Abstract
Background
The aim of this study is to assess for regional variation in the incidence of post-operative myocardial infarction (POMI) following nonemergent vascular surgery across the United States to identify potential areas for quality improvement initiatives.
Methods
We evaluated POMI rates across 17 regional Vascular Quality Initiative (VQI) groups that comprised 243 centers with 1,343 surgeons who performed 75,057 vascular operations from 2010 to 2014. Four procedures were included in the analysis: carotid endarterectomy (CEA, n = 39,118), endovascular abdominal aortic aneurysm (AAA) repair (EVAR, n = 15,106), infrainguinal bypass (INFRA, n = 17,176), and open infrarenal AAA repair (OAAA, n = 3,657). POMI was categorized by the method of diagnosis as troponin-only or clinical/ECG and rates were investigated in regions with ≥100 consecutive cases. Regions with significantly different POMI rates were defined as those >1.5 interquartile lengths beyond the 75th percentile of the distribution. Risk-adjusted rates of POMI were assessed using the VQI Cardiac Risk Index all-procedures prediction model to compare the observed versus expected rates for each region.
Results
Overall rates of POMI varied by procedure type: CEA 0.8%, EVAR 1.1%, INFRA 2.7%, and OAAA 4.2% (P < 0.001). Significant variation in POMI rates was observed between regions, resulting in differing ranges of POMI rates for each procedure: CEA 0.5–2.0% (P = 0.001), EVAR 0.3–3.1% (P < 0.001), INFRA 1.1–4.8% (P < 0.001), and OAAA 2.2–10.0% (P < 0.001). A single region in 3 of the 4 procedure-specific datasets was identified as a statistical outlier with a significantly higher POMI rate after CEA, EVAR, and OAAA; this region was identical for the EVAR and OAAA datasets but was a different region for the CEA dataset. No significant variation in POMI was noted between regions after INFRA. Procedure-specific clinical POMI rates (mean; range) were significantly different between regions for EVAR (0.4%; 0–1.1%, P = 0.01) and INFRA (1.4%; 0.5–2.9%, P = 0.01), but not for CEA (0.4%; 0–0.8%, P = 0.53) or OAAA (1.6%; 0–3.8%, P = 0.23). Procedure-specific troponin-only POMI rates (mean; range) were significantly different between regions for all procedures: CEA (0.4%; 0.1–1.2%, P < 0.001), EVAR (0.7%; 0–2.1%, P < 0.001), INFRA (1.3%; 0.4–2.5%, P = 0.001), and OAAA (2.5%; 0–8.5%, P < 0.001). After risk adjustment, regional variation was again noted with 3 regions having higher and 4 regions having lower than expected rates of POMI.
Conclusions
Significant variation in POMI rates following major vascular surgery exists across VQI regions even after risk adjustment. These findings may present an opportunity for focused regional quality improvement efforts.
INTRODUCTION
While major adverse cardiac events (MACEs) remain a leading cause of morbidity and mortality after vascular surgery, little is known about regional variation in cardiac event rates. The majority of the literature to date has focused on cardiac risk assessment, medical optimization, and the role of preoperative coronary revascularization before vascular surgery. Regional variability in admissions for acute myocardial infarction (AMI) has been previously re-ported.1 Regional variation has also been identified in the management and outcomes of AMI, including differences in medical management and coronary intervention.2–5 Recognizing these differences, we questioned if similar regional variability exists for postoperative myocardial infarction (POMI) after major vascular surgery.
The goal of this study was to investigate variability in POMI rates diagnosed by clinical/ECG or troponin criteria among regions within the Vascular Quality Initiative (VQI). POMI by troponin criteria was included because POMI often has an atypical presentation diagnosed primarily based on elevation in the cardiac biomarkers. In addition, troponin elevation has been demonstrated to be associated with increased short- and long-term mortality.6,7 Within the Vascular Study Group of New England (VSGNE), a postoperative troponin elevation and POMI predict a 26% and 55% lower 5-year survival, respectively.7 Because variation in POMI may be due to a number of factors including procedure type and patient comorbidities, notably renal insufficiency, diabetes, and preexisting coronary artery disease, risk adjustment was performed using the previously established VQI CRI (Cardiac Risk Index) all-procedures risk prediction model.8
METHODS
Study Design and Database
This is a retrospective analysis of data collected prospectively by the VQI, a national collaboration of regional quality groups established to collect and analyze data in an effort to improve patient care.9,10 Additional information is available at https://www.vascularweb.org/practiceresources/vascular-quality-initiative/Pages/default.aspx. Physicians, nurses, or clinical data abstractors prospectively enter clinical and demographic data at the time of patient discharge from the index hospitalization and at 1 year. Research analysts are blinded to patient, surgeon, center, and region identity.
We examined rates of POMI following nonemergent vascular surgery procedures, including carotid endarterectomy (CEA) (n = 39,118, 52%), infrainguinal bypass (INFRA) (n = 17,176, 23%), endovascular abdominal aortic aneurysm repair (EVAR) (n = 15,106, 20%), and open infrarenal AAA repair (OAAA) (n = 3,657, 5%) between January 1, 2010 and December 31, 2014. At the time of the analysis, the VQI included 17 regional groups encompassing 243 centers, which were comprised of 5–29 centers per region and 1343 physicians. Entry of consecutive procedures by each participating center is assured by annual audit against hospital claims data submitted by each center.11
For this analysis, VQI regions (1) with fewer than 3 centers participating in their regional procedure-specific dataset and (2) those VQI centers without a designated regional affiliation (n = 11) were combined into a single region. This group of centers was created for this analysis to maintain anonymity of data. Only VQI regions performing at least 100 procedure-specific cases were included in the analysis. Three VQI regions were identified as having performed <100 OAAA cases and thus were excluded from the OAAA analysis.
Outcome Measures
The primary outcome was the rate of POMI by region. POMI was classified according to a hierarchy of (1) none, (2) troponin elevation, or (3) clinical/ECG. Clinical POMI was defined as clinical symptoms such as angina and/or ECG changes in conjunction with cardiac biomarker abnormality consistent with infarction. The nature of the ECG changes was not standardized across all sites but was defined according to local parameters at each participating center. Troponin-only POMI was defined as troponin elevation beyond the normal upper limit without creatinine phosphokinase muscle brain elevation and without other clinical signs, symptoms, or ECG changes consistent with myocardial infarction (MI). The clinical POMI diagnosis superseded the troponin designation so that only one classification was assigned to each event. Screening for POMI was not prescribed but was left to the discretion of the clinicians at each center. Troponin levels were classified as normal or abnormal but absolute values were not recorded.
Covariates Examined
More than 100 demographic and clinical variables, which have been previously described, were collected prospectively for each procedure and entered into the VQI database (http://www.vascularqualityinitiative.org/about/procedures-collected). Clinical variables included comorbidities, perioperative medication, procedural details, and postoperative complications. Preoperative coronary artery disease (CAD) was classified as (1) none, (2) asymptomatic with history of MI ≥6 months, (3) stable angina or MI within 6 months, or as (4) unstable angina. Preoperative congestive heart failure (CHF) was categorized as (1) none, (2) asymptomatic with history of CHF, (3) mild, (4) moderate, or (5) severe based on the degree of physical limitation.
Postoperative CHF was defined as new pulmonary edema with requirement for monitoring or treatment in the intensive care or step-down unit. Postoperative dysrhythmia was defined as a new rhythm disturbance requiring treatment with medications or cardioversion. MACE included POMI, CHF, and/or dysrhythmia. Return to the operating room included any of the specified indications: bleeding, thrombosis, or infection.
Statistical Analysis
Univariate analyses were conducted with t-test for continuous variables and χ2 test analysis for categorical variables. Outlier regions were identified using Tukey’s method, defined as 1.5 interquartile lengths beyond the 75th percentile of distribution.12
The VQI CRI all-procedures risk prediction for POMI has been previously described.8
The VQI CRI all-procedures model includes the following predictors of POMI: procedure type, age, body mass index, history of renal insufficiency, diabetes, congestive heart failure or coronary artery disease, and results of preoperative stress testing. Complete details of the model are shown in the on-line Appendix. The VQI CRI all-procedures model was used to calculate an expected POMI rate for each patient in a given region based on that individual patient’s characteristics and procedure. The observed (O) mean rates of POMI were then recorded for each region and compared with expected (E) rates. An O/E ratio <1 indicates a lower than expected rate of POMI and an O/E >1 indicates a higher than expected rate of POMI. Chi-squared comparisons determined significance between O/E rates across regions.13 Using this method, we risk-adjusted regional POMI rates by procedure mix and important patient characteristics.
Analyses were conducted using SAS v9.4 (SAS Institute, Inc., Cary, NC) and Stata 13 (StataCorp, College Station, TX). The University of Vermont Institutional Review Board has approved the use of deidentified data from the VQI for research purposes and does not require written informed consent.
RESULTS
Patients with POMI had higher rates of CAD, CHF, diabetes, HTN, COPD, and renal insufficiency (Table I). Prior coronary revascularization was more common in patients with POMI as was an abnormal preoperative cardiac stress test. Aspirin and statin usage were similar between the 2 groups, while P2Y12 inhibitors and beta-blockers were more commonly used in patients with POMI.
Table I.
Preoperative characteristics of patients and procedures with and without POMI
| Preoperative patient characteristic | Postoperative myocardial infarction
|
P value | |
|---|---|---|---|
| No POMI n = 73,947 (98.5%) |
Yes POMI n = 1,110 (1.5%) |
||
| Age, mean (SD) | 70.1 (9.9) | 72.6 (9.3) | <0.001 |
| Sex | |||
| Male | 49,322 (98.5) | 737 (1.5) | 0.83 |
| Female | 24,620 (98.5) | 373 (1.5) | |
| Race | |||
| White | 66,215 (98.6) | 974 (1.4) | 0.003 |
| Black | 4,974 (98.6) | 73 (1.4) | |
| Other | 2,758 (97.8) | 63 (2.2) | |
| Ethnicity | |||
| Hispanic | 2,485 (98.5) | 39 (1.5) | 0.76 |
| Non-Hispanic | 70,719 (98.5) | 1,055 (1.5) | |
| BMI, mean (SD) | 28.0 (5.8) | 27.2 (5.6) | <0.001 |
| CAD | |||
| None | 52,788 (98.9) | 594 (1.1) | <0.001 |
| History, asymptomatic | 14,381 (97.8) | 327 (2.2) | |
| Stable angina | 4,914 (97.5) | 124 (2.5) | |
| Unstable angina or MI < 6 months | 1,739 (96.5) | 64 (3.5) | |
| CHF | |||
| None | 65,533 (98.8) | 825 (1.2) | <0.001 |
| History, asymptomatic | 4,956 (97.1) | 149 (2.9) | |
| Mild | 2,245 (96.3) | 86 (3.7) | |
| Moderate to severe | 1,153 (95.9) | 49 (4.1) | |
| Stress test | |||
| Not done | 47,651 (98.6) | 664 (1.4) | <0.001 |
| Normal | 19,615 (98.8) | 240 (1.2) | |
| Abnormal | 6,468 (97.0) | 203 (3.0) | |
| Diabetes | |||
| None | 48,825 (98.8) | 583 (1.2) | <0.001 |
| Noninsulin dependent | 15,466 (98.4) | 255 (1.6) | |
| Insulin dependent | 9,586 (97.3) | 271 (2.7) | |
| Creatinine >1.8 mg/dL | |||
| No | 67,749 (98.7) | 911 (1.3) | <0.001 |
| Yes | 3,971 (96.7) | 136 (3.3) | |
| Dialysis | |||
| No | 72,162 (98.6) | 1,038 (1.4) | <0.001 |
| Functioning transplant | 243 (95.7) | 11 (4.3) | |
| On dialysis | 1,511 (96.2) | 60 (3.8) | |
| Smoking status | |||
| Never | 14,709 (98.6) | 216 (1.4) | 0.001 |
| Prior | 34,806 (98.4) | 579 (1.6) | |
| Current | 24,333 (98.7) | 313 (1.3) | |
| HTN | |||
| No | 9,360 (99.1) | 89 (0.9) | <0.001 |
| Yes | 64,544 (98.4) | 1,020 (1.6) | |
| COPD | |||
| No | 55,723 (98.6) | 789 (1.4) | <0.001 |
| Not treated | 6,124 (97.9) | 129 (2.1) | |
| On meds | 10,023 (98.5) | 157 (1.5) | |
| On home oxygen | 1,989 (98.3) | 34 (1.7) | |
| Prior vascular surgery | |||
| No | 49,538 (98.9) | 571 (1.1) | <0.001 |
| Yes | 24,292 (97.8) | 536 (2.2) | |
| History of CABG | |||
| No | 44,041 (98.9) | 508 (1.1) | <0.001 |
| <5 years | 3,679 (98.5) | 56 (1.5) | |
| ≥5 years | 7,384 (97.3) | 204 (2.7) | |
| History of PCI | |||
| No | 43,748 (98.8) | 523 (1.2) | <0.001 |
| <5 years | 5,730 (97.8) | 130 (2.2) | |
| ≥5 years | 5,537 (98.0) | 111 (2.0) | |
| Preoperative medications | |||
| Aspirin | |||
| No | 17,937 (98.5) | 280 (1.5) | 0.46 |
| Yes | 55,938 (98.5) | 829 (1.5) | |
| P2Y12 antagonist | |||
| No | 56,268 (98.7) | 754 (1.3) | <0.001 |
| Yes | 17,579 (98.0) | 355 (2.0) | |
| Statin | |||
| No | 18,710 (98.6) | 265 (1.4) | 0.28 |
| Yes | 55,165 (98.5) | 844 (1.5) | |
| Beta-blocker | |||
| None | 29,060 (99.0) | 284 (1.0) | <0.001 |
| Day of surgery only | 2,166 (98.8) | 26 (1.2) | |
| Preoperative: 1–30 days | 3,768 (98.4) | 63 (1.6) | |
| Chronic: >30 days | 38,843 (98.1) | 736 (1.9) | |
| Urgent procedure | |||
| No | 63,679 (98.7) | 870 (1.3) | <0.001 |
| Yes | 10,268 (97.7) | 240 (2.3) | |
BMI, body mass index; CABG, coronary artery bypass graft; COPD, chronic obstructive pulmonary disease; HTN, hypertension; PCI, percutaneous coronary intervention; SD, standard deviation.
The POMI rate, across all regions, varied by procedure type with the mean (range) rate as follows: CEA 0.8% (0.5–2.0%, P = 0.001), EVAR 1.1% (0.3–3.1%, P < 0.001), INFRA 2.7% (1.1–4.8%, P < 0.001), and OAAA 4.2% (2.2–10.0%, P < 0.001) (P < 0.001) (Table II). Troponin-only POMI was more common than clinical POMI after EVAR (63 % vs. 37%) and OAAA (61% vs. 39%). Rates of troponin and clinical POMI were similar after CEA (50% vs. 50%) and INFRA (49% vs. 51%). Regional differences in POMI were noted (P < 0.001 across groups). The procedure-specific trend in POMI rate variability, as a function of time, between 2010 and 2014 was not statistically significant (CEA, P = 0.05; EVAR, P = 0.10; INFRA, P = 0.12; OAAA, P = 0.64), indicating no temporal influences.
Table II.
Postoperative myocardial infarction by procedure type and VQI region
| Variable | Postoperative myocardial infarction
|
P value | |
|---|---|---|---|
| No POMI n = 73,947 (98.5%) |
Yes POMI n = 1,110 (1.5%) |
||
| Procedure | |||
| CEA | 38,790 (99.2) | 328 (0.8) | <0.001 |
| EVAR | 14,931 (98.8) | 175 (1.2) | |
| INFRA | 16,721 (97.4) | 455 (2.6) | |
| OAAA | 3,505 (95.8) | 152 (4.2) | |
| Regiona | |||
| 1 | 3,773 (97.3) | 105 (2.7) | <0.001 |
| 2 | 4,217 (99.1) | 40 (0.9) | |
| 3 | 8,232 (99.1) | 74 (0.9) | |
| 4 | 1,489 (99.1) | 14 (0.9) | |
| 5 | 4,859 (98.9) | 54 (1.1) | |
| 6 | 5,291 (98.5) | 83 (1.5) | |
| 7 | 2,820 (99.1) | 27 (0.9) | |
| 8 | 5,105 (98.6) | 70 (1.4) | |
| 9 | 3,543 (98.3) | 62 (1.7) | |
| 10 | 3,332 (99.0) | 32 (1.0) | |
| 11 | 4,103 (97.5) | 106 (2.5) | |
| 12 | 969 (97.7) | 23 (2.3) | |
| 13 | 2,842 (98.7) | 38 (1.3) | |
| 14 | 2,790 (98.9) | 30 (1.1) | |
| 15 | 17,817 (98.4) | 296 (1.6) | |
| 16 | 688 (98.9) | 8 (1.1) | |
| 17 | 2,117 (97.8) | 48 (2.2) | |
VQI regions have been renumbered in ascending order for this analysis to maintain anonymity of data.
Regional Variation in Overall, Troponin-Only, and Clinical POMI Rates
A significant difference in overall regional POMI rates was noted for each procedure: CEA (mean 0.8%, P < 0.001), EVAR (mean 1.1%, P < 0.001), INFRA (mean 2.7%, P < 0.001), and OAAA (mean 4.2%, P < 0.001) (Fig. 1). The procedural regional variability in overall, troponin-only, and clinical POMI rates is illustrated in Figure 2. Significant differences in troponin-only POMI rates were noted across regions after the 4 procedures: CEA (mean 0.4%, P < 0.001), EVAR (mean 0.7%, P < 0.001), INFRA (mean 1.3%, P = 0.001), and OAAA (mean 2.5%, P < 0.001). Significant differences in clinical POMI rates were noted across regions after EVAR (mean 0.4%, P = 0.01) and INFRA (mean 1.4%, P = 0.01), but not after CEA (mean 0.4%, P = 0.53) or OAAA (mean 1.6%, P = 0.23).
Fig. 1.
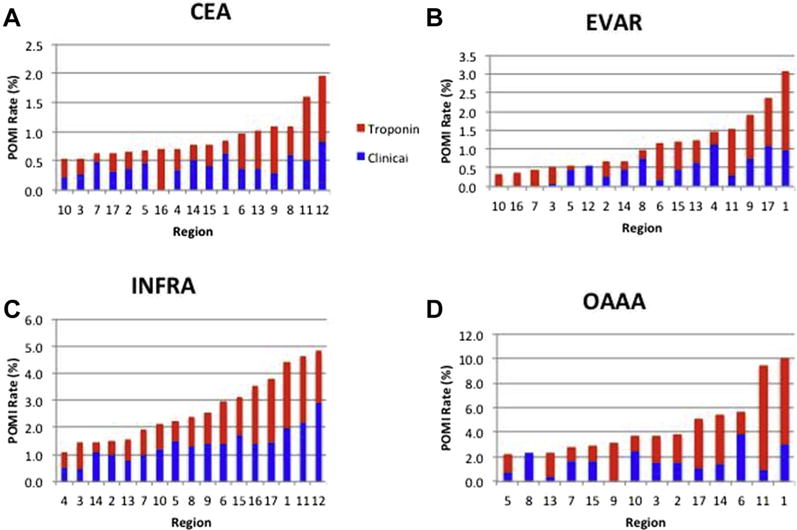
Regional variability in rates of POMI by procedure: (A) CEA, (B) EVAR, (C) INFRA, and (D) OAAA (3 regions excluded due to <100 procedures). Note difference in scale across procedures. Red indicates troponin-only POMI and blue indicates clinical/ECG POMI.
Fig. 2.
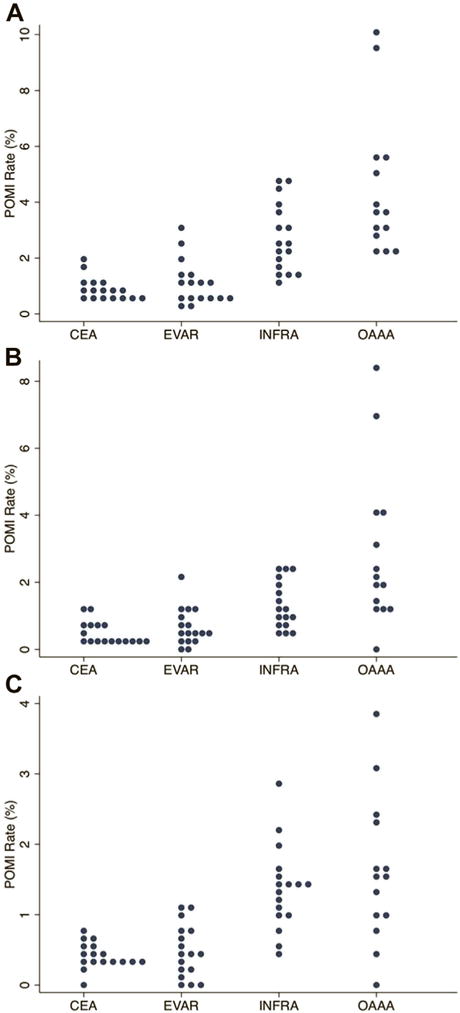
Dot plot of POMI rates by procedure: (A) overall, (B) troponin only, and (C) clinical/ECG. Each dot represents a unique VQI region. Note difference in scale across procedures.
Region 12 was identified as having a statistically significant higher overall rate of POMI (2.0%) after CEA (Fig. 3), defined as 1.5 interquartile lengths beyond the 75th percentile of distribution. Region 1 was identified as having statistically significant higher overall rates of POMI after EVAR (3.1%) and OAAA (10%). Region 1 had a statistically significant higher rate of troponin-only POMI (2.1%) after EVAR. Region 11 had a statistically significant higher rate of troponin-only POMI after OAAA (8.5%). Region 12 had a statistically significant higher rate of clinical POMI after CEA (0.8%) and INFRA (2.9%).
Fig. 3.
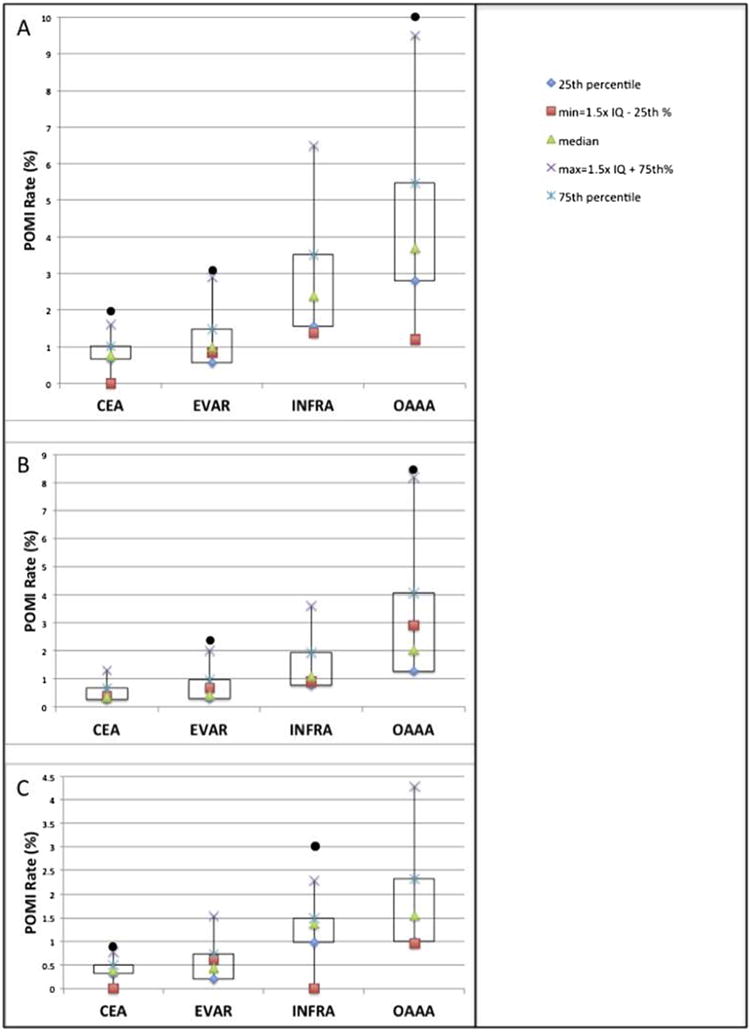
Box and whisker plot of (A) overall, (B) troponin only, and (C) clinical/ECG POMI rates by procedure. Each black circle represents a unique VQI region outlier with a POMI rate >1.5 interquartile lengths beyond the 75th percentile of distribution.
Risk-Adjusted Regional POMI Rates
After performing risk adjustment using the VQI CRI all-procedures prediction model, differences in observed versus expected POMI rates were noted, with 3 regions having statistically significant higher than expected rates (Table III). Four regions had statistically significant lower than expected POMI rates. Ten regions had observed POMI rates similar to expected. Interregional comparisons of O/E rates revealed numerous statistically significant differences (Table IV).
Table III.
Observed versus expected rates of POMI based on the VQI CRI all-procedures model
| VQI region | Observed POMI rate (%) | Expected POMI rate (%) | O/E | P value |
|---|---|---|---|---|
| 1 | 2.7 | 1.6 | 1.8 | <0.001 |
| 2 | 1.0 | 1.5 | 0.6 | 0.005 |
| 3 | 0.9 | 1.4 | 0.6 | <0.001 |
| 4 | 1.0 | 1.4 | 0.7 | 0.18 |
| 5 | 1.1 | 1.6 | 0.7 | 0.005 |
| 6 | 1.5 | 1.4 | 1.0 | 0.74 |
| 7 | 1.0 | 1.4 | 0.7 | 0.05 |
| 8 | 1.4 | 1.5 | 0.9 | 0.32 |
| 9 | 1.7 | 1.6 | 1.1 | 0.48 |
| 10 | 0.9 | 1.3 | 0.7 | 0.06 |
| 11 | 2.5 | 1.5 | 1.7 | <0.001 |
| 12 | 2.4 | 1.3 | 1.9 | 0.003 |
| 13 | 1.3 | 1.8 | 0.8 | 0.09 |
| 14 | 1.1 | 1.3 | 0.8 | 0.33 |
| 15 | 1.7 | 1.7 | 1.0 | 0.68 |
| 16 | 1.1 | 1.3 | 0.9 | 0.93 |
| 17 | 2.2 | 1.8 | 1.2 | 0.17 |
O/E, observed versus expected ratio >1 indicates higher than expected rate and <1 indicates lower than expected rate of POMI.
Table IV.
Regional comparisons of observed versus expected POMI rates
| Region | 2 | 3 | 7 | 5 | 4 | 10 | 13 | 14 | 8 | 16 | 15 | 6 | 9 | 17 | 11 | 1 | 12 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Region | O/E | 0.6 | 0.6 | 0.7 | 0.7 | 0.7 | 0.7 | 0.8 | 0.8 | 0.9 | 0.9 | 1.0 | 1.0 | 1.1 | 1.2 | 1.7 | 1.8 | 1.9 |
| 2 | 0.6 | * | * | * | * | * | * | * | ||||||||||
| 3 | 0.6 | * | * | * | * | * | * | * | ||||||||||
| 7 | 0.7 | * | * | * | * | * | ||||||||||||
| 5 | 0.7 | * | * | * | * | * | * | * | ||||||||||
| 4 | 0.7 | * | * | * | ||||||||||||||
| 10 | 0.7 | * | * | * | * | |||||||||||||
| 13 | 0.8 | * | * | * | * | |||||||||||||
| 14 | 0.8 | * | * | * | ||||||||||||||
| 8 | 0.9 | * | * | * | ||||||||||||||
| 16 | 0.9 | |||||||||||||||||
| 15 | 1.0 | * | * | * | * | * | * | |||||||||||
| 6 | 1.0 | * | * | * | * | * | * | |||||||||||
| 9 | 1.1 | * | * | * | * | * | * | * | ||||||||||
| 17 | 1.2 | * | * | * | * | * | * | |||||||||||
| 11 | 1.7 | * | * | * | * | * | * | * | * | * | * | * | * | |||||
| 1 | 1.8 | * | * | * | * | * | * | * | * | * | * | * | * | |||||
| 12 | 1.9 | * | * | * | * | * | * | * | * | * | * | * | * |
O/E, observed versus expected ratio.
P < 0.05.
DISCUSSION
Cardiac complications following vascular surgical procedures remain a primary cause of perioperative morbidity and mortality. Although regional variation in AMI admissions, management, and percutaneous coronary revascularization has been reported, relatively little information exists about regional differences in cardiac events after vascular surgery.4,5 The VQI structure presents an opportunity to analyze this and other variability in vascular surgery practice.9 Kalish et al. have previously identified VQI center differences in surgical site infections after INFRA.14 As a direct result of this work evaluating surgical site infection variability, the VQI initiated a reporting system, the Center Opportunity for Improvement Reports, designed to inform centers of their risk-adjusted outcomes and to identify possible areas for improvement in this and other areas. In another study, Arous et al.15 examined regional variability in imaging before CEA. In this study, we examined regional variability in POMI after common vascular operations.
While open vascular surgical procedures are considered high risk interventions, established POMI rates vary by procedure. In this study, we found that CEA and EVAR have a lower risk of POMI when compared with INFRA and OAAA, which led to our examination of regional POMI rates by procedure. Our results indicate that there is identifiable regional variation in POMI rates across the VQI following major vascular surgery procedures. While the variability at the regional level was not widespread, important differences were noted. One VQI region was identified as having a higher overall rate of POMI, >1.5 times the interquartile length beyond the 75th percentile of distribution, after CEA, EVAR, and OAAA.
Because the diagnosis of MI is not always straightforward in the postoperative setting, we examined regional variability according to POMI subtype (clinical versus troponin-only). Regional differences in clinical POMI were noted after EVAR and INFRA, but not after CEA or OAAA. Regional differences in troponin-only POMI were noted after CEA, INFRA, EVAR, and OAAA. The troponin-only POMI rates of 2 distinct regions—one in the EVAR cohort and a second distinct region in the OAAA cohort—were identified as outliers, with troponin-only POMI rates >1.5 times the interquartile lengths beyond the 75th percentile of distribution. These differences are clinically important because previous published literature indicates that elevated serum troponin, even in the absence of ECG and/or clinical manifestations of POMI, portends a worse short- and long-term prognosis.6,7,16–19
There are multiple factors that may influence the POMI rate following vascular surgery, including patient characteristics, differences in perioperative care, and practices regarding routine postoperative troponin testing. We used the VQI CRI all-procedures risk prediction model to risk-adjust for procedure type and important patient comorbidities. Using this method, we identified 3 regions with statistically significant higher than expected rates and 4 regions with statistically significant lower than expected POMI rates. While regional and procedure-specific variability in POMI rates has been identified, the etiology of these differences remains to be elucidated. We suspect that these differences are due to processes of care, which may include factors such as patient or procedure selection and variation in diagnosis or management of POMI.
Limitations
As with any observational study, there are certain limitations inherent to this analysis. First, the incidence of POMI is relatively low, making measurement of center differences difficult. The large sample size of POMI within the VQI only enables broad regional comparisons. Second, there are physiologic and treatment strategy differences between an ST segment elevation MI (STEMI) and a non-ST segment elevation MI (NSTEMI) and the VQI does not currently distinguish between the two. Third, while troponin is the most sensitive and specific marker for myocardial cell necrosis, elevation of this biomarker may be secondary to other conditions.20 For example, the etiology of an elevated serum troponin may be an acute coronary syndrome from obstructive coronary artery disease or a mismatch between increased myocardial oxygen demand and insufficient cardiac reserve. Troponin elevation may also be seen in other cardiac conditions such as CHF or noncardiac complications including exacerbations of emphysema or systemic sepsis. Finally, there are undoubtedly surgeon-, center-, and region-specific differences with regard to screening for postoperative cardiac complications. Furthermore, the rates of POMI may be influenced by the rigor of screening, such as routine troponin testing, which was not mandated or recorded in the registries.
Future Directions
Identification of this regional variation in POMI rates is the first step in quality assurance and improvement. These data may inform regional VQI groups and encourage a more in-depth look at factors such as cardiac risk stratification and practice patterns of POMI diagnosis at the center level. Investigation into regional differences in medical management of POMI after vascular surgery is planned to further this work.
CONCLUSION
Variation in POMI rates following major vascular surgery exists across VQI regions even after risk adjustment. Identification of variability warrants further examination of patient factors and processes of care that may influence these complications. Ultimately, a better understanding of the underlying causes of this variation is a potential target for center-specific and region-specific quality improvement efforts.
Supplementary Material
Footnotes
Presented at the 42nd Annual Meeting of the New England Society for Vascular Surgery, Newport, RI, October 4, 2015.
References
- 1.Wennberg DE, Birkmeyer JD. The Dartmouth Atlas of Cardiovascular Health Care. Chicago, IL: AHA Press; 1999. Specialty Care Series. Dartmouth Atlas of Cardiovascular Health Care Working Group; pp. 82–3. [Google Scholar]
- 2.Pilote L, Califf RM, Sapp S, et al. Regional variation across the United States in the management of acute myocardial infarction. GUSTO-1 Investigators. Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries. N Engl J Med. 1995;333:565–72. doi: 10.1056/NEJM199508313330907. [DOI] [PubMed] [Google Scholar]
- 3.Krumholz HM, Chen J, Rathore SS, et al. Regional variation in the treatment and outcomes of myocardial infarction: investigating New England’s advantage. Am Heart J. 2003;146:242–9. doi: 10.1016/S0002-8703(03)00237-0. [DOI] [PubMed] [Google Scholar]
- 4.Ko DT, Wang Y, Alter DA, et al. Regional variation in cardiac catheterization appropriateness and baseline risk after acute myocardial infarction. J Am Coll Cardiol. 2008;51:716–23. doi: 10.1016/j.jacc.2007.10.039. [DOI] [PubMed] [Google Scholar]
- 5.Kolte D, Khera S, Aronow WS, et al. Regional variation across the United States in management and outcomes of ST-elevation myocardial infarction: analysis of the 2003 to 2010 national inpatient sample database. Clin Cardiol. 2014;37:204–12. doi: 10.1002/clc.22250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Waes JA, Nathoe HM, de Graaff JC, et al. Cardiac Health After Surgery (CHASE) Investigators Myocardial injury after noncardiac surgery and its association with short-term mortality. Circulation. 2013;127:2264–71. doi: 10.1161/CIRCULATIONAHA.113.002128. [DOI] [PubMed] [Google Scholar]
- 7.Simons JP, Baril DT, Goodney PP, et al. Vascular Study Group of New England The effect of postoperative myocardial ischemia on long-term survival after vascular surgery. J Vasc Surg. 2013;58:1600–8. doi: 10.1016/j.jvs.2013.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bertges DJ, Neal D, Schanzer A, et al. The Vascular Quality Initiative Cardiac Risk Index for prediction of myocardial infarction after vascular surgery. J Vasc Surg. 2016;64:1411–1421.e1. doi: 10.1016/j.jvs.2016.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Woo K, Eldrup-Jorgensen J, Hallett JW, et al. for the Vascular Quality Initiative Regional quality groups in the Society for Vascular Surgery Vascular Quality Initiative. J Vasc Surg. 2013;57:884–90. doi: 10.1016/j.jvs.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 10.Cronenwett JL, Likosky DS, Russell MT, et al. A regional registry for quality assurance and improvement: the Vascular Study Group of Northern New England (VSGNNE) J Vasc Surg. 2007;46:1093–101. doi: 10.1016/j.jvs.2007.08.012. discussion 1101–1102. [DOI] [PubMed] [Google Scholar]
- 11.M2S Pathways Support. Available at: http://www.vascularqualityinitiative.org/wp-content/uploads/SVS-PSO-Data-Validation_0.pdf. Accessed July 15, 2015.
- 12.Zar J. Biostatistical Analysis. Upper Saddle River, NJ: Prentice-Hall; 2010. [Google Scholar]
- 13.Breslow NE, Day NE. Volume II – The Design and Analysis of Cohort Studies. Lyon: International Agency for Research on Cancer; 1987. Statistical Methods in Cancer Research; pp. 93–4. [PubMed] [Google Scholar]
- 14.Kalish JA, Farber A, Homa K, et al. Society for Vascular Surgery Patient Safety Organization Arterial Quality Committee Factors associated with surgical site infection after lower extremity bypass in the Society for Vascular Surgery (SVS) Vascular Quality Initiative (VQI) J Vasc Surg. 2014;60:1238–46. doi: 10.1016/j.jvs.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 15.Arous EJ, Simons JP, Flahive JM, et al. Vascular Quality Initiative National variation in preoperative imaging, carotid duplex ultrasound criteria, and threshold for surgery for asymptomatic carotid artery stenosis. J Vasc Surg. 2015;62:937–44. doi: 10.1016/j.jvs.2015.04.438. [DOI] [PubMed] [Google Scholar]
- 16.Devereaux PJ, Chan MT, Alonso-Coello P, et al. Vascular Events in Noncardiac Surgery Patients Cohort Evaluation (VISION) Study Investigators Association between postoperative troponin levels and 30-day mortality among patients undergoing noncardiac surgery. JAMA. 2012;307:2295–304. doi: 10.1001/jama.2012.5502. [DOI] [PubMed] [Google Scholar]
- 17.Devereaux PJ, Xavier D, Pogue J, et al. POISE (Peri-Operative ISchemic Evaluation) Investigators Characteristics and short-term prognosis of perioperative myocardial infarction in patients undergoing noncardiac surgery. Ann Intern Med. 2011;154:523–8. doi: 10.7326/0003-4819-154-8-201104190-00003. [DOI] [PubMed] [Google Scholar]
- 18.Redfern G, Rodseth RN, Biccard BM. Outcomes in vascular surgical patients with isolated postoperative troponin leak: a meta-analysis. Anaesthesia. 2011;66:604. doi: 10.1111/j.1365-2044.2011.06763.x. [DOI] [PubMed] [Google Scholar]
- 19.Bhatti UH, Manunga J, Kalra M, et al. Routine postoperative troponin testing following major vascular surgical procedures carries short-term and long-term prognostic significance. J Vasc Surg. 2015;61:155S–6S. [Google Scholar]
- 20.Beckman JA. Postoperative troponin screening: a cardiac Cassandra? Circulation. 2013;127:2253–6. doi: 10.1161/CIRCULATIONAHA.113.003195. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


