Abstract
Background
Subacromial corticosteroid injections are frequently performed for pain associated with supraspinatus tendinopathy. Glucose prolotherapy has been used clinically for multiple tendinopathies and is hypothesized to be an alternate injection therapy for supraspinatus tendinopathy.
Methods
A prospective, randomized, double blinded clinical trial was conducted. Thirty-six patients with supraspinatus tendinopathy were randomized into two groups: 17 received an ultrasound-guided injection of glucose into the tendinopathic parts of the supraspinatus tendon and 19 received an ultrasound-guided injection of corticosteroid into the subacromial bursa. Primary outcome was level of pain with overhead activities at 3 months. Secondary outcome measures included level and frequency of pain and function, shoulder range of motion, impingement tests, strength and tendon changes on ultrasound.
Results
Level of pain with overhead activities was significantly reduced at the 3-month follow-up in the prolotherapy group and at the 6-month follow-up for both the prolotherapy and corticosteroid groups. There were no significant differences between the groups at any time point.
Conclusions
Both glucose prolotherapy and corticosteroid were generally well tolerated; however, glucose prolotherapy offered no additional benefit over subacromial corticosteroid injection for supraspinatus tendinopathy.
Keywords: corticosteroid injection, glucose prolotherapy, rotator cuff dysfunction, supraspinatus tendinopathy
Introduction
Rotator cuff dysfunction secondary to tendinopathy of the supraspinatus tendon is a common cause of shoulder pain in adults and is characterized by painful functional limitation of the shoulder, especially with overhead activities. Histopathology of symptomatic rotator cuff tendons reveals mucoid degeneration and fibrocartilagnous metaplasia similar to changes found in painful Achilles and patella tendons.1
The American Academy of Orthopaedic Surgeons’ clinical practice guidelines for the management of rotator cuff problems suggest that patients with rotator cuff related symptoms, including tendinopathic changes to the supraspinatus tendon in the absence of full thickness tears, should initially be treated non-operatively using exercise and/or nonsteroidal anti-inflammatory drugs based on a moderate strength recommendations and cannot recommend being for against the use of subacromial corticosteroid injection based on inconclusive evidence.2 Despite this, subacromial corticosteroid injections are one of the most frequently used management tools in supraspinatus tendinopathy.
Prolotherapy involves the injection of a small amount of solution into tissues with the aim of inducing healing of the injured structure. Prolotherapy solutions are considered to induce the proliferation of cells to help strengthen, tighten and heal the tendon or other injured tissue.3
At concentrations greater than 10%, glucose is presumed to cause an osmotic gradient outside the cells where it is injected, causing some cells to lose water and lyse, leading to an influx of growth factors and inflammatory cells that are then assumed to initiate the wound-healing cascade in the local area, including the deposition of collagen.3 New collagen loses volume and contracts as it matures, leaving a more robust and tighter ligament or tendon.4
Prolotherapy has been studied for its use in the treatment of common extensor tendinopathy of the elbow, Achilles tendinopathy, plantar fasciitis and patella tendonopathies;5–8 however, there are no published papers evaluating its use in supraspinatus tendinopathy.
The present study therefore aimed to directly compare glucose prolotherapy injection into the supraspinatus tendon with corticosteroid injection into the subacromial bursa abutting the tendinopathic supraspinatus tendon for treatment of supraspinatus tendinopathy.
Materials and Methods
Study design
The present study comprised a prospective, double-blind, randomized clinical controlled trial to compare the injection of glucose prolotherapy into the supraspinatus tendon versus corticosteroid into the subacromial bursa for the treatment of symptomatic supraspinatus tendinopathy. The study was approved by the local Ethics Committee.
Setting and participants
We invited general practitioners in the local area to refer patients with symptoms and signs of rotator cuff dysfunction for the evaluation of the existence of supraspinatus tendinopathy and possible inclusion in the study. Advertisements for the study were sent to the local Surf Life Saving Clubs and posted on social media. All patients who presented to the rooms of the senior author were considered for inclusion in the study.
Eligbility criteria
To be included in the present study, participants had to present with symptomatic supraspinatus tendinopathy of at least 3 months in duration, as diagnosed on the basis of a history of shoulder pain with overhead activities, positive impingement signs, pain with supraspinatus testing and ultrasound evidence of abnormal hypoechoic areas or anechoic clefts or foci in the supraspinatus tendon suggesting tendinopathy. Ultrasound and X-ray were performed on all participants. All subjects were required to be over 18 years of age, and were excluded if they had previous shoulder surgery in the past 12 months, rotator cuff tears greater than 50% of the tendon thickness, calcific tendinitis, adhesive capsulitis, inflammatory arthritis, acromioclavicular joint pain, os acromiale, glenohumeral osteoarthritis, previous fracture in the past 6 months, bone tumours or osteonecrosis as seen on X-ray.
Power analysis
Based on data from a pilot study (n = 5 in each group) to determine the standard deviation for the change in level of pain (0.55), a sample size calculation was performed using an analysis of variance model with a minimum detectable difference of one step in the Likert scale (0 = none, 1 = mild, 2 = moderate, 3 = severe, 4 = very severe) and one level of difference was determined to be clinically significant. The calculation was performed using significant level alpha of 0.05 and 80% power. Using this calculation, it was determined that, to have a 95% chance of finding an 80% difference between the two groups for level of pain with overhead activity at 3 months after injection, the trial would require at least six patients in each group and 12 patients in total to be recruited.
Randomization and groups
After gaining informed consent, the patients were randomized by computer-generated randomization (Random Allocation Software, version 1.0.0; Isfahan University of Medical Sciences, Isfahan, Iran) to receive either an ultrasound-guided corticosteroid injection into the subacromial bursa adjacent to the area of supraspinatus tendinopathy (corticosteroid group) or an ultrasound-guided glucose prolotherapy injection into the area of supraspinatus tendinopathy (prolotherapy group). The injection fluid for the corticosteroid group contained 1 mL of 40 mg/mL methylprednisolone acetate (Depo-Medrol; Pfizer, West Ryde, NSW, Australia) and 1 mL of 1% lignocaine hydrochloride (Xylocaine; AstraZeneca, Macquarie Park, NSW, Australia). The injection fluid for the prolotherapy group contained 1 mL of 50% glucose (25 g/50 mL) (Glucose 50%; Phebra, Lane Cove West, NSW, Australia) and 1 mL of 1% lignocaine hydrochloride (Xylocaine, AstraZeneca) giving a 25% glucose prolotherapy solution.
Injection protocol
Ultrasound was performed by an experienced musculoskeletal sonographer using a Logiq E9 machine (General Electric, Boston, MA, USA) with a 6–15 MHz linear transducer. All patients received an ultrasound prior to, at the time of the injection and at the 3-month follow-up. The pathological area of the supraspinatus tendon was identified and graded using a five-point grading scale from zero (normal tendon) to four (partial thickness tear) (see Supplementary material, Appendix, Table A1). All injections were performed by a single surgeon with over 10 years of experience with respect to performing subacromial injections.
Both injections were performed using a lateral approach. With the patient in an upright sitting position, a 22-gauge needle was directed towards either the subacromial bursa adjacent to the tendinopathic area of supraspinatus (corticosteroid) or the hypoechoic/anechoic areas of the supraspinatus tendon (glucose) as guided by the sonographer until the tip of the needle was seen in the correct position. The corticosteroid was injected into the subacromial bursa. The glucose injection was performed in multiple areas of the supraspinatus tendon, depending on how many hypoechoic and anechoic clefts there were, with no more than 0.5 mL being injected into each discreet area of the tendon.
Patients and the outcome assessor were blinded to treatment group allocation and injection received.
Clinical outcomes
Baseline demographics and clinical characteristics were recorded prior to injection including age, sex, affected shoulder and duration of symptoms.
The main outcome measure was level of pain with overhead activities at 3 months scored on a patient-rated five-point Likert scale (very severe, severe, moderate, mild or none).
Secondary outcome measures included level of night pain, frequency of night pain, frequency of pain with activity, overall shoulder satisfaction, shoulder range of motion, impingement tests and shoulder strength.
A single examiner visually assessed passive shoulder range of motion in forward flexion, abduction and external rotation, as well as hand-held dynamometer measurements of muscle force in internal and external rotation at neutral with the elbow at 90°, supraspinatus position of 90° of shoulder abduction in the scapula plane and hand behind back lift off, as described and validated previously.9
Internal and external rotation impingement tests were performed at each follow-up. Ultrasound assessment of the supraspinatus tendon was performed at baseline and 3 months.
Treatment protocol
Patients were restricted from the use of any aspirin or anti-inflammatory medication for 4 weeks after injection. They were asked to refrain from any heavy lifting in the week following the injection and to use ice and paracetamol for any post-injection pain. They were advised to start a home rehabilitation programme at 2 weeks after injection consisting of but not limited to scapula retraction exercises, rowing and external rotation with yellow theraband performed at least twice daily. They were instructed on correct technique at initial visit and given written instructions for all exercises. Technique was reviewed at the 6-week and 3-month follow-up.
Statistical analysis
Statistical analysis using intention to treat was performed using SPSS, version 22 (IBM, Armonk, NY, USA) and Prism, version 6 (GraphPad Software, Inc., San Diego, CA, USA). For nonparametric data such as pain scores, the Mann–Whitney rank sum test was used to assess differences between groups at different time points. p < 0.05 was considered statistically significant. A Wilcoxon signed rank test was used to used to assess differences within each group between different time points.
For parametric data such as shoulder strength and range of motion, an unpaired Student’s t-test was used to assess differences between groups at different time points. p < 0.05 was considered statistically significant. A paired Students t-test was used to assess differences within each group between different time points.
Fisher’s exact test was use to assess dichotomous data, such as patient demographics and impingement signs.
Results
There were 36 shoulders in the present study: 17 in the prolotherapy group and 19 in the corticosteroid group. There were 27 males and nine females, with a mean age of 48 years (range 22 years to 78 years). Mean duration of symptoms was 26 months (range 3 months to 180 months). No significant difference was found between the groups for sex, age, duration of symptoms, shoulder involved, dominant side affected and workers compensation status (p > 0.05) (Table 1).
Table 1.
Patient demographics.
| Prolotherapy group | Corticosteroid group | p-value | |
|---|---|---|---|
| Number of shoulders | 17 | 19 | |
| Male : Female | 13:4 | 14:5 | 1 |
| Age (years), mean (SD) | 51 (16) | 46(15) | 0.29 |
| Duration of symptoms (months), mean (SD) | 23 (43) | 28 (25) | 0.67 |
| Left : Right shoulder | 7:10 | 7:12 | 1 |
| Dominant hand (Yes : No) | 10:7 | 11:8 | 1 |
| Workers compensation (Yes : No) | 1:16 | 3:16 | 0.61 |
| Pain scores | |||
| Overall shoulder satisfaction, mean (SEM)* | 1.9 (0.2) | 1.8 (0.2) | 0.89 |
| Frequency of shoulder pain with activity, mean (SEM)** | 3.2 (0.2) | 3.0 (0.2) | 0.47 |
| Frequency of shoulder pain during sleep, mean (SEM)** | 3.0 (0.2) | 2.7 (0.2) | 0.4 |
| Level of shoulder pain with activities above the head, mean (SEM)*** | 2.3 (0.2) | 2.6 (0.2) | 0.28 |
| Level of shoulder pain during sleep, mean (SEM)*** | 1.5 (0.3) | 2.0 (0.2) | 0.09 |
| Range of motion scores | |||
| Forward flexion (°), mean (SEM) | 167 (3) | 161 (7) | 0.43 |
| Abduction (°), mean (SEM) | 166 (5) | 153 (7) | 0.17 |
| External rotation (°), mean (SEM) | 67 (4) | 60 (4) | 0.23 |
| Strength | |||
| External rotation (N), mean (SEM) | 84 (7) | 83 (8) | 0.9 |
| Internal rotation (N), mean (SEM) | 89 (7) | 85 (9) | 0.76 |
| Supraspinatus (N), mean (SEM) | 59 (8) | 68 (8) | 0.41 |
| Lift off (N), mean (SEM) | 53 (5) | 50 (6) | 0.67 |
*Scored on a five-point Likert scale (very bad, bad, poor, fair, good) and converted to a numerical score from 0 to 4.
**Scored on a five-point Likert scale (never, monthly, weekly, daily, always) and converted to a numerical score from 0 to 4.
**Scored on a five-point Likert scale (none, mild, moderate, severe, very severe) and converted to a numerical score from 0 to 4.
There were no significant differences between groups in baseline pain scores, shoulder range of motion or strength values (p > 0.05) (Table 1).
All participants were followed up at 6 weeks, 3 months and 6 months after injection. There were three patients who were unable to attend the 6-week appointment (all in the corticosteroid group) and their subjective pain scores were taken by phone call or e-mail. Two patients (one in each group, different from the previous three) could not attend the 3-month follow-up and five missed the 6-month follow-up (three in the corticosteroid group, two in the prolotherapy group), all submitted subjective scores via phone or e-mail. Ultrasounds were performed at the 3-month follow-up on all patients who attended, and at the next available time for the two patients who could not attend the follow-up appointment (both performed prior to the 6-month follow-up).
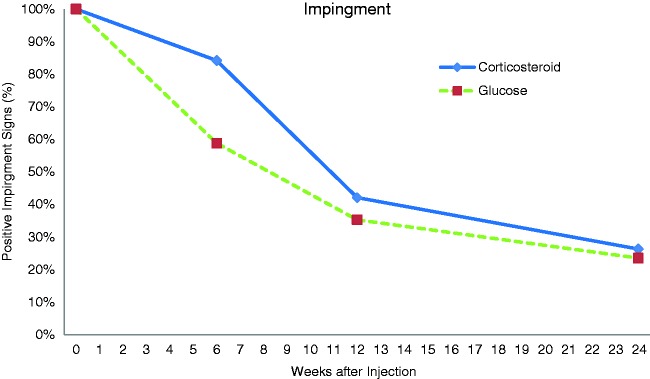
All patients (100%) had positive impingement tests at the beginning of the study. At 6-week follow-up, this had reduced to 59% in the prolotherapy group and 84% in the corticosteroid group (p = 0.13) (Fig. 1). There were less subjects with positive impingements tests at the 3-month follow-up (35% in the prolotherapy group, 42% in the corticosteroid group) (p = 0.74). At 6-month follow-up, there were further reductions in positive impingement signs in both groups (24% in the prolotherapy group, 26% in the corticosteroid group) with no significant difference between groups (p = 1).
Figure 1.
Percentage of subjects with positive impingement signs.
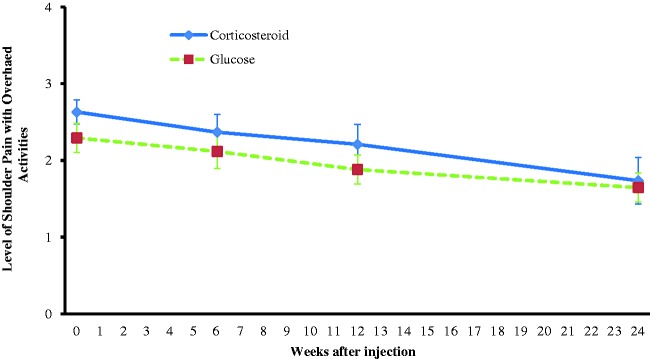
There was no significant difference between the groups at baseline for pain with overhead activity (moderate to severe range). Only the prolotherapy group had a significant reduction for pain with overhead activity at 3 months (mild–moderate) (P = 0.035); however, there remained no significant difference between the groups (p = 0.42) (Table 2). By 6 months, there was significantly less pain compared to baseline (mild to moderate) in both groups (prolotherapy p = 0.002; corticosteroid, p = 0.004) and there remained no significant difference between the groups (p = 0.99) (Fig. 2 and Table 2).
Table 2.
Pain scores over time.
| Baseline | 6 weeks | p-value (baseline to 6 weeks) | 3 months | p-value (baseline to 3 months) | 6 months | p-value (baseline to 6 months) | |
|---|---|---|---|---|---|---|---|
| Overall shoulder satisfaction, mean (SEM)* | |||||||
| Prolotherapy | 1.9 (0.2) | 2.2 (0.2) | 0.59 | 2.5 (0.3) | 0.013 | 2.8 (0.2) | 0.013 |
| Corticosteroid | 1.8 (0.2) | 1.8 (0.3) | 1 | 2.1 (0.3) | 0.236 | 2.4 (0.3) | 0.24 |
| p-value Prolotherapy versus Corticosteroid | 0.89 | 0.3 | 0.3 | 0.3 | |||
| Frequency of shoulder pain with activity, mean (SEM)** | |||||||
| Prolotherapy | 3.2 (0.2) | 2.9 (0.2) | 0.096 | 2.5 (0.2) | 0.004 | 2.5 (0.2) | 0.006 |
| Corticosteroid | 3.0 (0.2) | 2.9 (0.3) | 0.655 | 2.8 (0.2) | 0.493 | 2.3 (0.3) | 0.054 |
| p-value value Prolotherapy versus Corticosteroid | 0.47 | 0.96 | 0.28 | 0.79 | |||
| Frequency of shoulder pain during sleep, mean (SEM)** | |||||||
| Prolotherapy | 3.0 (0.2) | 2.5 (0.3) | 0.033 | 2.0 (0.3) | 0.004 | 2.2 (0.3) | 0.013 |
| Corticosteroid | 2.7 (0.2) | 2.6 (0.3) | 0.558 | 2.6 (0.3) | 0.453 | 2.0 (0.4) | 0.016 |
| p-value value Prolotherapy versus Corticosteroid | 0.4 | 0.74 | 0.16 | 0.62 | |||
| Level of shoulder pain with activities above the head, mean (SEM)*** | |||||||
| Prolotherapy | 2.3 (0.2) | 2.1 (0.2) | 0.317 | 1.9 (0.2) | 0.035 | 1.7 (0.2) | 0.002 |
| Corticosteroid | 2.6 (0.2) | 2.4 (0.2) | 0.166 | 2.2 (0.3) | 0.059 | 1.7 (0.3) | 0.004 |
| p-value Prolotherapy versus Corticosteroid | 0.28 | 0.5 | 0.42 | 0.99 | |||
| Level of shoulder pain during sleep, mean (SEM)*** | |||||||
| Prolotherapy | 1.5 (0.3) | 1.7 (0.3) | 0.102 | 1.4 (0.3) | 0.527 | 1.4 (0.2) | 0.564 |
| Corticosteroid | 2.0 (0.2) | 2.0 (0.3) | 0.813 | 1.6 (0.2) | 0.033 | 1.2 (0.3) | 0.005 |
| p-value Prolotherapy versus Corticosteroid | 0.09 | 0.69 | 0.37 | 0.53 |
*Scored on a five-point Likert scale (very bad, bad, poor, fair, good) and converted to a numerical score from 0 to 4.
**Scored on a five-point Likert scale (never, monthly, weekly, daily, always) and converted to a numerical score from 0 to 4.
**Scored on a five-point Likert scale (none, mild, moderate, severe, very severe) and converted to a numerical score from 0 to 4.
p-values shown in bold are statistically significant (p < 0.05).
Figure 2.
Level of shoulder pain with overhead activities.
Overall shoulder satisfaction increased throughout the trial for both groups; however, the increase was only significant for the prolotherapy group at 3 months and 6 months and there was no significant differences between groups at all time points (Table 2).
There was a significant decrease in the frequency of shoulder pain with activity in the prolotherapy group at 3 months and 6 months, although there was no significant difference between groups. There was a significant decrease in frequency of pain during sleep at 6 weeks, 3 months and 6 months in the prolotherapy group and 6 months in the corticosteroid group, although there were no significant differences between groups at any time point for frequency of pain during sleep (Table 2).
There was no significant difference between the two groups for ultrasound appearance at baseline (p = 0.35). The appearance of the supraspinatus tendon significantly improved compared to baseline for both the prolotherapy group (p = 0.006) and the corticosteroid group (p = 0.018) at 3 months, with no significant difference between the groups (p = 0.44). There was no individual correlation between tendon changes and pain and function scores at 3 months, with some of the most pathological tendons scoring mild to no pain with overhead activity and some normal tendons scoring severe to very severe pain with overhead activity.
There was a significant increase in supraspinatus strength in the prolotherapy group at 3 months (p = 0.02) that was maintained at 6 months; however, there was no significant difference in range of motion or strength between groups at either time point (Tables 3 and 4).
Table 3.
Range of motion scores over time.
| Baseline | 6 weeks | p-value (baseline to 6 weeks) | 3 months | p-value (baseline to 3 months) | 6 months | p-value (baseline to 6 months) | |
|---|---|---|---|---|---|---|---|
| Forward flexion (°), mean (SEM) | |||||||
| Prolotherapy | 167 (3) | 169 (3) | 0.163 | 173 (2) | 0.013 | 172 (2) | 0.07 |
| Corticosteroid | 161 (7) | 165 (4) | 0.525 | 172 (3) | 0.079 | 165 (7) | 0.592 |
| p-value Prolotherapy versus Corticosteroid | 0.43 | 0.38 | 0.7 | 0.31 | |||
| Abduction (°), mean (SEM) | |||||||
| Prolotherapy | 166 (5) | 168 (6) | 0.387 | 175 (0) | 0.06 | 175 (2) | 0.088 |
| Corticosteroid | 153 (8) | 158 (8) | 0.2311 | 163 (7) | 0.044 | 163 (8) | 0.106 |
| p-value Prolotherapy versus Corticosteroid | 0.17 | 0.3 | 0.1 | 0.15 | |||
| External rotation (°), mean (SEM) | |||||||
| Prolotherapy | 67 (4) | 55 (3) | 0.008 | 65 (3) | 0.68 | 61 (3) | 0.181 |
| Corticosteroid | 60 (4) | 58 (4) | 0.591 | 57 (5) | 0.578 | 63 (5) | 0.625 |
| p-value Prolotherapy versus Corticosteroid | 0.23 | 0.45 | 0.18 | 0.79 |
p-values shown in bold are statistically significant (p < 0.05).
Table 4.
Strength scores over time.
| Baseline | 6 weeks | p-value (baseline to 6 weeks) | 3 months | p-value (baseline to 3 months) | 6 months | p-value (baseline to 6 months) | |
|---|---|---|---|---|---|---|---|
| External rotation (N), mean (SEM) | |||||||
| Prolotherapy | 84 (7) | 96 (8) | 0.03 | 91 (10) | 0.34 | 99 (8) | 0.01 |
| Corticosteroid | 83 (8) | 85 (6) | 0.58 | 93 (8) | 0.02 | 96 (9) | 0.06 |
| p-value Prolotherapy versus Corticosteroid | 0.9 | 0.27 | 0.83 | 0.75 | |||
| Internal rotation (N), mean (SEM) | |||||||
| Prolotherapy | 89 (7) | 98 (7) | 0.11 | 98 (8) | 0.19 | 98 (7) | 0.16 |
| Corticosteroid | 85 (9) | 92 (8) | 0.04 | 97 (9) | 0.06 | 94 (9) | 0.14 |
| p-value Prolotherapy versus Corticosteroid | 0.76 | 0.54 | 0.92 | 0.71 | |||
| Supraspinatus (N), mean (SEM) | |||||||
| Prolotherapy | 59 (8) | 69 (9) | 0.09 | 77 (8) | 0.02 | 77 (8) | 0.02 |
| Corticosteroid | 68 (8) | 67 (8) | 0.75 | 72 (9) | 0.46 | 71 (9) | 0.61 |
| p-value Prolotherapy versus Corticosteroid | 0.41 | 0.9 | 0.72 | 0.65 | |||
| Lift off (N), mean (SEM) | |||||||
| Prolotherapy | 53 (5) | 65 (6) | 0.09 | 73 (6) | 0.00 | 72 (5) | 0.00 |
| Corticosteroid | 50 (6) | 60 (5) | 0.02 | 63 (6) | 0.03 | 62 (7) | 0.07 |
| p-value Prolotherapy versus Corticosteroid | 0.67 | 0.51 | 0.26 | 0.24 |
p-values shown in bold are statistically significant (p < 0.05).
Discussion
The present study showed a decreased level and frequency of pain, increased overall shoulder satisfaction and an improvement in appearance of the supraspinatus tendon in patients with supraspinatus tendinopathy for both those patients receiving glucose prolotherapy injection and those receiving cortisone injection. There was no difference between the two groups for any outcome measure at any time point.
The results of the present study suggest that both corticosteroid and glucose prolotherapy injections, in conjunction with a home exercise therapy programme, are effective in the management of symptomatic supraspinatus tendinopathy, with neither being superior to the other. Both groups showed improvements from baseline for pain and tendon appearance, although there was no significant difference between the groups at 3 months. By the 6-month follow-up, both groups had significantly decreased pain with overhead activities compared to baseline and there remained no significant difference between the groups.
To our knowledge, there are no previous studies evaluating glucose prolotherapy injections in the shoulder. However, there have, been numerous studies evaluating the use of corticosteroid injections for rotator cuff dysfunction. The most recent Cochrane review conducted on corticosteroid injections for shoulder pain was published in 2009.10 For rotator cuff disease, subacromial steroid injection was demonstrated to have a small benefit over placebo in some trials. The results of two trials involving a total of 45 participants that compared subacromial steroid injection with placebo in rotator cuff disease could be pooled and there was a small benefit of subacromial steroid injection over placebo at 4 weeks with respect to pain, function and range of active abduction. It was not possible to combine the results of the other five trials that compared subacromial steroid injection with placebo for rotator cuff disease, athough two of these trials reported some benefit of injection over placebo.
Prolotherapy involves the injection of solution at sites of painful ligament and tendon insertions.11 A review by Hauser et al.3 evaluated the use of dextrose (glucose) prolotherapy on human subjects in published papers prior to October 2011 and found 44 case series, two non-randomized controlled trials (RCTs) and nine RCTs. Of the nine RCTs, three evaluated intratendinous dextrose prolotherapy injections. Topal et al.12 evaluated dextrose prolotherapy in the treatment of Osgood-Schlatter disease, using injections of 12.5% dextrose injected over the apophysis and patella tendon origin. Yelland et al.13 evaluated dextrose prolotherapy injections for chronic low back pain using injections of 20% glucose into tender lumbopelvic ligaments. Yelland et al.14 also evaluated dextrose prolotherapy injections for Achilles tendinosis using 20% glucose into the tender points of the Achilles. Although all of these studies reported positive results for the prolotherapy groups and significant reductions in pain compared to placebo or usual care, there are flaws in the study designs that make the results difficult to interpret.
A study by Ryan et al.7 evaluated intratendinous injections of hyperosmolar dextrose into the Achilles tendon and observed changes in the sonographic appearance of the tendon. Using injections of 25% dextrose, abnormal hypoechoic areas and anechoic clefts or foci in the thickened portion of the Achilles tendon were targeted under ultrasound guidance. A significant reduction in the size of the intratendinous tearing was found for those who had pain in the midportion of the Achilles, as well as a reduction in the size of the hypoechoic region. It was concluded that these improvements are likely a result of positive tissue remodelling subsequent to a proinflammatory response after the injection. Although this make senses at a theoretical level, we discovered similar results in the present study in both groups, with a reduction of tear size, a reduction in hypoechoic areas and tissue remodelling occurring regardless of whether or not they had glucose injected into the target area or corticosteroid injected into the nearby bursa. These changes did not occur in all tendons, and it was not necessarily the ‘normalized’ tendons that had the best clinical results because there were patients in both groups who recorded no pain with overhead activity after 3 months to 6 months and who had the same appearance of their tendon at all time points with no defect filling and, by contrast, there were also patients who had normal looking tendons after 3 months who still had severe to very severe pain with over head activity despite this change in their tendon architecture. It remains to be determined whether there is a positive long-term clinical affect of tissue remodelling that is not seen initially and a minimum 2-year follow-up of our cohort may provide some answers to this question.
A strength of the present study was that all the injections were performed by a single experienced surgeon, who was guided by an ultrasound performed by an experienced sonographer, reducing the variability of injections between and within groups. Another strength was the RCT design, with both patients and outcome assessor being blinded to group selection. To our knowledge, this is the first RCT investigating prolotherapy for supraspinatus tendinopathy. A third strength was the use of validated patient-oriented outcome measures with minimal missing data.
The main weakness of the present study was the absence of a placebo injection control group. Another potential limitation of the present study was the limited number of injections given. Prolotherapy treatment protocols in the literature commonly consist of several injections every 2 weeks to 6 weeks over several months; however, there is no evidence for single versus multiple injections. At third weakness is the relatively short duration of follow-up.
Conclusions
To our knowledge, this is the first study comparing glucose prolotherapy with corticosteroid injection for the treatment of symptomatic supraspinatus tendinopathy. The results obtained suggest that glucose prolotherapy may be used as an alternative to corticosteroid injection, although it is not likely to provide any additional benefit over corticosteroid injection over the short term.
Supplementary Material
Supplementary Material
Supplementary material is available at: http://journals.sagepub.com/doi/suppl/10.1177/1758573217708199.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. The paper has not been presented at any society or meeting.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical Review and Patient Consent
All patients provided written consent for inclusion in this study.
References
- 1.Khan KM, Cook JL, Bonar F, et al. Histopathology of common tendinopathies. Sports Med 1999; 27: 188–201. [DOI] [PubMed] [Google Scholar]
- 2.American Academy of Orthopaedic Surgeons. AAOS clinical practice guideline: optimizing the management of rotator cuff problems. J Am Acad Orthop Surg 2011; 19: 380–383. [DOI] [PubMed] [Google Scholar]
- 3.Hauser RA, Hauser MA, Baird NM. Evidence-based use of dextrose prolotherapy for musculoskeletal pain: a scientific literature review. J Prolother 2011; 3: 765–789. [Google Scholar]
- 4.Banks AR. A rationale for prolotherapy. J Orthop Med 1991; 13: 55–59. [Google Scholar]
- 5.Distel LM, Best TM. Prolotherapy: a clinical review of its role in treating chronic musculoskeletal pain. Am Acad Physical Med Rehab 2011; 3: S78–S81. [DOI] [PubMed] [Google Scholar]
- 6.Ryan M, Wong A, Taunton J. Favorable outcomes after sonographically guided intratendinous injection of hyperosmolar dextrose for chronic insertional and midportion achilles tendinosis. Am J Roentgenol 2010; 194: 1047–1053. [DOI] [PubMed] [Google Scholar]
- 7.Ryan M, Wong A, Gillies J, et al. Sonographically guided intratendinous injections of hyperosmolar dextrose/lidociane: a pilot study for the treatment of chronic plantar fasciitis. Br J Sports Med 2009; 43: 303–306. [DOI] [PubMed] [Google Scholar]
- 8.Ryan M, Wong A, Rabago D, et al. Ultrasound-guided injections of hyperosmolar dextrose for overuse patella tendinopathy: a pilot study. Br J Sports Med 2011; 45: 972–977. [DOI] [PubMed] [Google Scholar]
- 9.Ronquillo JC, Szomor Z, Murrell GAC. Examination of the shoulder. Tech Shoulder Elb Surg 2011; 12: 116–125. [Google Scholar]
- 10.Buchbinder R, Green S, Youd JM. Corticosteroid injections for shoulder pain. Cochrane Database Syst Rev 2009; 1: CD004016–CD004016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rabago D, Slattengren A, Zgierska A. Prolotherapy in primary care practice. Prim Care 2010; 37: 65–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Topol GA, Podesta LA, Reeves KD, et al. Hyperosmolar dextrose injection for recalcitrant Osgood-Schlatter disease. Pediatrics 2011; 128: e1121–e1128. [DOI] [PubMed] [Google Scholar]
- 13.Yelland MJ, Glasziou PP, Bogduk N, et al. Prolotherapy injections, saline injections, and exercises for chronic low-back pain: a randomized trial. Spine 2003; 29: 9–16. [DOI] [PubMed] [Google Scholar]
- 14.Yelland MJ, Sweeting KR, Lyftogt JA, et al. Prolotherapy injections and eccentric loading exercises for painful Achilles tendinosis: a randomized trial. Br J Sports Med 2011; 45: 421–428. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.