Abstract
Head and neck cancer is the sixth most common cancer worldwide. It remains one of the leading causes of death, and its early detection is crucial. Liquid biopsy has emerged as a promising tool for detecting and monitoring the disease status of patients with early and advanced cancers. Circulating tumor DNA (ctDNA), circulating tumor cells (CTCs), and exosomal miRNAs have received enormous attention because of their apparent clinical implications. Analyses of these circulating biomarkers have paved the way for novel therapeutic approaches and precision medicine. A growing number of reports have implicated the use of circulating biomarkers for detection, treatment planning, response monitoring, and prognosis assessment. Although these new biomarkers can provide a wide range of possible clinical applications, no validated circulating biomarkers have yet been integrated into clinical practice for head and neck cancer. In this review, we summarize the current knowledge of circulating biomarkers in this field, focusing on their feasibility, limitations, and key areas of clinical applications. We also highlight recent advances in salivary diagnostics and their potential application in head and neck cancer.
Keywords: biomarker, circulating tumor DNA, circulating tumor cell, exosomal miRNA, salivary diagnostics, saliva-exosomics
Introduction
Head and neck cancer is the sixth leading malignancy worldwide (Jemal et al. 2011). The predominant histological type is squamous cell carcinoma (SCC) that mainly occurs in the oral cavity, oropharynx, hypopharynx, and larynx. Despite advanced surgery and therapeutic strategies, the overall survival of head and neck cancer patients has remained unchanged for decades. Traditional cancer-screening techniques such as imaging and protein biomarkers are not sufficient for early detection. The Cancer Genome Atlas Network recently provided a comprehensive catalog of somatic genomic alterations in 279 head and neck SCCs (HNSCCs) to understand the molecular basis, thus accelerating the development of novel strategies for diagnosis and targeted therapies (The Cancer Genome Atlas Network 2015). Liquid biopsy has been increasingly considered as an option for molecular characterization and detection of cancer as it can provide real-time information about cancer in a minimally invasive manner. Circulating tumor DNA (ctDNA), circulating tumor cells (CTCs), and exosomal miRNAs are emerging biomarkers that can be applied to cancer detection, treatment planning, and response monitoring (Siravegna et al. 2017). Notably, ctDNA and exosomal miRNAs have been shown to be present in multiple body fluids, including saliva, and are very promising biomarkers for cancer (Weber et al. 2010). In this review, we summarize the current knowledge about circulating biomarkers (ctDNA, CTCs, and exosomal miRNAs) and their potential clinical applications in head and neck cancer.
Circulating Tumor DNA and Circulating Tumor Cells
Early Detection
ctDNA mainly originates from apoptotic or necrotic tumor cells and contains the mutations present in the tumor (Fig. 1). Somatic mutations are tumor specific, and evaluation of these unique genetic changes offers the potential for better diagnostic accuracy. Several studies have demonstrated a high concordance of mutational profiles between plasma ctDNA and matched tumor samples in lung cancer (Newman et al. 2014), breast cancer (Beaver et al. 2014; Bettegowda et al. 2014), and colorectal cancer (Diehl et al. 2008; Thierry et al. 2014). Bettegowda et al. (2014) evaluated ctDNA in breast, colorectal, gastroesophageal, and pancreatic cancers and found that the overall ctDNA detection rates for patients with stage II, III, and IV cancers were 55%, 69%, and 82%, respectively. Importantly, 47% of patients with stage I cancers had detectable ctDNA, suggesting that ctDNA is a promising biomarker for early detection of cancer. Newman et al. (2014) detected ctDNA in 50% of patients with stage I lung cancer. Moreover, Beaver et al. (2014) detected ctDNA in patients with early stage breast cancer with a sensitivity of 93.3%, using droplet digital polymerase chain reaction (ddPCR).
Figure 1.
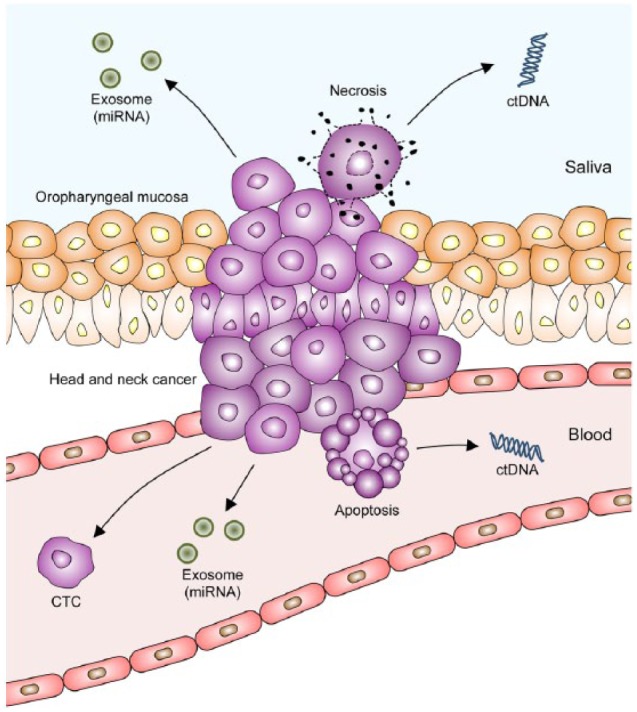
Circulating biomarkers in head and neck cancer. Circulating tumor DNA (ctDNA), circulating tumor cells (CTCs), and exosomal miRNAs are complementary biomarkers present in plasma and/or saliva. Apoptotic tumor cells release ctDNA into blood, whereas necrotic tumor cells shed ctDNA into saliva. Tumor cells release exosomal miRNAs into blood and saliva. Primary tumor and metastatic lesions release CTCs into blood.
A recent proof-of-principle study reported ctDNA to be a biomarker in head and neck cancer (Wang et al. 2015). In a cohort of 93 patients with HNSCC, including 20 cases of early stage cancer, plasma and saliva samples were screened for somatic mutations (TP53, PIK3CA, NOTCH1, FBXW7, CDKN2A, NRAS, and HRAS) and human papillomaviruses (HPV16 and 18) (Table). Plasma ctDNA was shown to be a more sensitive biomarker than salivary ctDNA for oropharynx, hypopharynx, and larynx cancer (plasma ctDNA: 86%–100% vs. salivary ctDNA: 47%–70%). However, salivary ctDNA showed better sensitivity than plasma ctDNA (100% vs. 80%) in oral cancer, indicating that oral cancer–derived DNA is more readily detected in saliva due to the close proximity of the tumor to saliva. Importantly, when both plasma and saliva were tested in combination, the overall ctDNA detection rate was 96%, irrespective of tumor location or stage. These findings demonstrate the importance of examining combination or appropriate bodily fluids according to tumor type to achieve the highest sensitivity.
Table.
Summary of Saliva and Plasma ctDNA Biomarker Profiles Identified in Head and Neck Squamous Cell Carcinoma.
| % of Positivity (No. Detected/No. Examined) |
|||||
|---|---|---|---|---|---|
| ctDNA | Saliva | Plasma | Saliva or Plasmaa | ||
| Site | Oral cavity | TP53 | 100 (36/36) | 85 (11/13) | 100 (13/13) |
| PIK3CA | 100 (2/2) | 50 (1/2) | 100 (2/2) | ||
| NOTCH1 | 100 (3/3) | NA | NA | ||
| CDKN2A | 100 (2/2) | NA | NA | ||
| Translocation | 100 (2/2) | NA | NA | ||
| HPV 16 DNA | 100 (1/1) | NA | NA | ||
| (Total) | 100 (46/46) | 80 (12/15) | 100 (15/15) | ||
|
|
|||||
| Oropharynx | TP53 | 80 (4/5) | 100 (1/1) | 100 (1/1) | |
| PIK3CA | 25 (2/8)b | 100 (5/5) | 100 (5/5) | ||
| FBXW7 | 67 (2/3) | 100 (3/3) | 100 (3/3) | ||
| HPV 16 DNA | 41 (7/17) | 92 (11/12) | 92 (11/12) | ||
| NRAS | 0 (0/1) | 0 (0/1) | 0 (0/1) | ||
| (Total) | 47 (16/34)b | 91 (20/22) | 91 (20/22) | ||
|
|
|||||
| Larynx | TP53 | 70 (7/10) | 86 (6/7) | 100 (7/7) | |
|
|
|||||
| Hypopharynx | TP53 | 67 (2/3) | 100 (3/3) | 100 (3/3) | |
|
| |||||
| Stage | Early (I + II) | TP53 | 100 (16/16) | 75 (6/8) | 100 (8/8) |
| HPV 16 DNA | 100 (2/2) | 100 (1/1) | 100 (1/1) | ||
| PIK3CA | 100 (1/1) | 0 (0/1) | 100 (1/1) | ||
| NOTCH1 | 100 (1/1) | NA | NA | ||
| (Total) | 100 (20/20) | 70 (7/10) | 100 (10/10) | ||
|
|
|||||
| Late (III + IV) | TP53 | 87 (33/38) | 94 (15/16) | 100 (16/16) | |
| PIK3CA | 33 (3/9)b | 100 (6/6) | 100 (6/6) | ||
| FBXW7 | 67 (2/3) | 100 (3/3) | 100 (3/3) | ||
| HPV16 DNA | 38 (6/16) | 91 (10/11) | 91 (10/11) | ||
| NOTCH1 | 100 (2/2) | NA | NA | ||
| CDKN2A | 100 (2/2) | NA | NA | ||
| Translocation | 100 (2/2) | NA | NA | ||
| NRAS | 0 (0/1) | 0 (0/1) | 0 (0/1) | ||
| (Total) | 70 (51/73)b | 92 (34/37) | 95 (35/37) | ||
|
| |||||
| HPV | HPV 16 | HPV16 DNA | 40 (12/30) | 86 (18/21) | 86 (18/21) |
|
| |||||
| Overall | 76 (71/93)b | 87 (41/47) | 96 (45/47) | ||
All biomarker data and detection rate were extracted from the results of the safe-sequencing system (Safe-SeqS) and digital polymerase chain reaction published by Wang et al. (2015).
ctDNA, circulating tumor DNA; HPV, human papillomavirus; NA, not applicable.
Detection rate in “saliva or plasma” was calculated only if patients’ data from both saliva and plasma were available.
One patient with PIK3CA-negative but HPV-positive saliva was counted in the total number.
For cancers with a viral etiology such as nasopharyngeal carcinoma, detection of the cancer-associated viral DNA may provide a good strategy for identifying individuals with early stage disease. Chan et al. (2013) screened asymptomatic volunteers for plasma Epstein-Barr virus (EBV) DNA and found 69 of the 1,318 participants (5.2%) had viral DNA, among whom 3 individuals were diagnosed with nasopharyngeal carcinoma. Early detection of cancer, particularly before metastatic spread, is crucial for early intervention and improving prognosis.
Primary and metastatic tumors release subsets of CTCs into the blood (Fig. 1). An important hypothesis is that CTCs mirror tumor heterogeneity, and increased CTC levels exhibit diagnostic features. CTCs have been tested in numerous studies for diagnosis of primary tumors and metastatic relapse (Alix-Panabières and Pantel 2016). Nichols et al. (2012) and He et al. (2013) reported that CTCs were detected in 6 of 15 (40.0%) and 3 of 9 (33.3%) patients with head and neck cancer, respectively. Buglione et al. (2012) found that CTCs were more frequently found in advanced stages of head and neck cancer than in its early stages. Moreover, Jatana et al. (2011) and Gröbe et al. (2014) reported that an increased number of CTCs was correlated with poorer prognosis, and the presence of CTCs was correlated with locoregional relapse. However, CTCs seem to be much less sensitive than ctDNA for early cancer detection. Bettegowda et al. (2014) found that no CTCs were detected in early stage bladder, breast, and colorectal cancers, whereas ctDNA was detected in 81% of these cancers. These findings suggest that CTC is more likely to be a prognostic marker rather than an early diagnostic marker in cancer.
Treatment Selection
CTCs can be exploited as surrogate biopsy specimens to investigate the presence of drug targets. Measuring cell surface expression of the epidermal growth factor receptor (EGFR) on CTCs provides critical information for planning anti-EGFR treatment. In support of this, cetuximab treatment was more effective in reducing EGFR-positive CTCs than conventional chemotherapy in HNSCC (Tinhofer et al. 2012). Moreover, detection of CTCs expressing programmed cell death ligand 1 (PD-L1) on their surface can be predictive of response for anti–PD-1 immunotherapy as PD-L1 is a key factor that suppresses T-cell function (Butt and Mills 2014). Mazel et al. (2015) reported that PD-L1–expressing CTCs were detected in 11 of 16 (68.8%) patients with breast cancer, suggesting its usefulness in treatment planning.
Detecting CTCs is challenging due to their extremely low levels. It is estimated that only 1 to 2 CTCs are present per 7.5 mL of blood, making them difficult to study (Nichols et al. 2012). Currently, the only Food and Drug Administration (FDA)–approved platform for isolating CTC is CellSearch (Riethdorf et al. 2007). CellSearch is a standardized, semiautomated system that enables positive selection of CTCs based on the expression of the epithelial marker EpCAM. Testing therapeutic targets on a small population of CTCs in patients with HNSCC is currently under investigation, but its clinical utility has not yet been established.
Monitoring Treatment Response
ctDNA can be used in monitoring response to cancer treatment. Compared with imaging, ctDNA offers the diagnostic advantage of real-time monitoring of treatment response (Haber and Velculescu 2014). A recent study reported an early spike in plasma ctDNA levels (increase in BRAF mutated DNA) in melanoma patients with T-cell transfer immunotherapy, reflecting transient tumor cell death (Xi et al. 2016). Another study found a reduction in ctDNA levels (EGFR mutations) in lung cancer patients after tyrosine kinase inhibitor (TKI) therapy, suggesting early indication of treatment response (Thress et al. 2015). A gatekeeper mutation (EGFR T790M) associated with TKI resistance was also detected during ctDNA monitoring. Moreover, ctDNA is more sensitive than CTCs or cancer antigen 15-3 (CA15-3) as a circulating biomarker for predicting treatment response in breast cancer (Dawson et al. 2013). Thus, detecting the differential early dynamics of mutations may predict treatment response in the context of systemic therapy, enabling earlier therapeutic intervention.
A clearance study investigating the half-life of plasma EBV DNA in nasopharyngeal carcinoma patients demonstrated that the median half-life during chemotherapy was 3.99 d (range, 1.85–28.29 d) (Wang et al. 2010). Another study reported that the half-life of plasma ctDNA (APC, KRAS, TP53, and PIK3CA) in colorectal cancer was 114 min after surgery, suggesting that ctDNA is an ideal biomarker to monitor rapid changes of tumor size because of its fast dynamics (Diehl et al. 2008). These findings suggest that viral DNA or ctDNA measurements could be used to reliably monitor tumor dynamics in cancer patients undergoing chemotherapy and/or surgery (Fig. 2A).
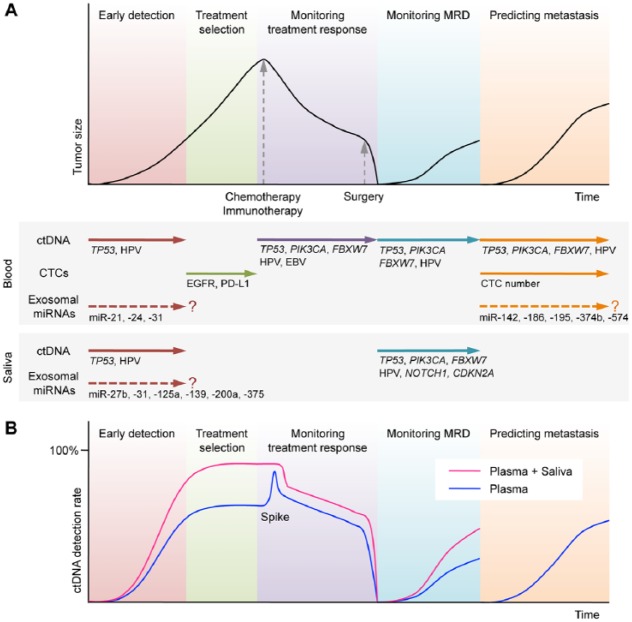
Figure 2.
Potential clinical applications of circulating biomarkers in the treatment of head and neck cancer. (A) Schematic time course of disease management and tumor size in head and neck cancer patients undergoing chemotherapy (or immunotherapy) and surgery. Plasma circulating tumor DNA (ctDNA) analysis allows early detection, monitoring treatment response, monitoring minimal residual disease (MRD), and predicting metastasis. Circulating tumor cell (CTC) analysis can assist the selection of targeted therapies. Exosomal miRNAs currently offer limited insight into clinical applications. Salivary ctDNA analysis can provide complementary information. (B) Use of plasma and salivary ctDNA in combination allows higher detection of cancer than use of plasma ctDNA alone. A spike in ctDNA level reflects transient tumor cell death by systemic therapy.
Monitoring Minimal Residual Disease
Recent studies have demonstrated that ctDNA levels can be exploited to monitor minimal residual disease (MRD) following surgery (Reinert et al. 2016). In principle, detection of ctDNA may be a more suitable approach than other circulating biomarkers for measuring MRD, as ddPCR has the highest sensitivity. Diehl et al. (2008) detected mutations as low as 0.01% in cell-free DNA in colorectal cancer patients, and those with MRD relapsed within 1 y after surgery. A prospective study of 230 patients with colorectal cancer demonstrated that relapse-free survival at 3 y after surgery was 90% for the ctDNA-negative group and 0% for the ctDNA-positive group (Tie et al. 2016). In a separate study of 55 patients with breast cancer, postoperative ctDNA detection predicted poor relapse-free survival with a high level of accuracy (Garcia-Murillas et al. 2015). Hamana et al. (2005) reported that ctDNAs were detected postoperatively by the use of microsatellite markers, predicting relapse in oral SCC. Stratification of patients into high- or low-risk groups on the basis of ctDNA levels would enable earlier rescue treatment after surgery. Although there is evidence to indicate that ctDNA is a promising biomarker for monitoring MRD, whether identifying MRD-positive patients could improve patient outcomes through early therapeutic intervention remains to be elucidated in large clinical trials.
Predicting Metastasis
The current use of ctDNA is based on the evidence that it shares common mutational profiles with primary or secondary tumors. This implies that we can obtain a signature of metastasis without the need for an invasive tissue biopsy (Dawson et al. 2013; Bettegowda et al. 2014). In support of this, numerous studies have reported ctDNA to be a highly sensitive biomarker for metastasis, reflecting tumor burden and heterogeneity in various cancers, including head and neck cancer (Dawson et al. 2013; Bettegowda et al. 2014; Lebofsky et al. 2015).
The prognostic value of CTC enumeration has also been demonstrated in various tumor types via large clinical trials (Groot Koerkamp et al. 2013; Ma et al. 2014; Wang et al. 2014). There is growing evidence that detection of CTCs correlates with poor survival in head and neck cancer patients (Jatana et al. 2010, 2011; Gröbe et al. 2014; Kulasinghe et al. 2015). In addition, CTC number was reported to be correlated with a higher incidence of regional metastasis in head and neck cancer (Hristozova et al. 2011). However, these studies were unable to provide threshold CTC values correlating with poor prognosis as CTC numbers are highly variable among individuals. Although the clinical value of CTC analysis remains controversial, there is evidence indicating that CTC numbers after surgery or systemic therapy can be predictive of treatment outcomes and metastasis (Toss et al. 2014).
The low numbers of CTCs make their detection challenging. CellSearch method selects for tumor cells expressing EpCAM; therefore, downregulation of EpCAM during the epithelial-mesenchymal transition may make CTCs undetectable. Actually, only one-third of CTCs are found to be EpCAM positive in metastatic breast cancer patients (de Albuquerqu et al. 2012). This limitation might be overcome by combining different technologies and using additional markers. With this in mind, we propose that the combined use of ctDNA and CTCs may be an ideal strategy to assess the risk for metastasis in head and neck cancer.
Circulating Exosomal miRNAs
Exosomes are small, cell-secreted vesicles that carry diverse cellular constituents from their parental cells, including DNA, RNA, and protein. Exosomes have important roles in exchanging molecular information between cells (Simons and Raposo 2009). Given their content, exosomes could potentially be exploited as cancer biomarkers. Within the complex cargo of exosomes, miRNAs are the most relevant constituents for cancer diagnosis as they are regulatory molecules of both oncogenes and tumor suppressor genes (Chen et al. 2012). miRNA profiles in plasma exosomes have been reported to correlate with those in tumors from which they originate (Mitchell et al. 2008; Rosenfeld et al. 2008). These characteristics make exosomal miRNAs promising biomarkers for cancers (Skog et al. 2008; Taylor and Gercel-Taylor 2008; Rabinowits et al. 2009). The use of miRNA signature as a diagnostic tool in head and neck cancer has been explored previously. Summerer et al. (2015) reported that high expression of circulating miR-142, miR-186, miR-195, miR-374b, and miR-574 represent prognostic biomarkers for head and neck cancer. Similarly, elevated levels of miR-21 and miR-24 were detected in plasma from head and neck cancer patients (Lin et al. 2010; Hsu et al. 2012). Moreover, amplified miR-31 was detected in the plasma of head and neck cancer patients and was observed to have reduced after tumor resection, suggesting its tumor origin (Liu et al. 2010). These reports provide considerable evidence that exosomal miRNAs can be exploited as a valuable tool in cancer diagnosis.
The miRNA database, miRandola, provides a comprehensive catalog of extracellular noncoding RNAs identified in various diseases, including cancer, and currently contains 3,283 entries with 1,002 miRNAs (http://mirandola.iit.cnr.it/) (Russo et al. 2017). Although a number of exosomal miRNAs have been proposed as diagnostic and prognostic markers, most have been investigated by inconsistent methods, and the heterogeneous results hamper the reliability of miRNAs in clinical diagnosis (Ono et al. 2015). Moreover, it is unclear whether immune cells make a strong contribution to circulating miRNA levels. Systemic or local inflammation may perturb miRNA expression and its reproducibility even within the same individual (Pritchard et al. 2012). The diagnostic performance of exosomal miRNAs in head and neck cancer remains inconsistent among studies; thus, more studies are needed to further characterize exosomal miRNAs. Validation in large clinical trials with standardized protocols is required to substantiate the value of exosomal miRNAs in a clinical setting.
Circulating Biomarkers in Saliva
Salivary ctDNA has been demonstrated to be a more sensitive biomarker than plasma ctDNA for early stage oral cancer (Table). A proof-of-principle study demonstrated that ctDNA can be detected in saliva in early stage oral cancer with 100% sensitivity (Wang et al. 2015). Even in patients with cancers at other sites (oropharynx, hypopharynx, and larynx), ctDNA was found in the saliva of 47% to 70% of these patient groups, making it a valuable biomarker for detecting head and neck cancer. Saliva is enriched with tumor DNA originating from the oropharyngeal cavity; thus, analyzing both saliva and plasma may be optimal for effective screening of head and neck cancer (Fig. 2B).
Circulating cell-free salivary miRNAs were first discovered and characterized in our laboratory (Park et al. 2009). Salivary miRNAs were found to be remarkably stable, and endogenous salivary miRNAs degrade at a much slower rate than exogenous miRNAs (Park et al. 2009). miRNA profiling demonstrated that salivary miRNAs are packaged into exosomes, rendering them resistant to degradation by RNases (Michael et al. 2010; Gallo et al. 2012). Since then, salivary miRNAs have been studied as potential biomarkers for head and neck cancer on the basis of their relative ease of collection and detection. We reported that miR-125a and miR-200a were significantly less in saliva collected from oral cancer patients than they were in controls (Park et al. 2009). Similarly, the expression of miR-139 and miR-375 was also found to be decreased in saliva collected from oral cancer patients compared with that obtained from normal controls (Wiklund et al. 2011; Duz et al. 2016). In addition, increased expression of miR-27b and miR-31 was observed in saliva obtained from oral cancer patients compared with that obtained from controls (Liu et al. 2012; Momen-Heravi et al. 2014). Importantly, expression levels of these miRNAs reverted to baseline after excision of the lesions, suggesting their potential use as diagnostic biomarkers (Liu et al. 2012; Duz et al. 2016). Although certain sets of salivary miRNAs may serve as putative biomarkers for head and neck cancer, further research is required to validate these findings and elucidate the molecular mechanisms involved.
Conclusion and Future Direction
A growing body of evidence implicates the clinical utility of circulating biomarkers extracted from multiple body fluids for cancer patients, focusing on patient stratification and monitoring disease status. Actively released ctDNA in plasma and saliva may be preferred for the early detection of head and neck cancer, whereas CTCs released from metastatic lesions may predict poor prognosis. Analyzing CTCs for surface expression of drug targets such as EGFR and PD-L1 can provide critical information for planning immunotherapy. Other circulating biomarkers such as exosomal miRNAs can provide additional layers of information; thus, targeting multiple types of biomarkers that have independent mechanisms of release may increase the specificity and sensitivity of cancer diagnosis.
The key question concerning all types of circulating biomarkers (ctDNA, CTCs, and exosomal miRNAs) is how representative they are of the whole tumor. In this regard, assessment of biomarkers should be considered in the context of integrity and inclusivity of all tumor features. For example, mutated DNA fragments such as TP53 and PIK3CA can only provide information about limited regulatory pathways. CTCs are considered to contain the complete cellular information; however, if a tumor is heterogeneous, they might represent just a small proportion of the tumor, thereby compromising their clinical relevance. In contrast, exosomes are expected to represent more of the tumor because they are thought to be derived from the whole tumor, reflecting its heterogeneous characteristic. However, the cargo of exosomes has been demonstrated to be selectively assembled through the trans-Golgi network and endosomal system; therefore, exosomal miRNAs can be only partially representative of whole cellular miRNAs. Further studies with large patient cohorts using standardized protocols are required to determine whether each approach, individually as well as combined, can improve overall survival.
Analysis of circulating biomarkers in multiple body fluids alongside plasma can provide complementary information and represent key milestones toward the implementation of liquid biopsies in personalized medicine. Saliva is a mixture of the secretions from the major salivary glands and numerous minor salivary glands. The combination of these secretions, including gingival crevicular fluid, is whole saliva, containing proteins, microorganisms, and cellular debris. The use of whole saliva is easy, noninvasive, and informative; thus, salivary diagnostics may fulfill the ambitions of precision medicine initiatives. Ductal saliva from individual salivary glands (parotid, submandibular, and sublingual) can be used for research studies but would be impractical for clinical utilities.
We first coined the term saliva-exosomics to describe next-generation salivaomics that studies salivary exosomes through the advanced “omics” technologies to better delineate their specific functions and biomarkers for cancer (Nonaka and Wong 2017). A unique subset of exosomes originating from tumors appears to be present in saliva, which might be due to processing and selection of tumor-derived exosomes in the salivary gland. Nevertheless, the amount and content of salivary exosomes are highly variable even in patients with the same tumor types and stages. A more in-depth understanding of the biology of salivary exosomes will provide the basis for biomarker development and new therapeutic avenues.
Establishing simple, rapid, and affordable technologies to analyze circulating biomarkers represents an important future challenge. Currently, ddPCR and next-generation sequencing play a central role in ctDNA and miRNA analysis. However, high cost and complicated data manipulation impede their applications in routine clinical care. We recently developed a novel diagnostic platform, EFIRM (electric field-induced release and measurement), that can detect ctDNA and exosomal RNA directly, only requiring 40 µL of body fluid (Wei et al. 2014; Tu et al. 2015; Pu et al. 2016). Further development and application of saliva-based point-of-care technologies will allow for better immediate clinical management, leading to earlier intervention and reduced morbidity and mortality.
Author Contributions
T. Nonaka, D.T.W. Wong, contributed to conception and design, drafted and critically revised the manuscript. Both authors gave final approval and agree to be accountable for all aspects of the work.
Footnotes
This work was supported by grants (CA206126 and TR000923) from the National Institutes of Health (NIH) to D.T.W. Wong. T. Nonaka was supported by the National Institute of Dental and Craniofacial Research (NIDCR/NIH) training grant (DE023057).
D.T.W. Wong is the cofounder of RNAmeTRIX, a molecular diagnostic company. D.T.W. Wong holds equity in RNAmeTRIX and serves as a company director and scientific advisor. The University of California also holds equity in RNAmeTRIX. Intellectual property that D.T.W. Wong invented and that was patented by the University of California has been licensed to RNAmeTRIX. D.T.W. Wong is a consultant to GlaxoSmithKlein, Wrigley, and Colgate-Palmolive. The authors declare no other potential conflicts of interest with respect to the authorship and/or publication of this article.
ORCID iD: T. Nonaka  https://orcid.org/0000-0002-9183-4734
https://orcid.org/0000-0002-9183-4734
References
- Alix-Panabières C, Pantel K. 2016. Clinical applications of circulating tumor cells and circulating tumor DNA as liquid biopsy. Cancer Discov. 6(5):479–491. [DOI] [PubMed] [Google Scholar]
- Beaver JA, Jelovac D, Balukrishna S, Cochran R, Croessmann S, Zabransky DJ, Wong HY, Toro PV, Cidado J, Blair BG, et al. 2014. Detection of cancer DNA in plasma of patients with early-stage breast cancer. Clin Cancer Res. 20(10):2643–2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettegowda C, Sausen M, Leary RJ, Kinde I, Wang Y, Agrawal N, Bartlett BR, Wang H, Luber B, Alani RM, et al. 2014. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci Transl Med. 6(224):224ra24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buglione M, Grisanti S, Almici C, Mangoni M, Polli C, Consoli F, Verardi R, Costa L, Paiar F, Pasinetti N, et al. 2012. Circulating tumour cells in locally advanced head and neck cancer: preliminary report about their possible role in predicting response to non-surgical treatment and survival. Eur J Cancer. 48(16):3019–3026. [DOI] [PubMed] [Google Scholar]
- Butt AQ, Mills KH. 2014. Immunosuppressive networks and checkpoints controlling antitumor immunity and their blockade in the development of cancer immunotherapeutics and vaccines. Oncogene. 33(38):4623–4631. [DOI] [PubMed] [Google Scholar]
- Chan KC, Hung EC, Woo JK, Chan PK, Leung SF, Lai FP, Cheng AS, Yeung SW, Chan YW, Tsui TK, et al. 2013. Early detection of nasopharyngeal carcinoma by plasma Epstein-Barr virus DNA analysis in a surveillance program. Cancer. 119(10):1838–1844. [DOI] [PubMed] [Google Scholar]
- Chen X, Liang H, Zhang J, Zen K, Zhang CY. 2012. Secreted microRNAs: a new form of intercellular communication. Trends Cell Biol. 22(3):125–132. [DOI] [PubMed] [Google Scholar]
- Dawson SJ, Tsui DW, Murtaza M, Biggs H, Rueda OM, Chin SF, Dunning MJ, Gale D, Forshew T, Mahler-Araujo B, et al. 2013. Analysis of circulating tumor DNA to monitor metastatic breast cancer. N Engl J Med. 368(13):1199–1209. [DOI] [PubMed] [Google Scholar]
- de Albuquerque A, Kaul S, Breier G, Krabisch P, Fersis N. 2012. Multimarker analysis of circulating tumor cells in peripheral blood of metastatic breast cancer patients: a step forward in personalized medicine. Breast Care (Basel). 7(1):7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehl F, Schmidt K, Choti MA, Romans K, Goodman S, Li M, Thornton K, Agrawal N, Sokoll L, Szabo SA, et al. 2008. Circulating mutant DNA to assess tumor dynamics. Nat Med. 14(9):985–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duz MB, Karatas OF, Guzel E, Turgut NF, Yilmaz M, Creighton CJ, Ozen M. 2016. Identification of miR-139-5p as a saliva biomarker for tongue squamous cell carcinoma: a pilot study. Cell Oncol (Dordr). 39(2):187–193. [DOI] [PubMed] [Google Scholar]
- Gallo A, Tandon M, Alevizos I, Illei GG. 2012. The majority of microRNAs detectable in serum and saliva is concentrated in exosomes. PLoS One. 7(3):e30679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Murillas I, Schiavon G, Weigelt B, Ng C, Hrebien S, Cutts RJ, Cheang M, Osin P, Nerurkar A, Kozarewa I, et al. 2015. Mutation tracking in circulating tumor DNA predicts relapse in early breast cancer. Sci Transl Med. 7(302):302ra133. [DOI] [PubMed] [Google Scholar]
- Gröbe A, Blessmann M, Hanken H, Friedrich RE, Schon G, Wikner J, Effenberger KE, Kluwe L, Heiland M, Pantel K, et al. 2014. Prognostic relevance of circulating tumor cells in blood and disseminated tumor cells in bone marrow of patients with squamous cell carcinoma of the oral cavity. Clin Cancer Res. 20(2):425–433. [DOI] [PubMed] [Google Scholar]
- Groot Koerkamp B, Rahbari NN, Buchler MW, Koch M, Weitz J. 2013. Circulating tumor cells and prognosis of patients with resectable colorectal liver metastases or widespread metastatic colorectal cancer: a meta-analysis. Ann Surg Oncol. 20(7):2156–2165. [DOI] [PubMed] [Google Scholar]
- Haber DA, Velculescu VE. 2014. Blood-based analyses of cancer: circulating tumor cells and circulating tumor DNA. Cancer Discov. 4(6):650–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamana K, Uzawa K, Ogawara K, Shiiba M, Bukawa H, Yokoe H, Tanzawa H. 2005. Monitoring of circulating tumour-associated DNA as a prognostic tool for oral squamous cell carcinoma. Br J Cancer. 92(12):2181–2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S, Li P, Long T, Zhang N, Fang J, Yu Z. 2013. Detection of circulating tumour cells with the CellSearch system in patients with advanced-stage head and neck cancer: preliminary results. J Laryngol Otol. 127(8):788–793. [DOI] [PubMed] [Google Scholar]
- Hristozova T, Konschak R, Stromberger C, Fusi A, Liu Z, Weichert W, Stenzinger A, Budach V, Keilholz U, Tinhofer I. 2011. The presence of circulating tumor cells (CTCs) correlates with lymph node metastasis in nonresectable squamous cell carcinoma of the head and neck region (SCCHN). Ann Oncol. 22(8):1878–1885. [DOI] [PubMed] [Google Scholar]
- Hsu CM, Lin PM, Wang YM, Chen ZJ, Lin SF, Yang MY. 2012. Circulating miRNA is a novel marker for head and neck squamous cell carcinoma. Tumour Biol. 33(6):1933–1942. [DOI] [PubMed] [Google Scholar]
- Jatana KR, Balasubramanian P, Lang JC, Yang L, Jatana CA, White E, Agrawal A, Ozer E, Schuller DE, Teknos TN, et al. 2010. Significance of circulating tumor cells in patients with squamous cell carcinoma of the head and neck: initial results. Arch Otolaryngol Head Neck Surg. 136(12):1274–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jatana KR, Lang JC, Chalmers JJ. 2011. Identification of circulating tumor cells: a prognostic marker in squamous cell carcinoma of the head and neck? Future Oncol. 7(4):481–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. 2011. Global cancer statistics. CA Cancer J Clin. 61(2):69–90. [DOI] [PubMed] [Google Scholar]
- Kulasinghe A, Perry C, Jovanovic L, Nelson C, Punyadeera C. 2015. Circulating tumour cells in metastatic head and neck cancers. Int J Cancer. 136(11):2515–2523. [DOI] [PubMed] [Google Scholar]
- Lebofsky R, Decraene C, Bernard V, Kamal M, Blin A, Leroy Q, Rio Frio T, Pierron G, Callens C, Bieche I, et al. 2015. Circulating tumor DNA as a non-invasive substitute to metastasis biopsy for tumor genotyping and personalized medicine in a prospective trial across all tumor types. Mol Oncol. 9(4):783–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SC, Liu CJ, Lin JA, Chiang WF, Hung PS, Chang KW. 2010. miR-24 up-regulation in oral carcinoma: positive association from clinical and in vitro analysis. Oral Oncol. 46(3):204–208. [DOI] [PubMed] [Google Scholar]
- Liu CJ, Kao SY, Tu HF, Tsai MM, Chang KW, Lin SC. 2010. Increase of microRNA miR-31 level in plasma could be a potential marker of oral cancer. Oral Dis. 16(4):360–364. [DOI] [PubMed] [Google Scholar]
- Liu CJ, Lin SC, Yang CC, Cheng HW, Chang KW. 2012. Exploiting salivary miR-31 as a clinical biomarker of oral squamous cell carcinoma. Head Neck. 34(2):219–224. [DOI] [PubMed] [Google Scholar]
- Ma X, Xiao Z, Li X, Wang F, Zhang J, Zhou R, Wang J, Liu L. 2014. Prognostic role of circulating tumor cells and disseminated tumor cells in patients with prostate cancer: a systematic review and meta-analysis. Tumour Biol. 35(6):5551–5560. [DOI] [PubMed] [Google Scholar]
- Mazel M, Jacot W, Pantel K, Bartkowiak K, Topart D, Cayrefourcq L, Rossille D, Maudelonde T, Fest T, Alix-Panabieres C. 2015. Frequent expression of PD-L1 on circulating breast cancer cells. Mol Oncol. 9(9):1773–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael A, Bajracharya SD, Yuen PS, Zhou H, Star RA, Illei GG, Alevizos I. 2010. Exosomes from human saliva as a source of microRNA biomarkers. Oral Dis. 16(1):34–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL, Peterson A, Noteboom J, O’Briant KC, Allen A, et al. 2008. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci U S A. 105(30):10513–10518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momen-Heravi F, Trachtenberg AJ, Kuo WP, Cheng YS. 2014. Genomewide study of salivary microRNAs for detection of oral cancer. J Dent Res. 93(7 Suppl):86S–93S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman AM, Bratman SV, To J, Wynne JF, Eclov NC, Modlin LA, Liu CL, Neal JW, Wakelee HA, Merritt RE, et al. 2014. An ultrasensitive method for quantitating circulating tumor DNA with broad patient coverage. Nat Med. 20(5):548–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols AC, Lowes LE, Szeto CC, Basmaji J, Dhaliwal S, Chapeskie C, Todorovic B, Read N, Venkatesan V, Hammond A, et al. 2012. Detection of circulating tumor cells in advanced head and neck cancer using the CellSearch system. Head Neck. 34(10):1440–1444. [DOI] [PubMed] [Google Scholar]
- Nonaka T, Wong DTW. 2017. Saliva-exosomics in cancer: molecular characterization of cancer-derived exosomes in saliva. Enzymes. 42:125–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono S, Lam S, Nagahara M, Hoon DS. 2015. Circulating microRNA biomarkers as liquid biopsy for cancer patients: pros and cons of current assays. J Clin Med. 4(10):1890–1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park NJ, Zhou H, Elashoff D, Henson BS, Kastratovic DA, Abemayor E, Wong DT. 2009. Salivary microRNA: discovery, characterization, and clinical utility for oral cancer detection. Clin Cancer Res. 15(17):5473–5477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard CC, Kroh E, Wood B, Arroyo JD, Dougherty KJ, Miyaji MM, Tait JF, Tewari M. 2012. Blood cell origin of circulating microRNAs: a cautionary note for cancer biomarker studies. Cancer Prev Res (Phila). 5(3):492–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pu D, Liang H, Wei F, Akin D, Feng Z, Yan Q, Li Y, Zhen Y, Xu L, Dong G, et al. 2016. Evaluation of a novel saliva-based epidermal growth factor receptor mutation detection for lung cancer: a pilot study. Thorac Cancer. 7(4):428–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinowits G, Gercel-Taylor C, Day JM, Taylor DD, Kloecker GH. 2009. Exosomal microRNA: a diagnostic marker for lung cancer. Clin Lung Cancer. 10(1):42–46. [DOI] [PubMed] [Google Scholar]
- Reinert T, Scholer LV, Thomsen R, Tobiasen H, Vang S, Nordentoft I, Lamy P, Kannerup AS, Mortensen FV, Stribolt K, et al. 2016. Analysis of circulating tumour DNA to monitor disease burden following colorectal cancer surgery. Gut. 65(4):625–634. [DOI] [PubMed] [Google Scholar]
- Riethdorf S, Fritsche H, Muller V, Rau T, Schindlbeck C, Rack B, Janni W, Coith C, Beck K, Janicke F, et al. 2007. Detection of circulating tumor cells in peripheral blood of patients with metastatic breast cancer: a validation study of the CellSearch system. Clin Cancer Res. 13(3):920–928. [DOI] [PubMed] [Google Scholar]
- Rosenfeld N, Aharonov R, Meiri E, Rosenwald S, Spector Y, Zepeniuk M, Benjamin H, Shabes N, Tabak S, Levy A, et al. 2008. MicroRNAs accurately identify cancer tissue origin. Nat Biotechnol. 26(4):462–469. [DOI] [PubMed] [Google Scholar]
- Russo F, Di Bella S, Vannini F, Berti G, Scoyni F, Cook HV, Santos A, Nigita G, Bonnici V, Lagana A, et al. 2017. miRandola 2017: a curated knowledge base of non-invasive biomarkers. Nucleic Acids Res. 46(D1):D354–D359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons M, Raposo G. 2009. Exosomes—vesicular carriers for intercellular communication. Curr Opin Cell Biol. 21(4):575–581. [DOI] [PubMed] [Google Scholar]
- Siravegna G, Marsoni S, Siena S, Bardelli A. 2017. Integrating liquid biopsies into the management of cancer. Nat Rev Clin Oncol. 14(9):531–548. [DOI] [PubMed] [Google Scholar]
- Skog J, Würdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves M, Curry WT, Jr, Carter BS, Krichevsky AM, Breakefield XO. 2008. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 10(12):1470–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summerer I, Unger K, Braselmann H, Schuettrumpf L, Maihoefer C, Baumeister P, Kirchner T, Niyazi M, Sage E, Specht HM, et al. 2015. Circulating microRNAs as prognostic therapy biomarkers in head and neck cancer patients. Br J Cancer. 113(1):76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor DD, Gercel-Taylor C. 2008. MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol Oncol. 110(1):13–21. [DOI] [PubMed] [Google Scholar]
- The Cancer Genome Atlas Network. 2015. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature. 517(7536):576–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thierry AR, Mouliere F, El Messaoudi S, Mollevi C, Lopez-Crapez E, Rolet F, Gillet B, Gongora C, Dechelotte P, Robert B, et al. 2014. Clinical validation of the detection of KRAS and BRAF mutations from circulating tumor DNA. Nat Med. 20(4):430–435. [DOI] [PubMed] [Google Scholar]
- Thress KS, Paweletz CP, Felip E, Cho BC, Stetson D, Dougherty B, Lai Z, Markovets A, Vivancos A, Kuang Y, et al. 2015. Acquired EGFR C797S mutation mediates resistance to AZD9291 in non-small cell lung cancer harboring EGFR T790M. Nat Med. 21(6):560–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tie J, Wang Y, Tomasetti C, Li L, Springer S, Kinde I, Silliman N, Tacey M, Wong HL, Christie M, et al. 2016. Circulating tumor DNA analysis detects minimal residual disease and predicts recurrence in patients with stage II colon cancer. Sci Transl Med. 8(346):346ra392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinhofer I, Hristozova T, Stromberger C, Keilhoiz U, Budach V. 2012. Monitoring of circulating tumor cells and their expression of EGFR/phospho-EGFR during combined radiotherapy regimens in locally advanced squamous cell carcinoma of the head and neck. I nt J Radiat Oncol Biol Phys. 83(5):e685–e690. [DOI] [PubMed] [Google Scholar]
- Toss A, Mu Z, Fernandez S, Cristofanilli M. 2014. CTC enumeration and characterization: moving toward personalized medicine. Ann Transl Med. 2(11):108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu M, Wei F, Yang J, Wong D. 2015. Detection of exosomal biomarker by electric field-induced release and measurement (EFIRM). J Vis Exp. (95):52439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Zheng G, Cheng B, Chen F, Wang Z, Chen Y, Wang Y, Xiong B. 2014. Circulating tumor cells (CTCs) detected by RT-PCR and its prognostic role in gastric cancer: a meta-analysis of published literature. PLoS One. 9(6):e99259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang WY, Twu CW, Chen HH, Jan JS, Jiang RS, Chao JY, Liang KL, Chen KW, Wu CT, Lin JC. 2010. Plasma EBV DNA clearance rate as a novel prognostic marker for metastatic/recurrent nasopharyngeal carcinoma. Clin Cancer Res. 16(3):1016–1024. [DOI] [PubMed] [Google Scholar]
- Wang Y, Springer S, Mulvey CL, Silliman N, Schaefer J, Sausen M, James N, Rettig EM, Guo T, Pickering CR, et al. 2015. Detection of somatic mutations and HPV in the saliva and plasma of patients with head and neck squamous cell carcinomas. Sci Transl Med. 7(293):293ra104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber JA, Baxter DH, Zhang S, Huang DY, Huang KH, Lee MJ, Galas DJ, Wang K. 2010. The microRNA spectrum in 12 body fluids. Clin Chem. 56(11):1733–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei F, Lin CC, Joon A, Feng Z, Troche G, Lira ME, Chia D, Mao M, Ho CL, Su WC, et al. 2014. Noninvasive saliva-based EGFR gene mutation detection in patients with lung cancer. Am J Respir Crit Care Med. 190(10):1117–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiklund ED, Gao S, Hulf T, Sibbritt T, Nair S, Costea DE, Villadsen SB, Bakholdt V, Bramsen JB, Sorensen JA, et al. 2011. MicroRNA alterations and associated aberrant DNA methylation patterns across multiple sample types in oral squamous cell carcinoma. PLoS One. 6(11): e27840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi L, Pham TH, Payabyab EC, Sherry RM, Rosenberg SA, Raffeld M. 2016. Circulating tumor DNA as an early indicator of response to T-cell transfer immunotherapy in metastatic melanoma. Clin Cancer Res. 22(22):5480–5486. [DOI] [PMC free article] [PubMed] [Google Scholar]