Abstract
Objectives
To assess the feasibility and inform design features of a fully powered randomized controlled trial (RCT) evaluating the effects of Tai Chi (TC) in Parkinson’s disease (PD) and to select outcomes most responsive to TC assessed during off-medication states.
Design
Two-arm, wait-list controlled RCT.
Settings
Tertiary care hospital.
Subjects
Thirty-two subjects aged 40–75 diagnosed with idiopathic PD within 10 years.
Interventions
Six-month TC intervention added to usual care (UC) versus UC alone.
Outcome Measures
Primary outcomes were feasibility-related (recruitment rate, adherence, and compliance). Change in dual-task (DT) gait stride-time variability (STV) from baseline to 6 months was defined, a priori, as the clinical outcome measure of primary interest. Other outcomes included: PD motor symptom progression (Unified Parkinson’s Disease Rating Scale [UPDRS]), PD-related quality of life (PDQ-39), executive function (Trail Making Test), balance confidence (Activity-Specific Balance Confidence Scale, ABC), and Timed Up and Go test (TUG). All clinical assessments were made in the off-state for PD medications.
Results
Thirty-two subjects were enrolled into 3 sequential cohorts over 417 days at an average rate of 0.08 subjects per day. Seventy-five percent (12/16) in the TC group vs 94% (15/16) in the UC group completed the primary 6-month follow-up assessment. Mean TC exposure hours overall: 52. No AEs occurred during or as a direct result of TC exercise. Statistically nonsignificant improvements were observed in the TC group at 6 months in DT gait STV (TC [20.1%] vs UC [−0.1%] group [effect size 0.49; P = .47]), ABC, TUG, and PDQ-39. UPDRS progression was modest and very similar in TC and UC groups.
Conclusions
Conducting an RCT of TC for PD is feasible, though measures to improve recruitment and adherence rates are needed. DT gait STV is a sensitive and logical outcome for evaluating the combined cognitive-motor effects of TC in PD.
Keywords: Tai Chi, Parkinson’s disease, gait analysis, dual-task performance, feasibility, randomized trial
Introduction
Parkinson’s disease (PD) is one of the most common neurodegenerative disorders.1,2 While primarily characterized by motor symptoms, such as rigidity, tremor, bradykinesia, and postural imbalance,2 PD is also associated with a number of non-motor symptoms (eg, reduced cognitive function and depression) which together lead to reduced ability to accomplish functional tasks, increased fall risk, and a reduced quality of life.3
Gait disturbances in particular are a prominent clinical manifestation of PD and are among its most disabling symptoms.4 In healthy individuals, motor performance and gait health depends on the interaction between automatic (unconscious) and volitional (cognitive) control of movement.5 In PD, the early and preferential loss of dopamine leads to diminished automatic and increased cognitive control of movements that include frontal lobe circuitry.6,7 Consequently, individuals with PD need to handle and sustain a larger cognitive load to execute motor tasks.6,8–11
The ability to walk while simultaneously performing an unrelated cognitive task, referred to here as cognitive dual tasking (DT), is essential to many activities of daily living. Such DT interferes with volitional postural control, a particular problem in PD where automaticity of gait is already impaired.8,9 In addition to reducing gait speed, DT in PD has been shown to increase gait variability,12 a marker of instability and fall risk.13,14 Measures of gait variability during DT are also associated with PD progression8,9 and are sensitive to therapeutic interventions.15,16
Tai Chi (TC) is a multicomponent mind-body exercise that is growing in popularity with demonstrated benefits for multiple aspects of physical and cognitive health. TC improves postural control in general17,18 and shows promise as an intervention for reducing the risk of falls and treating PD symptoms specifically. TC integrates balance, flexibility, and neuromuscular coordination training with a number of cognitive components––including heightened body awareness, focused mental attention, imagery, multitasking (cognitive-motor and motor-motor), and planned and goal-oriented training––which together may result in benefits to PD beyond conventional exercise.19
A recent meta-analysis supports a benefit of TC for reducing falls and improving balance for individuals with PD.20 One large-scale trial included in this meta-analysis demonstrated that, for mid- to later-stage PD patients evaluated while on PD medications, TC was superior to both resistance training and low-intensity stretching for outcomes related to fall risk, balance, and multiple domains of physical function.21 However, it is not known if TC’s impact on fall reduction and postural control, in this and other studies, is mediated by an improved ability to maintain healthy stride dynamics and cognitive-motor integration during DT. In addition, nearly all studies to date have evaluated the impact of TC while participants were on PD medications, in contrast to the off-medications state that allows a more direct measure of pathology.20 Moreover, very few studies have evaluated the impact of TC on newly diagnosed or early stage PD progression. Better understanding of the motor and cognitive processes through which TC alters gait and balance, especially assessed in the practically defined off-medication state in earlier stage PD patients, could better help inform the therapeutic potential of TC and contribute to the development of biomarkers and therapeutic targets for integrative PD treatments.
To inform the feasibility and design features of a fully powered RCT evaluating the effects of TC on DT stride time variability (STV) in early- to mid-stage PD and assessed in the off-medication state, this pilot study addressed two primary aims: (1) To assess the feasibility of recruiting and retaining individuals with PD into a 6-month RCT of TC exercise; and (2) to collect preliminary data on the efficacy of TC in reducing DT STV to inform sample size requirements for a future trial.
Materials and Methods
Overview of Study Design
We employed a 2-arm waitlist RCT. A total of 32 participants with idiopathic PD, aged 40–75, were randomized 1:1 to 6 months of TC training in addition to usual healthcare (TC group) or to usual healthcare alone (UC group). A permuted-block randomization scheme with randomly varying block sizes was utilized. Primary staff overseeing assessment and analysis of gait-related and functional outcomes were blinded to treatment assignment. Since this was a pilot study, the sample size calculation was selected to provide adequate precision in estimating feasibility. We aimed to identify the most sensitive gait parameter to measure in a future RCT. Our primary clinical outcome was change in DT gait variability evaluated from baseline to 6 months. A battery of secondary outcomes included motor and non-motor function assessments. The institutional review board at Brigham and Women’s Hospital approved this study (2013P002343). The trial was registered at ClinicalTrials.gov (NCT02418780). Our preliminary findings are reported following guidelines in the extension of CONSORT statement for randomized pilot and feasibility trials.22
Study Participants
Participants were recruited from Boston area neurology practices and PD support groups between March 2014 and August 2015. Individuals were eligible if they were recently diagnosed with idiopathic PD (≤10 years) and had limited disease progression (Modified Hoehn and Yahr [H–Y] stages 1–2.5);23 40–75 years of age; and willing to undergo baseline and follow-up testing while off PD-related medication for 12 h. Individuals were excluded for the following reasons: diagnosis of atypical parkinsonism; history of major neurological or psychiatric disease, orthopedic impairment, or other disease that could likely contribute to a gait disturbance; any severe, chronic condition or acute medical event for which participation in exercise programs was contraindicated; history of deep brain stimulation or other brain surgery; or significant TC experience (>6 months training in past 2 years). Written informed consent was obtained from all participants.
Interventions
Participants randomized to the TC group received 6 months of training. The PD-specific TC program was adapted from protocols used in prior study populations with balance and gait impairments,24,25 heart failure,26 and chronic obstructive pulmonary disease.27 The program targeted the symptoms of individuals with relatively early-stage PD and included balance, flexibility, agility, and moderate aerobic training combined with training in multiple cognitive components (mindfulness, focused attention, DT, and mindful breathing). Training included 2-person interactive exercises. Meditative music was played in all classes. Table 1 outlines the specific exercises included in the protocol.
Table 1.
Description of Parkinson’s Disease Specific Tai Chi Program.
| Week | Activities | Approximate Duration (in Minutes) |
|---|---|---|
| 1–2 | Check-in | 2 |
| Tai Chi warm-up exercises | 38 | |
| Tai Chi pouring | ||
| Tai Chi swinging and drumming the body | ||
| Standing meditation | ||
| Hip circles | ||
| Spiral lower extremity joints | ||
| Stretching arms and flanks | ||
| Fountain | ||
| Washing with Qi from the heaven | ||
| Renewing the body with the breath | ||
| Introduction to Tai Chi Movement #1 | 15 | |
| Raising the power | ||
| Tai Chi cool-down exercices | 5 | |
| Self-massage and meridian tapping | ||
| Washing with Qi from heavens | ||
| 3–8 | Check-in | 2 |
| Tai Chi warm-up exercises | 18 | |
| Review and practice Tai Chi Movement #1 | 5 | |
| Learn and practice Tai Chi Movements #2 and #3 | 30 | |
| Push and withdraw | ||
| Wave hands like clouds | ||
| Tai Chi cool-down exercices | 5 | |
| 9–12 | Check-in | 2 |
| Tai Chi warm-up exercises | 13 | |
| Review and practice Tai Chi Movements #1–3 | 15 | |
| Learn and practice Tai Chi Movements #4, #5, #6 | 25 | |
| Grasp the sparrow’s tail | ||
| Cross hands | ||
| Golden rooster stands on one leg | ||
| Tai Chi cool-down exercices | 5 | |
| 13–24 | Check-in | 2 |
| Tai Chi warm-up exercises | 13 | |
| Review and practice Tai Chi Movements #1–6 | 30 | |
| Agility and interactive Tai Chi training | 10 | |
| Tai Chi cool-down exercices | 5 | |
Classes took place at Brigham and Women's Hospital (Cohorts 1 and 2) and Boston Medical Center (Cohort 3). Participants were asked to attend 2 classes per week on average over the 6-month study. They were also asked to practice a minimum of 60 min per week outside of class, using a provided instructional DVD. Class attendance was recorded by instructors. Participants recorded frequency and duration of home practice using self-report logs collected monthly by study staff.
Procedures
All study assessments were performed at the Motion Analysis Laboratory at Spaulding Rehabilitation Hospital. Outcomes were assessed at baseline, 3 months (to study progression), and 6 months (ie, after the completion of 6 months of training). All study participants were asked to withhold anti-Parkinsonian medications for 12 h prior to testing visits.
Outcomes Assessed
Feasibility and safety (Aim 1)
In order to assess the feasibility of recruiting and retaining individuals with PD into a 6-month RCT of TC exercise, the following data were collected: Recruitment rate (number of subjects screened vs enrolled and average number enrolled per day), adherence to TC protocol (number of classes attended per subject and self-reports of hours of home-based exercise), compliance with the assessment protocol (number of study visits completed per subject), and adverse events (AEs) reported during the study.
Clinical (Aim 2)
To select the gait parameters most sensitive to TC, steady-state gait was assessed during 90 s of continuous over-ground walking at normal preferred speed, with and without the addition of DT challenges. Change in DT STV from baseline to 6 months was defined, a priori, as the clinical outcome measure of primary interest. Gait was assessed along a 15-m path that was wide enough to enable smooth turning. We used 2 cognitive DT challenges: serial subtractions (counting backwards by 3’s (DTcount)) and the Star Movement Task (DTstar) (a visuospatial task that requires subjects to visualize a star moving among 4 imaginary boxes arranged in a square according to verbal instructions and report the star’s final location).28 Stride times was assessed using 2 force sensing resistors® (model FSR 402, Interlink Electronics, Westlake Village, CA, USA) placed under the heel and the toe and recorded with the SHIMMER™ sensing platform (Dublin, Ireland).29
Stride times were calculated for each gait cycle as the time between initial heel strike of one foot and the subsequent heel strike of that same foot. STV was calculated from the stride time time-series as the coefficient of variation (CV, 100 multiplied by the SD of the stride times divided by the mean of each subject's stride times). DT costs were calculated using both absolute and proportional measures. Absolute DT costs (Abs. DT) were calculated for each participant as the difference in walking speed or STV between undisturbed single-task walking (ST) and DT walking. Proportional DT costs (% DT) were calculated for each participant’s walking speed or STV as follows: 100 × ([DT – ST]/ST). Both the number and the accuracy of serial subtractions during DTcount and the accuracy of the star movement during DTstar were recorded.
PD motor symptom progression was assessed with the 27 items of the UPDRS motor subscale (part 3); scores range from 0 to 108 (higher score indicates greater motor disability).30 Testing was conducted by a clinician with expertise in PD assessment. PD-related quality of life (QoL) was assessed using the PDQ-39, a 39-item self-report assessment of PD-specific health-related QoL over the last month (higher score indicates worse QoL).31 Executive cognitive function was assessed using the Trail Making Test (TMT). TMT A (number sequence only) assesses visual search. TMT B (alternating numbers and letters) evaluates executive control (lower score indicates higher executive function).32,33 The ratio between TMT B and A more accurately assesses executive function since it corrects for processing speed.33 Balance confidence was assessed using the Activity-specific Balance Confidence Scale (ABC) (higher score indicates greater self-confidence in physical functioning).34 The Timed Up and Go Test (TUG)35 was used to assess mobility. The average of 3 attempts of the time required to rise from a chair, walk 3 m, return, and sit down was collected (faster times indicate better mobility).
Data Synthesis and Statistical Analysis
Pre-specified hypotheses related to feasibility (Aim 1)
Feasibility was evaluated with respect to 4 attributes: recruitment, retention, attendance, and adherence. Recruitment success was evaluated by completion of planned enrollment, defined as randomization into the trial, and by evidence of an adequate rate of enrollment. Specifically, we tested the hypothesis that our expected rate of enrollment was noninferior to a rate of 0.25 randomizations per day during active recruitment based on a one-sided 90% lower confidence bound assuming Poisson-distributed enrollments per day. With our planned sample size of n = 20 per cohort and 2 cohorts, the study would have greater than 90% power if our true expected rate was at least 0.37 per day, equivalent to completing recruitment in 81 days for each cohort. Retention, attendance, and adherence were quantified by the proportion of subjects who completed the trial, the proportion of TC classes attended, and the proportion of assigned home practice completed, respectively. Study completion rates were compared between treatment groups by relative risk of noncompletion and tested by Fisher's exact test. Minimal adequate attendance and adherence were defined as attending at least 70% of TC classes and completing at least 70% of home practice sessions, respectively. We tested noninferiority of expected rates of attendance and adherence assuming beta-binomial distributed events. Noninferiority to our target attendance and adherence rates were evaluated using one-sided 90% lower confidence bounds on the estimated average rates across participants. Confidence bounds and one-tailed P values for testing noninferiority for all 4 measures of feasibility were obtained using maximum likelihood estimates of each rate and its standard error using a log link function.
Analysis of primary and secondary outcomes (Aim 2)
Efficacy outcomes were compared between TC and UC using repeated-measure ANOVA models with shared baseline, fixed effects of visit and treatment × post-baseline visit interaction, and unstructured covariance among repeated participant-specific observations over the 3 visits. The Wald statistic from a linear contrast over the treatment × visit interaction testing 6-month treatment-dependent response was used to test for any effect of TC and as a criterion for selecting efficacy outcomes that are potentially sensitive to TC. Two stages of selection were used, first to select the most responsive DT STV outcome (counting vs star) based on effect sizes. Effect sizes were calculated as the estimated treatment difference in 6-month change divided by the standard deviation of 6-month change among control group participants. A second stage of selection ranked additional gait-related and non–gait-related outcomes to identify the best measure for evaluating the efficacy of TC in a future trial. Selection of the optimal outcome was guided by consideration of clinical relevance and participant burden.
Data were analyzed using MATLAB (Mathworks, Natick, MA) and SAS (version 9.4 SAS Institute, Cary, NC).
Results
Participant Flow
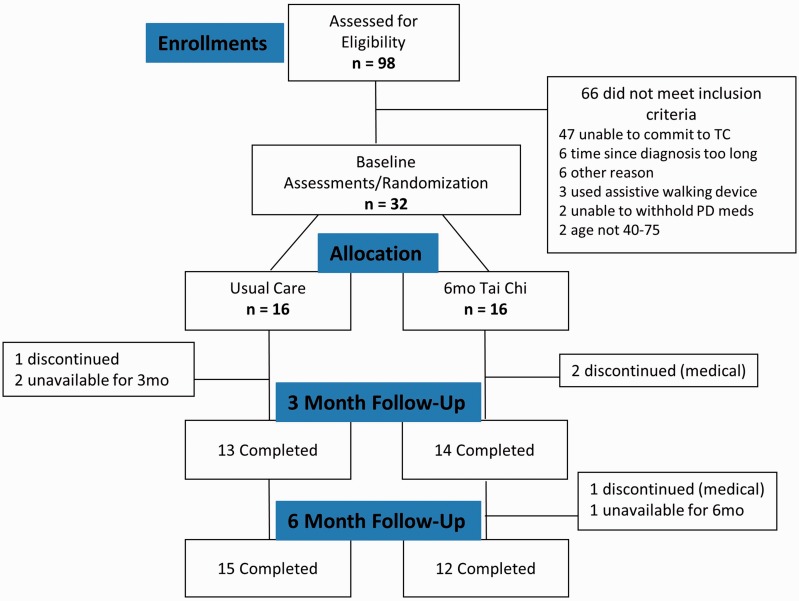
A CONSORT flowchart is shown in Figure 1.
Figure 1.
Flow of participants in randomization trial of subjects with Parkinson’s disease.
Baseline Characteristics
Participants randomized to TC or UC groups were relatively well matched at baseline (see Table 2). Mean (±SD) age was 63.8 ± 6.3 years, and 50% of participants were female. Mean PD duration was 2.9 ± 2.3 years. Seventy-five percent of participants were H–Y stage 2. Mean UPDRS motor score was 23.5 ± 9.5, with slightly worse function in the TC group.
Table 2.
Baseline Characteristics of Subjects by Groups.
| Tai Chi | Usual Care | |
|---|---|---|
| (n = 16) | (n = 16) | |
| Age (years), AVG ± SD | 65.7 ± 3.86 | 62 ± 7.77 |
| Gender, n (%) | ||
| Male | 9 (56.3%) | 7 (43.8%) |
| Female | 7 (43.8%) | 9 (56.3%) |
| Race, n (%) | ||
| White | 16 (100%) | 16 (100%) |
| Ethnicity, n (%) | ||
| Non-Hispanic | 16 (100%) | 14 (87.5%) |
| Unknown | 2 (12.5%) | |
| Education (years), AVG ± SD | 20.2 ± 8.46 | 17.56 ± 2.31 |
| Employment status, n (%) | ||
| Employed | 9 (56.3%) | 10 (62.5%) |
| Retired | 6 (37.5%) | 5 (31.3%) |
| Unemployed | 0 (0.0%) | 1 (6.3%) |
| Disabled | 1 (6.3%) | 0 (0.0%) |
| BMI (kg/m2), AVG ± SD | 28.2 ± 5.52 | 26.9 ± 5.82 |
| Physical activity status (0–10 scale), AVG ± SD | 4.1 ± 1.93 | 4.31 ± 2.73 |
| Duration of disease (years), AVG ± SD | 2.9 ± 2.38 | 2.9 ± 2.20 |
| Taking PD Meds, n (%) | 14 (87.5%) | 15 (93.8%) |
| Hoehn and Yahr stage, n (%) | ||
| 2 | 11 (68.8%) | 13 (81.3%) |
| 2.5 | 5 (31.3%) | 3 (18.8%) |
| UPDRS Motor Score (0–108 scale), AVG ± SD | 26.38 ± 9.64 | 20.63 ± 8.69 |
Abbreviation: AVG, average; BMI, body mass index; PD, Parkinson’s disease; SD, standard deviation; UPDRS, Unified Parkinson’s Disease Rating Scale.
Continuous variables: values provided are mean ± standard deviation.
Categorical variables: values provided are absolute frequency (relative frequency).
Feasibility Outcomes (Aim 1)
Recruitment
Participants were recruited over a 15-month interval. Recruitment was slower than expected. Ultimately, 32 subjects with idiopathic PD were enrolled into 3 sequential cohorts over 417 days at an average rate of 0.08 subjects per day (95% CI: 0.05–0.11, P > .99 for noninferiority to a rate of 0.25; recruitment rates for the 3 cohorts were 0.12, 0.09, and 0.04 subjects per day, respectively). We terminated our recruitment before achieving 40 subjects due to a combination of lower than expected recruitment rates and funding constraints.
Retention, attendance, and adherence
Of the 32 subjects randomized, 75% (12/16) in the TC group versus 94% (15/16) in the UC group completed the primary 6-month follow-up assessment (relative risk among TC subjects of early termination or loss to follow-up = 1.25, 95% CI: 0.88–1.94, P = .33). Of those randomized to the TC group, 3 officially withdrew participation due to unrelated medical reasons. One was lost to follow-up. Another completed all follow-up study visits but stopped attending classes after only 5 classes and did not do any home practice. One UC subject withdrew consent. Of the 16 subjects randomized to TC, attendance of the 48 scheduled TC classes ranged from 4% to 92% with a median of 68%. The estimated percentage of TC classes attended was 57% (95% CI: 44%–70%, P = .98 for noninferiority to 70%). Among the 12 TC subjects who completed the study, class attendance ranged from 10% to 92% with an estimated percentage of TC classes attended of 70% (95% CI: 57%–80%, P = .51). Including 2 TC subjects with missing home practice data who had withdrawn from the study, 75% (12/16) completed at least 70% of home practice (95% CI: 48%–93%, P = .45 for non-inferiority to 70%). Average amount of home practice was 30 h among the 14 TC subjects with data; among the 12 subjects who completed the study (not withdrawn or lost to follow-up), average amount of home practice was 32 h. Mean total TC exposure hours were 52 and 67 for all subjects and just completers, respectively.
Adverse events
No AEs occurred during or as a direct result of TC exercise. Over the course of the study, 12 AEs were reported by 9 subjects (4 in TC, 5 in UC group); all AEs were unrelated to the study and included: back pain (n = 5), falls/injuries (n = 4), illness (n = 2), and pain (n = 1).
Clinical Outcomes (Aim 2)
We analyzed gait related data of 16, 14, and 12 subjects at baseline, 3, and 6 months, respectively, in the TC group, and 16, 13, and 13, respectively, for the same time periods in the UC group.
STV increased with the addition of a DT challenge in both the TC and UC groups (Table 3). Repeated-measure ANOVA models adjusting for variation due to baseline score, age, gender, and UPDRS score revealed a statistically nonsignificant trend over 6 months towards a greater reduction in DTcount STV in the TC (20.1%) versus UC (−0.1%) group (effect size 0.49; P = .47; Figure 2). Similar patterns were observed for DTstar STV. Absolute and percent DT cost of STV also trended towards being lower in the TC group, but the differences were not statistically significant. A per-protocol analysis of DTcount STV limited TC participants who were at least 50% compliant with class attendance and home practice resulted in an effect size similar to the estimates including all participants (EF = 0.47; P = .55).
Table 3.
Clinical Outcome Measures.
| Clinical Outcomes Measures | Groups |
Statistical Result |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Tai Chi |
Usual Care |
Tai Chi |
Usual Care |
Tai Chi vs Usual Care |
|||||||
| Baseline (n = 16) |
3 Months (n = 14) |
6 Months (n = 12) |
Baseline (n = 16) |
3 Months (n = 13) |
6 Months (n = 13) |
Across visit |
Across visit |
Across groups |
|||
| Mean ± SDa | Slope (95% CI) baseline score, age, gender, UPDRSb | P valuec | Effect Sizec | ||||||||
| Gait-related outcomes | |||||||||||
| Stride time variability DTc (CV %) | 4.62 ± 3.44 | 4.02 ± 1.36 (n = 13)d | 3.69 ± 1.52 | 3.44 ± 1.49 | 4.69 ± 4.29 | 3.49 ± 2.10 | −0.62 (−1.93, 0.68) | −0.05 (−1.26, 1.15) | −0.57 (−2.14, 1.0) | .47 | 0.49 |
| Stride time variability DTs (CV %) | 3.48 ± 0.86 | 3.24 ± 0.89 | 3.53 ± 1.57 | 2.86 ± 1.09 | 2.59 ± 0.73 | 3.22 ± 1.69 | 0.22 (−0.88, 1.31) | 0.13 (−0.81, 1.06) | 0.09 (−1.38, 1.56) | .91 | 0.05 |
| Stride time variability ST (CV %) | 3.39 ± 0.89 | 3.07 ± 0.82 | 2.72 ± 0.78 | 2.87 ± 1.02 | 2.41 ± 0.52 | 2.59 ± 0.58 | −0.32 (−0.82, 0.18) | −0.49 (−0.93, 0.03) | 0.17 (−0.45, 0.79) | .58 | 0.17 |
| Absolute DTc cost variability | 1.23 ± 3.48 | 0.95 ± 1.38 | 0.97 ± 1.42 | 0.57 ± 1.30 | 2.29 ± 3.89 | 0.91 ± 1.75 | −0.28 (−1.54, 0.98) | 0.38 (−0.80, 1.56) | −0.66 (−2.07, 0.74) | .35 | 0.52 |
| % DTc cost variability | 40.84 ± 120.7 | 37.07 ± 53.94 | 38.66 ± 49.51 | 21.25 ± 35.3 | 78.35 ± 123.9 | 30.39 ± 53.3 | −3.51 (−46.8, 39.76) | 13.77 (−26.6, 54.18) | −17.3 (−63.6, 29.07) | .46 | 0.42 |
| Absolute DTs cost variability | 0.09 ± 0.87 | 0.16 ± 0.48 | 0.81 ± 1.37 | −0.01 ± 1.11 | 0.19 ± 0.41 | 0.63 ± 1.45 | 0.53 (−0.49, 1.55) | 0.61(−0.26, 1.48) | −0.08 (−1.40, 1.24) | .9 | 0.05 |
| % DTs cost variability | 4.67 ± 23.11 | 6.63 ± 18.75 | 31.06 ± 49.24 | 2.14 ± 26.84 | 7.80 ± 16.65 | 22.87 ± 50.24 | 19.82 (−15.7, 55.33) | 20.16 (−9.66, 49.98) | −0.34 (−47.1, 46.46) | .99 | 0.01 |
| Gait speed DTc (m/s) | 1.07 ± 0.21 | 1.09 ± 0.20 | 1.02 ± 0.19 | 1.19 ± 0.24 | 1.20 ± 0.25 | 1.13 ± 0.21 | −0.09 (−0.23, 0.05) | −0.03 (−0.15, 0.10) | −0.06 (−0.23, 0.11) | .47 | 0.21 |
| Gait speed DTs (m/s) | 1.13 ± 0.18 | 1.16 ± 0.22 | 1.07 ± 0.17 | 1.25 ± 0.18 | 1.28 ± 0.19 | 1.24 ± 0.24 | −0.07 (−0.17, 0.02) | −0.02 (−0.09, 0.06) | −0.06 (−0.18, 0.06) | .35 | 0.36 |
| Gait speed ST (m/s) | 1.26 ± 0.16 | 1.22 ± 0.19 | 1.20 ± 0.19 | 1.30 ± 0.18 | 1.33 ± 0.18 | 1.29 ± 0.18 | −0.05 (−0.15, 0.06) | −0.03 (−0.12, 0.07) | −0.02 (−0.16, 0.13) | .81 | 0.12 |
| Absolute DTc cost gait speed | −0.19 ± 0.17 | −0.16 ± 0.11 | −0.18 ± 0.13 | −0.11 ± 0.15 | −0.14 ± 0.15 | −0.15 ± 0.12 | −0.02 (−0.12, 0.07) | −0.01 (−0.10, 0.07) | −0.01 (−0.12, 0.09) | .8 | 0.05 |
| % DTc cost gait speed | −15.0 ± 13.44 | −12.7 ± 9.02 | −14.9 ± 10.10 | −8.73 ± 11.54 | −10.6 ± 12.45 | −12.1 ± 10.50 | −2.01 (−9.47, 5.44) | −1.03 (−7.83, 5.78) | −0.99 (−9.76, 7.78) | .82 | 0.06 |
| Absolute DTs cost gait speed | −0.13 ± 0.10 | −0.06 ± 0.07 | −0.13 ± 0.10 | −0.05 ± 0.12 | −0.05 ± 0.08 | −0.05 ± 0.12 | −0.05 (−0.10, 0.01) | 0.02 (−0.03, 0.07) | −0.07 (−0.14, 0.01) | .07 | 0.81 |
| % DTs cost gait speed | −10.3 ± 8.02 | −5.23 ± 6.15 | −10.5 ± 7.48 | −3.64 ± 9.25 | −3.81 ± 6.27 | −4.15 ± 9.53 | −3.91 (−7.91, 0.09) | 0.75 (−2.66, 4.17) | −4.67 (−9.87, 0.53) | .08 | 0.75 |
| Secondary outcomes | |||||||||||
| UPDRS motor score | 26.38 ± 9.644 | 27 ± 8.24 | 29.42 ± 8.76 | 20.63 ± 8.69 | 25.31 ± 9.89 | 26.21 ± 8.02 (n = 14)d | 4.53 (2.26, 6.79)e | 4.24 (2.34, 6.14)e | 0.28 (−2.75, 3.32) | .85 e | 0.08 |
| PDQ-39 (PDSI) | 17.38 ± 12.42 | 10.45 ± 6.89 | 12.47 ± 8.97 | 16.30 ± 11.21 | 11.52 ± 8.83 | 14.16 ± 11.59 (n = 15)d | −1.88 (−7.31, 3.55) | −2.75 (−7.69, 2.18) | 0.87 (−6.64, 8.39) | .82 | 0.10 |
| TMT A | 44.17 ± 1.76 | 36.43 ± 7.77 | 37.78 ± 11.14 | 37.73 ± 10.56 | 33.61 ± 5.69 | 31.94 ± 5.82 (n = 14)d | −4.08 (−8.83, 0.67) | −7.06 (−11.3, −2.85) | 2.99 (−2.66, 8.63) | .295 | 0.33 |
| TMT B | 84.80 ± 17.44 | 74.61 ± 14.56 | 80.22 ± 24.85 | 89.32 ± 61.60 | 96.00 ± 77.07 | 78.85 ± 87.25 (n = 14)d | 0.40 (−20.0, 20.81) | −15.4 (−33.4, 2.62) | 15.79 (−12.5, 44.10) | .27 | 0.40 |
| TMT B:A ratio | 2.03 ± 0.62 | 2.09 ± 0.38 | 2.18 ± 0.59 | 2.26 ± 0.93 | 2.70 ± 1.61 | 2.44 ± 2.42 (n = 14)d | 0.23 (−0.57, 1.03) | 0.03 (−0.70, 0.76) | 0.20 (−0.92, 1.32) | .72 | 0.12 |
| ABC scale | 80.00 ± 17 | 84.70 ± 15.4 | 85.60 ± 12.0 | 87.20 ± 11.6 | 86.70 ± 12.2 | 84.00 ± 14.2 (n = 15)d | 0.18 (−0.47, 0.83) | −0.13 (−0.69, 0.44) | 0.30 (−0.58, 1.19) | .49 | 0.29 |
| TUG (Average 3 trials) | 9.69 ± 2.71 | 9.20 ± 2.36 | 9.11 ± 2.53 | 9.72 ± 1.88 | 9.23 ± 1.28 | 9.72 ± 1.67 (n = 14)d | −0.35 (−1.09, 0.39) | 0.06 (−0.62, 0.74) | −0.41 (−1.44, 0.62) | .43 | 0.46 |
Abbreviations: ABC, Activity-Specific Balance Confidence; ANOVA, analysis of variance; CI, confidence interval; CV, coefficient of variation; DT, dual-task; PDQ-39, The 39-Item Parkinson’s Disease Questionnaire; PDSI, Parkinson’s Disease Summary Index; STV, stride-time variability; TMT, Trail Making Test; TUG, Timed Up and Go; UPDRS, Unified Parkinson’s Disease Rating Scale.
Values provided are unadjusted mean ± standard deviation (SD).
Values are estimated slope (lower 95% confidence interval, upper 95% confidence interval) for shared-baseline repeated-measure ANOVA models adjusted for age, gender, and UPDRS score.
Values are P values and effect sizes for comparing mean rates of change among Tai Chi versus usual care group for shared-baseline repeated-measure ANOVA models adjusted for age, gender, and UPDRS score.
Specified sample size.
Values are provided for shared-baseline repeated-measure ANOVA models adjusted for age and gender.
Compared to undisturbed walking, gait speed decreased during DT walking in both groups (Table 3). Group differences in this outcome, as well as derived differences in absolute and percent DT cost, were small and not statistically significant (see Table 3).
Performance on both cognitive tasks was comparable overall between groups. Accuracy of serial subtractions was equivalent while seated and during walking (93% and 94% in TC and UC, respectively). Accuracy of the Star Movement Task decreased slightly during gait (86% and 87% in TC and UC, respectively) compared to seated (95% and 90% in TC and UC, respectively).
Statistically nonsignificant trends at 6 months indicating greater improvements in the TC versus UC group were observed in PD-related QoL, ABC, and the TUG. Both groups improved slightly in TMT, but the results were not statistically significant. Repeated-measure ANOVA models adjusting for variation due to baseline score, age and gender revealed that UPDRS progression was modest and very similar in TC and UC groups.
Discussion
The present study aimed to assess the feasibility of conducting an RCT of TC training for individuals in the early stages of PD progression and assessing outcomes targeting cognitive-motor interdependence in the off-medication state. Based on the observed recruitment, adherence, and retention rates, a future RCT fully powered to evaluate the effectiveness of TC for PD patients would likely be achievable with modifications to improve recruitment and adherence rates. Furthermore, our ability to detect small clinical differences between the TC and usual care group allowed us to identify promising markers of improvement to use in future trials of this intervention.
Outcomes
A recent meta-analysis based on 15 RCTs supports a benefit of TC for reducing falls and improving balance for individuals with PD.20 However, the cognitive and motor mechanisms underlying these benefits remain poorly understood. A primary motivation for this pilot study was to lay the foundation for exploring the hypothesis that TC reduces the impact of a cognitive distraction on gait steadiness in PD (ie, DT gait STV). Studies have shown that DT STV is a promising discriminating metric for predicting falls and understanding PD disease progression36,37 and prior TC research has demonstrated improvements in DT STV in healthy older adults following 6 months of TC training.36 However, prior studies of TC for individuals with PD have not evaluated the impact of TC on DT STV in this population. While our study was not adequately powered to assess the efficacy of TC on DT gait STV, we observed a clear trend towards greater reductions in DTcount STV in the TC group when compared to the wait-list control. This finding supports that STV is a good outcome for evaluating cognitive-motor improvement in future studies, potentially informing the mechanism underlying improved postural control following TC training.
The prevailing neuropsychological theory used to explain the difficulties of performing 2 concurrent cognitive tasks posits that each task competes for shared brain resources.38 The extent to which performance is disrupted in one or both tasks thus depends upon resource availability,39 resource allocation,40,41 and/or the speed of information processing.42 Therefore, the observation that performing a cognitive task while walking disrupts locomotor control suggests that these tasks compete for shared resources involving specific neural networks. Age- and/or Parkinson’s-related increases in the cognitive task costs on locomotor control may arise in part from reduction in cortical resources, faulty allocation of resources,43 or slowing of the speed at which information is processed.41 Future studies that combine clinical DT gait assessments with targeted neuroimaging-based brain activation patterns and resting state neural network connectivity would significantly contribute to evaluating the neural basis underlying TC’s impact on cognitive-motor interactions in PD.
Other outcomes we observed to be sensitive to TC and to be considered in future trials include TUG and ABC. There was a trend toward the TC group improving in the TUG, although our observed magnitude of decrease was smaller than that found in a previous large RCT of TC training in PD.21 Statistically nonsignificant positive trends were also observed in ABC. Baseline scores for both these outcomes indicated relatively low levels of impairment compared to prior studies, which may be due to our population being less progressed in their PD. This, in turn, may have led to a potential ceiling effect precluding larger improvements. UPDRS scores worsened in both groups, as is expected over 6 months of PD progression. However, scores increased less in the TC group, suggesting potential slowing of motor symptom progression with TC training. In contrast, there were no observed trends in executive function evaluated with the TMT. Future studies should consider a wider battery of tests to evaluate executive and other domains of cognition.
Lessons Learned and Design of Future Trial
A total of 98 individuals were assessed for eligibility, demonstrating that recruitment efforts were successful in reaching people with PD and that the study was of interest. Nearly half (48%) of those screened did not meet eligibility criteria (Figure 1). Obstacles to commitment included class location, class start time, or a combination of both. Enrollment rates for a larger RCT could likely be enhanced by offering participants a choice of class site and start time. A longer recruitment window and use of multiple recruiting centers would also facilitate recruitment of a larger sample size.
Power calculations based on preliminary findings suggest that if the effect of TC on DTcount STV is as observed in this study (a magnitude associated with a clinically meaningful reduction in falls4,44), then a sample size of 134 (67 per group) would provide 80% power for a future trial when testing at 2-tailed P < .05. This magnitude of effect might be further increased by improved TC compliance and a longer exposure period. As the optimal dose of TC for PD is not known, it is possible that a longer period of practice may be necessary to observe clinically meaningful improvements. To sustain adherence over a longer intervention period, the use of incentives could be considered, as employed in other longer-term TC studies.45 Enhancement of the effect size of TC may also require adjustments to the training protocol. The optimal intensity level of exercise for individuals with PD is currently under investigation; however, some evidence suggests that higher intensity exercise might be beneficial.46–49 As our study participants were in the early stages of PD and relatively high functioning, perhaps they would have benefited from a more challenging training program. A future TC regimen might include a greater emphasis on, and an earlier introduction of, challenging training elements such as greater emphasis on single leg stance, dynamic agility, varying movement tempos, and adding additional sequences requiring more complex cognitive-motor coordination.50 While we conducted testing of subjects in the off state, prior studies of TC for PD have conducted testing of subjects while on their PD medications, which could potentially account for the differences in results. Testing in both on- and off-medication state in future studies should be considered.
Figure 2.
Mean stride-time variability (STV) during dual-task serial subtraction (DTcount) walking at baseline, 3, and 6 months by group.
To control for attention and expectation associated with the intervention, a future study could include an additional active control arm. For example, seated stretching has been shown to be a relatively inert comparison intervention in this population21 and might be considered.
Limitations
We recognize several limitations. The sample size of both groups was small and treatment duration was relatively short. Due to the nature of the intervention, participants could not be blinded to their treatment assignments, introducing bias and a potential impact of expectation on outcomes. The study also did not include an active control, which limits our ability to interpret the effects of TC and identify potential mechanisms.
Acknowledgments
The authors would like to thank all our study participants, our research team at Spaulding Rehabilitation Hospital, Stefano Sapienza, Eric Fabara, Matilde Bertoli, and Isabelle Pitteloud, and our TC instructors, Stanwood Chang, Jane Moss, and Nancy Couts, without whom the study would not have been possible.
Declaration of Conflicting Interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: PMW is the founder and sole owner of the Tree of Life Tai Chi Center. PMW’s interests were reviewed and managed by the Brigham and Women’s Hospital and Partner’s HealthCare in accordance with their conflict of interest policies. The other authors have no conflicts of interest to declare.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by grants from the Osher Center for Integrative Medicine and the Davis Phinney Foundation for Parkinson’s. PMW was supported by a NCCIH-funded K24 (AT009282) and GV-D has been supported by a fellowship from Alfonso Martin Escudero Foundation (Spain) and a grant from Real Colegio Complutense at Harvard. JGVM was supported by a fellowship from the Brazilian National Council of Research and Development (CNPQ, Brazil) (201499/2012-6).
Trial Registration
Trial registration number: Clinical Trials, protocol registration system: NCT02418780.
References
- 1.Ascherio A, Schwarzschild MA. The epidemiology of Parkinson’s disease: risk factors and prevention. Lancet Neurol. 2016;15(12):1257–1272. [DOI] [PubMed] [Google Scholar]
- 2.de Lau LM, Breteler MM. Epidemiology of Parkinson’s disease. Lancet Neurol. 2006;5(6):525–535. [DOI] [PubMed] [Google Scholar]
- 3.Neurological Disorders: Public Health Challenges. Geneva, Switzerland: World Health Organization; 2006.
- 4.Hausdorff JM. Gait dynamics in Parkinson’s disease: common and distinct behavior among stride length, gait variability, and fractal-like scaling. Chaos (Woodbury, N.Y.). 2009;19(2):026113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Redgrave P, Rodriguez M, Smith Y, et al. Goal-directed and habitual control in the basal ganglia: implications for Parkinson’s disease. Nat Rev Neurosci. 2010;11(11):760–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu T, Hallett M. Neural correlates of dual task performance in patients with Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2008;79(7):760–766. [DOI] [PubMed] [Google Scholar]
- 7.Vervoort G, Heremans E, Bengevoord A, et al. Dual-task-related neural connectivity changes in patients with Parkinson’ disease. Neuroscience. 2016;317: 36–46. [DOI] [PubMed] [Google Scholar]
- 8.Hausdorff JM, Balash J, Giladi N. Effects of cognitive challenge on gait variability in patients with Parkinson’s disease. J Geriatr Psychiatry Neurol. 2003;16(1):53–58. [DOI] [PubMed] [Google Scholar]
- 9.Maidan I, Rosenberg-Katz K, Jacob Y, et al. Altered brain activation in complex walking conditions in patients with Parkinson's disease. Parkinsonism Relat Disord. 2016;25:91–96. [DOI] [PubMed]
- 10.Maidan I, Bernad-Elazari H, Giladi N, Hausdorff JM, Mirelman A. When is higher level cognitive control needed for locomotor tasks among patients with Parkinson’s disease? Brain Topography. 2017;30(4):531–538. [DOI] [PubMed] [Google Scholar]
- 11.Sarter M, Albin RL, Kucinski A, Lustig C. Where attention falls: increased risk of falls from the converging impact of cortical cholinergic and midbrain dopamine loss on striatal function. Exp Neurol. 2014;257: 120–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yogev G, Giladi N, Peretz C, Springer S, Simon ES, Hausdorff JM. Dual tasking, gait rhythmicity, and Parkinson’s disease: which aspects of gait are attention demanding? Eur J Neurosci. 2005;22(5):1248–1256. [DOI] [PubMed] [Google Scholar]
- 13.Herman T, Mirelman A, Giladi N, Schweiger A, Hausdorff JM. Executive control deficits as a prodrome to falls in healthy older adults: a prospective study linking thinking, walking, and falling. J Gerontol A Biol Sci Med Sci. 2010;65(10):1086–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mirelman A, Gurevich T, Giladi N, Bar-Shira A, Orr-Urtreger A, Hausdorff JM. Gait alterations in healthy carriers of the LRRK2 G2019S mutation. Ann Neurol. 2011;69(1):193–197. [DOI] [PubMed] [Google Scholar]
- 15.Frenkel-Toledo S, Giladi N, Peretz C, Herman T, Gruendlinger L, Hausdorff JM. Treadmill walking as an external pacemaker to improve gait rhythm and stability in Parkinson’s disease. Mov Disord. 2005;20(9):1109–1114. [DOI] [PubMed] [Google Scholar]
- 16.Herman T, Giladi N, Gruendlinger L, Hausdorff JM. Six weeks of intensive treadmill training improves gait and quality of life in patients with Parkinson’s disease: a pilot study. Arch Phys Med Rehabil. 2007;88(9):1154–1158. [DOI] [PubMed] [Google Scholar]
- 17.Wayne PM, Gow BJ, Costa MD, et al. Complexity-based measures inform effects of Tai Chi Training on standing postural control: cross-sectional and randomized trial studies. PLoS One. 2014;9(12):e114731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Song R, Ahn S, So H, Lee EH, Chung Y, Park M. Effects of t’ai chi on balance: a population-based meta-analysis. J Altern Complement Med. 2015;21(3):141–151. [DOI] [PubMed] [Google Scholar]
- 19.Wayne PM, Fuerst ML. The Harvard Medical School Guide to Tai Chi, Boston, MA: Shambhala Publications Inc, 2013. [Google Scholar]
- 20.Song R, Grabowska W, Park M, et al. The impact of Tai Chi and Qigong mind-body exercises on motor and non-motor function and quality of life in Parkinson’s disease: a systematic review and meta-analysis. Parkinsonism Relat Disord. 2017;41: 3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li F, Harmer P, Fitzgerald K, et al. Tai chi and postural stability in patients with Parkinson’s disease. N Engl J Med. 2012;366(6):511–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eldridge SM, Chan CL, Campbell MJ, et al. CONSORT 2010 statement: extension to randomised pilot and feasibility trials. BMJ. 2016;355: i5239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goetz CG, Poewe W, Rascol O, et al. Movement Disorder Society Task Force report on the Hoehn and Yahr staging scale: status and recommendations. Mov Disord. 2004;19(9):1020–1028. [DOI] [PubMed] [Google Scholar]
- 24.McGibbon CA, Krebs DE, Parker SW, et al. Tai Chi and vestibular rehabilitation improve vestibulopathic gait via different neuromuscular mechanisms: preliminary report. BMC Neurol. 18;5(1):3. [DOI] [PMC free article] [PubMed]
- 25.Manor B, Lough M, Gagnon MM, Cupples A, Wayne PM, Lipsitz LA. Functional benefits of tai chi training in senior housing facilities. J Am Geriatr Soc. 2014;62(8):1484–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yeh GY, McCarthy EP, Wayne PM, et al. Tai chi exercise in patients with chronic heart failure: a randomized clinical trial. Arch Intern Med. 2011;171(8):750–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moy ML, Wayne PM, Litrownik D, et al. Long-term Exercise After Pulmonary Rehabilitation (LEAP): design and rationale of a randomized controlled trial of Tai Chi. Contemp Clin Trials. 2015;45(Pt B):458–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sturnieks DL, St George R, Fitzpatrick RC, Lord SR. Effects of spatial and nonspatial memory tasks on choice stepping reaction time in older people. J Gerontol A Biol Sci Med Sci. 2008;63(10):1063–1068. [DOI] [PubMed] [Google Scholar]
- 29.Shimmer Platform. http://www.shimmersensing.com/. Accessed April 4, 2017.
- 30.Perlmutter JS. Assessment of Parkinson disease manifestations. Curr Protoc Neurosci. 2009 Oct;Chapter 10:Unit 10.1. [DOI] [PMC free article] [PubMed]
- 31.Peto V, Jenkinson C, Fitzpatrick R. Determining minimally important differences for the PDQ-39 Parkinson’s disease questionnaire. Age Ageing. 2001;30(4):299–302. [DOI] [PubMed] [Google Scholar]
- 32.Bowie CR, Harvey PD. Administration and interpretation of the Trail Making Test. Nat Protoc. 2006;1(5):2277–2281. [DOI] [PubMed] [Google Scholar]
- 33.Arbuthnott K, Frank J. Trail making test, part B as a measure of executive control: validation using a set-switching paradigm. J Clin Exp Neuropsychol. 2000;22(4):518–528. [DOI] [PubMed] [Google Scholar]
- 34.Myers AM, Fletcher PC, Myers AH, Sherk W. Discriminative and evaluative properties of the activities-specific balance confidence (ABC) scale. J Gerontol A Biol Sci Med Sci. 1998;53(4):M287–M294. [DOI] [PubMed] [Google Scholar]
- 35.Nocera JR, Stegemoller EL, Malaty IA, Okun MS, Marsiske M, Hass CJ. Using the timed up & go test in a clinical setting to predict falling in Parkinson’s disease. Arch Phys Med Rehabil. 2013;94(7):1300–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wayne PM, Hausdorff JM, Lough M, et al. Tai Chi training may reduce dual task gait variability, a potential mediator of fall risk, in healthy older adults: cross-sectional and randomized trial studies. Front Hum Neurosci. 2015;9: 332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mirelman A, Herman T, Brozgol M, et al. Executive function and falls in older adults: new findings from a five-year prospective study link fall risk to cognition. PLoS One. 2012;7(6):e40297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pashler H. Dual-task interference in simple tasks: data and theory. Psychol Bull. 1994;116(2):220–244. [DOI] [PubMed] [Google Scholar]
- 39.Tombu M, Jolicoeur P. A central capacity sharing model of dual-task performance. J Exp Psychol Hum Percept Perform. 2003;29(1):3–18. [DOI] [PubMed] [Google Scholar]
- 40.Kahneman D. Attention and Effort, Englewood Cliffs, NJ: Prentice-Hall, 1973. [Google Scholar]
- 41.Navon D, Gopher D. On the economy of the human-processing system. Psychol Rev. 1979;86(3):214. [Google Scholar]
- 42.Dux PE, Tombu MN, Harrison S, Rogers BP, Tong F, Marois R. Training improves multitasking performance by increasing the speed of information processing in human prefrontal cortex. Neuron. 2009;63(1):127–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pashler H. Shifting visual attention and selecting motor responses: distinct attentional mechanisms. J Exp Psychol Hum Percep Perform. 1991;17(4):1023–1040. [DOI] [PubMed] [Google Scholar]
- 44.Henderson EJ, Lord SR, Brodie MA, et al. Rivastigmine for gait stability in patients with Parkinson’s disease (ReSPonD): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Neurol. 2016;15(3):249–258. [DOI] [PubMed] [Google Scholar]
- 45.Wayne PM, Gagnon MM, Macklin EA, et al. The Mind Body-Wellness in Supportive Housing (Mi-WiSH) study: design and rationale of a cluster randomized controlled trial of Tai Chi in senior housing. Contemp Clin Trials. 2017;60: 96–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kelly NA, Ford MP, Standaert DG, et al. Novel, high-intensity exercise prescription improves muscle mass, mitochondrial function, and physical capacity in individuals with Parkinson’s disease. J Appl Physiol. 2014;116(5):582–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morberg BM, Jensen J, Bode M, Wermuth L. The impact of high intensity physical training on motor and non-motor symptoms in patients with Parkinson’s disease (PIP): a preliminary study. NeuroRehabilitation. 2014;35(2):291–298. [DOI] [PubMed] [Google Scholar]
- 48.Alberts JL, Phillips M, Lowe MJ, et al. Cortical and motor responses to acute forced exercise in Parkinson’s disease. Parkinsonism Relat Disord. 2016;24: 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Beall EB, Lowe MJ, Alberts JL, et al. The effect of forced-exercise therapy for Parkinson’s disease on motor cortex functional connectivity. Brain Connect. 2013;3(2):190–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.King LA, Horak FB. Delaying mobility disability in people with Parkinson disease using a sensorimotor agility exercise program. Phys Ther. 2009;89(4):384–393. [DOI] [PMC free article] [PubMed] [Google Scholar]