described in the “Materials and Methods” section. The time of exposure of 20 min/day, 3×/week (Monday, Wednesday, and Friday) for 2 weeks, was chosen as the midrange time of exposure used in our previous experiments,4,5 and as shown by Shupak, N.15 Outcomes of cell viability (live/dead) assays for ELF-EMF-5-0.4 treated and control cells show no statistical significance (Figure 3).
Figure 3.
Cell Viability Assay. Cell Viability Assay Shows No Statistically Significant Difference Between CTRL/Sham ELF-EMF Treatment When Exposed to 5 Hz, 0.4 mT for 20 Min/Day, 3×/Week for 2 Weeks. CTRL, Control; EMF, Electromagnetic Field.
Effect of ELF-EMF-5-0.4 on Cell Proliferation
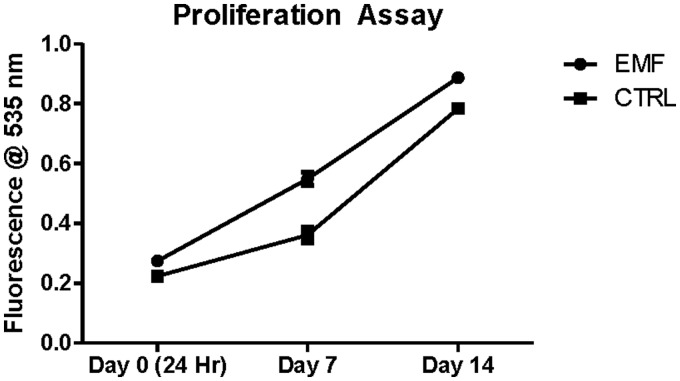
To determine whether ELF-EMF-5-0.4 had an effect on cell proliferation, cells were subjected to ELF-EMF fields as described above. ELF-EMF-5-0.4 exposed (n = 3) versus control (CTRL/sham, n = 3) cell groups were harvested, and data were calculated and plotted showing high fluorescence values correlating to greater total metabolic activity. Results in Figure 4 show no statistically significant difference between the MSCs exposed to EMF compared with CTRL after 2 weeks of ELF-EMF exposure, therefore when ELF-EMF is employed using these parameters does not alter cellular proliferation.
Figure 4.
Proliferation Assay. MSCs Exposed to ELF-EMF (Denoted by Circle) Show No Statistically Significant Change in Proliferation Rates Compared With CTRL (Denoted by Square) When Exposed to 5 Hz, 0.4 mT for 20 Min/Day, 3×/Week for 2 Weeks. CTRL, Control; EMF, Electromagnetic Field.
ELF-EMF-5-0.4 Exposure Is Not Genotoxic to MSCs/Pericytes
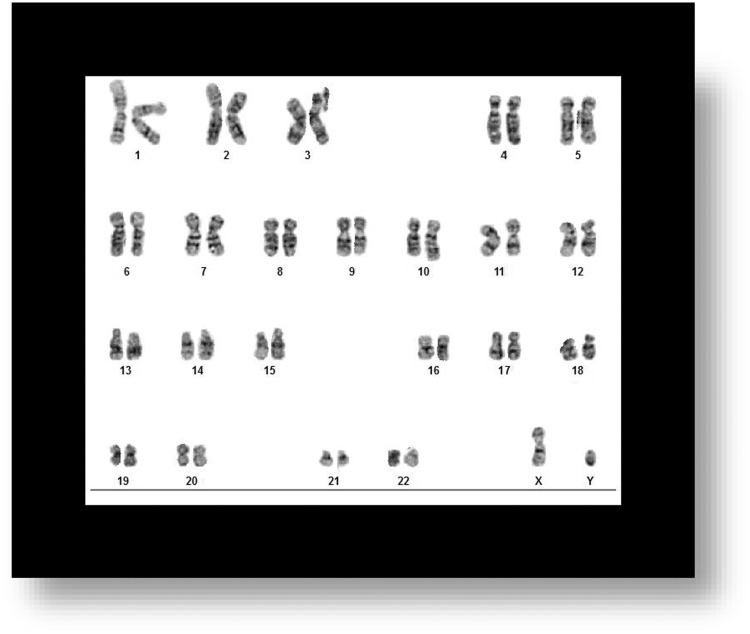
After determining that the MSC used had a normal karyotype (Figure 2), we exposed cells to 5 Hz, 0.4 mT ELF-EMF for 20 min/day, 3×/week (Monday, Wednesday, and Friday) for 2 weeks (n = 6 samples per group) and compared them with MSCs controls (sham, n = 6). We stained the (a) CTRL samples and (b) ELF-EMF-treated samples with Giemsa (mixed with Gurrs) to investigate the presence of chromosomal aberrations that could have occurred as a result of exposure to ELF-EMF. Euploid metaphases with 46 chromosomes were analyzed for the presence of chromosome aberrations, including chromatid breaks, isochromatid breaks, and chromatid exchanges. If present, chromatid discontinuances of lengths greater than the width of the chromatid were considered to be chromatid breaks. Chromatid discontinuances with lengths less than the chromatid width were considered to be chromatid caps and were not counted as aberrations in the present analysis. If present, we considered exchanges to include chromatid interchanges between 2 or more chromosomes, chromatid interchanges between arms of a chromosome, nonterminal deletions and aberrations resulting from the fusion of broken ends of chromatids from 1 arm of a chromosome. Neither (a) MSC control samples nor (b) MSC treatment samples showed chromatid breaks or discontinuances after being treated with ELF-EMF of 5 Hz, 0.4 mT for 20 min/day, 3×/week for 2 weeks (Figure 5).
Figure 5.
Karyotype Analysis After (a) CTRL/Sham Treatment and (b) ELF-EMF Treatment. Neither MSC Treatment Sample Show Chromatic Breaks or Discontinuance After Being Treated With ELF-EMF of 5 Hz, 0.4 mT for 20 Min/Day, 3×/Week for 2 Weeks.
Discussion
Nonionizing radiation refers to any type of electromagnetic radiation that does not carry enough energy to ionize atoms or molecules, meaning completely removing an electron from an atom or molecule, resulting in cell toxicity. ELF fields are in the range of 3–30 Hz and are designated as nonionizing radiation. We chose an ELF-EMF of 5 Hz, 0.4 mT field to test on MSCs/pericytes because we have used this magnetic field strength and frequency in past experiments to measure its effect on various cytokines and transcription factors involved in pain- and inflammation-related mechanisms in vitro.4,5 Although we demonstrated an anti-inflammatory secretion profile in cells treated with ELF-EMF, others have reported harmful cellular effects, depending on the energy state of the field.10 Bioelectromagnetics, the study of how living organisms interact with EMF, investigates the interaction between electrons, atoms, ions, and molecules present in all living matter, and how they are influenced by electromagnetic interactions.16 Faraday’s law of induction and Maxwell’s equations explain how an EMF is created: an electric field is generated whenever a charge (Q) is present, and a magnetic field arises from the electrical current flow. Units of measure are Gauss and Tesla (10 000 Gauss), which expresses the flux density/field strength produced by the EMF. Faraday’s law can be applied to electrical currents that already exist in the body (heart, brain, etc), which are capable of producing magnetic fields outside the body.17 These fields can be measured by electrocardiograph, electroencephalograph, and magnetoencephalography, which is a technique for mapping brain activity by recording magnetic fields produced by electrical currents occurring naturally in the brain. These endogenous fields can be affected by exogenous EMF stimulation, as can tissues and organs in the body, by modulating biochemical reactions and the behavior of charged molecules.18
Research shows the plasma membrane is one of the main locations where applied EMF acts on the cell.19,20 EMF exposure to the outside surface of the cell can alter ligand–receptor interactions11 known as mechanically gated ion channels.21 ELF-EMF can pass unobstructed through living tissue, with frequencies close to the resonant patterns of calcium (Ca2+), sodium (Na+), and other ions.22 Due to this direct cellular interaction, EMF are reported to increase healing rates much quicker than other therapies, as they more quickly permeate tissue immediately after insult.23 EMF quickly restores equilibrium between free radicals and antioxidants to stop the cascade of inflammatory progression and biochemical degradation in traumatized tissue.24 EMF therapies not only have the potential to restore equilibrium in reactive oxygen species related to free radical/antioxidative chemistry, they also induce currents that stabilize cytosolic Ca2+ that is activated by oxidative stress.25 ELF-EMF has also been reported to upregulate classes of protective and restorative gene loci as well as downregulate dysregulatory and apoptotic gene loci.23 Although the energy in nonionizing radiation is not strong enough to break ion bonds in atoms and molecules,26,27 numerous studies have investigated the effects of EMF on adult stem cells, demonstrating that changes in proliferation rates, depend upon cell culture conditions and/or EMF parameters such as frequency, field strength, and time of exposure.28–30 Here, we show that ELF-EMF-5-0.4 did not alter MSCs/pericytes’ viability or proliferation ability. Furthermore, we demonstrated that no breakages, fusions, or translocations were present in MSCs/pericytes after 2 weeks exposure to ELF-EMF of 5 Hz, 0.4 mT.
Investigations of cytotoxic or genotoxic effects of EMF on other cells lines include blood cells and immune cells. Previous investigations of comet assay, sister chromatid exchanges, and chromosome aberrations along with micronucleus tests were also conducted to study genotoxic effects of ELF-EMF on blood cells. Results showed the absence of genotoxicity.31 Previous reports of the effect of EMF exposure to human lymphoid cells and human peripheral lymphocytes show EMF produced no genotoxic effect either.32,33 Ames test analysis was conducted to investigate effects of EMF exposure on 4 strains of Salmonella typhimurium (TA97a, TA98, TA100, and TA102) to test whether EMF would increase their rate of mutation. Results showed a lack of EMF-induced genotoxic effect.34
Conclusion
The ongoing debate regarding harmful or beneficial effects of ELF-EMF has created both positive and negative arguments for whether ELF-EMF could result in pathological alterations in humans. ELF-EMF has been investigated for decades to determine its effect on different cell types and subcellular functions, with conflicting results.35 In many, if not most cases, these conflicting results are due to differences in dosimetry (frequency, field strength, and time of exposure). In order to reduce concerns that our ELF-EMF treatment could cause cytotoxicity or chromosomal damage, we tested it on human MSCs/pericytes, a heterogeneous, tissue-specific population of cells located in perivascular areas throughout the body. Based on the results of cell viability tests, proliferation rate assays, and karyotype analysis, data show that a 5 Hz, 0.4 mT EMF does not cause either cytotoxic effects or genotoxic chromosome breaks in MSCs/pericytes after 2 weeks exposure.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Wake Forest Center for Integrative Medicine, grant no. WFBHS-63313-740-120330-00000-668195.
References
- 1.Traina G, Fontanesi G, Costa P, et al. Effect of electromagnetic stimulation on patients suffering from non-union. A retrospective study with a control group. Electromagn Biol Med. 1991;10(1–2):101–117. [Google Scholar]
- 2.Sutbeyaz S, Sezer N, Koseoglu F, Kibar S. Low-frequency pulsed electromagnetic field therapy in fibromyalgia: a randomized, double-blind, sham-controlled clinical study. Clin J Pain. 2009;25(8):722–728. [DOI] [PubMed] [Google Scholar]
- 3.Pilla A. Nonthermal electromagnetic fields: From first messenger to therapeutic applications. Electromagn Biol Med. 2013;32(2):123–136. [DOI] [PubMed] [Google Scholar]
- 4.Ross C, Harrison BS. Effect of pulsed electromagnetic field on inflammatory pathway markers in RAW 264.7 murine macrophages. J Inflamm Res. 2013;6:45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ross C, Harrison BS. Effect of electromagnetic field on cyclic adenosine monophosphate in a human mu-opioid receptor cell model. Electromagn Biol Med. 2014;7:1–7. [DOI] [PubMed] [Google Scholar]
- 6.Nelson F, Zvirbulis R, Pilla AA. Non-invasive electromagnetic field therapy produces rapid and substantial pain reduction in early knee osteoarthritis: a randomized double-blind pilot study. Rheumatol Int. 2013;33(8):2169–2173. [DOI] [PubMed] [Google Scholar]
- 7.Iannitti T, Fistetto G, Esposito A, Rottigni V, Palmieri B. Pulsed electromagnetic field therapy for management of osteoarthritis-related pain, stiffness and physical function: clinical experience in the elderly. Clin Interv Aging. 2013;8:1289–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ross C, Siriwardane ML, Almeida-Porada G, et al. The effect of low-frequency electromagnetic field on human bone-marrow derived mesenchymal stem/progenitor cell differentiation. Stem Cell Res. 2015;15(1):96–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ross C. The use of electric, magnetic, and electromagnetic field for directed cell migration and adhesion in regenerative medicine. Biotechnol Prog. 2017;33(1):5–16. [DOI] [PubMed] [Google Scholar]
- 10.Maziarz A, Kocan B, Bester M, et al. How electromagnetic fields can influence adult stem cells: positive and negative impacts. Stem Cell Res Ther. 2016;7(1):54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Funk R, Monsees T. Effects of electromagnetic fields on cells: physiological and therapeutical approaches and molecular mechanisms of interaction. A review. Cells Tissues Organs. 2006;182(2):59–78. [DOI] [PubMed] [Google Scholar]
- 12.Carpenter DE, Ayrapetyan S, eds. Biological Effects of Electric and Magnetic Fields: Beneficial and Harmful Effects. 1st ed. New York, NY: Academic Press; 1994.
- 13.de Girolamo L, Stanco D, Galliera E, et al. Low frequency pulsed electromagnetic field affects proliferation, tissue-specific gene expression, and cytokines release of human tendon cells. Cell Biochem Biophys. 2013;66(3):697–708. [DOI] [PubMed] [Google Scholar]
- 14.Chamberlain J, Yamagami T, Colletti E, et al. Efficient generation of human hepatocytes by the intrahepatic delivery of clonal human mesenchymal stem cells in fetal sheep. Hepatology. 2007;46(6):1935–1945. [DOI] [PubMed] [Google Scholar]
- 15.Shupak N. Therapeutic uses of pulsed magnetic field exposure. Radio Science Bulletin. 2003;307:9–32.
- 16.Bartschata K, Kushnerb MJ. Electron collisions with atoms, ions, molecules, and surfaces: Fundamental science empowering advances in technology. Proc Natl Acad Sci U S A. 2016;113(26):7016–7034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Becker R, Marino A. Electromagnetism and Life, Albany, NY: State University of New York Press, pp. 1982. [Google Scholar]
- 18.Blank M, Goodman R. Do electromagnetic fields interact directly with DNA? Bioelectromagnetics. 1997;18(2):111–115. [DOI] [PubMed] [Google Scholar]
- 19.Bawin S, Adey WR, Sabbot IM. Ionic factors in release of 45Ca2+ from chicken cerebral tissue by electromagnetic fields. Proc Natl Acad Sci U S A. 1978;75(12):6314–6318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Romeo S, Zeni L, Sarti M, et al. DNA electrophoretic migration patterns change after exposure of Jurkat cells to a single intense nanosecond electric pulse. PLoS One. 2011;6(12):e28419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Irmak M. Multifunctional Merkel cells: their roles in electromagnetic reception, finger-print formation, Reiki, epigenetic inheritance and hair form. Med Hypotheses. 2010;75:162–168. [DOI] [PubMed] [Google Scholar]
- 22.Liboff A. The cyclotron resonance hypothesis: experimental evidence and theoretical constraints. In: Bengt Norden and Claes Ramal ed. Interaction Mechanisms of Low-level Electromagnetic Fields With Living Systems. London, England: Oxford University Press; 1991: 130–147.
- 23.Gordon G. Designed electromagnetic pulsed therapy: clinical applications. J Cell Physiol. 2007;212(3):579–582. [DOI] [PubMed] [Google Scholar]
- 24.Han J, Shuvaev VV, Muzykantovv VR. Targeted interception of signaling reactive oxygen species in the vascular endothelium. Ther Deliv. 2012;3(2):263–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duong C, Kim JY. Exposure to electromagnetic field attenuates oxygen-glucose deprivation-induced microglial cell death by reducing intracellular Ca(2+) and ROS. Int J Radiat Biol. 2016;92(4):195–201. [DOI] [PubMed] [Google Scholar]
- 26.Tenforde T, Kaune W. Interaction of extremely low-frequency electric and magnetic fields with humans. Health Phys. 1987;53:585–606. [DOI] [PubMed] [Google Scholar]
- 27.Ng K-H. Non-ionizing radiations—sources, biological effects, emissions and exposures. In: Proceedings of the International Conference on Non-ionizing Radiation at UNITEN ICNIR2003 Electromagnetic Fields and Our Health; October 20–22, 2003. http://www.who.int/peh-emf/meetings/archive/en/keynote3ng.pdf. Accessed May 4, 2018.
- 28.Tsai M, Li WJ, Tuan RS, Chang WH. Modulation of osteogenesis in human mesenchymal stem cells by specific pulsed electromagnetic field stimulation. J Orthop Res. 2009;27(9):1169–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kang K-S, Hong J-M, Kang J-A, Rhie J-W, Jeong Y-J, Cho D-W. Regulation of osteogenic differentiation of human adipose-derived stem cells by controlling electromagnetic field conditions. Exp Mol Med. 2013;45:e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cho H, Seo YK, Yoon HH, et al. Neural stimulation on human bone marrow-derived mesenchymal stem cells by extremely low frequency electromagnetic fields. Biotechnol Prog. 2012;28(5):1329–1335. [DOI] [PubMed] [Google Scholar]
- 31.Stronati L, Testa A, Villani P, et al. Absence of genotoxicity in human blood cells exposed to 50 Hz magnetic fields as assessed by comet assay, chromosome aberration, micronucleus, and sister chromatid exchange analyses. Bioelectromagnetics. 2004;25(1):41–48. [DOI] [PubMed] [Google Scholar]
- 32.Cohen M, Kunska A, Astemborski JA, McCulloch D, Paskewitz DA. Effect of low level, 60-Hz electromagnetic fields on human lymphoid cells: mitotic rate and chromosome breakage in human peripheral lymphocytes. Bioelectromagnetics. 1986;7(4):415–423. [DOI] [PubMed] [Google Scholar]
- 33.Rosenthal M, Obe G. Effects of 50-Hz electromagnetic fields on proliferation and chromosomal alterations in human peripheral lymphocytes untreated or pretreated with chemical mutagens. Mutat Res. 1989;210(2):329–335. [DOI] [PubMed] [Google Scholar]
- 34.Morandi M, Pak CM, Caren RP, Caren LD. Lack of an EMF-induced genotoxic effect in the Ames assay. Life Sci. 1996;59(3):263–271. [DOI] [PubMed] [Google Scholar]
- 35.Singh S, Kapoor N. Health implications of electromagnetic fields, mechanisms of action, and research needs. Adv Biol. 2014;2014(Article ID 198609):24. [Google Scholar]