Short abstract
Oxidative stress plays an important role in the onset and progression of Parkinson disease. Although released dopamine at the synaptic terminal is mostly reabsorbed by dopaminergic neurons, some dopamine is presumably taken up by astroglia. This study examined the dopamine-induced astroglial protective function through the activation of the pentose-phosphate pathway (PPP) to reduce reactive oxygen species (ROS). In vitro experiments were performed using striatal neurons and cortical or striatal astroglia prepared from Sprague-Dawley rats or C57BL/6 mice. The rates of glucose phosphorylation in astroglia were evaluated using the [14C]deoxyglucose method. PPP activity was measured using [1-14C]glucose and [6-14C]glucose after acute (60 min) or chronic (15 hr) exposure to dopamine. ROS production was measured using 2′,7′-dichlorodihydrofluorescein diacetate. The involvement of the Kelch-like ECH-associated protein 1 (Keap1) or nuclear factor-erythroid-2-related factor 2 (Nrf2) system was evaluated using Nrf2 gene knockout mice, immunohistochemistry, and quantitative reverse transcription polymerase chain reaction analysis for heme oxygenase-1. Acute exposure to dopamine elicited increases in astroglial glucose consumption with lactate release. PPP activity in astroglia was robustly enhanced independently of Na+-dependent monoamine transporters. In contrast, chronic exposure to dopamine induced moderate increases in PPP activity via the Keap1/Nrf2 system. ROS production from dopamine increased gradually over 12 hr. Dopamine induced neuronal cell damage that was prevented by coculturing with astroglia but not with Nrf2-deficient astroglia. Dopamine-enhanced astroglial PPP activity in both acute and chronic manners may possibly reduce neuronal oxidative stress.
Keywords: astrocytes, dopamine, glucose, glutamate, glutathione, reactive oxygen species
Introduction
Parkinson disease (PD) is characterized by a progressive degeneration of dopaminergic neurons in the substantia nigra (SN; Halliday et al., 1996). Although the exact mechanism by which dopaminergic neurons undergo cell death has not been elucidated, oxidative stress and mitochondrial impairment may play important roles. In fact, early studies clearly showed deficiencies of mitochondrial complex I in autopsied brains from patients with idiopathic PD (Mizuno et al., 1989; Schapira et al., 1989). In addition, genetic defects that fail to maintain normal mitochondrial function are known to lead to the early onset of PD. Lewy bodies, a hallmark of PD, consist of α-synuclein and can lead to mitochondrial dysfunction (Martin and Teismann, 2009); in turn, mitochondrial dysfunction triggers α-synuclein accumulation (Esteves et al., 2011).
ATP is generated in mitochondria through the oxidative metabolism of glucose. The brain utilizes large amounts of oxygen and glucose to generate ATP, most of which is consumed by Na+,K+-ATPase to maintain cellular ionic gradients (Dienel, 2009). These ionic gradients are essential for the generation of action potentials; thus, greater neuronal excitation leads to the consumption of more ATP, and mitochondrial overload can occur because of the demand for ATP production (Clarke and Sokoloff, 1999; Dienel, 2009). Therefore, the region of the brain where continuous neuronal firing occurs like SN can be a potential locus of neuronal damage. In fact, dopaminergic neurons possess a pace-making activity of 2 to 4 Hz (Grace and Bunney, 1983) that maintains a proper concentration of dopamine (DA) in the striatum (Romo and Schultz, 1990). Dopaminergic neurons in the SN, therefore, are constantly exposed to mitochondrial oxidative stress (Venkateshappa et al., 2012). Although less than 1% of all oxygen that is consumed is a source of reactive oxygen species (ROS) under normal conditions, larger amounts of ROS are generated once mitochondrial dysfunction occurs; this, in turn, accelerates further mitochondrial damage (Zhang and Gutterman, 2007). Therefore, genetic defects affecting mitochondrial quality control can result in the early onset and rapid progression of this vicious cycle (Palacino et al., 2004; Clark et al., 2006; Andres-Mateos et al., 2007).
Another source of oxidative stress to dopaminergic neurons is DA itself. DA is auto-oxidized to form dopamine quinone (Graham et al., 1978) and ROS (Fridovich, 1975) when it is released from presynaptic terminals. Therefore, DA can also damage dopaminergic neurons. To avoid the production of dopamine quinone and ROS, DA is taken up immediately after synaptic release by DA transporters (DAT) expressed on the nerve terminals of dopaminergic neurons in the striatum (Wheeler et al., 1993; Torres et al., 2003). Importantly, however, DA-derived ROS can also affect cellular function intracellularly after transportation into the dopaminergic neurons by DA transporters (Lohr et al., 2014; Choi et al., 2015; Masoud et al., 2015). In contrast to dopaminergic neurons, glutamatergic neurons do not recycle glutamate (Glu) directly. Although Glu itself does not produce ROS, Glu can cause excitatory cell death unless Glu exists in the synaptic cleft after it is released from presynaptic glutamatergic neurons. Glu is taken up mainly by astroglia that envelope synapses and is recycled back to neurons as glutamine (Danbolt, 2001; Zhou and Danbolt, 2014). Astroglia express sodium-dependent Glu transporters (GLT-1 or GLAST) that enable the immediate clearance of Glu via an inward sodium gradient in astroglia that is, in turn, generated by astroglial Na+,K+-ATPase (Pines et al., 1992; Storck et al., 1992).
Although the means by which ATP is generated in astroglia with regard to glucose metabolism has not yet been elucidated, glycolytic metabolism could play a major role (Robinson et al., 1998). In fact, exposure to Glu at concentrations of 500 to 1,000 µM, which are similar to those in the synaptic cleft in vivo, enhanced astroglial glucose utilization in vitro (Pellerin and Magistretti, 1994; Takahashi et al., 1995). The huge amount of lactate that is released from astroglia upon exposure to Glu can be interpreted as evidence supporting the hypothesis that Glu-induced ATP production is mostly dependent on glycolysis (Pellerin and Magistretti, 1994; Takahashi et al., 1995). Early in vitro studies have reported that DA induces a similar phenomenon in cultured astroglia (Semenoff and Kimelberg, 1985). Namely, DA induces the Na+-dependent accumulation of DA in astroglia and increases glucose utilization. The astroglial uptake of DA was later found to be more dependent on norepinephrine transporter (NET) or serotonin transporter (SERT) than on DAT (Dave and Kimelberg, 1994; Inazu et al., 2001; Takeda et al., 2002; Inazu et al., 2003). Although at least three different Na+-dependent monoamine transporters can be involved in the uptake of DA by astroglia (Nishijima and Tomiyama, 2016), a recent study has revealed that murine striatal astroglia express DAT in vivo (Asanuma et al., 2014). To the contrary, the generation of DAT-Cre mice has allowed confirming that only DA neurons in the mouse brain turn on the DAT gene (Zhuang et al., 2005; Turiault et al., 2007).
Glu uptake induces increase in glucose utilization and glycolytic activation (Dienel, 2009). We previously reported that cultured astroglia are more glycolytic than neurons (Abe et al., 2006a), and active glycolytic metabolism in astroglia is related to flux in the pentose-phosphate pathway (PPP) that branches during the middle of glycolysis, producing NADPH. NADPH is utilized to produce a reduced form of glutathione (GSH), which is consumed by glutathione peroxidase to eliminate ROS and to form an oxidized form of glutathione (GSSG). Therefore, astroglia may play a protective role against ROS-induced neurotoxicity if Glu and DA enhance glycolysis through a Na+-dependent transporter. These different types of neurons (i.e., dopaminergic and glutamatergic) may be the target of a protective function provided by astroglia.
Several reports have indicated that astroglial protection against oxidative stress may play important roles against the initiation and progression of PD (Chen et al., 2009; Asanuma et al., 2010; Lastres-Becker et al., 2012). To our knowledge, however, only a few reports have addressed DA-induced metabolic changes and the possible protective function of astroglia induced by DA. Both DA and Glu can induce the astroglial activation of glycolysis through Na+-dependent transporters and PPP activation. Of note, DA generates ROS, while Glu does not. Therefore, astroglia respond to DA and Glu in different manners. In fact, astroglia can respond to ROS through the Kelch-like ECH-associated protein 1 (Keap1) or nuclear factor-erythroid-2-related factor 2 (Nrf2) system, by which ROS induces astroglial protection through PPP activation, since the rate-limiting enzyme of PPP, glucose-6 phosphate dehydrogenase (G6PDH), is transcriptionally regulated by Nrf2 (Thimmulappa et al., 2002; Lee et al., 2003).
In this study, we examined the effects of acute and chronic exposure to DA and Glu on glucose metabolism and ROS production using cultured astroglia. We found that DA can induce astroglial PPP activation directly through ROS derived from DA and that both acute and chronic exposure to DA can induce PPP activation without necessity of Na+-dependent monoamine transporters. Chronic exposure to DA induced cell damage only in the absence of astroglia. Astroglia cocultured with neurons, however, protected neurons through the Nrf2-dependent mechanism.
Materials and Methods
Animals
Timed-pregnant Sprague-Dawley rats were obtained from Japan SLC, Inc. (Hamamatsu, Japan). Male C57BL/6 (wild-type [WT]) mice (Japan SLC, Inc.) and Nrf2 gene knockout (KO) mice (on C57BL/6 background, originally generated by Dr. Masayuki Yamamoto) were used in this study. Nrf2-KO mice were genotyped by polymerase chain reaction (PCR) amplification of genomic DNA extracted from tail snips. All the animal procedures were performed in accordance with “The Animal Experimentation Guidelines” of Keio University School of Medicine and were approved by the Committee on Animal Care and Use, Keio University.
Chemicals
Chemicals and materials were obtained from the following sources: 2-deoxy-D-[1-14C]glucose ([14C]deoxyglucose; specific activity, 2.13 GBq/mmol), Insta-Fluor Plus, and hyamine hydroxide 10-X were obtained from Perkin-Elmer Life Sciences (Boston, MA, USA); D-[1-14C]glucose (specific activity, 2.035 GBq/mmol) and D-[6-14C]glucose (specific activity, 2.035 GBq/mmol) were obtained from American Radiolabeled Chemicals, Inc. (St. Louis, MO, USA); normal goat serum was obtained from Jackson ImmunoResearch (West Grove, PA, USA); rabbit anti-DAT antibody and rabbit anti-NET were obtained from Alomone Labs (Jerusalem, Israel); mouse anti-SERT antibody was obtained from Chemicon International (Temecula, CA, USA); mouse monoclonal anti-glial fibrillary acidic protein (GFAP) was obtained from Sigma (St. Louis, MO, USA); rabbit anti-GFAP antibody was obtained from DAKO (Glostrup, Denmark); rhodamine-conjugated goat anti-mouse (for GFAP), anti-rabbit (for GFAP) IgG antibody, and fluorescein-isothiocyanate-conjugated goat anti-rabbit (for DAT and NET) and anti-mouse (for SERT) antibody were obtained from Santa Cruz Biochemistry (Delaware, CA, USA); Dulbecco’s modified Eagle media (DMEM) with or without glucose, penicillin, and streptomycin were obtained from Life Technologies (Grand Island, NY, USA); defined fetal bovine serum was obtained from HyClone Laboratories (Logan, UT, USA); and 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA) and monochlorobimane (MCB) were obtained from Molecular Probe Inc. (Eugene, OR, USA); and alamarBlue was obtained from Thermo Fisher Scientific (Waltham, MA, USA). All other chemicals were obtained from Sigma (St. Louis, MO, USA).
Preparation of Cells
Primary astroglial cultures were prepared from either the cerebral cortex of rats or mice at 24 to 48 hr after birth (Takahashi et al., 1995) or the striatum of fetal rats on embryonic Day 16 (Takahashi et al., 2000; Abe et al., 2006b). The dissociated cells from the cerebral cortices (2.5 × 105 cells/mL for rat and 5.0 × 105 cells/mL for mouse) or striata (5.0 × 105 cells/mL) were plated (15 mL/flask) in uncoated 75-cm2 culture flasks (Sumitomo Bakelite, Tokyo, Japan) and cultured in a glucose-containing medium (final concentration, 12 mmol/L of D-glucose) composed of DMEM with 10% (v/v) fetal bovine serum, penicillin (100 U/mL), and streptomycin (100 µg/mL) at 37°C in humidified air containing 21% O2 and 7% CO2 (Day 0). The culture medium was changed every 2 days until the cultures reached confluence. On Day 11, the adherent cells were treated with trypsin-EDTA solution, suspended in fresh high (12 mmol/L)-glucose medium and placed in uncoated 12-well culture plates (1.0 mL/well, respectively; Nalge Nunc, Rochester, NY, USA) or in 25-cm2 culture flasks (5 mL/flask; Nalge Nunc) or in 12-well cell culture inserts (0.25 mL/insert well; Greiner Bio-One International GmbH, Kremsmünster, Austria). For immunohistochemical staining, the cells were plated (2 mL/dish) on 35-mm glass-bottomed dishes (Matsunami Glass, Industry, Osaka, Japan) precoated with poly-L-lysine (5 µg/mL). Some cells were grown on glass-bottomed 24-well culture plates (EZView culture plate LB, Iwaki, Tokyo Japan) for fluorometric assay. From the day after subculturing, the cells were cultured in DMEM, fetal bovine serum, penicillin, and streptomycin and a final D-glucose concentration of 12 mmol/L for 10 days. The culture medium was changed twice a week, and the cells were used once they had reached confluence (on Day 21 in vitro).
The primary neuronal cultures were prepared from the striatum of fetal rats on embryonic Day 16 and mice on embryonic Day 14, as described previously (Takahashi et al., 1995). Mechanically dissociated cells were placed in 12-well culture plates (1.0 mL/well, respectively) or 25-cm2 culture flasks (5 mL/flask) coated with poly-L-lysine (5 µg/mL). For the neuronal cultures, viable cells (1.5 × 106 cells per mL) that excluded Trypan blue were placed in the cultures, and cytosine arabinoside (10 µmol/L) was added 72 hr later to induce the mitotic arrest of the astroglia. The cells were cultured in a glucose medium (final concentration, 12 mmol/L D-glucose) at 37°C in humidified air containing 21% O2 and 7% CO2. In vitro assays were performed using cultures that were 7 or 8 days old for rat neurons and 5 days for mouse neurons. The nutrient medium remained untouched until the experiments were initiated.
Two different models of neuronal-astroglial coculture were used. When neurons were grown on astroglia in 25-cm2 culture flasks, neuronal cells were seeded on the astroglial cell layer on culture Day 21, and cytosine arabinoside was added 72 hr later. When neurons were seeded on poly-L-lysine-coated 12-well culture plate (Greiner Bio-One International GmbH, Kremsmünster, Austria), astroglia that had been grown on 12-well cell culture inserts (Greiner Bio-One International GmbH, Kremsmünster, Austria) were placed on the neuronal wells on Day 21 and cytosine arabinoside was added 60 to 72 hr later. After exposure to DA, neuronal cell viability can be evaluated separately by removing astroglial cell culture inserts.
Experimental Protocol
To assess the effects of DA or Glu on astroglial glucose metabolism, ROS production, and immunohistochemistry, the nutrient medium (12 mmol/L glucose concentration) was removed and fresh nutrient medium containing DA (0, 10, or 100 µmol/L) or Glu (0, 100, or 500 µmol/L) was added, and the cultures were kept in a CO2 incubator for 15 hr. As for the neuronal cultures, because medium changes per se can damage cells through Glu toxicity (Driscoll et al., 1993), DA and Glu were added directly to the culture media of the cells so as to reach the final concentrations described earlier. For acute exposure to DA, Glu, or hydrogen peroxide (H2O2), the drugs were added directly to the 14C assay medium. For inhibitors of Na+-dependent monoamine transporters (GBR12909 [10 µmol/L] for DAT [Miyazaki et al., 2011], desipramine [1 µmol/L] for NET [Takeda et al., 2002], and fluoxetine [10 µmol/L] for SERT [Inazu et al., 2001]), the cells were exposed to culture media containing the appropriate inhibitors for 30 to 120 min in advance; the inhibitors were also added to the 14C assay medium.
For the assessment of neuronal damage induced by DA exposure, neurons or neurons grown on astroglial cell layer were exposed to DA (100 µmol/L) for 30 to 48 hr. Total oxidation of [6-14C]glucose, which reflects the tricarboxylic acid (TCA) cycle metabolism, was measured and subtracted by astroglial [6-14C]glucose oxidation. Since 14CO2 production from [6-14C]glucose is almost exclusively from cultured neurons (Takahashi et al., 2012), neuronal damage can be detected sensitively. Some neurons were exposed to DA with or without 12-well cell culture inserts where astroglia had been grown. After removal of astroglial inserts, neuronal viability was measured using alamarBlue reduction assay (Abe et al., 2002).
Measurement of the Rate of D-Glucose Consumption and L-Lactate Production
The rates of glucose consumption and lactate production were determined by measuring the changes in glucose and lactate concentrations in culture media after standardization using the protein contents of the wells. The lactate concentration was measured based on the colorimetric reaction of N-ethyl-N-(3-methylphenyl)-N′-acetylethylenediamine using the lactate oxidase-peroxidase method (Shimojo et al., 1991). The rates of glucose consumption and lactate production were normalized according to the cellular protein content of each well.
Measurement of the Rate of 2-Deoxy-D-[1-14C]Glucose Phosphorylation
The rates of glucose phosphorylation in astroglia were evaluated using a modification (Smith et al., 1985; Takahashi et al., 1995) of the [14C]deoxyglucose method (Sokoloff et al., 1977). Cells were cultured in a 12-well culture plate and then washed twice with phosphate-buffered saline (PBS) containing no glucose. An assay solution consisting of Dulbecco’s balanced salt solution (DBSS) containing 110 mmol/L NaCl, 5.4 mmol/L KCl, 1.8 mmol/L CaCl2, 0.8 mmol/L MgSO4, 0.9 mmol/L NaH2PO4, and 44 mmol/L NaHCO3 in addition to 2 mmol/L of D-glucose and DA (0, 10, 100 µmol/L) labeled with 12.5 µL/mL of [14C]deoxyglucose (original concentration: 3.7 MBq/mL) was added to each assay solution, and incubation was continued for 60 min at 37°C in 7% CO2. At the end of the incubation period, the DBSS was replaced with a fresh reaction mixture lacking [14C]deoxyglucose, and incubation was continued at 37°C in 7% CO2 for 5 min to allow the efflux of the residual [14C]deoxyglucose from the cells (Takahashi et al., 1995; Abe et al., 2006a). This procedure allows the evaluation of glucose phosphorylation activity instead of simple glucose uptake activity. The cell carpets were washed quickly three times with ice-cold PBS and were digested in 1.0 mL of 0.1 mM NaOH at room temperature. The cell digests were then assayed for protein content using the bicinchoninic acid method (Smith et al., 1985), and the 14C count was measured using a liquid scintillation counter (Tri-Carb 3100TR; Perkin-Elmer Life Sciences, Boston, MA, USA). The rates of glucose phosphorylation (pmol glucose/µg protein/60 min) based on the conversion from [14C]deoxyglucose phosphorylation over 60 min were then calculated from the specific activity of [14C]deoxyglucose (i.e., mBq [14C]deoxyglucose/mmol glucose) in the reaction mixture.
Measurement of the Rate of D-[1-14C]Glucose and D-[6-14C]Glucose Oxidation to 14CO2
The rate of [14C]glucose oxidation to 14CO2 was measured using a modification of a previously described method (Abe et al., 2006a). Cells cultured in 25-cm2 culture flasks were washed twice with PBS containing no glucose. Assay solutions consisting of DBSS in addition to 2 mmol/L of D-glucose with DA (0, 10, 100 µmol/L), Glu (0, 100, or 500 µmol/L), or H2O2 (0, 10, 100 µmol/L) labeled with 1.0 µL/mL of D-[1-14C]glucose or D-[6-14C]glucose (original concentrations: 3.7 MBq/mL) were added, and the flasks were capped with rubber stoppers containing a center well and incubated at 37°C for 60 min. When Na+-free assay medium was used, the Na+ in DBSS was substituted with choline chloride. The 14CO2 that was produced was trapped using a cotton ball placed in the center well containing 100 µL of hyamine hydroxide 10-X. The reactions were terminated by the injection of 250 µL of 60% perchloric acid through the rubber stopper, and the flasks were kept at 4°C overnight to trap the 14CO2. The center wells were then transferred to 20-mL glass scintillation counter vials, and 500 µL of ethanol and 10 mL of Insta-Fluor Plus were added. The 14C contents of the vials were evaluated using a liquid scintillation counter. The assay solutions consisted of 2.5 mL of DBSS containing 2 mmol/L D-glucose; these solutions were labeled by adding 1.0 µL/mL of D-[1-14C]glucose or D-[6-14C]glucose (original concentrations: 3.7 MBq/mL).
Waniewski and Martin (2004) reported that substantial 14C counts were obtained from a flask without cells. Therefore, the 14C count obtained from a flask without cells in which the reaction had been stopped at 60 min was regarded as the background value and was subtracted in our studies. The cell carpets remaining in the incubation flasks after the removal of the reaction mixtures were then digested with 5 mL of 0.1 mol/L NaOH, and their protein contents were determined. The rates of total glucose oxidation (pmol glucose/µg protein/60 min) based on the conversion from [14C]glucose to 14CO2 over 60 min were measured.
Measurement of PPP Activity in Cultured Astroglia and Neurons
PPP activity was measured using a modification of the method described by Hothersall et al. (1979). Briefly, cells were incubated with glucose with tracer doses of [1-14C]glucose or [6-14C]glucose for 60 min. The PPP rate was calculated as the difference between the 14CO2 derived from [1-14C]glucose (metabolized by both PPP and the TCA cycle), while the value derived from [6-14C]glucose (metabolized by only the TCA cycle) was regarded as an indicator of PPP activity(Takahashi et al., 2012).
Measurement of ROS Production
The production of ROS (mainly H2O2) in cells was assessed using H2DCFDA (Suematsu et al., 1993; Gomes et al., 2005) and semiquantitative fluorometric measurements. Just prior to the assay, the nutrient medium was removed, and the cells were washed twice with PBS without glucose. Then, DBSS containing 10 µmol/L of H2DCFDA dissolved in dimethyl sulfoxide (final volume of dimethyl sulfoxide: 0.1%) supplemented with 2 mmol/L glucose was added, and the cells were incubated at 37°C in humidified air with 7% CO2 for 30 min. After loading the H2DCFDA for 30 min, the cells were washed twice again with PBS without glucose and DBSS containing 2 mmol/L D-glucose. The cells were further incubated for 60 min, and the fluorescent level indicating intracellular ROS production was measured at 0 and 60 min using a fluorescent microplate reader (Infinite 200 PRO; Tecan Japan, Kanagawa, Japan) with excitation at 485 nm and emission at 530 nm. As the fluorescent signals increased linearly for up to 60 min (data not shown), the results were expressed as the percent increase in the fluorescent signal at 60 min compared with that at 0 min.
Measurement of Total Glutathione Content Using a Fluorometric Method
The intracellular content of total glutathione in astroglia was assessed using MCB, which forms an adduct with glutathione via an enzymatic reaction catalyzed by glutathione transferases (Chatterjee et al., 1999). Cells were incubated with MCB (50 μmol/L) for 30 min. The intracellular formation of the GSH–MCB adduct was then assessed using a microplate reader with excitation at 360 nm and emission at 465 nm. The results were expressed as the fluorescence signal standardized by the cellular protein content.
Assessment of Neuronal Damage Induced by DA Using alamarBlue Assay
alamarBlue is a redox indicator that exhibits both fluorescence and colorimetric change in response to metabolic activity (Abe et al., 2002). alamarBlue is supposed to be reduced at a site in mitochondrial respiration and the redox potential of alamarBlue allows for the respiratory chain to function to near completion, which will provide a more accurate and sensitive indication of mitochondrial function (Abe et al., 2002). After removal of nutrient medium from the 12-well culture plate, cells were washed once with 1.0 ml PBS without glucose, then 1.0 ml DBSS with containing alamarBlue (10%) supplemented with 2 mmol/L glucose was added to each well and incubated for 60 min. Fluorescence level was measured at 0 min and 60 min using a fluorescent microplate reader (Infinite 200 PRO; Tecan Japan, Kanagawa, Japan) with excitation at 530 nm and emission at 590 nm. alamarBlue reduction activity was expressed by fluorescent value at 60 min over at 0 min after standardization by protein content of each well.
Immunohistochemistry
Astroglial cells that were grown on a glass-bottomed, 35-mm dish were fixed with 4% paraformaldehyde for 10 min on ice. Then, the cells were permeabilized with 0.5% Triton X-100 for 10 min at room temperature, followed by postfixation with 4% paraformaldehyde for 5 min on ice. For immunostaining, the cells were washed with PBS containing MgCl2 and CaCl2 twice, and nonspecific IgG binding sites were blocked by incubating the cells in PBS containing 3% bovine serum albumin and 3% normal goat serum for 30 min at room temperature. Cells were incubated with the primary antibodies (mouse anti-DAT [1:100; Alomone Labs, #AMT-003], anti-NET [1:100; Alomone Labs, #AMT-002], or rabbit ant-SERT [1:200; Chemicon International, MAB1564] antibody, mouse anti-GFAP [1:200; Sigma-Aldrich, #G3893], or rabbit anti-GFAP [1:200; DAKO, #Z0334]) for 2 hr at room temperature. Then, the cells were incubated with the secondary antibodies (rhodamine-conjugated goat anti-rabbit or anti-mouse IgG antibody or fluorescein-isothiocyanate-conjugated goat anti-mouse IgG antibody; 1:200) with 4′,6-diamino-2-phenylindole (1:1,000) for nuclear staining for 1 hr. The cells were examined using a laser confocal microscopy system (Leica TCS SP5; Leica Microsystems, Exton, PA, USA).
Evaluation of Nrf2 Activation by Quantitative Reverse Transcription PCR Analysis for mRNA of Heme Oxygenase-1
Total RNA from cultured cells was isolated using an RNeasy mini kit (Qiagen, Valencia, CA) with DNase (Qiagen, Valencia, CA) treatment. First-stranded cDNA was synthesized using a PrimeScript RT reagent Kit (TaKaRa) according to the manufacturer’s instructions. Quantitative reverse transcription PCR (qRT-PCR) was performed using predesigned gene-specific TaqMan Gene Expression Assays (Applied Biosystems) with the StepOnePlus real-time PCR system (Applied Biosystems). The rat heme oxygenase-1 (HO-1) TaqMan probe and primers (cat# Rn00561387_m1) and the rat glyceraldehyde 3-phosphate dehydrogenase TaqMan probe and primers (cat# Rn01775763_g1) were obtained from Applied Biosystems. The housekeeping gene glyceraldehyde 3-phosphate dehydrogenase was used for normalization. The fold difference between the levels of transcripts was calculated using the ΔΔCT method.
Statistical Analyses
Statistical comparisons among the values obtained for each group were performed using grouped t tests or a one-way analysis of variance followed by the Dunnett test or Tukey test for multiple comparisons. A p value of <.05 was considered statistically significant.
Results
Secondary Astroglial Cultures Obtained From Sprague-Dawley Rats Express DAT, NET, and SERT
Earlier studies have reported that cultured astroglia express several Na+-dependent monoamine transporters. As shown in Figure 1, cultured astroglia prepared from newborn Sprague-Dawley rat cortex (Figure 1(a), (c), and (e)) and embryonic striatum (Figure 1(b), (d), and (f)) showed DAT (Figure 1(a) and (b)) and NET (Figure 1(c) and (d)) immunoreactivity. However, SERT (Figure (e) and (f)) immunoreactivity was weak.
Figure 1.
Expression of dopamine transporter (DAT), norepinephrine transporter (NET), and serotonin transporter (SERT) in astroglial cultures prepared from Sprague-Dawley newborn cortex (a), (c), (e) or embryonic striatum (b), (d), (f). Confocal laser-scanning microscopic images showing double immunostaining for DAT (fluorescein-isothiocyanate, green) and GFAP (rhodamine, red) with nuclear staining (4′,6-diamino-2-phenylindole, blue; (a) and (b)), NET (fluorescein-isothiocyanate, green) and GFAP (rhodamine, red) with nuclear staining (4′,6-diamino-2-phenylindole, blue; (c) and (d)), or SERT (fluorescein-isothiocyanate, green) and GFAP (rhodamine, red) with nuclear staining (4′,6-diamino-2-phenylindole, blue; (e) and (f)) in cortical or striatal astroglia prepared from Sprague-Dawley rats. Scale bar = 20 µm.
Acute Exposure to DA Induced Increases in Glucose Utilization and Lactate Production
To mimic the phasic release of DA in vivo, we examined the effects of acute exposure (60 min) to DA on glucose metabolism in cortical astroglia. Figure 2(a) shows the effects of DA on [14C]deoxyglucose phosphorylation (an index of glucose utilization), and Figure 2(b) shows lactate released in the medium (an index of lactate production). As shown in Figure 2(a), DA at a concentration of 100 µmol/L induced significant increases in glucose utilization. In addition, lactate production, as shown in Figure 2(b), was also significantly increased by both 10 and 100 µmol/L of DA.
Figure 2.
Effect of acute exposure to dopamine (DA) on glucose consumption and lactate production in cortical astroglia. Glucose consumption (pmol/µg protein/60 min) was not significantly altered by 10 µmol/L of DA (123.3 ± 6.4, 108%) but was increased significantly by 100 µmol/L of DA (130.4 ± 11.5, 115%) after 60 min. Values are the mean ± SD (n = 4; (a)). Lactate production (pmol/µg protein/60 min) was increased significantly by 10 µmol/L (1094.9 ± 112.3, 117%) and 100 µmol/L of DA (1580.7 ± 127.5, 169%) after 60 min. Values are the mean ± SD (n = 6). *p < .05, ***p < .001 (analysis of variance followed by Dunnett test; (b)). ns = not significant; DA = dopamine.
Acute Exposure to DA and Glu Induced PPP Activation in Both Cortical and Striatal Astroglia
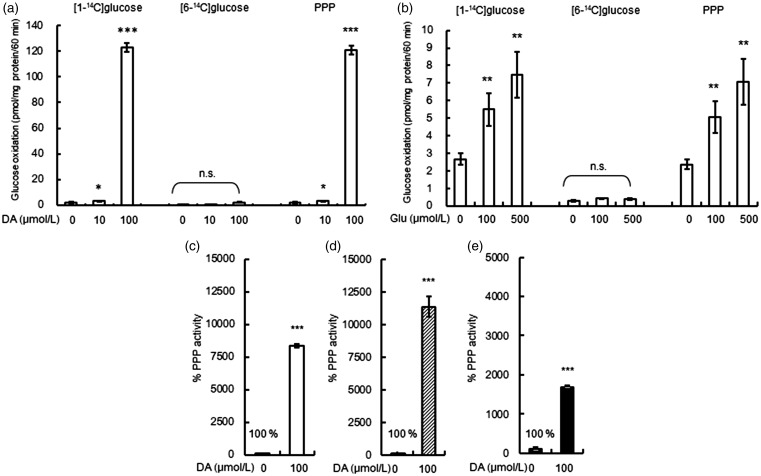
Since DA exposure to astroglia induced glycolytic metabolism in cortical astroglia, we measured the PPP flux from glycolysis during acute exposure to DA (10 and 100 µmol/L). Figure 3(a) shows the DA-induced alteration in the rates of oxidation of [1-14C]glucose and [6-14C]glucose in cortical astroglia. The difference between [1-14C]glucose and [6-14C]glucose indicates the PPP flux, revealing marked increases in PPP flux in astroglia treated with 100 µmol/L of DA for 60 min. Compared with DA, acute exposure to Glu (100 and 500 µmol/L) induced smaller increases in PPP flux, as shown in Figure 3(b).
Figure 3.
Effect of acute exposure to dopamine (DA) or glutamate (Glu) on pentose-phosphate pathway (PPP) flux. The difference in the rates of 14CO2 oxidation (pmol/µg protein/60 min) of [1-14C] glucose and [6-14C] glucose indicates the PPP flux. The rate of the PPP flux in cortical astroglia was increased significantly by both 10 µmol/L (2.9 ± 0.4, 166%) and by 100 µmol/L (120.7 ± 3.4, 6873%) of DA after 60 min (a). The rate of the PPP activity in astroglia was increased significantly by both 100 µmol/L (5.1 ± 0.9, 212%) and by 500 µmol/L of Glu (7.1 ± 1.3, 299%) after 60 min (b). Values are the mean ± SD of four flasks. *p < .05, **p < .01, *** p < .001 (analysis of variance followed by Dunnett test for multiple comparisons). The percentage changes in the PPP flux by 100 µmol/L of DA for 60 min based on the control values are shown. Exposure to DA for 60 min induced marked PPP activation in striatal astroglia (11463%; (d)), compared with cortical astroglia (8235%; (c)). The activation of the PPP flux in the neurons was relatively weak (1665%; (e)). Values are the mean ± SD of four flasks. *p < 0.05, **p < .01, ***p < .001 versus control (grouped t test). ns = not significant; PPP = pentose-phosphate pathway; DA = dopamine.
To compare the PPP flux induced by acute exposure to 100 µmol/L of DA in cortical and striatal astroglia as well as striatal neurons, the percentage changes compared with the control values for PPP flux are shown in Figure 3(c) to (e). Both cortical (Figure 3(c)) and striatal (Figure 3(d)) astroglia showed marked elevations of PPP flux, while the neuronal PPP (Figure 3(e)) showed a smaller elevation. To confirm that these marked increases in PPP flux induced by DA were genuine cellular biological responses, we measured 14CO2 derived from [1-14C]glucose in a flask without cells. 14CO2 production by DA was negligible when cells were absent, confirming that DA activates a cellular biological response, and not just a chemical reaction, between 14C-labeled glucose and DA (data not shown).
Acute Exposure to DA Induced PPP Activation Independent of Na+-Dependent Monoamine Transporters in Striatal Astroglia
DA-induced robust increases in PPP flux in both cortical and striatal astroglia. We speculated that these increases were passive increases in PPP flux resulting from increased glucose consumption, since DA uptake needs ATP generated by glucose metabolism. Thus, we evaluated which Na+-dependent monoamine transporters (DAT, NET, or SERT) are responsible. It has been reported that DA uptake by astroglia is dependent on these transporters (Dave and Kimelberg, 1994; Inazu et al., 2001; Takeda et al., 2002; Inazu et al., 2003). The simultaneous application of three inhibitors at the concentrations in this study suppressed [3H]DA uptake significantly by our cultured astroglia (data not shown). Figure 4 shows the effects of the simultaneous inhibition of these three transporters that are known to be expressed in cultured astroglia. The PPP activation in cortical astroglia induced by DA seemed to be inhibited by the inhibitors, as expected (Figure 4(a)). Importantly, however, basal glucose oxidation increased in the presence of the antagonists alone and this might have affected the results. Surprisingly, however, even after the inhibition of these three transporters, PPP flux induced by DA was not affected in striatal astroglia (Figure 4(b)). A possible explanation is that DA-derived ROS in extracellular space activated PPP flux directly (see later).
Figure 4.
Effects of the simultaneous inhibition of three Na+-dependent monoamine transporters (DAT, NET, and SERT) on dopamine (DA)-induced increases in pentose-phosphate pathway (PPP) flux in cortical (a) and striatal (b) astroglia. Values are the mean ± SD of four flasks. *p < .05 (analysis of variance followed by Tukey test for multiple comparisons). DA = dopamine; ns = not significant.
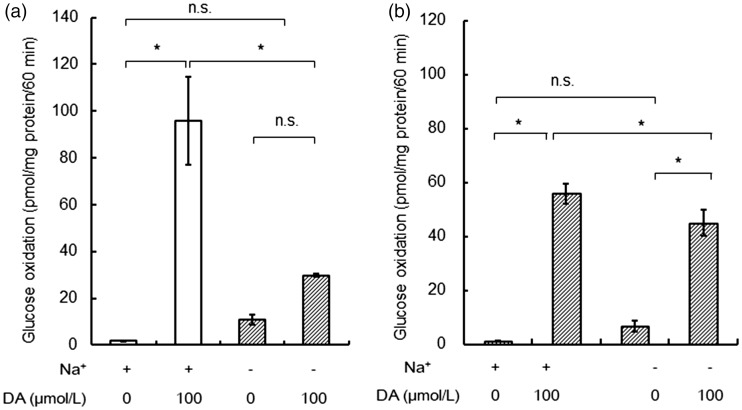
Na+-Depletion in the Assay Solution Reduced DA-Induced PPP Flux but Not H2O2-Induced PPP Flux in Astroglia
Next, we examined the effect of Na+ depletion from the assay solution. DAT, NET, and SERT are known to take up DA in a Na+-dependent manner. Interestingly, Na+-depletion suppressed, but did not abolish, DA-induced increases in PPP flux both in cortical (Figure 5(a)) and striatal (Figure 5(b)) astroglia. Since the increases in PPP flux induced by DA in cortical astroglia were inhibited by Na+-dependent transporters (Figure 4(a)), these results seem to be reasonable. However, Na+ depletion did not eliminate DA-induced PPP activation completely, suggesting the involvement of a mechanism other than Na+-dependent DA uptake.
Figure 5.
Effects of Na+-depletion from the assay solution on dopamine (DA)-induced increases in pentose-phosphate pathway (PPP) flux in cortical (a) and striatal (b) astroglia. Values are the mean ± SD of four flasks. **p < .05 (analysis of variance followed by Tukey test for multiple comparisons). DA = dopamine; ns = not significant.
DA May Induce an Increased PPP Flux Directly Through ROS Production
We evaluated other mechanisms by which DA induces robust increases in PPP flux without the involvement of Na+-dependent monoamine transporters. DA is known to release ROS through auto-oxidation, and ROS can penetrate the cell membrane without requiring a specific transporter. Inside the cell, ROS accelerates the consumption of NADPH, and the change in the REDOX state induces the direct activation of PPP flux. First, we evaluated the effect of H2O2 on PPP flux in astroglia. As shown in Figure 6, H2O2 induced robust increases in PPP flux in both cortical (Figure 6(a)) and striatal (Figure 6(b)) astroglia, respectively. Second, we evaluated the effect of Na+ depletion on these H2O2-induced increases in PPP flux. Figure 6 shows that Na+-depletion did not affect H2O2-induced increases in PPP flux both in cortical (Figure 6(a)) and striatal (Figure 6(b)) astroglia, supporting that H2O2 that penetrates cell membrane without specific transporters can activated PPP flux. These results implicate that part of the DA-induced PPP activation is not dependent on transporters, since DA can generate ROS both intracellularly and extracellularly.
Figure 6.
Effect of hydrogen peroxide (H2O2) on pentose-phosphate pathway (PPP) flux in cortical (a) and striatal (b) astroglia. Values are the mean ± SD of four flasks. *p < .05 (analysis of variance followed by Tukey test for multiple comparisons). H2O2 = hydrogen peroxide; ns = not significant.
Chronic Exposure to DA Induced PPP Activation in Both Cortical and Striatal astroglia, While Glu Did Not
To mimic the tonic release of DA in vivo, we examined the effects of chronic exposure (15 hr) to DA on the PPP flux in cortical astroglia. As shown in Figure 7(a), PPP flux in cortical astroglia increased after 15 hr of exposure to DA (10 and 100 µmol/L) in a dose-dependent manner. Compared with DA, chronic exposure to Glu (100 and 500 µmol/L) did not alter PPP flux in astroglia, as shown in Figure 7(b).
Figure 7.
Effects of chronic exposure to dopamine (DA) or glutamate (Glu) on pentose-phosphate pathway (PPP) activities. The difference in the rates of 14CO2 oxidation (pmol/µg protein/60 min) of [1-14C] glucose and [6-14C] glucose indicates the PPP flux. The rate of the PPP activity in astroglia was increased by 10 µmol/L (2.0 ± 0.1, 118%) and by 100 µM (2.7 ± 0.1, 163%) of DA after 15 hr (a). The rate of the PPP activity was not affected by either 100 µM (3.1 ± 0.3, 111%) or 500 µmol/L (3.0 ± 0.4, 107%) of Glu after 15 hr (b). Values are the mean ± SD of four flasks. *p < .05, **p < .01 (analysis of variance followed by Dunnett test). The percentage changes of the PPP flux for 60 min after 15 hr of exposure to 100 µmol/L of DA based on the control values are shown. Exposure to DA for 15 hr induced marked PPP activation in the striatal astroglia (225%; (d)), compared with the cortical astroglia (159%; (c)). The activation of the PPP flux in the neurons was weak (115%; (e)). Values are the mean ± SD of four flasks. *p < .05, ***p < .001 versus control (grouped t test). PPP = pentose-phosphate pathway; ns = not significant; DA = dopamine.
To compare the PPP flux induced by chronic exposure to 100 µmol/L of DA in cortical and striatal astroglia as well as striatal neurons, the percentage changes compared with the control values of PPP fluxes are shown in Figure 7(c) to (e). Both cortical (Figure 7(c)) and striatal (Figure 7(d)) astroglia showed significant elevations of PPP flux, while neuronal PPP (Figure 7(e)) showed no change in the PPP flux.
DA Exposure to Astroglia Enhanced the Transcription of HO-1
To explore the possible involvement of Nrf2 in the mechanism by which chronic exposure to DA induced PPP activation, we measured the transcriptional activity of the HO-1 gene, which is a well-known gene under the regulation of the Keap1 or Nrf2 system, using qRT-PCR analysis. Both in cortical (Figure 8(a)) and striatal (Figure 8(b)) astroglia, HO-1 expression was markedly increased after DA exposure for 12 hr, while Glu exposure (100 or 500 µmol/L) did not induce augmentation of HO-1 transcription, as expected (Figure 8(c)). To further clarify a possible involvement of DA transporters, the effects of inhibiting monoamine transporters on HO-1 transcription in cortical astroglia by DA were examined (Figure 8(d)). DA-induced augmentation of HO-1 transcription was not affected by combination of three inhibitors of monoamine transporters (DAT, NET, and SERT), suggesting that ROS production from DA in the extracellular space is sufficient.
Figure 8.
Effect of chronic exposure to dopamine (DA) and glutamate (Glu) on transcriptional activity of heme oxygenase-1 (HO-1) in cortical and striatal astroglia. The mRNA expression of HO-1 in cortical (a) and striatal (b) astroglia after exposure to DA was analyzed using quantitative real-time polymerase chain reaction. The HO-1-fold change was calculated relative to each control (mean ± SD, n = 4). ***p < .001 versus each control (grouped t test). Effects of Glu exposure on HO-1 expression (c) and effects of monoamine transporter inhibitors on DA-induced activation of HO-1 expression were examined (d). The HO-1-fold change was calculated relative to each control (mean ± SD, n = 3). *p < .05 (analysis of variance followed by Tukey test for multiple comparisons). HO-1 = heme oxygenase-1; DA = dopamine; Glu = glutamate; ns = not significant.
ROS Production From DA and Glu in an In Vitro Cell-Free Environment
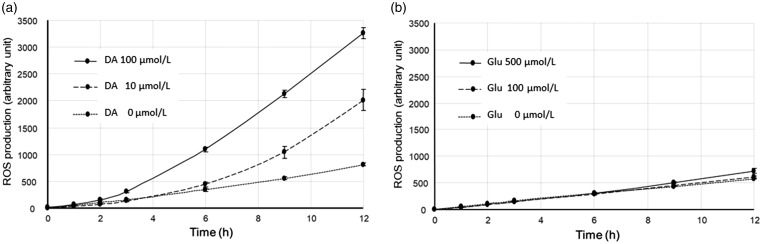
The auto-oxidation of DA has been postulated to induce ROS production, which plays detrimental roles in neuronal damage and in turn activates the Keap1 or Nrf2 system. We applied the dichlorofluorescein assay in an in vitro cell-free environment. DA induced the marked production of ROS from the culture medium without cells present (Figure 9(a)), while Glu did not (Figure 9(b)). These results indicate that DA could potentially trigger Nrf2 activation through ROS production.
Figure 9.
Production of reactive oxygen species (ROS) by dopamine (DA) and glutamate (Glu) in the absence of cells. The amount of ROS production by 10 and 100 µmol/L of DA increased gradually over 12 hr even in the absence of cells (a). In contrast, the amount of ROS production following exposure to 100 and 500 µmol/L of Glu was not different from that in the control (b). Values are the mean ± SD of four wells. ROS = reactive oxygen species; DA = dopamine; Glu = glutamate.
DA-Induced Suppression of ROS Production by Astroglia and DA-Induced Activation of GSH Synthesis
PPP activation can increase GSH and reduce oxidative stress. We examined the effects of both acute and chronic exposure to DA on ROS production by astroglia. As shown in Figure 10, both acute (Figure 10(a)) and chronic (Figure 10(b)) exposure to DA reduced ROS production in cortical astroglia significantly. Similar results were obtained in striatal astroglia (Figure 10(c) and (d)).
Figure 10.
Acute and chronic exposure to dopamine (DA) reduces reactive oxygen species (ROS) production rate in cortical and striatal astroglia. The ROS production rates (%) arising from different concentrations of DA (0, 10, 100 µmol/L) in the presence of cortical astroglia exposed to DA during 60-min measurement of ROS production are shown (111.2% ± 2.8%, 45.4% ± 4.3%, 35.3% ± 4.4%, respectively; (a)). The ROS production rates (%) in cortical astroglia exposed for 15 hr before the 60-min measurement of ROS production are also shown (123.3% ± 28.2%, 96.2% ± 15.0%, 96.5% ± 7.7%, respectively; (b)). The ROS production rates (%) arising from different concentrations of DA (0, 10, 100 µmol/L) in the presence of striatal astroglia exposed to DA during a 60-min measurement of ROS production are shown (124.4% ± 9.2%, 62.8% ± 4.5%, 70.4% ± 5.5%, respectively; (c)). The ROS production rates (%) in striatal astroglia exposed for 15 hr before the 60-min measurement of ROS production are also shown (149.8% ± 3.3%, 127.7% ± 4.1%, 115.9% ± 4.9%, respectively; (d)). Values are the mean ± SD of four wells. **p < .01 versus control (analysis of variance followed by Dunnett test for multiple comparisons). ROS = reactive oxygen species; DA = dopamine.
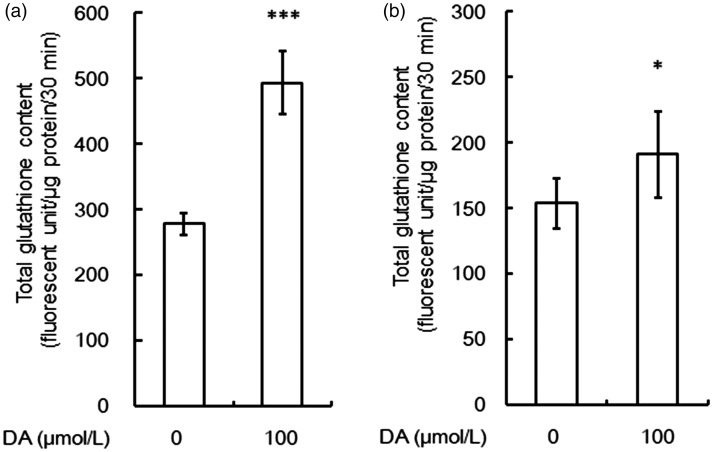
As for acute exposure to DA, increases in PPP flux can increase the GSH or GSSG ratio irrespective of the unaltered total amount of glutathione (GSH + GSSG). However, chronic exposure to DA can induce the de novo synthesis of glutathione, since the Keap1 or Nrf2 system that is activated by DA-derived ROS regulates glutathione synthesis (Lee et al., 2003). Figure 11 shows that the glutathione content, as determined using a fluorometric MCB assay, increased after 12 hr of exposure to DA.
Figure 11.
Effect of chronic exposure to dopamine (DA) on total glutathione contents in cortical (a) and striatal (b) astroglia. Values are the mean ± SD of four wells. *p < .05, ***p < .001 versus control (grouped t test). DA = dopamine.
DA-Induced Activation of Astroglial PPP Is Nrf-2 Dependent
To confirm the role of Nrf2 in the induction of PPP activation by DA exposure, PPP flux was measured using astroglial cells prepared from WT and Nrf2-KO mice. Both astroglia responded to acute exposure to DA by increasing PPP flux. However, chronic exposure to DA failed to activate PPP flux only in Nrf2-deficient astroglia (Figure 12).
Figure 12.
Effect of acute and chronic exposure to dopamine (DA) on pentose-phosphate pathway (PPP) flux in wild-type (WT) and Nrf2-knockout (KO) mice. The rate of the PPP flux in WT astroglia was increased significantly by 100 µmol/L of DA after 60 min (a) and 15 hr (b). In contrast, the rate of the PPP activity in Nrf2-KO astroglia increased significantly only acutely (a) and (b). Values are the mean ± SD of four flasks. *p < .05 (analysis of variance followed by Tukey test for multiple comparisons). DA = dopamine.
Astroglial Neuroprotective Effects Induced by DA
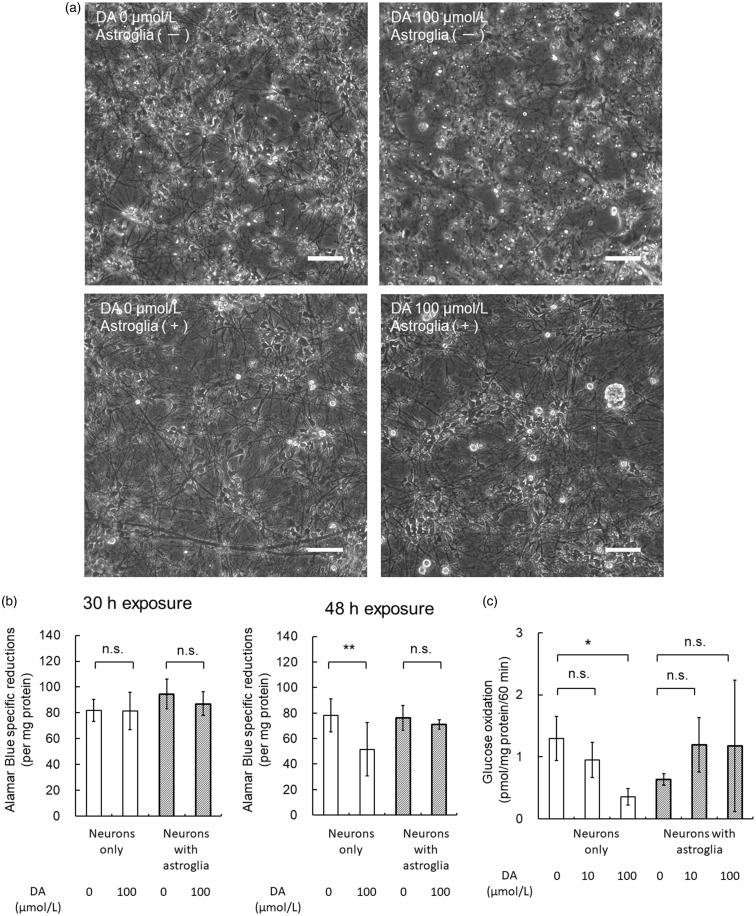
Finally, DA-induced neuroprotective effects provided by astroglia were evaluated using two models of neuronal-astroglial coculture (a) neurons grown on astroglial cell layer or (b) neurons cocultured with astroglial cell inserts. DA-exposure (100 µmol/L) for 30 hr did not induced changes in neuronal morphology (data not shown). After 48-hr exposure, however, damages of neuronal processes were evident when neurons were exposed to DA without astroglia (Figure 13(a)). In contrast, neurons cultured with astroglia and exposed to DA did not exhibit any morphological changes (Figure 13(a)). We assessed neuronal damage using alamarBlue assay. alamarBlue reduction activity in neurons without astroglia was decreased significantly at 48-hr exposure, while that in neurons with astroglia was not affected (Figure 13(b)). Moreover, neuronal [6-14C]glucose oxidation that reflects the TCA cycle metabolism was reduced significantly by 48-hr exposure to DA. In contrast, total neuronal-astroglial oxidation of [6-14C]glucose that reflects mainly neuronal TCA cycle metabolism remained unaltered after DA exposure after 40 hr (Figure 13(c)).
Figure 13.
Astroglia prepared from rat cortex provide neuroprotective effects against DA. Neurons cultured and exposed to DA (100 µmol/L) without astroglia exhibited morphological changes after 48 hr. In contrast, neurons that had been cultured on astroglial cell layer and exposed to DA were remained unaltered (a). Scale bar = 100 µm. Neuronal alamarBlue reduction activity was assessed after 30- or 48-hr exposure to DA (100 µmol/L) with or without rat astroglial cell inserts. alamarBlue reduction activities of neurons were unaltered regardless of the presence or absence of astroglial inserts at 30 hr. At 48 hr, however, alamarBlue reduction activity of neurons without astroglia was decreased significantly, while that of neurons with astroglia was not affected (b). Values are the mean ± SD of six wells. **p < .01 (analysis of variance followed by Tukey test for multiple comparisons). Neuronal [6-14C]glucose oxidation that reflects the TCA cycle metabolism was reduced significantly by 40-hr exposure to DA. In contrast, total neuronal-astroglial oxidation of [6-14C]glucose that reflects mainly neuronal TCA cycle metabolism was unaltered after DA exposure after 40 hr (c). Values are the mean ± SD of four flasks. *p < .05 versus control (analysis of variance followed by Dunnett test for multiple comparisons). DA = dopamine; ns = not significant.
Astroglial Neuroprotective Effects Induced by DA Are Dependent on Nrf2
We examined whether astroglial protective effects are Nrf2 dependent or not using astroglia prepared from WT or Nrf2-KO mice. As shown in Figure 14, after 36-hr exposure to DA (100 µmol/L), mouse neurons that had been prepared from WT mice were protected by coculturing with WT astroglia as assessed by alamarBlue assay. However, these protective effects disappeared when neurons were cocultured with Nrf2-defficient astroglia.
Figure 14.
Astroglia prepared from Nrf2-knock out (KO) mice do not provide neuroprotective effects against dopamine (DA). Mouse neurons that had been cultured without astroglia were damaged after 36-hr exposure to DA (100 µmol/L). Neurons that had been cocultured with (wild-type) WT astroglia using cell inserts and exposed to DA exhibited no damages, while neurons that had been cocultured with Nrf2-knockout (KO) astroglia were severely damaged (a). Scale bar = 100 µm. Mouse neuronal alamarBlue reduction activity was assessed after 36-hr exposure to DA (100 µmol/L) with or without mouse WT astroglial cell inserts (b) or Nrf2-KO astroglial cell inserts (c). alamarBlue reduction activities of neurons were unaltered in the presence of mouse astroglial inserts prepared from wild type at 36 hr. In contrast, alamarBlue reduction activity of neurons with astroglia prepared from Nrf2-KO mice was decreased significantly. Values are the mean ± SD of six wells. *p < .05 (analysis of variance followed by Tukey test for multiple comparisons). ns = not significant.
Discussion
This study showed that both acute and chronic exposure to DA induced PPP activation in cultured astroglia, accompanied by the suppression of ROS production. We found that DA modulated glucose metabolism in astroglia when applied both acutely and chronically via two different mechanisms. However, neither of these mechanisms was dependent on Na+-dependent monoamine transporters. ROS derived from DA seems to play important roles, with ROS activating PPP flux acutely via a cellular REDOX state change and chronically through Nrf2 activation. Unfortunately, however, we have to emphasize that no direct evidence was obtained supporting that DA-derived ROS played a causative role. Furthermore, whether ROS acted extracellularly or intracellularly after penetrating into the cells remains to be clarified. Notably, however, monoamine transporters may have some roles in cortical astroglia, since inhibitors of these transporters reduced PPP flux in response to acute DA exposure. The exact reason why an inhibition of monoamine transporters did not affect PPP flux in striatal astroglia is not readily clear. We speculate that the expression of monoamine transporters is weaker in striatal astroglia than in cortical astroglia, as our immunostaining suggested. Importantly, however, DA can activate astroglial PPP acutely through the ROS production outside the cells regardless of being taken up.
PD is a progressive neurodegenerative disease that causes motor symptoms as well as nonmotor symptoms, including cognitive, autonomic nervous system, and psychiatric disorders (Deutch, 2013). The cardinal symptom of the disease, motor dysfunction, can mostly, if not completely, be ascribed to the paucity of DA in the striata. DA is synthesized and released from dopaminergic neurons arising from the SN. The dopaminergic neurons in PD patients are progressively lost by undetermined mechanisms that have been associated with mitochondrial dysfunction (Mizuno et al., 1989; Schapira et al., 1989; Martin and Teismann, 2009). ROS production plays a major role in mitochondrial dysfunction, since mitochondria produces large amounts of ATP through the oxidative metabolism of glucose, resulting in unavoidable ROS production. In the brain, most of the ATP is consumed to generate action potentials, and DA neurons exhibit a pace-making activity of 2 to 4 Hz (Grace and Bunney, 1983), which maintains an optimal DA concentration in the striatum. Thus, mitochondria in dopaminergic neurons are constantly overloaded, leading to ROS production.
Another source of ATP consumption by DA neurons might be related to DA reuptake, which is mediated by DAT expressed in the nerve terminals of theses neurons in the striatum. DAT is a Na+-dependent cotransporter (Wheeler et al., 1993; Torres et al., 2003) and thus requires an inward gradient of Na+ across the cell membrane, which is generated by the consumption of ATP by Na+,K+-ATPase. This mechanism seems to be similar to the Glu reuptake system (Danbolt, 2001; Zhou and Danbolt, 2014). Glu released in the synaptic cleft is highly neurotoxic and must be removed immediately after neurotransmission (Driscoll et al., 1993). Most of the Glu, however, is taken up by astroglia surrounding neuronal synapses through similar Na+-dependent Glu transporters (GLT-1 and GLAST; Pines et al., 1992; Storck et al., 1992). Thus, astroglial ATP production is necessary for Glu reuptake. As for astroglial ATP production, glucose plays a prime role as an energy substrate. Whether glycolysis or mitochondrial oxidation plays a major role in ATP production has been a controversial topic for decades. We have shown that Glu induces an increase in astroglial glucose utilization and lactate production, indicating that glycolysis is dominant in cultured astroglia (Takahashi et al., 1995; Abe et al., 2006). In this study, we showed that the activity of the PPP, a glycolysis shunt pathway, was also increased. However, these increases seemed to be disproportionately high as a result of increased glucose consumption. DA is reportedly taken up by astroglia via transporters that are similar to those in DA neurons. In fact, we showed that astroglial cultures prepared from both the cortex and the striatum exhibited DAT, NET, and SERT in vitro. These results are consistent with both in vitro results (Semenoff and Kimelberg, 1985; Inazu et al., 2003) and in vivo results (Asanuma et al., 2014) reported by others, indicating that DA released from DA neurons might be taken up, at least in part, by astroglia.
Consequently, acute DA exposure to astroglia increased both glucose metabolism and PPP flux in cultured astroglia, as expected. Previous reports have shown that 50 µmol/L of DA induced DA uptake by cultured astroglia through NET in a Na+-dependent manner (Takeda et al., 2002; Inazu et al., 2003). This study showed that 100 µmol/L of DA also caused glycolytic activation associated with robust increases in PPP flux in cultured astroglia. The PPP is a glucose metabolism pathway that plays a major role in converting NADP+ to NADPH, which is necessary to maintain GSH in a reduced form against oxidative stress (Takahashi et al., 2012). Thus, it seems reasonable that DA released from DA neurons triggers the activation of astroglial glucose metabolism to reduce oxidative stress in the striatum. Surprisingly, however, these activations of PPP did not rely on Na+-dependent uptake by monoamine transporters. Indeed, H2O2 can act as a direct activator of PPP. Moreover, the H2O2-induced PPP activation was not Na+-dependent. We speculated that ROS derived from DA can act in a similar manner, consuming cellular NADPH and leading to the activation of PPP flux, since changes in the REDOX state are strong regulators of the rate-limiting enzyme of PPP flux (G6PDH; Clarke and Sokoloff, 1999; Dienel, 2009).
In the pathogenesis of PD, DA-derived ROS might also induce mitochondrial dysfunction, since DA is auto-oxidized to form dopamine quinone (Graham et al., 1978) and ROS (Fridovich, 1975). In this study, we showed that DA, but not Glu, produced ROS from the culture medium alone in the absence of cells. As mentioned earlier, acute DA exposure induced PPP activation in astroglia. DA-derived ROS did, indeed, decrease in the presence of astroglia. Importantly, astroglial PPP is known to be regulated chronically by a transcriptional mechanism through the Keap1 or Nrf2 system (Surh et al., 2008; Vargas and Johnson, 2009). Nrf2, a transcriptional factor, initiates G6PDH transcription and translation by numerous triggers of various stress signals (Surh et al., 2008; Vargas and Johnson, 2009). Oxidative stress, especially ROS, is thought to be a potent activator of the Keap1 or Nrf2 system (Surh et al., 2008; Vargas and Johnson, 2009). ROS modifies the cysteine residues of Keap1 and induces a conformational change, leading to the dissociation of Nrf2 from Keap1, and free Nrf2 translocates into the nucleus. This study showed that chronic DA exposure (15 hr) induced the nuclear translocation of Nrf2 in astroglia, while Glu did not. These results were confirmed by qRT-PCR for HO-1 gene transcription. The PPP flux was increased only in astroglia exposed to DA for 15 hr but not in astroglia exposed to Glu for 15 hr. Furthermore, DA-induced ROS production in the absence of astroglial cells disappeared in the presence of cells, suggesting an astroglial protective function in response to DA. We showed clearly that DA-exposure caused neuronal damages without astroglia and coculturing with astroglia prevented these damages via Nrf2-dependent mechanisms. One limitation of our study may be the concentration of DA. Although the exact concentration of DA in the brain is not known, 50 µmol/L of DA that was used in the past reports (Allen et al., 2013) and 100 µmol/L of DA in this study might be too high.
The exact mechanisms for the degeneration of dopaminergic neurons in PD have not been clarified, and the roles of astroglia and other glial cells have been controversial. The inflammatory response initiated by these glial cells might be another important issue (Teismann et al., 2003; Halliday and Stevens, 2011). Regarding the classical hypothesis of mitochondria dysfunction and ROS, however, astroglial cells may play protective roles against oxidative stress. These actions of astroglia in concert with dopaminergic neurons seem to be unique, since they are not seen with Glu neurons. The activation of an astroglial protective function could lead to a new strategy for preventing the onset and progression of PD.
Under both acute and chronic exposure to DA, astroglia possess systems to decrease oxidative stress. We can conclude that the release of DA from dopaminergic neurons can damage neurons themselves and can also enhance astroglial neuroprotective roles in the brain, possibly reducing oxidative stress. The enhancement of these protective roles of astroglia may represent an important therapeutic strategy for PD.
Summary
Dopamine-induced astroglial anti-oxidative function through the PPP was examined. Cultured astroglia expressed Na+-dependent monoamine transporters. Acute DA exposure enhanced PPP flux independently of monoamine transporters. Chronic exposure induced PPP activation via the Keap1 or Nrf2 system and protected neurons against DA toxicity.
Acknowledgments
Dr. Masayuki Yamamoto (Professor, Graduate School of Medicine, Tohoku University, Sendai, Japan) kindly provided Nrf2 knockout mice.
Author Contributions
S. T., K. M., and M. K. designed the experiments. K. M., Y. I., K. M., N. T., T. H., and S. T. performed the experiments. S. T., K. M., T. A., M. S., and N. S. analyzed the data and wrote the manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by a Grant-in-Aid for Scientific Research (C) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan: 24591276 (to S. T.) and 15K09324 (to S. T.).
References
- Abe T., Takahashi S., Fukuuchi Y. (2002). Reduction of Alamar Blue, a novel redox indicator, is dependent on both the glycolytic and oxidative metabolism of glucose in rat cultured neurons. Neurosci Lett, 326, 179–182. [DOI] [PubMed] [Google Scholar]
- Abe T., Takahashi S., Suzuki N. (2006. a). Metabolic properties of astrocytes differentiated from rat neurospheres. Brain Res, 1101, 5–11. [DOI] [PubMed] [Google Scholar]
- Abe T., Takahashi S., Suzuki N. (2006. b). Oxidative metabolism in cultured rat astroglia: Effects of reducing the glucose concentration in the culture medium and of D-aspartate or potassium stimulation. J Cereb Blood Flow Metab, 26, 153–160. [DOI] [PubMed] [Google Scholar]
- Allen G. F., Ullah Y., Hargreaves I. P., Land J. M., Heales S. J. (2013). Dopamine but not l-dopa stimulates neural glutathione metabolism. Potential implications for Parkinson’s and other dopamine deficiency states. Neurochem Int, 62, 684–694. [DOI] [PubMed] [Google Scholar]
- Andres-Mateos E., Perier C., Zhang L., Blanchard-Fillion B., Greco T. M., Thomas B., Ko H. S., Sasaki M., Ischiropoulos H., Przedborski S., Dawson T. M., Dawson V. L. (2007). DJ-1 gene deletion reveals that DJ-1 is an atypical peroxiredoxin-like peroxidase. Proc Natl Acad Sci U S A, 104, 14807–14812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asanuma M., Miyazaki I., Diaz-Corrales F. J., Kimoto N., Kikkawa Y., Takeshima M., Miyoshi K., Murata M. (2010). Neuroprotective effects of zonisamide target astrocyte. Ann Neurol, 67, 239–249. [DOI] [PubMed] [Google Scholar]
- Asanuma M., Miyazaki I., Murakami S., Diaz-Corrales F. J., Ogawa N. (2014). Striatal astrocytes act as a reservoir for L-DOPA. PLoS One, 9, e106362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee S., Noack H., Possel H., Keilhoff G., Wolf G. (1999). Glutathione levels in primary glial cultures: Monochlorobimane provides evidence of cell type-specific distribution. Glia, 27, 152–161. [PubMed] [Google Scholar]
- Chen P. C., Vargas M. R., Pani A. K., Smeyne R. J., Johnson D. A., Kan Y. W., Johnson J. A. (2009). Nrf2-mediated neuroprotection in the MPTP mouse model of Parkinson’s disease: Critical role for the astrocyte. Proc Natl Acad Sci U S A, 106, 2933–2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S. J., Panhelainen A., Schmitz Y., Larsen K. E., Kanter E., Wu M., Sulzer D., Mosharov E. V. (2015). Changes in neuronal dopamine homeostasis following 1-methyl-4-phenylpyridinium (MPP+) exposure. J Biol Chem, 290, 6799–6809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke D. D., Sokoloff L. (1999). Circulation and energy metabolism of the brain In Siegel G., Agranoff B., Albers R. W., Fisher S. (Eds.), Basic neurochemistry: Molecular, cellular, and medical aspects (6th ed., pp. 637–669). Philadelphia, PA: Lippincott-Raven. [Google Scholar]
- Clark I. E., Dodson M. W., Jiang C., Cao J. H., Huh J. R., Seol J. H., Yoo S. J., Hay B. A., Guo M. (2006). Drosophila pink1 is required for mitochondrial function and interacts genetically with parkin. Nature, 441, 1162–1166. [DOI] [PubMed] [Google Scholar]
- Danbolt N. C. (2001). Glutamate uptake. Prog Neurobiol, 65, 1–105. [DOI] [PubMed] [Google Scholar]
- Dave V., Kimelberg H. K. (1994). Na(+)-dependent, fluoxetine-sensitive serotonin uptake by astrocytes tissue-printed from rat cerebral cortex. J Neurosci, 14, 4972–4986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutch A. Y. (2013). Parkinson’s disease redefined. Lancet Neurol, 12, 422–423. [DOI] [PubMed] [Google Scholar]
- Dienel G. A. (2009). Energy metabolism in the brain In Byrne J. H. Roberts J. L. (Eds.), From molecules to networks: An introduction to cellular and molecular neuroscience (2nd ed., pp. 49–110). London, England: Academic Press. [Google Scholar]
- Driscoll B. F., Deibler G. E., Law M. J., Crane A. M. (1993). Damage to neurons in culture following medium change: Role of glutamine and extracellular generation of glutamate. J Neurochem, 61, 1795–1800. [DOI] [PubMed] [Google Scholar]
- Esteves A. R., Arduino D. M., Silva D. F., Oliveira C. R., Cardoso S. M. (2011). Mitochondrial dysfunction: The road to alpha-synuclein oligomerization in PD. Parkinsons Dis, 2011, 693761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridovich I. (1975). Superoxide dismutases. Ann Rev Biochem, 44, 147–159. [DOI] [PubMed] [Google Scholar]
- Gomes A., Fernandes E., Lima J. L. (2005). Fluorescence probes used for detection of reactive oxygen species. J Biochem Biophys Methods, 65, 45–80. [DOI] [PubMed] [Google Scholar]
- Grace A. A., Bunney B. S. (1983). Intracellular and extracellular electrophysiology of nigral dopaminergic neurons–2. Action potential generating mechanisms and morphological correlates. Neuroscience, 10, 317–331. [DOI] [PubMed] [Google Scholar]
- Graham D. G., Tiffany S. M., Vogel F. S. (1978). The toxicity of melanin precursors. J Invest Dermatol, 70, 113–116. [DOI] [PubMed] [Google Scholar]
- Halliday G. M., McRitchie D. A., Cartwright H., Pamphlett R., Hely M. A., Morris J. G. (1996). Midbrain neuropathology in idiopathic Parkinson’s disease and diffuse Lewy body disease. J Clin Neurosci, 3, 52–60. [DOI] [PubMed] [Google Scholar]
- Halliday G. M., Stevens C. H. (2011). Glia: Initiators and progressors of pathology in Parkinson’s disease. Mov Disord, 26, 6–17. [DOI] [PubMed] [Google Scholar]
- Hothersall J. S., Baquer N., Greenbaum A. L., McLean P. (1979). Alternative pathways of glucose utilization in brain. Changes in the pattern of glucose utilization in brain during development and the effect of phenazine methosulfate on the integration of metabolic routes. Arch Biochem Biophys, 198, 478–492. [DOI] [PubMed] [Google Scholar]
- Inazu M., Takeda H., Ikoshi H., Sugisawa M., Uchida Y., Matsumiya T. (2001). Pharmacological characterization and visualization of the glial serotonin transporter. Neurochem Int, 39, 39–49. [DOI] [PubMed] [Google Scholar]
- Inazu M., Takeda H., Matsumiya T. (2003). Functional expression of the norepinephrine transporter in cultured rat astrocytes. J Neurochem, 84, 136–144. [DOI] [PubMed] [Google Scholar]
- Lastres-Becker I., Ulusoy A., Innamorato N. G., Sahin G., Rabano A., Kirik D., Cuadrado A. (2012). α- Synuclein expression and Nrf2 deficiency cooperate to aggravate protein aggregation, neuronal death and inflammation in early-stage Parkinson’s disease. Hum Mol Genet, 21, 3173–3192. [DOI] [PubMed] [Google Scholar]
- Lee J. M., Calkins M. J., Chan K., Kan Y. W., Johnson J. A. (2003). Identification of the NF-E2-related factor-2-dependent genes conferring protection against oxidative stress in primary cortical astrocytes using oligonucleotide microarray analysis. J Biol Chem, 278, 12029–12038. [DOI] [PubMed] [Google Scholar]
- Lohr K. M., Bernstein A. I., Stout K. A., Dunn A. R., Lazo C. R., Alter S. P., Wang M., Li Y., Fan X., Hess E. J., Yi H., Vecchio L. M., Goldstein D. S., Guillot T. S., Salahpour A., Miller G. W. (2014). Increased vesicular monoamine transporter enhances dopamine release and opposes Parkinson disease-related neurodegeneration in vivo. Proc Natl Acad Sci U S A, 111, 9977–9982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin H. L., Teismann P. (2009). Glutathione—A review on its role and significance in Parkinson’s disease. FASEB J, 23, 3263–3272. [DOI] [PubMed] [Google Scholar]
- Masoud S. T., Vecchio L. M., Bergeron Y., Hossain M. M., Nguyen L. T., Bermejo M. K., Kile B., Sotnikova T. D., Siesser W. B., Gainetdinov R. R., Wightman R. M., Caron M. G., Richardson J. R., Miller G. W., Ramsey A. J., Cyr M., Salahpour A. (2015). Increased expression of the dopamine transporter leads to loss of dopamine neurons, oxidative stress and l-DOPA reversible motor deficits. Neurobiol Dis, 74, 66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki I., Asanuma M., Kikkawa Y., Takeshima M., Murakami S., Miyoshi K., Sogawa N., Kita T. (2011). Astrocyte-derived metallothionein protects dopaminergic neurons from dopamine quinone toxicity. Glia, 59, 435–451. [DOI] [PubMed] [Google Scholar]
- Mizuno Y., Ohta S., Tanaka M., Takamiya S., Suzuki K., Sato T., Oya H., Ozawa T., Kagawa Y. (1989). Deficiencies in complex I subunits of the respiratory chain in Parkinson’s disease. Biochem Biophys Res Commun, 163, 1450–1455. [DOI] [PubMed] [Google Scholar]
- Nishijima H., Tomiyama M. (2016). What mechanisms are responsible for the reuptake of levodopa-derived dopamine in Parkinsonian striatum? Front Neurosci, 10, 575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacino J. J., Sagi D., Goldberg M. S., Krauss S., Motz C., Wacker M., Klose J., Shen J. (2004). Mitochondrial dysfunction and oxidative damage in parkin-deficient mice. J Biol Chem, 279, 18614–18622. [DOI] [PubMed] [Google Scholar]
- Pellerin L., Magistretti P. J. (1994). Glutamate uptake into astrocytes stimulates aerobic glycolysis: A mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci U S A, 91, 10625–10629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pines G., Danbolt N. C., Bjoras M., Zhang Y., Bendahan A., Eide L., Koepsell H., Storm-Mathisen J., Seeberg E., Kanner B. I. (1992). Cloning and expression of a rat brain L-glutamate transporter. Nature, 360, 464–467. [DOI] [PubMed] [Google Scholar]
- Robinson S. R., Schousboe A., Dringen R., Magistretti P., Coles J., Hertz L. (1998). Metabolic trafficking between neurons and glia In Laming P. R., Sykova E., Reichenbach A., Hatton G. I., Bauer H. (Eds.), Glial cells: Their role in behaviour (pp. 83–106). New York, NY: Cambridge University Press. [Google Scholar]
- Romo R., Schultz W. (1990). Dopamine neurons of the monkey midbrain: Contingencies of responses to active touch during self-initiated arm movements. J Neurophysiol, 63, 592–606. [DOI] [PubMed] [Google Scholar]
- Schapira A. H., Cooper J. M., Dexter D., Jenner P., Clark J. B., Marsden C. D. (1989). Mitochondrial complex I deficiency in Parkinson’s disease. Lancet, 1, 1269. [DOI] [PubMed] [Google Scholar]
- Semenoff D., Kimelberg H. K. (1985). Autoradiography of high affinity uptake of catecholamines by primary astrocyte cultures. Brain Res, 348, 125–136. [DOI] [PubMed] [Google Scholar]
- Shimojo N., Fujino K., Kitahashi S., Nakao M., Naka K., Okuda K. (1991). Lactate analyzer with continuous blood sampling for monitoring blood lactate during physical exercise. Clin Chem, 37, 1978–1980. [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. (1985). Measurement of protein using bicinchoninic acid. Anal Biochem, 150, 76–85. [DOI] [PubMed] [Google Scholar]
- Sokoloff L., Reivich M., Kennedy C., Des Rosiers M. H., Patlak C. S., Pettigrew K. D., Sakurada O., Shinohara M. (1977). The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization: Theory, procedure, and normal values in the conscious and anesthetized albino rat. J Neurochem, 28, 897–916. [DOI] [PubMed] [Google Scholar]
- Storck T., Schulte S., Hofmann K., Stoffel W. (1992). Structure, expression, and functional analysis of a Na(+)-dependent glutamate/aspartate transporter from rat brain. Proc Natl Acad Sci U S A, 89, 10955–10959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suematsu M., Schmid-Schönbein G. W., Chavez-Chavez R. H., Yee T. T., Tamatani T., Miyasaka M., Delano F. A., Zweifach B. W. (1993). In vivo visualization of oxidative changes in microvessels during neutrophil activation. Am J Physiol, 264(3 Pt 2), H881–H891. [DOI] [PubMed] [Google Scholar]
- Surh Y. J., Kundu J. K., Na H. K. (2008). Nrf2 as a master redox switch in turning on the cellular signaling involved in the induction of cytoprotective genes by some chemopreventive phytochemicals. Planta Med, 74, 1526–1539. [DOI] [PubMed] [Google Scholar]
- Takahashi S., Driscoll B. F., Law M. J., Sokoloff L. (1995). Role of sodium and potassium ions in regulation of glucose metabolism in cultured astroglia. Proc Natl Acad Sci U S A, 92, 4616–4620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi S., Izawa Y., Suzuki N. (2012). Astroglial pentose phosphate pathway rates in response to high-glucose environments. ASN Neuro, 4. doi:10.1042/AN20120002 [DOI] [PMC free article] [PubMed]
- Takahashi S., Shibata M., Gotoh J., Fukuuchi Y. (2000). Astroglial cell death induced by excessive influx of sodium ions. Eur J Pharmacol, 408, 127–135. [DOI] [PubMed] [Google Scholar]
- Takeda H., Inazu M., Matsumiya T. (2002). Astroglial dopamine transport is mediated by norepinephrine transporter. Naunyn Schmiedebergs Arch Pharmacol, 366, 620–623. [DOI] [PubMed] [Google Scholar]
- Teismann P., Tieu K., Cohen O., Choi D. K., Wu D. C., Marks D., Vila M., Jackson-Lewis V., Przedborski S. (2003). Pathogenic role of glial cells in Parkinson’s disease. Mov Disord, 18, 121–129. [DOI] [PubMed] [Google Scholar]
- Thimmulappa R. K., Mai K. H., Srisuma S., Kensler T. W., Yamamoto M., Biswal S. (2002). Identification of Nrf2-regulated genes induced by the chemopreventive agent sulforaphane by oligonucleotide microarray. Cancer Res, 62, 5196–5203. [PubMed] [Google Scholar]
- Torres G. E., Gainetdinov R. R., Caron M. G. (2003). Plasma membrane monoamine transporters: Structure, regulation and function. Nat Rev Neurosci, 4, 13–25. [DOI] [PubMed] [Google Scholar]
- Turiault M., Parnaudeau S., Milet A., Parlato R., Rouzeau J. D., Lazar M., Tronche F. (2007). Analysis of dopamine transporter gene expression pattern—Generation of DAT-iCre transgenic mice. FEBS J, 274, 3568–3577. [DOI] [PubMed] [Google Scholar]
- Vargas M. R., Johnson J. A. (2009). The Nrf2-ARE cytoprotective pathway in astrocytes. Expert Rev Mol Med, 11, e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkateshappa C., Harish G., Mythri R. B., Mahadevan A., Bharath M. M., Shankar S. K. (2012). Increased oxidative damage and decreased antioxidant function in aging human substantia nigra compared to striatum: Implications for Parkinson’s disease. Neurochem Res, 37, 358–369. [DOI] [PubMed] [Google Scholar]
- Waniewski R. A., Martin D. L. (2004). Astrocytes and synaptosomes transport and metabolize lactate and acetate differently. Neurochem Res, 29, 209–217. [DOI] [PubMed] [Google Scholar]
- Wheeler D. D., Edwards A. M., Chapman B. M., Ondo J. G. (1993). A model of the sodium dependence of dopamine uptake in rat striatal synaptosomes. Neurochem Res, 18, 927–936. [DOI] [PubMed] [Google Scholar]
- Zhang D. X., Gutterman D. D. (2007). Mitochondrial reactive oxygen species-mediated signaling in endothelial cells. Am J Physiol Heart Circ Physiol, 292, H2023–H2031. [DOI] [PubMed] [Google Scholar]
- Zhou Y., Danbolt N. C. (2014). Glutamate as a neurotransmitter in the healthy brain. J Neural Transm, 121, 799–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang X., Masson J., Gingrich J. A., Rayport S., Hen R. (2005). Targeted gene expression in dopamine and serotonin neurons of the mouse brain. J Neurosci Methods, 143, 27–32. [DOI] [PubMed] [Google Scholar]