Abstract
The recent Food and Drug Administration’s approval of monoclonal antibodies targeting immune checkpoint receptors (ICRs) for recurrent or metastatic head and neck squamous cell carcinoma (HNSCC) offers exciting promise to improve patient outcome and reduce morbidities. A favorable response to ICR blockade relies on an extensive collection of preexisting tumor-specific T cells in the tumor microenvironment (TME). ICR blockade reinvigorates exhausted CD8+ T cells and enhances immune killing. However, resistance to ICR blockade is observed in about 85% of patients with HNSCC, therefore highlighting the importance of characterizing the mechanisms underlying HNSCC immune escape and exploring combinatorial strategies to sensitize hypoimmunogenic cold HNSCC to ICR inhibition. Cancer vaccines are designed to bypass the cold TME and directly deliver cancer antigens to antigen-presenting cells (APCs); these vaccines epitomize a priming strategy to synergize with ICR inhibitors. Cancer cells are ineffective antigen presenters, and poor APC infiltration as well as the M2-like polarization in the TME further dampens antigen uptake and processing, both of which render ineffective innate and adaptive immune detection. Cancer vaccines directly activate APC and expand the tumor-specific T-cell repertoire. In addition, cancer vaccines often contain an adjuvant, which further improves APC function, promotes epitope spreading, and augments host intrinsic antitumor immunity. Thus, the vaccine-induced immune priming generates a pool of effectors whose function can be enhanced by ICR inhibitors. In this review, we summarize the major HNSCC immune evasion strategies, the ongoing effort toward improving HNSCC vaccines, and the current challenges limiting the efficacy of cancer vaccines.
Keywords: mouth neoplasms, papillomavirus infections, tumor escape, immunization, immunotherapy, immunomodulation
Introduction
Head and neck squamous cell carcinoma (HNSCC) is cancer originating in the squamous epithelium lining the upper aerodigestive tract. The standard treatment for HNSCC is surgery with or without adjuvant chemoradiation, which frequently results in significant morbidities (Yom et al. 2017). The recent development of effective immunotherapy for patients with advanced disease offers an exciting new therapeutic strategy to potentially deescalate treatment and improve quality of life for previously untreated patients with HNSCC. The first monoclonal antibody approved for HNSCC was the epidermal growth factor receptor (EGFR)–targeted cetuximab. In 2016, the immune checkpoint receptor (ICR) blockers anti-PD-1 pembrolizumab (Keytruda; Merck) and nivolumab (Opdivo; Bristol-Myers Squibb) achieved Food and Drug Administration approval to treat advanced HNSCC by restoring the function of exhausted cytotoxic T lymphocytes (CTLs; Ferris et al. 2016; Seiwert et al. 2016). However, cetuximab and ICR inhibitors show objective response in only about 15% of patients (Reeves et al. 2011; Ferris et al. 2016; Bauman et al. 2017). To maximize the potential of immunotherapy, significant progress has been made toward understanding the mechanisms underlying HNSCC immune escape. Importantly, an efficient generation and infiltration of cancer-specific T cells is indispensable for the success of ICR blockade (Zou et al. 2016). Hence, strategies that encourage the expansion of tumor antigen–specific effector pool are among the most promising approaches to prime patients for ICR inhibition.
Cancer vaccines have shown remarkable potential in synergizing with ICR blockade to maximally expand tumor-specific CD8+ CTLs and sustain their function (Ott et al. 2017). This approach can mitigate cancer immune escape by enhancing the functions of antigen-presenting cells (APCs). Although tumor antigens could stem from normal proteins, such as melanoma-associated antigen A (the expression of which is otherwise limited to germ cells), the arguable majority of tumor-specific antigens without central tolerance are neoantigens, which are derived from somatic mutations or viral proteins. Indeed, cancer mutation–associated neoantigen load and cytolytic markers are positive prognosticators (Van Allen et al. 2015). In agreement, responders to ICR blockade demonstrate decreased mutations and neoantigen load after therapy (Riaz et al. 2017). This review focuses on the immune escape mechanisms developed by HNSCC and the ongoing effort in generating more effective therapeutic HNSCC vaccines.
Mechanisms of Immune Evasion in HNSCC
Immune detection and elimination of HNSCC entail the orchestration of the following events: 1) Damaged or dying cancer cells release danger-associated molecular patterns (DAMPs), such as fragments of cancer genomic and mitochondrial DNA, into the tumor microenvironment (TME; Deng, Liang, Xu, et al. 2014; Xu et al. 2017), which encourages chemotaxis and activation of APCs through DAMP-sensing machinery. 2) Neoantigens are sampled and processed by APCs to expand and cross-prime effector CTLs. TH1 cytokines and chemokines in the TME suppress immune cell subsets that smother CTL-centered inflammation, such as regulatory T cells (Tregs) and myeloid-derived suppressor cells (MDSCs). 3) Activated CTLs are functionally robust and lack high levels of ICR expression, which are markers of chronic exhaustion. 4) Activated effectors, including natural killer (NK) cells and CTLs, release perforin 1 to deliver granzyme B into the target HNSCC cells and elicit irreversible extrinsic apoptosis. Unfortunately, distinct immune escape mechanisms interfere with all of the aforementioned events. In this section, we summarize the current known HNSCC strategies that target these steps (Table 1, Fig. 1).
Table 1.
Factors Underpinning Immune Evasion in Head and Neck Squamous Cell Carcinoma.
| Mechanisms | References |
|---|---|
| Inhibition of chemotactic recruitment of antigen-presenting cells and effector cells | Leibowitz et al. 2013; Li et al. 2015; Lei, Xie, et al. 2016; Nguyen et al. 2016 |
| Overactivation of the suppressor populations and metabolic insufficiency in effector cells | Costa et al. 2013; Davis et al. 2016; Scharping et al. 2016 |
| Engagement of the immune checkpoint receptor signaling and suppression of effector function | Lyford-Pike et al. 2013; Li et al. 2015; Bauman et al. 2017; Kansy et al. 2017 |
| Alterations to autophagy | Baginska et al. 2013; Lei, Kansy, et al. 2016 |
| Cancer stem cell-mediated immunosuppression | Hu et al. 2016; Lee et al. 2016 |
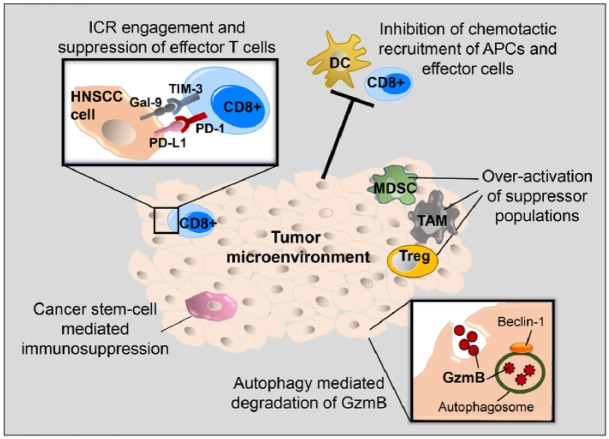
Figure 1.
Immune escape mechanisms in head and neck squamous cell carcinoma (HNSCC). Five mechanisms contribute to the immunosuppressive tumor microenvironment in HNSCC. 1) The engagement of immune checkpoint receptors (ICRs), such as PD-1 and TIM-3, with their ligands results in suppression of effector T-cell function. 2) Reduced chemokine production inhibits the recruitment of antigen-presenting cells (APCs) and effector T cells to the tumor. 3) The overactivation of immune suppressor populations, such as myeloid-derived suppressor cells (MDSCs), tumor-associated macrophages (TAMs), and regulatory T cells (Tregs), leads to a hostile tumor microenvironment. 4) Beclin 1 facilitates autophagosome formation, resulting in autophagy, which enhances the turnover of endocytosed granzyme B (GzmB). 5) Cancer stem cells in HNSCC marked by CD44 expression have increased PD-L1 expression, resulting in T-cell exhaustion.
Inhibition of Chemotactic Recruitment of APCs and Effector Cells
Downregulation of TH1 chemokines, such as CCL5, CXCL9, and CXCL10, is commonly observed in solid cancers. This process significantly dampens the infiltration of CD8+ CTLs into the tumor parenchyma, and restoration of TH1 chemokine levels improves host antitumor immune response (Lei, Xie, et al. 2016; Nguyen et al. 2016). Thus, mechanisms underpinning the inhibition of these chemokines are potential novel drug targets to improve cancer response to immunotherapy. TH1 chemokines are part of the interferon (IFN)–stimulated genes network and typically concordant with the levels of IFNs in the TME. IFNs bind to their receptors on cell surface and activate STAT1 (signal transducer and activator of transcription 1) to launch the transcription of IFN-stimulated genes. The protein tyrosine phosphatase, nonreceptor type 11 (PTPN11, also known as SHP-2) is a well-characterized inhibitor of STAT1. Notably, PTPN11 is overexpressed in HNSCC, and depletion of PTPN11 results in increased production of T cell–attracting chemokines CCL5 and CXCL10 (Leibowitz et al. 2013). PTPN11 is expressed in T cells in addition to cancer cells, and its expression levels are much higher in tumor-infiltrating lymphocytes (TILs) than matched peripheral blood lymphocytes. Inhibition of PTPN11 improves TH1 immune infiltration (Li et al. 2015), suggesting that restoration of HNSCC-mediated inhibition of TH1 chemokines is a promising strategy to enhance cancer immune detection.
The source of IFNs in the TME is complex. While type II IFN (IFN-γ) is predominantly secreted by activated NK cells and CTLs in HNSCC (Concha-Benavente et al. 2015), which may require preexisting signals to accumulate in the tumor, type I IFNs can be produced by a variety of cells in the TME, including cancer cells, endothelial cells, and APCs. The stimulator of IFN genes (STING) was recently identified as a pivotal pattern recognition receptor (PRR) that translates cytoplasmic DNA insults to type I IFN induction. DNA from damaged cancer cells can be engulfed by other tumor cells and APCs in the TME, which subsequently activates STING to mount type I IFN-dependent TH1 chemokine production. Activation of the STING pathway by its agonist cyclic dinucleotides improves effector T-cell tumor infiltration and shows promise in melanoma and colon cancer models (Deng, Liang, Burnette, et al. 2014; Woo et al. 2014; Fu et al. 2015; Xu et al. 2017). However, the hydrophilic nature of cyclic dinucleotides make their passive entry into the cytosol highly unlikely, which may explain their being less effective in the more aggressive HNSCC models, such as MOC2 (Moore et al. 2016), which is syngeneic to immunocompetent C57BL/6 mice and exhibits similar mutations as the human disease (Chalivendra et al. 2015).
Cancer cells frequently suppress the type I IFN induction pathway to encourage immunoevasion. The mechanisms underpinning the inhibition of type I IFN pathway in HNSCC remain elusive. Multiple inhibitory proteins have been identified to dampen cytoplasmic PRR-induced type I IFN induction in immune cells. Several of these proteins belong to the nucleotide-binding, leucine-rich repeat (NLR) family, such as NLRX1 (Lei et al. 2012). NLRX1 has a broad expression profile in normal tissues and, interestingly, is also expressed in HNSCC (Lei, Kansy, et al. 2016), which raises the question whether these intrinsic inhibitors of type I IFN shape the immune microenvironment of HNSCC.
Overactivation of the Suppressor Immune Populations and Metabolic Insufficiency in Effector Cells
The suppressor immune cell subsets in HNSCC parenchyma include at least CD4+FoxP3+ Tregs, CD11b+Gr-1+ MDSCs, and M2-skewed macrophages (Davis et al. 2016). MDSCs are associated with increased recurrence and metastasis and produce arginase I, which inhibits the proliferation and function of effector T cells. Depletion of MDSCs in HNSCC mouse models has also led to reversal of resistance to CTL-associated antigen 4 (CTLA-4) blockade (Clavijo et al. 2017). A reduction of MDSCs and Tregs in HNSCC increases in antigen-specific T cells (Weed et al. 2015). Tumor-associated macrophages in HNSCC are another class of suppressors that produce immunosuppressive TGF-β and IL-10 and exhibit a protumor M2-like phenotype (Costa et al. 2013). Metabolic sufficiency and increased oxidative activity are hallmarks of activated effector T cells. But CTLs among TILs of HNSCC show significantly reduced mitochondrial mass, which is a marker of metabolic insufficiency (Scharping et al. 2016). Thus, reprogramming the TME by reducing suppressors and improving metabolic state is a promising strategy to improve cancer response to ICR blockers.
Engagement of the ICR Signaling and Suppression of Effector Function
In addition to the interaction of MHC with T-cell receptor for antigen presentation, the activation of effector T cells depends on the sum of stimulatory and inhibitory signals. The expression of ICR prevents overzealous immune response, particularly against self-antigens. In the TME, activation of ICRs leads to rapid T-cell exhaustion. TILs of HNSCC express high levels of ICRs, such as PD-1, CTLA-4, and TIM-3 (T-cell immunoglobulin and mucin-domain containing 3; Lyford-Pike et al. 2013; Li et al. 2015; Jie et al. 2017; Kansy et al. 2017; Shayan et al. 2017). In fact, the ICR expression levels on TILs are significantly higher than T cells from the patient’s peripheral blood (Jie et al. 2017). Consistent with many solid tumors, TILs that are positive for 2 ICRs, such as PD-1 and TIM-3, are more functionally exhausted (Shayan et al. 2017). This phenotype also explains why ICR blockade is a promising strategy to reinvigorate CTLs in HNSCC. But a critical limitation of this approach is its dependence on an extensive preexisting neoantigen-specific CTLs. Although HNSCC features high mutation rates, with human papillomavirus (HPV)–negative tumors exhibiting twice as many as HPV+ HNSCCs (4.8 and 2.3 mutations/Mb, respectively; Stransky et al. 2011), the majority of HNSCCs are nonresponsive to ICR inhibitors. This phenotype suggests that HNSCC immunogenicity is only in part determined by neoantigen load. Neoantigens can contribute to CTL activation only when enough APCs can sample and cross-prime CD8+ T cells. Thus, inhibition of chemotactic trafficking of APCs and effectors results in another important mechanism of HNSCC immune escape.
Alterations to Autophagy
Autophagy has dichotomous roles in cancer initiation and response to treatment: it protects hosts from developing new tumors, but it can also provide remarkable adaptability to established cancer cells against nutrient deprivation. Targeting EGFR in HNSCC potently induces ER stress, which is a robust stimulus for autophagy (Lei, Kansy, et al. 2016). Inhibition of autophagy in HNSCC substantially sensitizes it to EGFR-targeted therapy, including cetuximab. Notably, autophagy defect mediated by Beclin 1 deficiency also increases HNSCC susceptibility to cetuximab-induced NK cell–mediated immune killing (Lei, Kansy, et al. 2016). A plausible mechanism is that autophagy facilitates the turnover of cytotoxic enzymes such as granzyme B, which delivers immune cytotoxicity to target cells (Baginska et al. 2013).
Cancer Stem Cell–Mediated Immunosuppression
A group of CD44+ALDHhigh cancer stem cells (CSCs) in HNSCC exhibits much more robust tumorigenic potential and resistance to chemotherapy (Prince et al. 2007). Recent studies also suggested that CD44+ cells have increased levels of PD-L1 than CD44− cells and promote an immunosuppressive TME by directly engaging the ICR signaling (Lee et al. 2016). A CSC-targeted vaccine significantly reduces tumor recurrence and metastasis (Hu et al. 2016). Although toxicity of the CSC vaccines in preclinical models appears modest and well tolerated, a more in-depth safety study is necessary to encourage the optimal dosing of this promising approach.
Rational Design of HNSCC Vaccines
The success of ICR inhibitors has also revitalized the enthusiasm for cancer vaccines, which deliver unique appeals in enhancing immune detection of cancer. The collective experiences of using vaccines in infectious diseases show that vaccine can induce robust antigen-specific T-cell expansion. Vaccines are safe and easy to administer in an outpatient setting. In addition, disease recurrence and metastasis are the leading causes of HNSCC-related death. Vaccines are highly attractive in a “minimal disease” setting after definitive surgical debulking to prevent recurrence and promote cure. However, due to the immunosuppressive TME in advanced-stage tumors, vaccine-induced effector T cells may rapidly become exhausted. Under such circumstances, vaccine alone is less likely to achieve long-term tumor regression, and a combination of tumor-specific vaccine and ICR blockade is warranted. To improve cancer vaccines, it is essential to design and optimize its 3 components: antigen, adjuvant, and delivery method.
Selection of HNSCC-Specific Antigens
Targeting tumor antigens with a vaccine approach likely differs between HPV+ and HPV− diseases because of their distinct tumor antigen repertoires. Notably, although HPV+ HNSCC tends to respond to chemoradiation better than HPV− disease, there seems to be little difference in the response rates to ICR blockade between the groups (Ferris et al. 2016; Bauman et al. 2017), suggesting that vaccine-potentiated immune priming would likely benefit both groups of patients.
In HPV− HNSCC, one antigen class is the epigenetically dysregulated genes abnormally expressed in tumor tissue but not in healthy tissues. This includes melanoma-associated antigen A, which is overexpressed in 75% of tumors (Atanackovic et al. 2006). However, this class of antigens is associated central immunologic tolerance. Similar to most solid tumors, neoantigens in HPV− HNSCC largely stem from mutations. The only mutation-associated neoantigen identified in human HNSCC specimens is derived from CASP8 and presented by HLA-B*3503 (Mandruzzato et al. 1997). CASP8 is frequently mutated in HNSCC and responsible for activating the extrinsic apoptotic pathway, which underlies the efficacy of immune killing of the target cells (Cancer Genome Atlas Network 2015). However, this neoantigen appears to be a sporadic mutation, and additional validation and discovery of the neoantigen pool are needed.
In HPV+ tumors, the consistent and unique expression of HPV oncoproteins makes more economic off-the-shelf therapeutic vaccines feasible. Although there are currently 3 HPV prophylactic vaccines approved by the Food and Drug Administration, including Gardasil and Cervarix, these vaccines are targeting the major capsid protein L1 to prevent infection of host cells. They are unlikely to yield a protective response against established HPV+ cancer, because the expression of L1 antigen is lost once HPV completes its integration into the host genome (Hernandez et al. 2011). Hence, new vaccine formulations targeting HPV proteins that are consistently expressed in established cancer are in urgent need. Among these proteins, HPV E6 and E7 produce highly immunogenic epitopes, constituting ideal vaccine targets: 1) these proteins are uniquely and consistently expressed by cancer cells, and 2) these viral antigens have not been subjected to central thymic tolerance.
A common caveat of a single antigen-targeted approach is its susceptibility to tumor immune editing, which eventually allows the growth of cancer cells with low target antigen expression. With the emerging neoantigen discovery pipelines, the expanded tumor antigen repertoire for HNSCC could direct multivalent vaccine designs. A potential pitfall of a strong CD8+ T-cell-targeted vaccine is that it typically lacks CD4+ T-helper response, which helps to sustain CD8+ T-cell proliferation and expands memory T cells (Klebanoff et al. 2006). Thus, a balance between CD4+ and CD8+ T-cell epitope in vaccine designs is likely needed to yield a robust and durable host response. In addition, as we will discuss later, including a robust adjuvant can improve functions and tumor-homing of APCs, resulting in better epitope spreading for a more diverse pool of tumor-specific effectors.
Codelivery of Adjuvant to Improve APC Function
APCs such as dendritic cells (DCs) are central to the linkage of tumor detection with the activation of CD8+ CTLs and CD4+ helper T cells. Adjuvant provides essential costimulatory signals to enhance antigen processing and cross-priming of CD8+ T cells. In the absence of adjuvant, at least in animal models, exposure to a high concentration of antigens leads to immune tolerance (Pozsgay et al. 2017). To avoid antigen-specific anergy, DCs that process tumor antigens must also receive costimulatory signals to polarize toward a TH1 phenotype. Hence, codelivery of antigen and adjuvant to the same cell may limit the number of APCs that potentiate antigen-specific tolerance. Common adjuvants for therapeutic cancer vaccines are usually PAMPs (pathogen-associated molecular patterns) or DAMPs, which are ligands for PRRs in innate immune cells, such as the Toll-like receptors (TLRs) and STING. CpG oligodeoxynucleotides are often used as an cancer vaccine adjuvant in mouse models, where its receptor Tlr9 is extensively expressed in the myeloid compartment. However, in humans, TLR9 expression is limited to B cells and plasmacytoid DCs (Hochrein and Wagner 2004). This critical difference will likely result in differences in responses. Hence, testing vaccine adjuvant that provides costimulatory signal for evolutionarily conserved PAMP or DAMP pathways in the preclinical model would have a more promising translational impact. Recent evidence suggests that mouse and human DCs exhibit heterogeneity, with the mouse CD8α+ DCs and human CD141+ DCs demonstrating much more robust potential in cross-presenting epitopes on MHC class I molecules and producing IL-12 to stimulate effector T-cell generation (Shortman and Heath 2010). Hence, engineering vaccines to target specific DC subsets may provide additional tools to fine-tune TH1/Tc1-skewed responses.
Selection of Vaccine Delivery Methods
Depending on the delivery vehicles for tumor antigens, the HNSCC vaccines in clinical trials can be generally classified as follows: peptide vaccine, nucleic acid vaccine, pathogen vector vaccine, and cell-based vaccine (Table 2). Highly desirable features for an effective vaccine include 1) delivery of optimal 3-dimentional density antigens with a balanced repertoire for CD4+ and CD8+ T-cell epitopes, 2) ability to deliver antigen and adjuvant into the same cells to limit the number of APCs undergoing antigen-specific tolerance, 3) effective homing to the lymph nodes, and 4) minimal side effect or safety concerns. Although each vaccine system has its merits, no vaccine can achieve all the aforementioned goals.
Table 2.
List of Anti-HNSCC Vaccines.
| Vaccine | Targeting Antigens | Clinical Trial ID | References |
|---|---|---|---|
| Peptide-based | |||
| GL-0810/0817 | MAGE-A3/HPV16 | NCT00257738 | Zandberg et al. 2015 |
| DPX-E7 vaccine | HPV16-E7 | NCT02865135 | Karkada et al. 2013 |
| HESPECTA | HPV E6 | NCT02821494 | Slingerland et al. 2016 |
| ISA101 | HPV16 E6/E7 | NCT02426892 | Kenter et al. 2008 |
| Anti-MUC1 | MUC1 | NCT02544880 | Weed et al. 2015 |
| TAA peptides | LY6K, CDCA1, and IMP3 | Phase II trial | Yoshitake et al. 2015 |
| p16(INK4a) vaccine | p16 | NCT01462838 | Reuschenbach et al. 2016 |
| Nucleic acid–based | |||
| INO-3112/INO-9012 | HPV16 /18 E6/7 | NCT02163057 | Bauml et al. 2016 |
| Allovectin-7 | Restore HLA-B7 / β2 | NCT00050388 | Gleich et al. 2001 |
| Pathogen-based | |||
| TG4001 | HPV16 E6/7 | NCT03260023 | N/A |
| ADXS11-001 | HPV16 E7 | NCT02002182 | Wallecha et al. 2012 |
| TRICOM | CEA and/or MUC1 | NCT00021424 | N/A |
| Cell-based | |||
| CSC-DC | ALDHhigh | Preclinical | Hu et al. 2016 |
| DC vaccine | p53 | NCT00404339 | Schuler et al. 2014 |
| MVX-ONCO-1 | Autologous tumor cells | NCT02999646 | Mach et al. 2016 |
ALDH, aldehyde dehydrogenase; β2, β2 microglobulin; CDCA1, cell division cycle associated 1; CEA, carcinoembryonic antigen; CSC-DC, cancer stem-like cell-based dendritic cell; DC, dendritic cell; HLA-B7, human leukocyte antigen B7; HNSCC, head and neck squamous cell carcinoma; HPV, human papilloma virus; IMP3, U3 small nucleolar ribonucleoprotein; LY6K, lymphocyte antigen 6 family member K; MAGE-A3, melanoma-specific antigen-A3; MUC1, mucin 1; N/A, not applicable; TAA, tumor-associated antigen.
Peptide-Based Vaccines
In addition to the ease of synthesizing neoantigen peptides and having off-the-shelf properties, peptide-based vaccines are generally safe and well-tolerated (Slingluff 2011). However, due to human leukocyte antigen (HLA) restriction, peptides bind only to certain HLA types, thereby restricting the use of these peptides to a subset of patients. In addition to HPV E6/E7 proteins, HPV+ cancers often overexpress p16, which makes it a potential target (Reuschenbach et al. 2016). In a phase 1/2a trial, a P16_37-63 peptide in emulsion was administered, and cellular and humoral immune responses were detected (NCT01462838). A common limitation associated with the peptides in emulsion is its low intracellular delivery efficiency (Kenter et al. 2009). Antigen density in a vaccine controls the number of activated effector and memory T cells (Bullock et al. 2003). Thus, a delivery system that can precisely control antigen 3-dimensional density and increase intracellular delivery likely improves vaccine efficacy.
Nucleic Acid–Based Vaccines
Nucleic acid–based cancer vaccines, which introduce plasmid DNA expressing a neoantigen, are developed because they inherently activate PRRs, such as STING and TLRs, and use the backbone as an adjuvant (Delaloye et al. 2009). For example, the INO-3112 vaccine combines 2 plasmids encoding HPV16/18 E6/7 and IL-12 (INO-9012). A phase 1b/2a clinical trial combining MEDI0457 (INO-3112/INO-9012) with anti-PD-L1 (durvalumab) has started recruiting.
Pathogen-Vector Vaccines
Bacterial and viral pathogens are used for vaccine delivery because of their intrinsic capability to activate the innate immune response as an adjuvant. The ADXS11-001 vaccine consists of a live-attenuated strain of Listeria monocytogenes encoding HPV 16 E7 fused to a nonhemolytic listeriolysin O protein and was tested in a phase 2 trial for HPV+ HNSCC patients (Wallecha et al. 2012). A phase 1/2 trial for ADXS11-001 with anti-PD-L1 in patients with HPV+ HNSCC is in progress (NCT02291055). Modified vaccinia virus-based delivery of HPV E6/E7 (TG4001 vaccine) is also combined with anti-PD-L1 for recurrent or metastatic HNSCC, with pending results (NCT03260023).
Cell-Based Vaccines
DC-based vaccines involve autologous monocyte extraction, ex vivo expansion with tumor antigen peptides in the presence of appropriate cytokines for maturation, and reintroduction into the host. For HNSCC, a DC vaccine pulsed with p53 peptides completed phase 1 clinical trials and is deemed safe (NCT00404339), but there is little immunologic responses to anti–wild type p53 (Schuler et al. 2014). Recent DC vaccines targeting CSCs were used in an adjuvant setting and showed significant improvement in host survival and tumor control in a preclinical squamous cell carcinoma model (Hu et al. 2016). The MVX-ONCO-1 vaccine delivers irradiated patient tumor cells in a capsule that has an allogeneic cell line modified to release granulocyte-macrophage colony-stimulating factor (Mach et al. 2016). A phase 1 clinical trial reported responses in >50% of patients and a good safety profile.
Challenges and Future Directions to Improve Anti-HNSCC Vaccines
Whole exome sequencing and bioinformatics prediction pipelines are integral to the generation of personalized vaccines (Fig. 2). Due to the aggressive nature and short life span of patients with recurrent or metastatic HPV− HNSCC, logistical support, time, and cost associated with neoantigen discovery and vaccine production are major limitations. But HNSCC vaccines represent a highly promising strategy to prevent cancer recurrence and de-escalate treatment for patients with HPV+ HNSCC (Fig. 2). Notably, many of the new tumor-specific CTLs will inevitably enter into exhaustion in the TME. A combination with ICR inhibitors likely encourages more durable and effective responses for patients with advanced tumors.
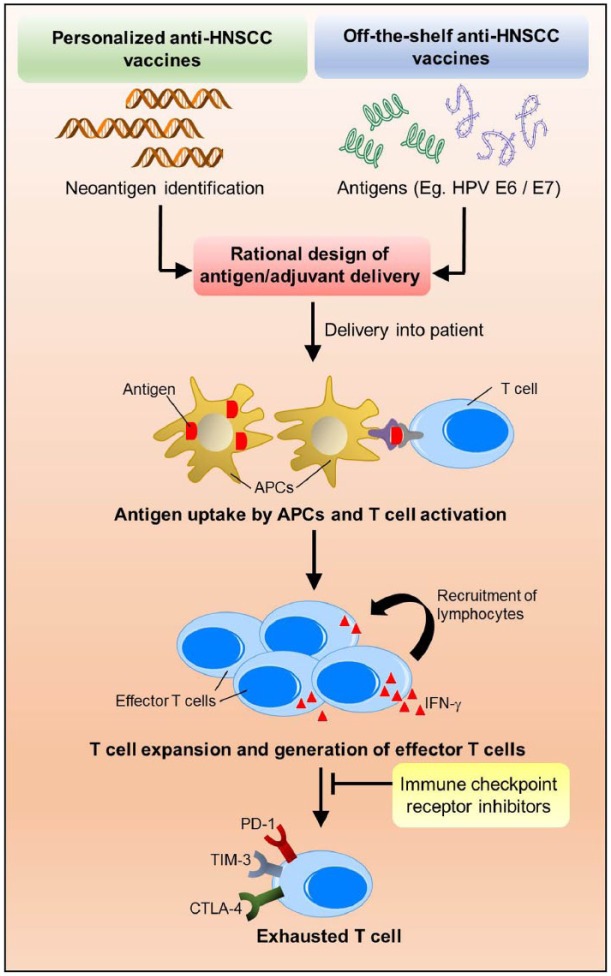
Figure 2.
A combination of head and neck squamous cell carcinoma (HNSCC) vaccines with immune checkpoint receptor (ICR) inhibitors to promote durable and effective responses in patients with HNSCC. HNSCC vaccines may be personalized vaccines based on whole exome sequencing–dependent neoantigen identification or off-the-shelf vaccines based on unique antigens, such as human papillomavirus (HPV) E6/E7 oncoproteins. Next, rational designs to enhance the intracellular delivery are essential to reprogram antigen-presenting cell–mediated immune detection of cancer. Activated antigen-presenting cells will enhance vaccine-specific T-cell expansion and epitope spreading to build a more diverse effector T-cell repertoire. However, some of the newly generated effector T cells will inevitably become exhausted with significantly high expression levels of ICR. Hence, a rational combination with ICR-targeted therapies likely sustains the function of the effector T cells and improves durability of the response.
Highly desirable vaccine features include good safety profile, activation of cellular and humoral responses, cost-effectiveness, and manufacture standardization. To date, no vaccine delivery method is fully successful in achieving these goals. For example, the cell-based vaccines are immunogenic, but the manufacturing costs are high and labor-intensive and lack standard quality control. Pathogen vector vaccines have safety concerns especially for immunocompromised patients, including individuals with HIV+ and those who receive myelosuppressive chemotherapy. Nanoparticles protect vaccine components from rapid degradation and show outstanding lymph node–homing property, which renders this class of delivery vehicles highly promising. But many nanovaccines utilize CpG to elicit robust antitumor immune responses in mouse models. Due to the lack of TLR9 expression in human APCs, additional validation is needed. In addition to adjuvant selection, other key vaccine features—such as 3-dimensional antigen density, proper delivery of appropriate ratios of CD4+ and CD8+ T-cell epitopes, and different combinatorial protocols—all need to be optimized to materialize the potential of cancer vaccines.
Overall, the future development of individualized cancer vaccines epitomizes cancer genomics–informed precision immunotherapy for HNSCC. The incidence rate of HPV+ HNSCC is projected to increase until 2060. Better vaccine delivery vehicles for HPV oncoprotein–targeted vaccines hold the key to complement the current treatments to reduce morbidities and enhance overall cure rates. In conjunction with the faster and more accurate neoantigen discovery pipeline, cancer vaccines will likely synergize with ICR-targeted therapies to elicit more robust and durable anti-HNSCC immunity.
Author Contributions
Y.S. Tan, Y.L. Lei, contributed to conception and design, drafted and critically revised the manuscript; K. Sansanaphongpricha, contributed to conception and design, drafted the manuscript; M.E.P. Prince, D. Sun, G.T. Wolf, contributed to conception and design, critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Acknowledgments
Due to the word limit, we apologize to authors whose work is not cited in this review.
Footnotes
This work is supported by the National Institutes of Health (grants R01 DE026728 and R00 DE024173; to Y.L.L.), U-M Comprehensive Cancer Center Fund for Discovery (to Y.L.L.), and POM Clinical Research Supplement (to Y.L.L.).
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
ORCID iD: G.T. Wolf  https://orcid.org/0000-0002-3219-9515
https://orcid.org/0000-0002-3219-9515
References
- Atanackovic D, Blum I, Cao Y, Wenzel S, Bartels K, Faltz C, Hossfeld DK, Hegewisch-Becker S, Bokemeyer C, Leuwer R. 2006. Expression of cancer-testis antigens as possible targets for antigen-specific immunotherapy in head and neck squamous cell carcinoma. Cancer Biol Ther. 5(9):1218–1225. [DOI] [PubMed] [Google Scholar]
- Baginska J, Viry E, Berchem G, Poli A, Noman MZ, van Moer K, Medves S, Zimmer J, Oudin A, Niclou SP, et al. 2013. Granzyme B degradation by autophagy decreases tumor cell susceptibility to natural killer-mediated lysis under hypoxia. Proc Natl Acad Sci U S A. 110(43):17450–17455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauman JE, Cohen E, Ferris RL, Adelstein DJ, Brizel DM, Ridge JA, O’Sullivan B, Burtness BA, Butterfield LH, Carson WE, et al. 2017. Immunotherapy of head and neck cancer: emerging clinical trials from a national cancer institute head and neck cancer steering committee planning meeting. Cancer. 123(7):1259–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauml JM, Cohen RB, Aggarwal C. 2016. Immunotherapy for head and neck cancer: latest developments and clinical potential. Ther Adv Med Oncol. 8(3):168–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullock TN, Mullins DW, Engelhard VH. 2003. Antigen density presented by dendritic cells in vivo differentially affects the number and avidity of primary, memory, and recall CD8+ T cells. J Immunol. 170(4):1822–1829. [DOI] [PubMed] [Google Scholar]
- Cancer Genome Atlas Network. 2015. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature. 517(7536):576–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalivendra V, Kanchi KL, Onken MD, Winkler AE, Mardis E, Uppaluri R. 2015. Genomic analysis to define molecular basis of aggressiveness in a mouse model of oral cancer. Genom Data. 3:61–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavijo PE, Moore EC, Chen J, Davis RJ, Friedman J, Kim Y, Van Waes C, Chen Z, Allen CT. 2017. Resistance to CTLA-4 checkpoint inhibition reversed through selective elimination of granulocytic myeloid cells. Oncotarget. 8(34):55804–55820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Concha-Benavente F, Srivastava RM, Trivedi S, Lei Y, Chandran U, Seethala RR, Freeman GJ, Ferris RL. 2015. Identification of the cell-intrinsic and -extrinsic pathways downstream of EGFR and IFNγ that induce PD-L1 expression in head and neck cancer. Cancer Res. 76(5):1031–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa NL, Valadares MC, Souza PP, Mendonca EF, Oliveira JC, Silva TA, Batista AC. 2013. Tumor-associated macrophages and the profile of inflammatory cytokines in oral squamous cell carcinoma. Oral Oncol. 49(3):216–223. [DOI] [PubMed] [Google Scholar]
- Davis RJ, Van Waes C, Allen CT. 2016. Overcoming barriers to effective immunotherapy: MDSCs, TAMs, and Tregs as mediators of the immunosuppressive microenvironment in head and neck cancer. Oral Oncol. 58:59–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaloye J, Roger T, Steiner-Tardivel QG, Le Roy D, Knaup Reymond M, Akira S, Petrilli V, Gomez CE, Perdiguero B, Tschopp J, et al. 2009. Innate immune sensing of modified vaccinia virus Ankara (MVA) is mediated by TLR2-TLR6, MDA-5 and the NALP3 inflammasome. PLoS Pathog. 5(6):e1000480. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Deng L, Liang H, Burnette B, Beckett M, Darga T, Weichselbaum RR, Fu YX. 2014. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest. 124(2):687–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng L, Liang H, Xu M, Yang X, Burnette B, Arina A, Li XD, Mauceri H, Beckett M, Darga T, et al. 2014. STING-dependent cytosolic DNA sensing promotes radiation-induced type I interferon-dependent antitumor immunity in immunogenic tumors. Immunity. 41(5):843–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris RL, Blumenschein G, Jr, Fayette J, Guigay J, Colevas AD, Licitra L, Harrington K, Kasper S, Vokes EE, Even C, et al. 2016. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 375(19):1856–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J, Kanne DB, Leong M, Glickman LH, McWhirter SM, Lemmens E, Mechette K, Leong JJ, Lauer P, Liu W, et al. 2015. STING agonist formulated cancer vaccines can cure established tumors resistant to PD-1 blockade. Sci Transl Med. 7(283):283ra52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleich LL, Gluckman JL, Nemunaitis J, Suen JY, Hanna E, Wolf GT, Coltrera MD, Villaret DB, Wagman L, Castro D, et al. 2001. Clinical experience with HLA-B7 plasmid DNA/lipid complex in advanced squamous cell carcinoma of the head and neck. Arch Otolaryngol Head Neck Surg. 127(7):775–779. [PubMed] [Google Scholar]
- Hernandez J, Elahi A, Siegel E, Coppola D, Riggs B, Shibata D. 2011. HPV L1 capsid protein detection and progression of anal squamous neoplasia. Am J Clin Pathol. 135(3):436–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochrein H, Wagner H. 2004. Of men, mice and pigs: looking at their plasmacytoid dendritic cells [corrected]. Immunology. 112(1):26–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Lu L, Xia Y, Chen X, Chang AE, Hollingsworth RE, Hurt E, Owen J, Moyer JS, Prince ME, et al. 2016. Therapeutic efficacy of cancer stem cell vaccines in the adjuvant setting. Cancer Res. 76(16):4661–4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jie HB, Srivastava RM, Argiris A, Bauman JE, Kane LP, Ferris RL. 2017. Increased PD-1+ and TIM-3+ TILs during cetuximab therapy inversely correlate with response in head and neck cancer patients. Cancer Immunol Res. 5(5):408–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kansy BA, Concha-Benavente F, Srivastava RM, Jie HB, Shayan G, Lei Y, Moskovitz J, Moy J, Li J, Brandau S, et al. 2017. PD-1 status in CD8+ T cells associates with survival and anti-PD-1 therapeutic outcomes in head and neck cancer. Cancer Res. 77(22):6353–6364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karkada M, Quinton T, Blackman R, Mansour M. 2013. Tumor inhibition by DepoVax-based cancer vaccine is accompanied by reduced regulatory/suppressor cell proliferation and tumor infiltration. ISRN Oncol. 2013:753427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenter GG, Welters MJ, Valentijn AR, Lowik MJ, Berends-van der Meer DM, Vloon AP, Drijfhout JW, Wafelman AR, Oostendorp J, Fleuren GJ, et al. 2008. Phase I immunotherapeutic trial with long peptides spanning the E6 and E7 sequences of high-risk human papillomavirus 16 in end-stage cervical cancer patients shows low toxicity and robust immunogenicity. Clin Cancer Res. 14(1):169–177. [DOI] [PubMed] [Google Scholar]
- Kenter GG, Welters MJ, Valentijn AR, Lowik MJ, Berends-van der Meer DM, Vloon AP, Essahsah F, Fathers LM, Offringa R, Drijfhout JW, et al. 2009. Vaccination against HPV-16 oncoproteins for vulvar intraepithelial neoplasia. N Engl J Med. 361(19):1838–1847. [DOI] [PubMed] [Google Scholar]
- Klebanoff CA, Gattinoni L, Restifo NP. 2006. CD8+ T-cell memory in tumor immunology and immunotherapy. Immunol Rev. 211:214–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Shin JH, Longmire M, Wang H, Kohrt HE, Chang HY, Sunwoo JB. 2016. CD44+ cells in head and neck squamous cell carcinoma suppress T-cell-mediated immunity by selective constitutive and inducible expression of PD-L1. Clin Cancer Res. 22(14):3571–3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei Y, Kansy BA, Li J, Cong L, Liu Y, Trivedi S, Wen H, Ting JP, Ouyang H, Ferris RL. 2016. EGFR-targeted mAb therapy modulates autophagy in head and neck squamous cell carcinoma through NLRX1-TUFM protein complex. Oncogene. 35(36):4698–4707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei Y, Wen H, Yu Y, Taxman DJ, Zhang L, Widman DG, Swanson KV, Wen KW, Damania B, Moore CB, et al. 2012. The mitochondrial proteins NLRX1 and TUFM form a complex that regulates type I interferon and autophagy. Immunity. 36(6):933–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei Y, Xie Y, Tan YS, Prince ME, Moyer JS, Nör J, Wolf GT. 2016. Telltale tumor infiltrating lymphocytes (TIL) in oral, head and neck cancer. Oral Oncol. 61:159–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibowitz MS, Srivastava RM, Andrade Filho PA, Egloff AM, Wang L, Seethala RR, Ferrone S, Ferris RL. 2013. SHP2 is overexpressed and inhibits pSTAT1-mediated APM component expression, T-cell attracting chemokine secretion, and CTL recognition in head and neck cancer cells. Clin Cancer Res. 19(4):798–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Jie HB, Lei Y, Gildener-Leapman N, Trivedi S, Green T, Kane LP, Ferris RL. 2015. PD-1/SHP-2 inhibits Tc1/Th1 phenotypic responses and the activation of T cells in the tumor microenvironment. Cancer Res. 75(3):508–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyford-Pike S, Peng S, Young GD, Taube JM, Westra WH, Akpeng B, Bruno TC, Richmon JD, Wang H, Bishop JA, et al. 2013. Evidence for a role of the PD-1: PD-L1 pathway in immune resistance of HPV-associated head and neck squamous cell carcinoma. Cancer Res. 73(6):1733–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mach N, Vernet R, Belkouch MC, Luy P, Ancrenaz V, Teta P, Blazek N, Grandjean N, Wasem J, Grogg J, et al. 2016. MVX-ONCO-1 phase 1 final results of the first personalized cell-based immunotherapy using cell encapsulation technology. Ann Oncol. 27 Suppl 6:1058P. [Google Scholar]
- Mandruzzato S, Brasseur F, Andry G, Boon T, van der Bruggen P. 1997. A CASP-8 mutation recognized by cytolytic T lymphocytes on a human head and neck carcinoma. J Exp Med. 186(5):785–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore E, Clavijo PE, Davis R, Cash H, Van Waes C, Kim Y, Allen C. 2016. Established T cell-inflamed tumors rejected after adaptive resistance was reversed by combination STING activation and PD-1 pathway blockade. Cancer Immunol Res. 4(12):1061–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen N, Bellile E, Thomas D, McHugh J, Rozek L, Virani S, Peterson L, Carey TE, Walline H, Moyer J, et al. ; Head and Neck SPORE Program Investigators. 2016. Tumor infiltrating lymphocytes and survival in patients with head and neck squamous cell carcinoma. Head Neck. 38(7):1074–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott PA, Hu Z, Keskin DB, Shukla SA, Sun J, Bozym DJ, Zhang W, Luoma A, Giobbie-Hurder A, Peter L, et al. 2017. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature. 547(7662):217–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozsgay J, Szekanecz Z, Sarmay G. 2017. Antigen-specific immunotherapies in rheumatic diseases. Nat Rev Rheumatol. 13(9):525–537. [DOI] [PubMed] [Google Scholar]
- Prince ME, Sivanandan R, Kaczorowski A, Wolf GT, Kaplan MJ, Dalerba P, Weissman IL, Clarke MF, Ailles LE. 2007. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc Natl Acad Sci U S A. 104(3):973–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves TD, Hill EG, Armeson KE, Gillespie MB. 2011. Cetuximab therapy for head and neck squamous cell carcinoma: a systematic review of the data. Otolaryngol Head Neck Surg. 144(5):676–684. [DOI] [PubMed] [Google Scholar]
- Reuschenbach M, Pauligk C, Karbach J, Rafiyan MR, Kloor M, Prigge ES, Sauer M, Al-Batran SE, Kaufmann AM, Schneider A, et al. 2016. A phase 1/2a study to test the safety and immunogenicity of a p16(INK4a) peptide vaccine in patients with advanced human papillomavirus–associated cancers. Cancer. 122(9):1425–1433. [DOI] [PubMed] [Google Scholar]
- Riaz N, Havel JJ, Makarov V, Desrichard A, Urba WJ, Sims JS, Hodi FS, Martin-Algarra S, Mandal R, Sharfman WH, et al. 2017. Tumor and microenvironment evolution during immunotherapy with nivolumab. Cell. 171(4):934–949.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharping NE, Menk AV, Moreci RS, Whetstone RD, Dadey RE, Watkins SC, Ferris RL, Delgoffe GM. 2016. The tumor microenvironment represses T cell mitochondrial biogenesis to drive intratumoral T cell metabolic insufficiency and dysfunction. Immunity. 45(2):374–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuler PJ, Harasymczuk M, Visus C, Deleo A, Trivedi S, Lei Y, Argiris A, Gooding W, Butterfield LH, Whiteside TL, et al. 2014. Phase I dendritic cell p53 peptide vaccine for head and neck cancer. Clin Cancer Res. 20(9):2433–2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiwert TY, Burtness B, Mehra R, Weiss J, Berger R, Eder JP, Heath K, McClanahan T, Lunceford J, Gause C, et al. 2016. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): an open-label, multicentre, phase 1b trial. Lancet Oncol. 17(7):956–965. [DOI] [PubMed] [Google Scholar]
- Shayan G, Srivastava R, Li J, Schmitt N, Kane LP, Ferris RL. 2017. Adaptive resistance to anti-PD1 therapy by Tim-3 upregulation is mediated by the PI3K-Akt pathway in head and neck cancer. Oncoimmunology. 6(1):e1261779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shortman K, Heath WR. 2010. The CD8+ dendritic cell subset. Immunol Rev. 234(1):18–31. [DOI] [PubMed] [Google Scholar]
- Slingerland M, Speetjens F, Welters M, Gelderblom H, Roozen I, Velden LAVD, Melief CJ, Zandvliet M, Burg SVD, Ossendorp F. 2016. A phase I study in patients with a human papillomavirus type 16 positive oropharyngeal tumor treated with second generation synthetic long peptide vaccine conjugated to a defined adjuvant. J Clin Oncol. 34 Suppl 15:TPS3113. [Google Scholar]
- Slingluff CL., Jr. 2011. The present and future of peptide vaccines for cancer: single or multiple, long or short, alone or in combination? Cancer J. 17(5):343–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stransky N, Egloff AM, Tward AD, Kostic AD, Cibulskis K, Sivachenko A, Kryukov GV, Lawrence MS, Sougnez C, McKenna A, et al. 2011. The mutational landscape of head and neck squamous cell carcinoma. Science. 333(6046):1157–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Allen EM, Miao D, Schilling B, Shukla SA, Blank C, Zimmer L, Sucker A, Hillen U, Foppen MHG, Goldinger SM, et al. 2015. Genomic correlates of response to CTLA-4 blockade in metastatic melanoma. Science. 350(6257):207–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallecha A, French C, Petit R, Singh R, Amin A, Rothman J. 2012. Lm-LLO-based immunotherapies and HPV-associated disease. J Oncol. 2012:542851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weed DT, Vella JL, Reis IM, De la, Fuente AC, Gomez C, Sargi Z, Nazarian R, Califano J, Borrello I, Serafini P. 2015. Tadalafil reduces myeloid-derived suppressor cells and regulatory T cells and promotes tumor immunity in patients with head and neck squamous cell carcinoma. Clin Cancer Res. 21(1):39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo SR, Fuertes MB, Corrales L, Spranger S, Furdyna MJ, Leung MY, Duggan R, Wang Y, Barber GN, Fitzgerald KA, et al. 2014. Sting-dependent cytosolic DNA sensing mediates innate immune recognition of immunogenic tumors. Immunity. 41(5):830–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu MM, Pu Y, Han D, Shi Y, Cao X, Liang H, Chen X, Li XD, Deng L, Chen ZJ, et al. 2017. Dendritic cells but not macrophages sense tumor mitochondrial DNA for cross-priming through signal regulatory protein alpha signaling. Immunity. 47(2):363–373.e365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yom SS, Mallen-St Clair J, Ha PK. 2017. Controversies in postoperative irradiation of oropharyngeal cancer after transoral surgery. Surg Oncol Clin N Am. 26(3):357–370. [DOI] [PubMed] [Google Scholar]
- Yoshitake Y, Fukuma D, Yuno A, Hirayama M, Nakayama H, Tanaka T, Nagata M, Takamune Y, Kawahara K, Nakagawa Y, et al. 2015. Phase II clinical trial of multiple peptide vaccination for advanced head and neck cancer patients revealed induction of immune responses and improved OS. Clin Cancer Res. 21(2):312–321. [DOI] [PubMed] [Google Scholar]
- Zandberg DP, Rollins S, Goloubeva O, Morales RE, Tan M, Taylor R, Wolf JS, Schumaker LM, Cullen KJ, Zimrin A, et al. 2015. A phase I dose escalation trial of MAGE-A3- and HPV16-specific peptide immunomodulatory vaccines in patients with recurrent/metastatic (RM) squamous cell carcinoma of the head and neck (SCCHN). Cancer Immunol Immunother. 64(3):367–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou W, Wolchok JD, Chen L. 2016. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: mechanisms, response biomarkers, and combinations. Sci Transl Med. 8(328):328rv4. [DOI] [PMC free article] [PubMed] [Google Scholar]