Abstract
Calprotectin (S100A8/A9), a heterodimeric complex of calcium-binding proteins S100A8 and S100A9, is encoded by genes mapping to the chromosomal locus 1q21.3 of the epidermal differentiation complex. Whereas extracellular calprotectin shows proinflammatory and antimicrobial properties by signaling through RAGE and TLR4, intracytoplasmic S100A8/A9 appears to be important for cellular development, maintenance, and survival. S100A8/A9 is constitutively expressed in myeloid cells and the stratified mucosal epithelia lining the oropharyngeal and genitourinary mucosae. While upregulated in adenocarcinomas and other cancers, calprotectin mRNA and protein levels decline in head and neck squamous cell carcinoma (HNSCC). S100A8/A9 is also lost during head and neck preneoplasia (dysplasia). Calprotectin decrease does not correlate with the clinical stage (TNM) of HNSCC. When expressed in carcinoma cells, S100A8/A9 downregulates matrix metalloproteinase 2 expression and inhibits invasion and migration in vitro. S100A8/A9 regulates cell cycle progression and decelerates cancer cell proliferation by arresting at the G2/M checkpoint in a protein phosphatase 2α–dependent manner. In HNSCC, S100A8 and S100A9 coregulate with gene networks controlling cellular development and differentiation, cell-to-cell signaling, and cell morphology, while S100A8/A9 appears to downregulate expression of invasion- and tumorigenesis-associated genes. Indeed, tumor formation capacity is attenuated in S100A8/A9-expressing carcinoma cells in vivo. Hence, intracellular calprotectin appears to function as a tumor suppressor in head and neck carcinogenesis. When compared with S100A8/A9-low HNSCC based on analysis of TCGA, S100A8/A9-high HNSCC shows significant upregulation of apoptosis-related genes, including multiple caspases. Accordingly, S100A8/A9 facilitates DNA damage responses in HNSCC, promotes apoptotic cell death, and confers sensitivity to cisplatin and X-radiation in vitro. In the tumor milieu, loss of S100A8/A9 strongly associates with poor squamous differentiation and higher tumor grading, EGFR upregulation, increased DNA methylation, and, finally, poorer overall survival for patients with HNSCC. Hence, intracellular calprotectin shows a multifaceted protective role against the development of HNSCC.
Keywords: S100A8/A9, oral squamous cell carcinoma, oropharyngeal squamous cell carcinoma, esophageal squamous cell carcinoma, nasopharyngeal squamous cell carcinoma, thyroid adenocarcinoma
Role of Calprotectin in Cellular Functions
Calprotectin (S100A8/A9) belongs to the S100 superfamily of EF-hand calcium-binding proteins, which contains >20 members (Donato 2001; Itou et al. 2002). Formed as a heterodimeric protein complex of S100A8 (MRP8 or calgranulin A; 8 kDa) and S100A9 (MRP14 or calgranulin B; 14 kDa) and encoded by genes that map to the human epidermal differentiation complex (EDC) on chromosomal locus 1q21.3, calprotectin is implicated in calcium-dependent regulation of cellular differentiation, proliferation, motility, and gene expression (Donato 2001; Itou et al. 2002). Genes located within the EDC on chromosome 1q21, including S100A8 and S100A9, are crucial to maintain normal epithelial phenotype, tissue development, and repair (Kypriotou et al. 2012; Abhishek and Palamadai Krishnan 2016). As a part of the EDC, S100A8/A9 and other proteins appear to regulate epithelial maturation, differentiation, and growth (Hsu et al. 2009).
S100A8/A9 is also essential for myeloid cell differentiation. S100A8, S100A9 and calprotectin complex are expressed during early stages of differentiation and cellular infiltration and are involved in regulation of casein kinase I and II (Donato 2001; Hessian and Fisher 2001; Bhattacharya et al. 2004). Later in development, S100A8/A9 is typically produced and released by infiltrating cells of the immune system, including polymorphonuclear leukocytes and macrophages, secretory cells, and damaged epithelial cells (Hessian and Fisher 2001; Hsu et al. 2009). Like polymorphonuclear leukocytes, normal mucosal squamous epithelial cells constitutively express S100A8/A9 in the cytoplasm (Bhattacharya et al. 2004). Within epithelial cells, calprotectin can activate NADPH oxidase to generate antibacterial, reactive-oxygen species and activate nuclear factor-kappaB (NF-κB) signaling (Benedyk et al. 2007; Berthier et al. 2012). Activation of NADPH oxidase appears to depend on binding of arachidonic acid at the HHH domain of the C-terminal region of S100A9 and phosphorylation at Thr113 (Fig. 1; Benedyk et al. 2007).
Figure 1.
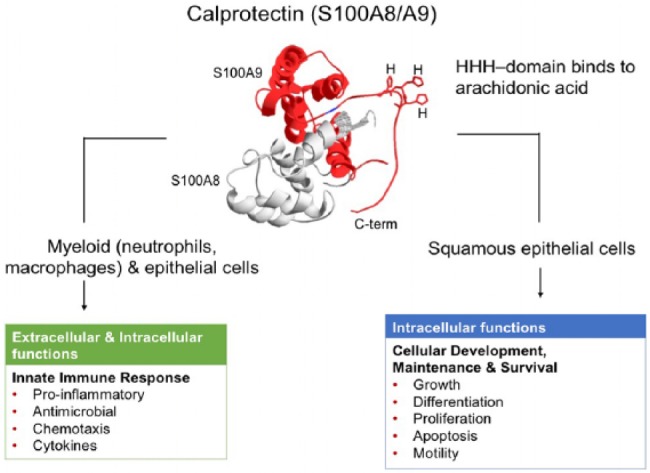
Putative structure and functions of calprotectin (S100A8/A9). HHH-domain binds arachidonic acid, as indicated in the C-terminal region (C-term) of the S100A9 subunit of the calprotectin complex.
During acute and chronic inflammatory responses, released calprotectin functions as an innate immune response regulatory or effector molecule (Iotzova-Weiss et al. 2015; Narumi et al. 2015). In the extracellular environment, calprotectin represents a key antimicrobial protein found in neutrophil extracellular traps (Urban et al. 2009). Extracellular S100A8/A9 is a biomarker of inflammatory disorders such periodontitis and inflammatory bowel disease (Kido et al. 2004; Baldassarre et al. 2007). Functioning as an intracellular antimicrobial protein, S100A8/A9 enhances epithelial cell resistance to invasion by oral and enteric bacterial pathogens, including Porphyromonas gingivalis, Salmonella Typhimurium, and Listeria monocytogenes (Nisapakultorn et al. 2001; Champaiboon et al. 2009).
S100A8/A9 in tissue spaces is considered an “alarmin,” signaling a proinflammatory response through the receptor for advanced glycation end products (RAGE) and toll-like receptor 4 (TLR4). By engaging calprotectin, TLR4 signaling amplifies innate inflammatory responses, including that associated with solid tumors (Ehrchen et al. 2009). RAGE is a cell surface molecule representing a multiligand receptor of the immunoglobulin superfamily. Binding of RAGE by S100A8/A9 triggers the activation of downstream cellular pathways, including mitogen-activated protein kinases (MAPKs), Cdc42/Rac, and NF-κB signaling pathways, which control cell survival, cell motility, and inflammatory responses (Taguchi et al. 2000; Hermani et al. 2006). Released calprotectin may act as a chemotactic agent and contribute to the recruitment of human monocytes and granulocytes to sites of inflammation (Eue et al. 2000). In septic patients, S100A8/A9 and RAGE are significantly elevated in peripheral blood leukocytes (Hofer et al. 2016)
Our understanding about how intracellular calprotectin may contribute to carcinogenesis is hitherto limited. In stratified squamous epithelium lining the oral mucosa, calprotectin expression is higher in the superficial spinous and keratin cell layers; in the deeper suprabasal and basal cells, detection is absent (Martinsson et al. 2005; Funk et al. 2015). Hence, calprotectin appears to contribute to differentiation and maturation of oral keratinocytes in epithelia. Consistent with a role in epithelial maturation and renewal, S100A8/A9 induces autophagy and apoptosis (Ghavami et al. 2004; Ghavami et al. 2008) and significantly increases caspase 3/caspase 7 activity in multiple human cell types (Ghavami et al. 2010). In prostate carcinoma cells, calprotectin appears to induce cell death by downregulating the antiapoptotic protein survivin and increasing reactive-oxygen species (Sattari et al. 2014). S100A9 alone promoted apoptosis and compromised growth of acute promyelocytic leukemia cells (Zhu et al. 2017). Similarly, treatment of cervical squamous cell carcinoma cells (CaSki cells) with purified recombinant S100A8 and S100A9 proteins also induced apoptosis and inhibited cell migration (Qin et al. 2010), suggesting that released calprotectin can function in an autocrine manner. Figure 1 summarizes the putative structure and functional properties of the S100A8/A9 complex.
Expression and Tissue Distribution of S100A8/A9 in Human Cancer
During carcinogenesis, inflammatory cells release S100A8/A9 into the tumor microenvironment (Gebhardt et al. 2002; Gebhardt et al. 2006). Extracellular calprotectin may contribute to inflammation-induced tumor initiation and progression (Gebhardt et al. 2002). In human malignancies—including prostate, gastric, colorectal, breast, bladder, and oropharyngeal carcinomas—S100A8/A9 expression levels in neoplastic cells and/or tumor-associated macrophages may reflect prospects for the progression of the disease and patient overall survival (Yao et al. 2007; Fan, Zhang, et al. 2012; Kim et al. 2014; Tidehag et al. 2014; Funk et al. 2015; Bao et al. 2016; Khammanivong et al. 2016; Moris et al. 2016).
Calprotectin is expressed in a cell- and tissue-specific manner in mature and differentiating normal tissues (Donato 2003). Expression of S100A8 and S100A9 can also be influenced by epigenetic factors. Normal human epithelial tissues that do not endogenously express calprotectin include skin (Gebhardt et al. 2006), breast (Moon et al. 2008; Rodriguez-Barrueco et al. 2015), thyroid (Ito et al. 2005; Ito et al. 2009), liver (Németh et al. 2009), gastric mucosa (Turovskaya et al. 2008; Fan, Zhang, et al. 2012), prostate (Hermani et al. 2006), ovary (Ødegaard et al. 2008), bladder (Yao et al. 2007), and lung (Arai et al. 2001). In primary malignant neoplasms derived from these tissues, primarily adenocarcinomas, S100A8/A9 expression is generally induced (Fig. 2, red arrow). Whether increased calprotectin expression observed in these malignant neoplasms is a sequela of carcinogenesis or actually drives tumor development and progression is unclear.
Figure 2.
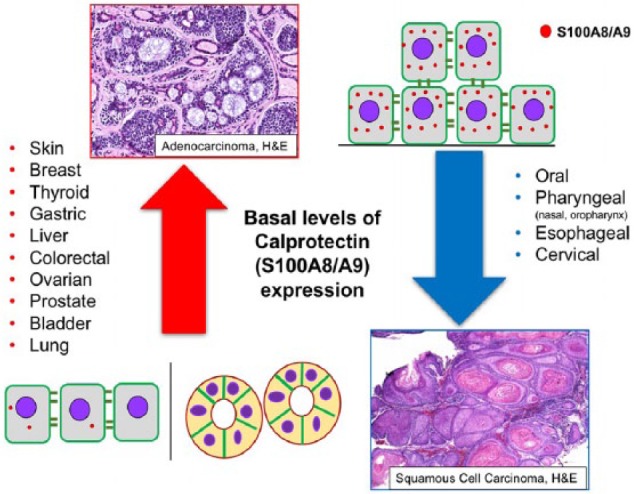
S100A8/A9 expression is differentially regulated in human malignancies. In tissues that express limited or no endogenous calprotectin (left side), such as the glandular breast epithelium or epidermis of the skin, calprotectin levels increase (red arrow) when tumorigenesis occurs; these neoplasms are generally diagnosed as adenocarcinomas. In tissues that constitutively express higher levels of calprotectin, including the oral mucosal epithelium (right side), squamous cell carcinomas show reduced calprotectin expression (blue arrow). Basal level of expression represents constitutive calprotectin expression in normal tissues. H&E, hematoxylin and eosin.
In contrast, S100A8/A9 is constitutively expressed in normal mucosal tissues lined by stratified squamous epithelia (Gonzalez et al. 2003; Funk et al. 2015; Khammanivong et al. 2016). In oral, oropharyngeal, nasopharyngeal, esophageal (Kong et al. 2004; Wang et al. 2004), and cervical (Coleman and Stanley 1994; Tugizov et al. 2005) squamous cell carcinomas, calprotectin is significantly downregulated (Fig. 2, blue downward arrow). The differential control of calprotectin expression in normal epithelia and tissue-specific malignancies suggests epigenetic regulation during epithelial development and dysregulation associated with the initiation of certain cancers.
Structural Biology and Regulation of S100A8/A9
In humans, S100A8 and S100A9 typically form heterodimers (S100A8/A9); homodimers are not normally detectable but can form under certain conditions (Leukert et al. 2005). Monomers and homotrimeric or homotetrameric complexes are also possible (Nacken et al. 2003). As mentioned, S100A8/A9 expression is associated with early stages of myeloid cell differentiation and inflammation. Normally localized to the suprabasal spinous and keratin cell layers of stratified squamous mucosal epithelia (Hayashi et al. 2007), expression is most likely differentially regulated during each stage of cell differentiation.
The S100A8 and S100A9 genes encode no upstream signaling peptides, which are normally required to target proteins for export. Hence, the calprotectin complex is predicted to reside in the cytoplasm and is unlikely to be secreted by epithelium into extracellular space under normal conditions. Calprotectin has been reported to be released from aberrantly differentiated metaplastic primary human squamous tracheobronchial cells (Kim et al. 2007). In response to chronic exogenous stress, including inflammation, epithelial progenitor cells could therefore undergo metaplasia and release S100A8/A9. Some workers describe release as noncanonical secretion. Other than release during tissue and cell apoptosis or necrosis, secretion of extracellular calprotectin from normal epithelium has not been reported.
S100A8/A9, involucrin, and filaggrin genes are upregulated in human gingival keratinocytes in response to interleukin 1α (IL-1α) and calcium—2 factors known to promote keratinocyte differentiation (Hayashi et al. 2007). In contrast, transforming growth factor β (TGF-β), which inhibits proliferation and differentiation, downregulates calprotectin expression (Hayashi et al. 2007). Similarly, keratinocyte growth factor produced by mesenchymal cells appears to inhibit calprotectin expression (Bando et al. 2010), whereas the transcription factors C/EBPα and GLI, which regulate cellular growth and differentiation, appear to be essential for S100A8 and S100A9 expression (Cammenga et al. 2003; Tavor et al. 2003; Hayashi et al. 2007). In HaCaT keratinocytes, IL-1α is also suggested to induce S100A9 expression by signaling through the IL-1 receptor and p38 MAPK, which increases the binding activity of C/EBPβ (Bando et al. 2013).
Regulation of calprotectin in epithelial cells may differ from other cell lineages. For example, in fibroblasts, transcriptional regulation of S100A8 appears to be mediated by fibroblast growth factor 2 (FGF-2), IL-1β, and TGF-β (Rahimi et al. 2005), whereas S100A9 expression in myeloid cells appears to be regulated by a myeloid-related regulatory element in the upstream promoter region; myeloid-related regulatory element is known to bind poly(ADP-ribose) polymerase 1 and the Ku70/Ku80 transcriptional complex (Kerkhoff et al. 2002; Grote et al. 2006). These several regulatory pathways could explain how calprotectin is differentially regulated depending on the cell lineage.
Calprotectin appears to be essential for development. Whereas not all cell types constitutively express calprotectin, mutation of the S100A8 subunit of the calprotectin complex causes rapid resorption of the mouse embryo by day 9.5 of development (Passey et al. 1999). Mutation of the S100A9 subunit also abrogates S100A8 protein production (but not gene expression), impairs myeloid cell function, and results in an S100A8/A9null mutation (Hobbs et al. 2003; Manitz et al. 2003). Therefore, S100A8 appears to be critical for physiologic development and cellular function.
Role of Calprotectin in Head and Neck Carcinogenesis
Calprotectin and Oral and Oropharyngeal Squamous Cell Carcinoma
Head and neck cancer includes oral squamous cell carcinoma (OSCC) and oropharyngeal squamous cell carcinoma (OPSCC) and is the sixth-most prevalent cancer worldwide; incidence appears to be increasing in the last decade, primarily in association with human papilloma virus (HPV) infection (El-Naggar et al. 2017). More than 90% of malignancies affecting the oral and oropharyngeal mucosae are SCCs (El-Naggar et al. 2017). In OSCC and OPSCC, S100A8 and S100A9 mRNAs (Roesch Ely et al. 2005; Sapkota et al. 2008; Khammanivong et al. 2016; Reckenbeil et al. 2016) and translated proteins (Funk et al. 2015) significantly decrease when compared with nonneoplastic stratified squamous oral and oropharyngeal epithelium. Approximately 90% of OSCC and OPSCC specimens show combined loss of S100A8 and S100A9 expression based on data from The Cancer Genome Atlas (TCGA; Khammanivong et al. 2016). Interestingly, S100A8 and S100A9 downregulation is similar across all tumor grades (T), independent of nodal involvement (N) or distant metastasis (M; Khammanivong et al. 2016), but is related to locoregional recurrence (Harris et al. 2015). Given that calprotectin expression and clinical staging (TNM) appear mutually independent, dysregulation of S100A8/A9 may be a feature of the initiation of oral and oropharyngeal tumorigenesis. Indeed, S100A8/A9 expression in oral premalignant (precancerous) epithelial dysplasia is lower than in otherwise healthy oral mucosal tissues, suggesting progressive loss of S100A8/A9 during oral carcinogenesis (Argyris et al. manuscript in preparation).
When expressed endogenously or ectopically, intracellular calprotectin appears to regulate the malignant characteristics of OSCC cells. Based on a well-differentiated human OSCC cell line (TR146 cells), cellular MMP-2 activity, cell invasion, and migration in vitro were increased when endogenous S100A8 and S100A9 expression was silenced via shRNA, as we reported (Silva et al. 2014). Conversely, when S100A8 and S100A9 were overexpressed with transfection of KB cells (S100A8/A9-negative HeLa-like carcinoma cell line), MMP-2 expression, invasiveness, and migratory potential were significantly inhibited (Silva et al. 2014). Hence, S100A8/A9 suppresses malignant features of carcinoma cells in vitro that are typically associated with tumor initiation and spread (Fig. 3).
Figure 3.
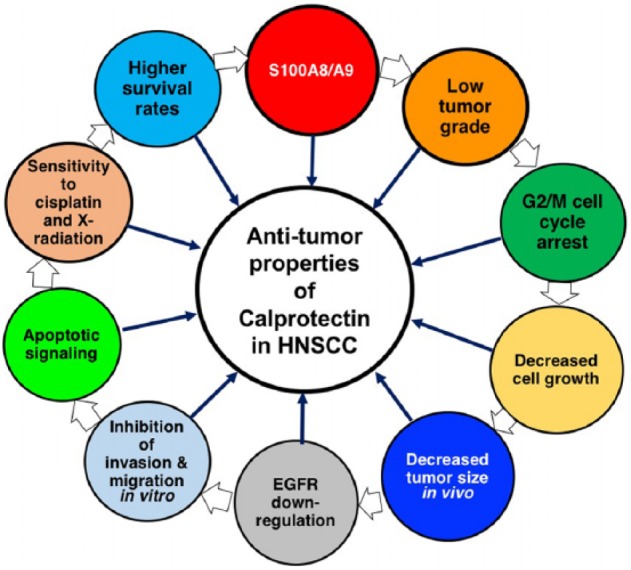
The multifaceted tumor-suppressive role of calprotectin in head and neck squamous cell carcinoma (HNSCC). When expressed in HNSCC, specifically oral and oropharyngeal, S100A8/A9 is associated with greater squamous differentiation (lower tumor grading) and activation of the G2/M DNA damage cell cycle checkpoint. These changes appear to cause attenuated cell growth in vitro and decreased tumor formation in vivo. In addition, epidermal growth factor receptor (EGFR), a negative prognostic factor, sustains proliferative signaling in the tumor milieu and is downregulated in S100A8/A9-expressing cell lines and tissues. Furthermore, calprotectin inhibits cancer cell migration and invasion, facilitates recruitment of DNA damage response proteins, and appears to promote apoptotic DNA fragmentation, thus conferring sensitivity to cisplatin and X-radiation. Finally, patients with S100A8/A9-high HNSCCs survive longer than patients with S100A8/A9-low or S100A8/A9-negative tumors. The white arrows do not necessarily indicate successive events but interconnected functions that collectively contribute (black arrows) to the antitumor properties of the calprotectin complex in HNSCC.
When tested in vivo, calprotectin-negative KB and KB-EGFP cells formed significantly larger tumors than KB-S100A8/A9 cells in a mouse xenograft tumorigenesis model (Khammanivong et al. 2016). Indeed, in OSCC and OPSCC more globally, S100A8 and S100A9 coregulate with gene networks controlling cellular development and differentiation, cell-to-cell signaling and interaction, and cell morphology; S100A8/A9 appears to downregulate expression of genes associated with invasion and tumorigenesis (Khammanivong et al. 2016). Loss of S100A8/A9 in OSCC is associated with poor tumor differentiation (Argyris et al. manuscript in preparation) and increased DNA methylation (Khammanivong et al. 2016; Fig. 3).
Cytoplasmic calprotectin also functions in vitro to control the cell cycle DNA damage checkpoint at G2/M and impede proliferation (Khammanivong et al. 2013). Overexpression of S100A8/A9 enhances protein phosphatase 2A (PP2A) activity and activates phosphorylation of p-Chk1 at Ser345. PP2A appears to target and dephosphorylate mitotic p-Cdc25C at Thr48, allowing p-Chk1 (Ser345) to phosphorylate Cdc25C at the inhibitory residue, Ser216. Phosphorylated and inactivated p-Cdc25C (Ser216) is targeted by 14-3-3β, which translocates both proteins for accumulation in the cytoplasm. As a consequence, the G2/M cyclin B1/p-Cdc2 (Thr14/Tyr15) complex remains inactive, arresting cell cycle at the G2/M DNA damage checkpoint (Khammanivong et al. 2013). Silencing of S100A8/A9 expression in the TR146 OSCC cells increased anchorage-dependent and anchorage-independent growth. S100A8/A9- mediated control of the G2/M DNA damage checkpoint is therefore a likely tumor-suppressive mechanism in human OSCC (Fig. 3).
Mutations of the tumor-suppressor TP53 are detected in up to 72% of the HNSCC cases (Network 2015). Other frequently mutated genes include CDKN2A, PIK3CA, NOTCH1, PTEN, and HRAS (Network 2015; El-Naggar et al. 2017). Given that S100A8 and S100A9 are not frequently mutated in OSCC and OPSCC, epigenetic events may explain decreased S100A8/A9 expression in these neoplasms. Furthermore, epidermal growth factor receptor (EGFR) is highly upregulated in OSCC and OPSCC; EGFR expression is considered a negative prognostic indicator for disease progression and survival outcome (Mitsudomi and Yatabe 2010). In vitro and ex vivo experiments showed that S100A8 and S100A9 levels were inversely correlated to membranous and cytoplasmic expression of EGFR (Argyris et al. manuscript in preparation). Given that calprotectin increases caspase 3/caspase 7 activity (Viemann et al. 2007; Ghavami et al. 2010) and that caspases 3 and 7 proteolytically cleave EGFR at the C-terminal region (Bae et al. 2001; Zhuang et al. 2003; He et al. 2006), S100A8/A9 may downregulate EGFR posttranslationally in OSCC and OPSCC through a caspase 3/caspase 7–dependent mechanism.
Consistent with a role for caspase activity causing downregulation of EGFR, S100A8/A9-high SCCs of the head and neck upregulate >363 apoptosis-related genes significantly more than S100A8/A9-low neoplasms, including CASP1, -3, -4, -5, -7, -8, -9, -10, and -14, based on analysis of data from TCGA. Furthermore, intracellular calprotectin appears to promote DNA fragmentation and cell death following radio- and chemotherapy, conferring S100A8/A9-expressing OSCC cells more sensitive to cisplatin and X-radiation (Argyris et al. manuscript in preparation). Notably, overall patient survival rates appear affected by calprotectin status; patients with S100A8- and/or S100A9-high HNSCC survive longer than patients with S100A8/A9-low tumors (Funk et al. 2015; Khammanivong et al. 2016; Fig. 3).
In S100A8/A9-high OSCCs and OPSCCs negative for HPV, calprotectin appears to function as a tumor suppressor, attenuating the malignant phenotype of carcinoma cells; our knowledge remains limited about the role of calprotectin in HPV-driven tumors. Calprotectin may inhibit viral oncogenic activity by regulating CKII-mediated E7 phosphorylation in vitro (Tugizov et al. 2005). Based on TCGA data, S100A8 and S100A9 expression tended to decrease more (nonsignificant) in HPV+ than in HPV– HNSCC samples (Khammanivong et al. 2016); the biological and prognostic significance of this observation is currently unknown. S100A8 and S100A9, however, do not appear to be the only members of EDC regulated by HPV. Involucrin (IVL) and loricrin (LOR) are transcriptionally downregulated by E6 and E7 HPV oncoproteins in proliferating and differentiating human foreskin keratinocytes (Lehr and Brown 2003; Gyöngyösi et al. 2012). Specifically, involucrin is indirectly suppressed by E6 oncoprotein through HPV-mediated downregulation of transcription factor C/EBPα (Marthaler et al. 2017). C/EBPα, which regulates cellular growth and differentiation, appears to be essential for S100A8 and S100A9 expression (Cammenga et al. 2003; Tavor et al. 2003). HPV-driven decrease in C/EBPα may therefore also be responsible for S100A8/A9 downregulation in HPV+ HNSCC.
Calprotectin and Esophageal Squamous Cell Carcinoma
Esophageal squamous cell carcinoma (ESCC) represents the eighth-most common type of human cancer and is characterized by poor prognosis; 5-y survival is 19% for all ESCC cases and only 0.9% for advanced tumors (Testa et al. 2017). The role of S100A8 and S100A9 in the initiation and progression of esophageal carcinogenesis has been described in in vitro and in vivo studies. Epidemiologic studies of ESCC suggest that zinc deprivation may be a major etiologic factor (Yang 1980; van Rensburg 1981). With special diets, zinc-deprived hyperplastic and zinc-replenished rat esophageal tissues were modeled (Taccioli et al. 2009). The hyperplastic zinc-deficient esophagi showed a distinct gene expression signature with 57- and 5-fold greater expression of S100A8 and S100A9 mRNA levels, respectively, than the zinc-replenished tissues (Taccioli et al. 2009). Nutritional replenishment of zinc levels restored S100A8 and S100A9 gene expression and the physiologic esophageal phenotype in vivo.
ESCC from 4-nitroquinoline 1-oxide (4-NQO)–treated, zinc-deficient p53+/- mice demonstrated increased S100A8 immunoreactivity, while nonneoplastic control zinc-sufficient p53+/- esophagi displayed weak S100A8 expression. Prolonged zinc deficiency in rats (21 wk) combined with noncarcinogenic low doses of N-nitrosamethylbenzylamine elicited a 66.7% incidence of ESCC (Taccioli et al. 2012). Dysplastic (precancerous) and neoplastic zinc-deficient esophagi showed 2-fold greater upregulation of S100A8 and S100A9 than the zinc-sufficient rat esophagi (Taccioli et al. 2012). When 4-NQO was applied topically to induce oral-esophageal tumorigenesis in zinc-deficient COX-2-/- mice, both S100A8 and S100A9 were upregulated in the precancerous forestomach (Wan et al. 2011). In these mice, the RAGE-S100A8/A9 inflammatory axis appeared activated in premalignant and carcinomatous lesions of the forestomach and tongue (Wan et al. 2011). Hence, zinc deficiency in this model appears to regulate S100A8 and S100A9 expression and modulates the link between S100A8/A9-RAGE interaction and downstream NF-κB/COX-2 signaling, driving esophageal cell proliferation and carcinogenesis. How zinc chelation by calprotectin (Clark et al. 2016) might contribute is unknown. Interestingly, signaling through the S100A8/A9-TLR4 pathway could contribute to the early development of esophageal cancer in a RAGE-independent manner, since RAGE-/- mice showed increased Toll-like receptor 4 (TLR4) mRNA and protein levels (Mark et al. 2013).
In human ESCC tissues ex vivo, S100A8 was detected in all specimens (n = 16); adjacent nonneoplastic stratified squamous esophageal epithelium showed negative or low S100A8 immunoreactivity (Taccioli et al. 2009). S100A9 protein was expressed in 89.5% of human ESCC samples (n = 57) and only 66.7% of nonneoplastic epithelium according to immunohistochemistry (Fan, Gao, et al. 2012). Whereas S100A8 and S100A9 were reported to be upregulated in human ESCC (Taccioli et al. 2009; Fan, Gao, et al. 2012), 11 of 16 S100 genes, including S100A8 and S100A9, have been reported to be downregulated in 62 ESCC cases when compared with adjacent nonneoplastic esophageal epithelium (Ji et al. 2004). Specifically, S100A8 and S100A9 expression was downregulated in 82.3% (51 of 62) and 77.4% (48 of 62) of ESCC, respectively (Ji et al. 2004). Similarly, S100A8, S100A9, and cytokeratins (KRT4 and KRT13), genes implicated in squamous cell differentiation and maturation, were coordinately downregulated in ESCC (n = 5) in comparison with adjacent nonneoplastic esophageal mucosal tissues, according to cDNA microarray and Northern blot analysis (Luo et al. 2004). Based on immunohistochemistry, S100A8 and S100A9 were decreased in 87% and 84% (n = 31) of human ESCC tumors, respectively; increased S100A8/A9 expression levels were associated with greater differentiation (Luo et al. 2004). In a Chinese cohort (n = 64), S100A9 expression in ESCCs decreased in 91% of the specimens (Wang et al. 2004). This ex vivo data underscores the lack of consensus about the role of S100A8 and S100A9 proteins in human esophageal carcinogenesis.
Calprotectin and Nasopharyngeal and Laryngeal Squamous Cell Carcinoma
The role of S100A8/A9 in nasopharyngeal, pharyngeal, and laryngeal cancer is underinvestigated. In nasopharyngeal squamous cell carcinoma (NSCC), S100A8 and S100A9 expression was suppressed in vitro in 7 carcinoma cell lines as evidenced by cDNA array hybridization and reverse transcription polymerase chain reaction analysis (Fung et al. 2000). S100A8 and S100A9 protein expression was apparently absent in pharyngeal epithelial malignancies but expressed in the superficial layer of nonneoplastic pharyngeal epithelium according to ProteinChip arrays (Melle et al. 2004). In contrast, S100A9 protein levels appeared significantly greater in the stroma of NSCC cases (n = 66) than normal nasopharyngeal epithelial tissue (Li et al. 2009). S100A9 immunoreactivity varied in stromal inflammatory cells in all NSCC cases, whereas the carcinoma cells and normal pharyngeal epithelial cells were uniformly negative for S100A9 (Li et al. 2009). In more advanced NSCC with increased regional lymph node metastasis, S100A9 was upregulated in the inflamed tumor microenvironment but not in the carcinoma cells per se (Li et al. 2009).
In laryngeal squamous cell carcinoma (LSCC) specimens (n = 2), S100A9 protein levels were lower than in adjacent nonneoplastic tissues as assayed with 2-dimensional gel electrophoresis and mass spectroscopy (Sewell et al. 2007). In Hep-2 LSCC cells, the 3′UTR of S100A8 harbors a specific binding site for miR-24 in-vitro (Guo et al. 2012). Whereas ectopic expression of miR-24 had no significant effect on S100A8 mRNA levels, S100A8 protein significantly decreased when Hep-2 cells were transfected with miR-24 (Guo et al. 2012). miR-24 therefore appeared to negatively regulate S100A8 expression via translational repression. In contrast to OSCC and OPSCC, S100A8 protein may be associated with tumor invasion in LSCC. After S100 antibody blockade, miR-24 significantly inhibited invasion of Hep2 cells in vitro (Guo et al. 2012). Hence, the role of S100A8, S100A9, and calprotectin complex in LSCC requires further investigation.
Calprotectin and Thyroid Adenocarcinoma
In thyroid cancer, S100A8/A9 does not appear to play a significant role except for an anaplastic and clinically aggressive subtype, although the literature is sparse. Nonneoplastic follicular cells of the thyroid gland do not express S100A8 or S100A9 proteins, and both proteins are absent in normal thyroid tissue and benign neoplasms, including follicular adenoma (Ito et al. 2005; Ito et al. 2009). Thyroid malignancies, including follicular and papillary carcinomas with conventional tumor architecture, are also uniformly S100A8 and S100A9 negative. A few follicular and papillary thyroid carcinomas with atypical growth patterns show S100A9 immunoreactivity in <5% of neoplastic cells (Ito et al. 2005; Ito et al. 2009). In contrast, thyroid adenocarcinomas with high-grade transformation (dedifferentiated, anaplastic), poorer prognosis, and more aggressive tumor behavior showed significantly increased S100A8/A9 immunohistochemical positivity (Ito et al. 2005; Ito et al. 2009). Elevated calprotectin protein levels, therefore, may drive dedifferentiation and high-grade transformation in thyroid cancer.
In anaplastic thyroid carcinoma (ATC), S100A8/A9 mRNA and protein levels are greater than in nonneoplastic thyroid tissue and well-differentiated thyroid tumors (Reeb et al. 2015). ATC cell lines proliferate in response to exogenous S100A8 in vitro (Reeb et al. 2015). Knockdown of endogenous S100A8 through shRNA techniques inhibited S100A8-mediated ATC cell proliferation in vitro and tumor formation in vivo; animal survival improved (Reeb et al. 2015). S100A8 appears to stimulate cell growth by interacting with RAGE and activating 3 downstream MAPK pathways: p38, ERK1/2, and JNK (Reeb et al. 2015). Interestingly, the oncogenic and metastatic potential of ATC cells was independent of the status of S100A9. Hence, therapeutic targeting of S100A8 may prove beneficial for patients with ATC.
In patients with papillary thyroid carcinoma, the oxidative stress index, serum lipid hydroperoxides, and calprotectin levels correlate and appear to increase (Tabur et al. 2015). After total thyroidectomy, the levels of serum S100A8/A9 substantially decrease (Tabur et al. 2015). Hence, calprotectin may be a useful biomarker for this type of thyroid malignancy.
Conclusion
In contrast to malignant neoplasms of other anatomic sites, intracellular S100A8A/A9 appears to play a multifaceted tumor-suppressive role in HNSCC, specifically oral and oropharyngeal tumors, by regulating tumor differentiation, restoring the G2/M cell cycle checkpoint, and inhibiting invasion and migration in vitro and tumor formation in vivo. Conversely, loss of calprotectin expression associates with upregulation of the negative prognosticator EGFR, resistance of carcinoma cells to chemotherapeutics and X-irradiation, and poorer patient survival rates. Notably, decrease or loss of calprotectin at the stage of preneoplasia by a subset of dysplastic cells may accelerate cell proliferation and induce expression of cancer-promoting genes, therefore contributing to transformation toward HNSCC. The reviewed anticancer properties of calprotectin in HNSCC (Fig. 3) suggest targets for new therapeutic strategies. Indeed, calprotectin itself may represent a therapeutic target. We speculate that restoration of endogenous levels of S100A8/A9 in dysplastic cells of oral premalignant epithelial lesions or existing tumors could prevent or decelerate malignant transformation and invasion of the basement membrane, reduce cell proliferation via arrest of the cell cycle at G2, indirectly promote EGFR cleavage, augment the DNA damage repair response, and increase the efficacy of established therapeutic regimens, including radio- and chemotherapy.
Author Contributions
P.P. Argyris, contributed to conception, design, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript; Z.M. Slama, contributed to data acquisition and analysis, critically revised the manuscript; K.F. Ross, M.C. Herzberg, contributed to conception, design, data analysis, and interpretation, critically revised the manuscript; A. Khammanivong, contributed to conception, design, data acquisition, analysis, and interpretation, critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Acknowledgments
The authors thank Dr. Brian Guenther for developing the ribbon diagram of S100A8/A9 (Fig. 1).
Footnotes
Related work in the authors’ laboratory is supported by the National Institutes of Health (R01DE021206 to M.C.H. and R90DE023058 to P.P.A.).
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Abhishek S, Palamadai Krishnan S. 2016. Epidermal differentiation complex: a review on its epigenetic regulation and potential drug targets. Cell J. 18(1):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai K, Teratani T, Nozawa R, Yamada T. 2001. Immunohistochemical investigation of S100A9 expression in pulmonary adenocarcinoma: S100A9 expression is associated with tumor differentiation. Oncol Rep. 8(3):591–596. [DOI] [PubMed] [Google Scholar]
- Bae SS, Choi JH, Oh YS, Perry DK, Ryu SH, Suh PG. 2001. Proteolytic cleavage of epidermal growth factor receptor by caspases. FEBS Lett. 491(1–2):16–20. [DOI] [PubMed] [Google Scholar]
- Baldassarre ME, Altomare MA, Fanelli M, Carbone D, Di Bitonto G, Mautone A, Laforgia N. 2007. Does calprotectin represent a regulatory factor in host defense or a drug target in inflammatory disease? Endocr Metab Immune Disord Drug Targets. 7(1):1–5. [DOI] [PubMed] [Google Scholar]
- Bando M, Hiroshima Y, Kataoka M, Herzberg MC, Ross KF, Shinohara Y, Yamamoto T, Nagata T, Kido J. 2010. Modulation of calprotectin in human keratinocytes by keratinocyte growth factor and interleukin-1alpha. Immunol Cell Biol. 88(3):328–333. [DOI] [PubMed] [Google Scholar]
- Bando M, Zou X, Hiroshima Y, Kataoka M, Ross KF, Shinohara Y, Nagata T, Herzberg MC, Kido J. 2013. Mechanism of interleukin-1α transcriptional regulation of S100A9 in a human epidermal keratinocyte cell line. Biochim Biophys Acta. 1829(9):954–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao YI, Wang A, Mo J. 2016. S100A8/A9 is associated with estrogen receptor loss in breast cancer. Oncol Lett. 11(3):1936–1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedyk M, Sopalla C, Nacken W, Bode G, Melkonyan H, Banfi B, Kerkhoff C. 2007. HaCat keratinocytes overexpressing the S100 proteins S100A8 and S100A9 show increased NADPH oxidase and NF-kappaB activities. J Invest Dermatol. 127(8):2001–2011. [DOI] [PubMed] [Google Scholar]
- Berthier S, Nguyen MV, Baillet A, Hograindleur MA, Paclet MH, Polack B, Morel F. 2012. Molecular interface of S100A8 with cytochrome B558 and NADPH oxidase activation. PLoS One. 7(7):e40277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya S, Bunick CG, Chazin WJ. 2004. Target selectivity in EF-hand calcium binding proteins. Biochim Biophys Acta. 1742(1–3):69–79. [DOI] [PubMed] [Google Scholar]
- Cammenga J, Mulloy JC, Berguido FJ, MacGrogan D, Viale A, Nimer SD. 2003. Induction of C/EBPalpha activity alters gene expression and differentiation of human CD34+ cells. Blood. 101(6):2206–2214. [DOI] [PubMed] [Google Scholar]
- Champaiboon C, Sappington KJ, Guenther BD, Ross KF, Herzberg MC. 2009. Calprotectin S100A9 calcium-binding loops I and II are essential for keratinocyte resistance to bacterial invasion. J Biol Chem. 284(11):7078–7090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark HL, Jhingran A, Sun Y, Vareechon C, de Jesus Carrion S, Skaar EP, Chazin WJ, Calera JA, Hohl TM, Pearlman E. 2016. Zinc and manganese chelation by neutrophil S100A8/A9 (calprotectin) limits extracellular aspergillus fumigatus hyphal growth and corneal infection. J Immunol. 196(1):336–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman N, Stanley MA. 1994. Expression of the myelomonocytic antigens CD36 and L1 by keratinocytes in squamous intraepithelial lesions of the cervix. Hum Pathol. 25(1):73–79. [DOI] [PubMed] [Google Scholar]
- Donato R. 2001. S100: a multigenic family of calcium-modulated proteins of the EF-hand type with intracellular and extracellular functional roles. Int J Biochem Cell Biol. 33(7):637–668. [DOI] [PubMed] [Google Scholar]
- Donato R. 2003. Intracellular and extracellular roles of S100 proteins. Microsc Res Tech. 60(6):540–551. [DOI] [PubMed] [Google Scholar]
- Ehrchen JM, Sunderkötter C, Foell D, Vogl T, Roth J. 2009. The endogenous Toll-like receptor 4 agonist S100A8/S100A9 (calprotectin) as innate amplifier of infection, autoimmunity, and cancer. J Leukoc Biol. 86(3):557–566. [DOI] [PubMed] [Google Scholar]
- El-Naggar AK, Chan JKC, Grandis JR, Takata T, Slootweg PJ. 2017. Tumours of the oral cavity and mobile tongue. In: El-Naggar AK, Chan JKC, Grandis JR, Takata T, Slootweg PJ, editors. World Health Organization (WHO) classification of head and neck tumours; International Agency for Research on Cancer (IARC). 4th ed. Lyon: IARCPress; p. 105–115. [Google Scholar]
- Eue I, Pietz B, Storck J, Klempt M, Sorg C. 2000. Transendothelial migration of 27E10+ human monocytes. Int Immunol. 12(11):1593–1604. [DOI] [PubMed] [Google Scholar]
- Fan B, Zhang LH, Jia YN, Zhong XY, Liu YQ, Cheng XJ, Wang XH, Xing XF, Hu Y, Li YA, et al. 2012. Presence of S100A9-positive inflammatory cells in cancer tissues correlates with an early stage cancer and a better prognosis in patients with gastric cancer. BMC Cancer. 12:316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan NJ, Gao CF, Wang CS, Zhao G, Lv JJ, Wang XL, Chu GH, Yin J, Li DH, Chen X, et al. 2012. Identification of the up-regulation of TP-alpha, collagen alpha-1(VI) chain, and S100A9 in esophageal squamous cell carcinoma by a proteomic method. J Proteomics. 75(13):3977–3986. [DOI] [PubMed] [Google Scholar]
- Fung LF, Lo AK, Yuen PW, Liu Y, Wang XH, Tsao SW. 2000. Differential gene expression in nasopharyngeal carcinoma cells. Life Sci. 67(8):923–936. [DOI] [PubMed] [Google Scholar]
- Funk S, Mark R, Bayo P, Flechtenmacher C, Grabe N, Angel P, Plinkert PK, Hess J. 2015. High S100A8 and S100A12 protein expression is a favorable prognostic factor for survival of oropharyngeal squamous cell carcinoma. Int J Cancer. 136(9):2037–2046. [DOI] [PubMed] [Google Scholar]
- Gebhardt C, Breitenbach U, Tuckermann JP, Dittrich BT, Richter KH, Angel P. 2002. Calgranulins S100A8 and S100A9 are negatively regulated by glucocorticoids in a c-Fos-dependent manner and overexpressed throughout skin carcinogenesis. Oncogene. 21(27):4266–4276. [DOI] [PubMed] [Google Scholar]
- Gebhardt C, Németh J, Angel P, Hess J. 2006. S100A8 and S100A9 in inflammation and cancer. Biochem Pharmacol. 72(11):1622–1631. [DOI] [PubMed] [Google Scholar]
- Ghavami S, Eshragi M, Ande SR, Chazin WJ, Klonisch T, Halayko AJ, McNeill KD, Hashemi M, Kerkhoff C, Los M. 2010. S100A8/A9 induces autophagy and apoptosis via ROS-mediated cross-talk between mitochondria and lysosomes that involves BNIP3. Cell Res. 20(3):314–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghavami S, Kerkhoff C, Chazin WJ, Kadkhoda K, Xiao W, Zuse A, Hashemi M, Eshraghi M, Schulze-Osthoff K, Klonisch T, et al. 2008. S100A8/9 induces cell death via a novel, RAGE-independent pathway that involves selective release of Smac/DIABLO and Omi/Htra2. Biochim Biophys Acta. 1783(2):297–311. [DOI] [PubMed] [Google Scholar]
- Ghavami S, Kerkhoff C, Los M, Hashemi M, Sorg C, Karami-Tehrani F. 2004. Mechanism of apoptosis induced by S100A8/A9 in colon cancer cell lines: the role of ROS and the effect of metal ions. J Leukoc Biol. 76(1):169–175. [DOI] [PubMed] [Google Scholar]
- Gonzalez HE, Gujrati M, Frederick M, Henderson Y, Arumugam J, Spring PW, Mitsudo K, Kim HW, Clayman GL. 2003. Identification of 9 genes differentially expressed in head and neck squamous cell carcinoma. Arch Otolaryngol Head Neck Surg. 129(7):754–759. [DOI] [PubMed] [Google Scholar]
- Grote J, Konig S, Ackermann D, Sopalla C, Benedyk M, Los M, Kerkhoff C. 2006. Identification of poly(ADP-ribose)polymerase-1 and Ku70/Ku80 as transcriptional regulators of S100A9 gene expression. BMC Mol Biol. 7:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Fu W, Chen H, Shang C, Zhong M. 2012. miR-24 functions as a tumor suppressor in Hep2 laryngeal carcinoma cells partly through down-regulation of the S100A8 protein. Oncol Rep. 27(4):1097–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyöngyösi E, Szalmás A, Ferenczi A, Kónya J, Gergely L, Veress G. 2012. Effects of human papillomavirus (HPV) type 16 oncoproteins on the expression of involucrin in human keratinocytes. Virol J. 9:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris TM, Du P, Kawachi N, Belbin TJ, Wang Y, Schlecht NF, Ow TJ, Keller CE, Childs GJ, Smith RV, et al. 2015. Proteomic analysis of oral cavity squamous cell carcinoma specimens identifies patient outcome-associated proteins. Arch Pathol Lab Med. 139(4):494–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi N, Kido J, Kido R, Wada C, Kataoka M, Shinohara Y, Nagata T. 2007. Regulation of calprotectin expression by interleukin-1alpha and transforming growth factor-beta in human gingival keratinocytes. J Periodontal Res. 42(1):1–7. [DOI] [PubMed] [Google Scholar]
- He YY, Huang JL, Chignell CF. 2006. Cleavage of epidermal growth factor receptor by caspase during apoptosis is independent of its internalization. Oncogene. 25(10):1521–1531. [DOI] [PubMed] [Google Scholar]
- Hermani A, De Servi B, Medunjanin S, Tessier PA, Mayer D. 2006. S100A8 and S100A9 activate MAP kinase and NF-kappaB signaling pathways and trigger translocation of RAGE in human prostate cancer cells. Exp Cell Res. 312(2):184–197. [DOI] [PubMed] [Google Scholar]
- Hessian PA, Fisher L. 2001. The heterodimeric complex of MRP-8 (S100A8) and MRP-14 (S100A9): antibody recognition, epitope definition and the implications for structure. Eur J Biochem. 268(2):353–363. [DOI] [PubMed] [Google Scholar]
- Hobbs JA, May R, Tanousis K, McNeill E, Mathies M, Gebhardt C, Henderson R, Robinson MJ, Hogg N. 2003. Myeloid cell function in MRP-14 (S100A9) null mice. Mol Cell Biol. 23(7):2564–2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer S, Uhle F, Fleming T, Hell C, Schmoch T, Bruckner T, Weigand MA, Brenner T. 2016. RAGE-mediated inflammation in patients with septic shock. J Surg Res. 202(2):315–327. [DOI] [PubMed] [Google Scholar]
- Hsu K, Champaiboon C, Guenther BD, Sorenson BS, Khammanivong A, Ross KF, Geczy CL, Herzberg MC. 2009. Anti-infective protective properties of S100 calgranulins. Antiinflamm Antiallergy Agents Med Chem. 8(4):290–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iotzova-Weiss G, Dziunycz PJ, Freiberger SN, Läuchli S, Hafner J, Vogl T, French LE, Hofbauer GF. 2015. S100A8/A9 stimulates keratinocyte proliferation in the development of squamous cell carcinoma of the skin via the receptor for advanced glycation-end products. PLoS One. 10(3):e0120971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y, Arai K, Nozawa R, Yoshida H, Hirokawa M, Fukushima M, Inoue H, Tomoda C, Kihara M, Higashiyama T, et al. 2009. S100A8 and S100A9 expression is a crucial factor for dedifferentiation in thyroid carcinoma. Anticancer Res. 29(10):4157–4161. [PubMed] [Google Scholar]
- Ito Y, Arai K, Ryushi, Nozawa, Yoshida H, Tomoda C, Uruno T, Miya A, Kobayashi K, Matsuzuka F, et al. 2005. S100A9 expression is significantly linked to dedifferentiation of thyroid carcinoma. Pathol Res Pract. 201(8–9):551–556. [DOI] [PubMed] [Google Scholar]
- Itou H, Yao M, Fujita I, Watanabe N, Suzuki M, Nishihira J, Tanaka I. 2002. The crystal structure of human MRP14 (S100A9), a Ca(2+)-dependent regulator protein in inflammatory process. J Mol Biol. 316(2):265–276. [DOI] [PubMed] [Google Scholar]
- Ji J, Zhao L, Wang X, Zhou C, Ding F, Su L, Zhang C, Mao X, Wu M, Liu Z. 2004. Differential expression of S100 gene family in human esophageal squamous cell carcinoma. J Cancer Res Clin Oncol. 130(8):480–486. [DOI] [PubMed] [Google Scholar]
- Kerkhoff C, Hofmann HA, Vormoor J, Melkonyan H, Roth J, Sorg C, Klempt M. 2002. Binding of two nuclear complexes to a novel regulatory element within the human S100A9 promoter drives the S100A9 gene expression. J Biol Chem. 277(44):41879–41887. [DOI] [PubMed] [Google Scholar]
- Khammanivong A, Sorenson BS, Ross KF, Dickerson EB, Hasina R, Lingen MW, Herzberg MC. 2016. Involvement of calprotectin (S100A8/A9) in molecular pathways associated with HNSCC. Oncotarget. 7(12):14029–14047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khammanivong A, Wang C, Sorenson BS, Ross KF, Herzberg MC. 2013. S100A8/A9 (calprotectin) negatively regulates G2/M cell cycle progression and growth of squamous cell carcinoma. PLoS One. 8(7):e69395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kido J, Kido R, Suryono, Kataoka M, Fagerhol MK, Nagata T. 2004. Induction of calprotectin release by porphyromonas gingivalis lipopolysaccharide in human neutrophils. Oral Microbiol Immunol. 19(3):182–187. [DOI] [PubMed] [Google Scholar]
- Kim SW, Cheon K, Kim CH, Yoon JH, Hawke DH, Kobayashi R, Prudkin L, Wistuba II, Lotan R, Hong WK, et al. 2007. Proteomics-based identification of proteins secreted in apical surface fluid of squamous metaplastic human tracheobronchial epithelial cells cultured by three-dimensional organotypic air-liquid interface method. Cancer Res. 67(14):6565–6573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim WT, Kim J, Yan C, Jeong P, Choi SY, Lee OJ, Chae YB, Yun SJ, Lee SC, Kim WJ. 2014. S100A9 and EGFR gene signatures predict disease progression in muscle invasive bladder cancer patients after chemotherapy. Ann Oncol. 25(5):974–979. [DOI] [PubMed] [Google Scholar]
- Kong JP, Ding F, Zhou CN, Wang XQ, Miao XP, Wu M, Liu ZH. 2004. Loss of myeloid-related proteins 8 and myeloid-related proteins 14 expression in human esophageal squamous cell carcinoma correlates with poor differentiation. World J Gastroenterol. 10(8):1093–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kypriotou M, Huber M, Hohl D. 2012. The human epidermal differentiation complex: cornified envelope precursors, S100 proteins and the “fused genes” family. Exp Dermatol. 21(9):643–649. [DOI] [PubMed] [Google Scholar]
- Lehr E, Brown DR. 2003. Infection with the oncogenic human papillomavirus type 59 alters protein components of the cornified cell envelope. Virology. 309(1):53–60. [DOI] [PubMed] [Google Scholar]
- Leukert N, Sorg C, Roth J. 2005. Molecular basis of the complex formation between the two calcium-binding proteins S100A8 (MRP8) and S100A9 (MRP14). Biol Chem. 386(5):429–434. [DOI] [PubMed] [Google Scholar]
- Li MX, Xiao ZQ, Liu YF, Chen YH, Li C, Zhang PF, Li MY, Li F, Peng F, Duan CJ, et al. 2009. Quantitative proteomic analysis of differential proteins in the stroma of nasopharyngeal carcinoma and normal nasopharyngeal epithelial tissue. J Cell Biochem. 106(4):570–579. [DOI] [PubMed] [Google Scholar]
- Luo A, Kong J, Hu G, Liew CC, Xiong M, Wang X, Ji J, Wang T, Zhi H, Wu M, et al. 2004. Discovery of Ca2+-relevant and differentiation-associated genes downregulated in esophageal squamous cell carcinoma using cDNA microarray. Oncogene. 23(6):1291–1299. [DOI] [PubMed] [Google Scholar]
- Manitz MP, Horst B, Seeliger S, Strey A, Skryabin BV, Gunzer M, Frings W, Schonlau F, Roth J, Sorg C, et al. 2003. Loss of S100A9 (MRP14) results in reduced interleukin-8-induced CD11b surface expression, a polarized microfilament system, and diminished responsiveness to chemoattractants in vitro. Mol Cell Biol. 23(3):1034–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark R, Bermejo JL, Bierhaus A, Plinkert PK, Angel P, Hess J. 2013. The receptor for advanced glycation end products is dispensable in a mouse model of oral and esophageal carcinogenesis. Histol Histopathol. 28(12):1585–1594. [DOI] [PubMed] [Google Scholar]
- Marthaler AM, Podgorska M, Feld P, Fingerle A, Knerr-Rupp K, Grässer F, Smola H, Roemer K, Ebert E, Kim YJ, et al. 2017. Identification of C/EBPα as a novel target of the HPV8 E6 protein regulating miR-203 in human keratinocytes. PLoS Pathog. 13(6):e1006406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinsson H, Yhr M, Enerbäck C. 2005. Expression patterns of S100A7 (psoriasin) and S100A9 (calgranulin-B) in keratinocyte differentiation. Exp Dermatol. 14(3):161–168. [DOI] [PubMed] [Google Scholar]
- Melle C, Ernst G, Schimmel B, Bleul A, Koscielny S, Wiesner A, Bogumil R, Möller U, Osterloh D, Halbhuber KJ, et al. 2004. A technical triade for proteomic identification and characterization of cancer biomarkers. Cancer Res. 64(12):4099–4104. [DOI] [PubMed] [Google Scholar]
- Mitsudomi T, Yatabe Y. 2010. Epidermal growth factor receptor in relation to tumor development: EGFR gene and cancer. FEBS J. 277(2):301–308. [DOI] [PubMed] [Google Scholar]
- Moon A, Yong HY, Song JI, Cukovic D, Salagrama S, Kaplan D, Putt D, Kim H, Dombkowski A, Kim HR. 2008. Global gene expression profiling unveils S100A8/A9 as candidate markers in H-ras-mediated human breast epithelial cell invasion. Mol Cancer Res. 6(10):1544–1553. [DOI] [PubMed] [Google Scholar]
- Moris D, Spartalis E, Angelou A, Margonis GA, Papalambros A, Petrou A, Athanasiou A, Schizas D, Dimitroulis D, Felekouras E. 2016. The value of calprotectin S100A8/A9 complex as a biomarker in colorectal cancer: a systematic review. J BUON. 21(4):859–866. [PubMed] [Google Scholar]
- Nacken W, Roth J, Sorg C, Kerkhoff C. 2003. S100A9/S100A8: myeloid representatives of the S100 protein family as prominent players in innate immunity. Microsc Res Tech. 60(6):569–580. [DOI] [PubMed] [Google Scholar]
- Narumi K, Miyakawa R, Ueda R, Hashimoto H, Yamamoto Y, Yoshida T, Aoki K. 2015. Proinflammatory proteins S100A8/S100A9 activate NK cells via interaction with RAGE. J Immunol. 194(11):5539–5548. [DOI] [PubMed] [Google Scholar]
- Németh J, Stein I, Haag D, Riehl A, Longerich T, Horwitz E, Breuhahn K, Gebhardt C, Schirmacher P, Hahn M, et al. 2009. S100A8 and S100A9 are novel nuclear factor kappa B target genes during malignant progression of murine and human liver carcinogenesis. Hepatology. 50(4):1251–1262. [DOI] [PubMed] [Google Scholar]
- Network CGA. 2015. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature. 517(7536):576–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisapakultorn K, Ross KF, Herzberg MC. 2001. Calprotectin expression in vitro by oral epithelial cells confers resistance to infection by Porphyromonas gingivalis. Infect Immun. 69(7):4242–4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ødegaard E, Davidson B, Elgaaen BV, Fagerhol MK, Engh V, Onsrud M, Staff AC. 2008. Circulating calprotectin in ovarian carcinomas and borderline tumors of the ovary. Am J Obstet Gynecol. 198(4):418.e1–e7. [DOI] [PubMed] [Google Scholar]
- Passey RJ, Williams E, Lichanska AM, Wells C, Hu S, Geczy CL, Little MH, Hume DA. 1999. A null mutation in the inflammation-associated S100 protein S100A8 causes early resorption of the mouse embryo. J Immunol. 163(4):2209–2216. [PubMed] [Google Scholar]
- Qin F, Song Y, Li Z, Zhao L, Zhang Y, Geng L. 2010. S100A8/A9 induces apoptosis and inhibits metastasis of CasKi human cervical cancer cells. Pathol Oncol Res. 16(3):353–360. [DOI] [PubMed] [Google Scholar]
- Rahimi F, Hsu K, Endoh Y, Geczy CL. 2005. FGF-2, IL-1beta and TGF-beta regulate fibroblast expression of S100A8. FEBS J. 272(11):2811–2827. [DOI] [PubMed] [Google Scholar]
- Reckenbeil J, Kraus D, Probstmeier R, Allam JP, Novak N, Frentzen M, Martini M, Wenghoefer M, Winter J. 2016. Cellular distribution and gene expression pattern of Metastasin (S100A4), Calgranulin A (S100A8), and Calgranulin B (S100A9) in oral lesions as markers for molecular pathology. Cancer Invest. 34(6):246–254. [DOI] [PubMed] [Google Scholar]
- Reeb AN, Li W, Sewell W, Marlow LA, Tun HW, Smallridge RC, Copland JA, Spradling K, Chernock R, Lin RY. 2015. S100A8 is a novel therapeutic target for anaplastic thyroid carcinoma. J Clin Endocrinol Metab. 100(2):E232–E242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Barrueco R, Yu J, Saucedo-Cuevas LP, Olivan M, Llobet-Navas D, Putcha P, Castro V, Murga-Penas EM, Collazo-Lorduy A, Castillo-Martin M, et al. 2015. Inhibition of the autocrine IL-6-JAK2-STAT3-calprotectin axis as targeted therapy for HR-/HER2+ breast cancers. Genes Dev. 29(15):1631–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roesch Ely M, Nees M, Karsai S, Mägele I, Bogumil R, Vorderwülbecke S, Ruess A, Dietz A, Schnölzer M, Bosch FX. 2005. Transcript and proteome analysis reveals reduced expression of calgranulins in head and neck squamous cell carcinoma. Eur J Cell Biol. 84(2–3):431–444. [DOI] [PubMed] [Google Scholar]
- Sapkota D, Bruland O, Bøe OE, Bakeer H, Elgindi OA, Vasstrand EN, Ibrahim SO. 2008. Expression profile of the S100 gene family members in oral squamous cell carcinomas. J Oral Pathol Med. 37(10):607–615. [DOI] [PubMed] [Google Scholar]
- Sattari M, Pazhang Y, Imani M. 2014. Calprotectin induces cell death in human prostate cancer cell (LNCaP) through survivin protein alteration. Cell Biol Int. 38(11):1311–1320. [DOI] [PubMed] [Google Scholar]
- Sewell DA, Yuan CX, Robertson E. 2007. Proteomic signatures in laryngeal squamous cell carcinoma. ORL J Otorhinolaryngol Relat Spec. 69(2):77–84. [DOI] [PubMed] [Google Scholar]
- Silva EJ, Argyris PP, Zou X, Ross KF, Herzberg MC. 2014. S100A8/A9 regulates MMP-2 expression and invasion and migration by carcinoma cells. Int J Biochem Cell Biol. 55:279–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabur S, Korkmaz H, Özkaya M, Elboğa U, Tarakçıoglu M, Aksoy N, Akarsu E. 2015. Serum calprotectin: a new potential biomarker for thyroid papillary carcinoma. Tumour Biol. 36(10):7549–7556. [DOI] [PubMed] [Google Scholar]
- Taccioli C, Chen H, Jiang Y, Liu XP, Huang K, Smalley KJ, Farber JL, Croce CM, Fong LY. 2012. Dietary zinc deficiency fuels esophageal cancer development by inducing a distinct inflammatory signature. Oncogene. 31(42):4550–4558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taccioli C, Wan SG, Liu CG, Alder H, Volinia S, Farber JL, Croce CM, Fong LY. 2009. Zinc replenishment reverses overexpression of the proinflammatory mediator S100A8 and esophageal preneoplasia in the rat. Gastroenterology. 136(3):953–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi A, Blood DC, del Toro G, Canet A, Lee DC, Qu W, Tanji N, Lu Y, Lalla E, Fu C, et al. 2000. Blockade of RAGE-amphoterin signalling suppresses tumour growth and metastases. Nature. 405(6784):354–360. [DOI] [PubMed] [Google Scholar]
- Tavor S, Park DJ, Gery S, Vuong PT, Gombart AF, Koeffler HP. 2003. Restoration of C/EBPalpha expression in a BCR-ABL+ cell line induces terminal granulocytic differentiation. J Biol Chem. 278(52):52651–52659. [DOI] [PubMed] [Google Scholar]
- Testa U, Castelli G, Pelosi E. 2017. Esophageal cancer: genomic and molecular characterization, stem cell compartment and clonal evolution. Medicines (Basel). 4(3):E67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidehag V, Hammarsten P, Egevad L, Granfors T, Stattin P, Leanderson T, Wikström P, Josefsson A, Hägglöf C, Bergh A. 2014. High density of S100A9 positive inflammatory cells in prostate cancer stroma is associated with poor outcome. Eur J Cancer. 50(10):1829–1835. [DOI] [PubMed] [Google Scholar]
- Tugizov S, Berline J, Herrera R, Penaranda ME, Nakagawa M, Palefsky J. 2005. Inhibition of human papillomavirus type 16 E7 phosphorylation by the S100 MRP-8/14 protein complex. J Virol. 79(2):1099–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turovskaya O, Foell D, Sinha P, Vogl T, Newlin R, Nayak J, Nguyen M, Olsson A, Nawroth PP, Bierhaus A, et al. 2008. RAGE, carboxylated glycans and S100A8/A9 play essential roles in colitis-associated carcinogenesis. Carcinogenesis. 29(10):2035–2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban CF, Ermert D, Schmid M, Abu-Abed U, Goosmann C, Nacken W, Brinkmann V, Jungblut PR, Zychlinsky A. 2009. Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans. PLoS Pathog. 5(10):e1000639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rensburg SJ. 1981. Epidemiologic and dietary evidence for a specific nutritional predisposition to esophageal cancer. J Natl Cancer Inst. 67(2):243–251. [PubMed] [Google Scholar]
- Viemann D, Barczyk K, Vogl T, Fischer U, Sunderkötter C, Schulze-Osthoff K, Roth J. 2007. MRP8/MRP14 impairs endothelial integrity and induces a caspase-dependent and -independent cell death program. Blood. 109(6):2453–2460. [DOI] [PubMed] [Google Scholar]
- Wan SG, Taccioli C, Jiang Y, Chen H, Smalley KJ, Huang K, Liu XP, Farber JL, Croce CM, Fong LY. 2011. Zinc deficiency activates S100A8 inflammation in the absence of COX-2 and promotes murine oral-esophageal tumor progression. Int J Cancer. 129(2):331–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Cai Y, Xu H, Zhao J, Xu X, Han YL, Xu ZX, Chen BS, Hu H, Wu M, et al. 2004. Expression of MRP14 gene is frequently down-regulated in Chinese human esophageal cancer. Cell Res. 14(1):46–53. [DOI] [PubMed] [Google Scholar]
- Yang CS. 1980. Research on esophageal cancer in China: a review. Cancer Res. 40(8 Pt 1):2633–2644. [PubMed] [Google Scholar]
- Yao R, Lopez-Beltran A, Maclennan GT, Montironi R, Eble JN, Cheng L. 2007. Expression of S100 protein family members in the pathogenesis of bladder tumors. Anticancer Res. 27(5A):3051–3058. [PubMed] [Google Scholar]
- Zhu Y, Zhang F, Zhang S, Deng W, Fan H, Wang H, Zhang J. 2017. Regulatory mechanism and functional analysis of S100A9 in acute promyelocytic leukemia cells. Front Med. 11(1):87–96. [DOI] [PubMed] [Google Scholar]
- Zhuang S, Ouedraogo GD, Kochevar IE. 2003. Downregulation of epidermal growth factor receptor signaling by singlet oxygen through activation of caspase-3 and protein phosphatases. Oncogene. 22(28):4413–4424. [DOI] [PubMed] [Google Scholar]


