Abstract
Background
Society for Vascular Surgery practice guidelines recommend 1- and 12-month follow-up with computed tomography imaging for the year after endovascular aneurysm repair (EVAR). We describe the incidence, risk factors, and outcomes of EVAR patients who are lost to follow-up (LTF).
Methods
All patients undergoing elective EVAR in the Vascular Quality Initiative (VQI) data set (January 2003-December 2015) were stratified according to long-term follow-up method (in-person vs phone call vs LTF). Mortality was captured for all patients by linkage with the Social Security Death Index. Univariable statistics, Kaplan-Meier estimated survival curves, and Cox proportional hazard modeling were used to compare groups. Coarsened exact matching analysis was then performed to refine the association between LTF and risk of post-EVAR death.
Results
During the study period, 11,309 patients underwent elective EVAR (78% in-person follow-up, 11% phone call follow-up, 11% LTF). On univariable analysis, LTF patients had larger baseline aneurysms, higher American Society of Anesthesiologists scores, more comorbidities, and worse baseline functional status compared to patients with in-person or phone call follow-up (P ≤ .05). Procedural factors (contrast material volume, blood transfusions, postoperative vasopressor use) were higher in the LTF group, as was the incidence of postoperative complications (P ≤ .05). Accordingly, LTF patients had longer postoperative lengths of stay and were less frequently discharged to home (P < .001). Five-year survival was lower for LTF vs phone call follow-up vs in-person follow-up (62% vs 68% vs 84%; P < .001). On multivariable analysis correcting for baseline differences between groups, there was a significantly higher risk of death for both the LTF group (hazard ratio, 6.45; 95% confidence interval, 4.89-8.51) and phone call follow-up group (hazard ratio, 3.48; 95% confidence interval, 2.66-4.57) compared with patients who followed up in person (P < .001). After coarsened exact matching on 30 preoperative and perioperative variables, 5-year survival after EVAR for LTF vs phone call follow-up vs in-person follow-up was 84.9% vs 84.8% vs 91.9%, respectively (log-rank, P < .001). Notably, patients with phone call follow-up had a lower prevalence of documented postoperative imaging compared with patients with in-person follow-up (56.1% vs 85.1%; P < .001).
Conclusions
EVAR patients with more comorbidities and a higher incidence of in-hospital complications tend to be more frequently LTF and ultimately have worse survival outcomes. In-person follow-up is associated with better post-EVAR survival and a higher rate of postoperative imaging. Phone follow-up confers a mortality risk equivalent to lack of follow-up, possibly as a result of inadequate postoperative imaging. Surgeons should stress the importance of office-based postoperative follow-up to all EVAR patients, particularly those with poor baseline health and functional status and more complicated perioperative courses.
Society for Vascular Surgery (SVS) practice guidelines recommend 1- and 12-month follow-up with computed tomography imaging for the year after endovascular aneurysm repair (EVAR) to identify endoleaks and aneurysmal enlargement.1 These recommendations are based primarily on expert opinion as high-quality evidence on this topic does not currently exist.2 There are a few retrospective studies evaluating the utility of these surveillance guidelines. Using an institutional database of 188 patients, Wu et al recently reported that follow-up surveillance is incomplete for more than half of patients who undergo EVAR.3 Interestingly, compliant patients had worse survival compared with patients lost to follow-up (LTF). Similarly, Garg et al demonstrated that more than half of Medicare beneficiaries undergoing EVAR fail to meet current surveillance guidelines4 and that patients with incomplete surveillance have lower complications and lower aneurysm-related and overall mortality rates.5
These findings have led to some discussion about the currently recommended post-EVAR surveillance regimen.2 Although some patients may require close follow-up because of anatomic complexity and real concerns for endoleak, some physicians argue that straightforward cases may not require such regimented follow-up. One alternative to the classic approach of regular in-person follow-up visits with imaging may be phone call follow-up. However, there are minimal data describing outcomes among EVAR patients with in-person vs phone call vs no follow-up.
In the current study, we aimed to address this knowledge gap. We describe the incidence, risk factors, and outcomes of EVAR patients who are LTF compared with those with in-person and phone call follow-up. We hypothesize that elective EVAR patients with in-person follow-up will have better survival than those patients with either phone call or no follow-up. By better understanding how different follow-up strategies affect post-EVAR mortality, we may be better informed about the appropriateness for different follow-up surveillance regimens in this group of patients.
METHODS
Data source
All patients undergoing elective EVAR in the Vascular Quality Initiative (VQI) data set between January 1, 2003, and December 31, 2015, were analyzed according to long-term follow-up method. Patients were excluded from analysis if they underwent EVAR for symptomatic or ruptured abdominal aortic aneurysm or if they were missing a value for mortality status. Patients were also excluded if they were missing a value for their follow-up variable in the VQI data set because the follow-up status of those patients could not be determined.
Follow-up method was classified according to the VQI variable dictionary6 and included in-person follow-up, phone call follow-up, and LTF (ie, follow-up information could not be collected). Follow-up is an independently recorded variable within the VQI data set that is separate from postoperative imaging and other variables. Patients are recorded as having only a single follow-up classification in the data set; multiple classifications are not provided. If a patient had multiple follow-up visits, the latest recorded follow-up status was used.
The Institutional Review Board approved this study before its initiation. No patient consent was obtained for this study as the data are sourced from a publically available database.
Outcomes
The primary outcome of the study was mortality. Secondary outcomes were analyzed for the in-person and phone call follow-up groups only and included documented postoperative imaging, endoleak, need for secondary interventions, and conversion to open repair. All outcomes were obtained from the VQI database. Mortality was captured for all patients by linkage with the Social Security Death Index.
Statistical methods
Descriptive statistics are expressed as mean ± standard error of the mean or percentage with count (number), as appropriate. Univariable statistics were used to describe baseline differences of in-person vs phone call vs LTF groups, including t-tests for continuous variables and analysis of variance for categorical variables.
Multivariable Cox proportional hazard modeling was then performed to compare risk of death after EVAR for each of the three groups. Stepwise model construction in backward fashion was used to determine associations between patient-, surgeon-, and hospital-level characteristics as well as factors contributing to renal dysfunction and mortality. Any variable demonstrating a P value ≥ .1 was deemed nonsignificant; these were removed from the model sequentially beginning with the highest P value. Cox models were run in multiple combinations to assess surgeon and center as covariates or clustering influences. Model discrimination was assessed with a Harrell C statistic.
Because of numerous baseline differences between groups, we then performed a coarsened exact matching (CEM) analysis to create matched cohorts for the in-person, phone call, and LTF groups. CEM is a form of matching that allows best-neighbor matching to minimize between-group heterogeneity while maximizing the number of available patients for analysis.7 Patients in each group were matched on 30 baseline and perioperative variables determined a priori. Mortality was then compared for the matched cohorts using Kaplan-Meier estimated survival curves with log-rank tests.
All analyses were performed using Stata 13.1 (StataCorp, College Station, Tex) with P < .05 set as significant.
RESULTS
Study cohort
A total of 22,644 patients underwent EVAR during the 12-year study period, of which 11,309 patients underwent elective EVAR and had a recorded value for their follow-up variable in the VQI data set (Fig 1); 78.2% (n = 8848) had in-person follow-up, 10.8% (n = 1222) had phone call follow-up, and 11.0% (n = 1239) were LTF.
Fig 1.
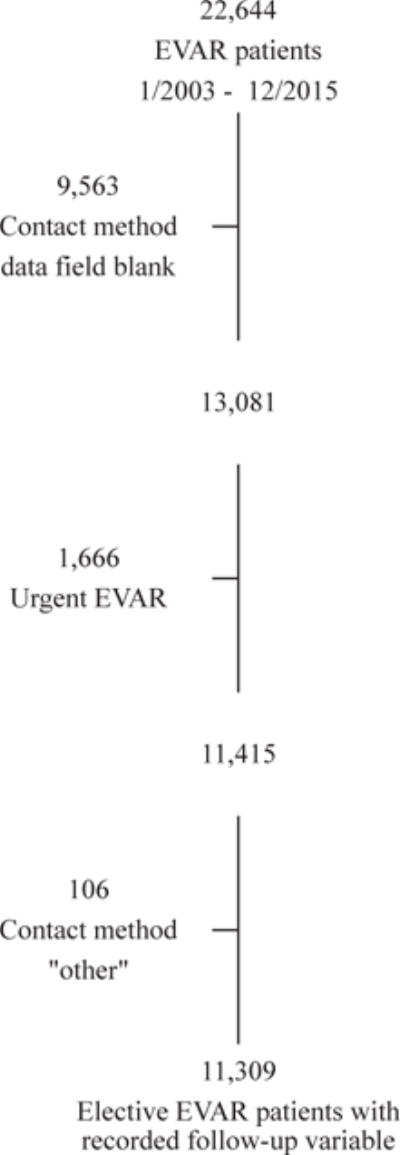
Study cohort. EVAR, Endovascular aneurysm repair.
Patients who were LTF were older (74.3 ± 0.3 vs 74.2 ± 0.2 vs 73.5 ± 0.1 years; P = .02), less likely to be male (78.7% vs 81.3% vs 82.0%; P = .02), and had larger baseline aneurysms (57.5 ± 0.5 mm vs 55.8 ± 0.3 mm vs 55.6 ± 0.2 mm; P < .001) compared with patients with phone call or in-person follow-up, respectively (Table I). LTF patients also had a higher prevalence of active smoking (33.7% vs 31.6% vs 29.5%; P = .02), higher American Society of Anesthesiologists scores (P < .001), and more comorbidities including coronary artery disease (31.8% vs 27.1% vs 29.6%; P = .04), congestive heart failure (14.8% vs 10.5% vs 10.6%; P < .001), chronic obstructive pulmonary disease (34.4% vs 31.7% vs 30.3%; P = .01), and dialysis dependence (2.2% vs 1.1% vs 0.9%; P = .002). Accordingly, LTF patients had worse functional status compared with phone call or in-person follow-up patients; they were less independently ambulatory (87.1% vs 92.5% vs 93.6%; P < .001), more commonly resided in a nursing home (2.4% vs 1.6% vs 1.0%; P < .001), and were more frequently classified as being unfit for open surgery (21.1% vs 19.3% vs 16.3%; P < .001).
Table I.
Baseline characteristics
| Variable | Follow-up method | P value | ||
|---|---|---|---|---|
| In-person (n = 8848) | Phone call (n = 1222) | LTF (n = 1239) | ||
| Age, years | 73.5 ± 0.1 | 74.2 ± 0.2 | 74.3 ± 0.3 | .02 |
| Male gender | 82.0 (7252) | 81.3 (993) | 78.7 (974) | .02 |
| Race | .02 | |||
| White | 92.8 (8213) | 93.3 (1138) | 91.9 (1137) | |
| Black | 3.90 (345) | 2.38 (29) | 5.17 (64) | |
| Asian | 0.99 (88) | 1.15 (14) | 0.65 (8) | |
| Other | 0.24 (21) | 0.25 (3) | 0.08 (1) | |
| Unknown | 1.03 (180) | 2.95 (36) | 2.18 (27) | |
| Body mass index, kg/m2 | 28.3 ± 0.1 | 27.5 ± 0.2 | 27.5 ± 0.2 | .48 |
| Maximum AP aortic diameter, mm | 55.6 ± 0.2 | 55.8 ± 0.3 | 57.5 ± 0.5 | <.001 |
| Insurance status | <.001 | |||
| Medicare | 64.6 (3627) | 63.8 (491) | 66.5 (470) | |
| Medicaid | 1.46 (82) | 1.69 (13) | 2.26 (16) | |
| Commercial | 30.9 (1733) | 28.8 (222) | 27.3 (193) | |
| Military/VA | 0.53 (30) | 1.43 (11) | 1.84 (13) | |
| None | 1.73 (97) | 3.12 (24) | 0.57 (4) | |
| Self-pay | 0.80 (45) | 1.17 (9) | 1.56 (11) | |
| ASA class | <.001 | |||
| 1 | 0.35 (28) | 0 (0) | 0.25 (3) | |
| 2 | 9.32 (752) | 5.94 (66) | 7.59 (90) | |
| 3 | 70.2 (5662) | 70.1 (779) | 68.1 (807) | |
| 4 | 20.1 (1622) | 24.0 (267) | 23.9 (283) | |
| 5 | 0.06 (5) | 0 (0) | 0.17 (2) | |
| Unfit for open surgery | 16.3 (1439) | 19.3 (235) | 21.1 (260) | <.001 |
| Comorbidities | ||||
| Hypertension | 83.9 (7415) | 82.4 (1006) | 84.1 (1039) | .38 |
| Diabetes | 19.9 (1756) | 17.9 (218) | 19.9 (245) | .25 |
| Smoking | .02 | |||
| Never | 14.2 (1252) | 12.4 (151) | 13.6 (168) | |
| Prior | 56.3 (4974) | 56.1 (685) | 52.8 (652) | |
| Current | 29.5 (2606) | 31.6 (386) | 33.7 (416) | |
| CAD | 29.6 (2619) | 27.1 (331) | 31.8 (391) | 0.04 |
| CHF | 10.6 (932) | 10.5 (128) | 14.8 (183) | <0.001 |
| COPD | 30.3 (2687) | 31.7 (387) | 34.4 (425) | 0.01 |
| Dialysis | 0.94 (83) | 1.06 (13) | 2.18 (27) | 0.002 |
| Pertinent surgical history | ||||
| Prior CABG or PCI | 36.3 (2053) | 34.3 (257) | 36.5 (255) | 0.53 |
| Prior aortic surgery | 3.56 (315) | 5.08 (62) | 4.29 (53) | 0.001 |
| Prior aneurysm repair | 3.19 (282) | 3.11 (38) | 3.80 (47) | .51 |
| Prior PVI | 5.38 (475) | 3.93 (48) | 4.68 (58) | .07 |
| Living status | ||||
| Home | 99.0 (8745) | 98.4 (1202) | 97.5 (1205) | <.001 |
| Nursing home | 0.96 (85) | 1.56 (19) | 2.43 (30) | |
| Homeless | 0.03 (3) | 0 (0) | 0.08 (1) | |
| Functional status | <.001 | |||
| Ambulatory | 93.6 (4979) | 92.5 (682) | 87.1 (626) | |
| Ambulatory with assistance | 5.56 (296) | 7.19 (53) | 10.4 (75) | |
| Wheelchair dependent | 0.75 (40) | 0.27 (2) | 2.23 (16) | |
| Bedridden | 0.13 (7) | 0 (0) | 0.28 (2) | |
AP, Anteroposterior; ASA, American Society of Anesthesiologists; CABG, coronary artery bypass graft; CAD, coronary artery disease; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; LTF, lost to follow-up; PCI, percutaneous coronary intervention; PVI, peripheral vascular intervention; VA, Veterans Administration.
Categorical data are presented as percentage (number). Continuous data are presented as mean ± standard error.
Procedural factors, including intraoperative type I endoleak (3.9% vs 3.4% vs 2.9%; P = .02), contrast material volume (109 ± 1.9 mL vs 106 ± 1.8 vs 105 mL ± 0.7 mL; P = .04), blood transfusions (0.57 ± 0.06 unit vs 0.26 ± 0.03= unit vs 0.18 ± 0.01 unit; P < .001), and postoperative vasopressor use (6.82% vs 5.57% vs 3.61%; P < .001) were higher in the LTF group compared with the phone call and in-person follow-up groups (Table II). LTF patients also had a higher incidence of postoperative complications, including myocardial infarction, heart failure, respiratory complications, renal dysfunction, lower extremity thrombosis, wound complications, and stroke (all, P ≤ .006; Table II). Consistent with these findings, LTF patients had longer postoperative lengths of stay (mean 3.72 ± 0.33 days vs 2.75 ± 0.32 days vs 2.29 ± 0.07 days; P < .001) and were less frequently discharged to home (86.1% vs 93.3% vs 95.1%; P < .001).
Table II.
Perioperative details
| Variable | Follow-up method | P value | ||
|---|---|---|---|---|
| In-person (n = 8848) | Phone call (n = 1222) | LTF (n = 1239) | ||
| Anesthesia | .13 | |||
| Local | 4.27 (375) | 2.71 (33) | 4.87 (60) | |
| Regional | 5.30 (468) | 4.84 (59) | 4.63 (57) | |
| General | 90.3 (1112) | 92.3 (1125) | 90.3 (1112) | |
| Procedure time, minutes | 143 ± 0.7 | 142 ± 2.0 | 144 ± 2.3 | .10 |
| Contrast material volume, mL | 105 ± 0.7 | 106 ± 1.8 | 109 ± 1.9 | .04 |
| Total RBC units transfused | 0.18 ± 0.01 | 0.26 ± 0.03 | 0.57 ± 0.06 | <.001 |
| Endoleak at completion | .02 | |||
| None | 73.8 (6511) | 70.8 (865) | 74.7 (916) | |
| Type I | 2.90 (256) | 3.36 (41) | 3.91 (48) | |
| Type II | 21.2 (1870) | 23.3 (285) | 18.4 (226) | |
| Type III | 0.42 (37) | 0.65 (8) | 0.41 (5) | |
| Postoperative vasopressors | 3.61 (318) | 5.57 (68) | 6.82 (84) | <.001 |
| Postoperative complication | ||||
| Myocardial infarction | 0.80 (71) | 1.23 (15) | 1.70 (21) | .005 |
| Heart failure | 0.66 (58) | 1.23 (15) | 1.95 (24) | <.001 |
| Respiratory | 0.98 (87) | 1.72 (21) | 2.67 (33) | <.001 |
| Kidney injury | 4.26 (375) | 4.60 (54) | 6.09 (75) | <.001 |
| Acute kidney injury | 2.26 (199) | 3.91 (46) | 5.03 (62) | |
| Temporary dialysis | 0.11 (10) | 0.09 (1) | 0.41 (5) | |
| Permanent dialysis | 1.89 (166) | 0.60 (7) | 0.65 (8) | |
| Lower extremity thrombosis | 0.69 (61) | 0.98 (12) | 1.54 (19) | .006 |
| Wound complication | 0.53 (47) | 0.98 (12) | 1.30 (16) | .003 |
| Return to OR | 1.52 (134) | 2.13 (26) | 3.89 (48) | <.001 |
| Stroke | 0.22 (17) | 0.19 (2) | 0.74 (8) | .004 |
| Minor | 0.20 (15) | 0.19 (2) | 0.46 (5) | |
| Major | 0.03 (2) | 0 (0) | 0.28 (3) | |
| ICU length of stay, days | 0.54 ± 0.01 | 0.73 ± 0.06 | 1.09 ± 0.10 | <.001 |
| Postoperative length of stay, days | 2.29 ± 0.07 | 2.75 ± 0.32 | 3.72 ± 0.33 | <.001 |
| Discharge status | <.001 | |||
| Home | 95.1 (8401) | 93.3 (1140) | 86.1 (1064) | |
| Rehabilitation | 2.75 (243) | 3.60 (44) | 5.26 (65) | |
| Nursing home | 1.99 (176) | 3.03 (37) | 7.44 (92) | |
| Other hospital | 0.12 (11) | 0.08 (1) | 1.13 (14) | |
| Homeless | 0.01 (1) | 0 (0) | 0.08 (1) | |
| Discharge other than to home | 4.88 (431) | 6.71 (82) | 13.9 (172) | <.001 |
ICU, Intensive care unit; LTF, lost to follow-up; OR, operating room; RBC, red blood cell.
Categorical data are presented as percentage (number). Continuous data are presented as mean values ± standard error.
Primary outcome: survival
Overall survival after EVAR for LTF vs phone call follow-up vs in-person follow-up was 67.4% vs 75.9% vs 91.3%, respectively (P < .001; Table III). Estimated 5-year survival was lower for LTF vs phone call follow-up vs in-person follow-up (62.0% vs 68.0% vs 84.0%; log-rank, P < .001; Fig 2, A).
Table III.
Univariable outcomes
| Outcome | Follow-up method | P value | ||
|---|---|---|---|---|
| In-person (n = 8848) | Phone call (n = 1222) | LTF (n = 1239) | ||
| Mortality (overall) | 8.66 (766) | 24.1 (294) | 32.6 (404) | <.001 |
| Survival, months | 36.6 ± 0.3 | 32.3 ± 0.7 | 32.2 ± 0.6 | <.001 |
| Follow-up, months | 13.1 ± 0.1 | 13.5 ± 0.2 | 1.2 ± 0.1 | <.001 |
| Documented postoperative imaging at last follow-up | 85.1 (7530) | 56.1 (685) | 19.4 (240) | <.001 |
| Secondary intervention required | 2.9 (257/8848) | 2.0 (24/1222) | .06 | |
LTF, Lost to follow-up.
Categorical data are presented as percentage (number). Continuous data are presented as mean values ± standard error.
Fig 2.
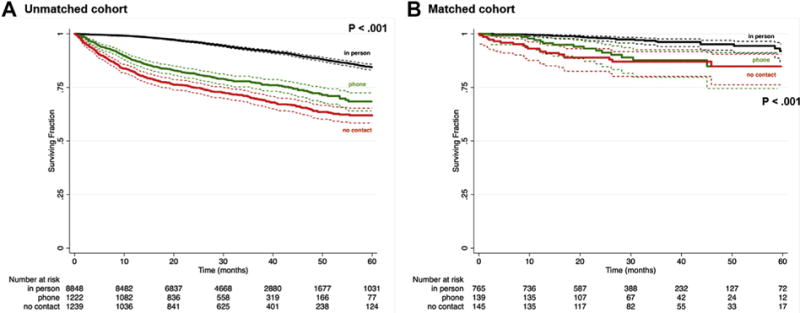
Kaplan-Meier curves showing survival after endovascular aneurysm repair (EVAR) among patients with in-person follow-up vs phone call follow-up vs no follow-up using the aggregate unmatched cohort (A) and matched cohort (B).
On multivariable analysis correcting for baseline differences between groups, there was a significantly higher risk of death for both the LTF group (hazard ratio [HR], 6.45; 95% confidence interval [CI], 4.89-8.51) and phone call follow-up group (HR, 3.48; 95% CI, 2.66-4.57) compared with patients who followed up in person (both, P <.001). Other independent predictors of post-EVAR death included age, self-pay insurance, congestive heart failure, chronic obstructive pulmonary disease, dialysis dependence, classification as unfit for open surgery, postoperative renal failure, and discharge to a nursing home (all, P ≤ .04; Table IV). The Harrell C statistic for the model was 0.81, connoting excellent discrimination.
Table IV.
Multivariable analysisa
| Variable | HR (95% CI) | P value |
|---|---|---|
| Follow-up method | ||
| In-person | Reference | — |
| Phone call | 3.48 (2.66-4.57) | <.001 |
| None | 6.45 (4.89-8.51) | <.001 |
| Age (per year) | 1.05 (1.04-1.06) | <.001 |
| Black race | 0.62 (0.37-1.04) | .07 |
| Primary insurance, self-pay | 2.61 (1.18-5.78) | .02 |
| Unfit for open surgery | 1.60 (1.28-2.00) | <.001 |
| Smoking (any) | 1.27 (0.97-1.68) | .09 |
| CHF | 1.60 (1.26-2.03) | <.001 |
| COPD | 1.25 (1.02-1.54) | .03 |
| Dialysis | 2.68 (1.56-4.62) | <.001 |
| Postoperative renal failure | ||
| Acute kidney injury | 1.66 (1.06-2.62) | .03 |
| Temporary dialysis | 5.80 (2.44-13.8) | <.001 |
| Discharge to nursing home | 2.01 (1.39-2.90) | .04 |
CHF, Congestive heart failure; CI, confidence interval; COPD, chronic obstructive pulmonary disease; HR, hazard ratio.
C statistic = 0.81.
CEM
To refine the association between follow-up method and risk of post-EVAR death, we matched 1049 patients (765 in-person follow-up, 139 phone call follow-up, and 145 LTF) on 30 baseline and perioperative variables (Table V). The matched cohorts were relatively well balanced, especially with respect to those variables found to be independent predictors of mortality after EVAR on multivariable analysis (Table IV).
Table V.
Matched cohort: Baseline characteristics and perioperative details
| Variable | Follow-up method | P value | ||
|---|---|---|---|---|
| In-person (n = 765) | Phone call (n = 139) | None (n = 145) | ||
| Baseline characteristics | ||||
| Age, years | 69.1 ± 0.30 | 71.0 ± 0.74 | 70.9 ± 0.77 | .27 |
| Male gender | 98.8 (756) | 95.7 (133) | 95.9 (139) | .007 |
| White race | 100 (765) | 100 (139) | 100 (145) | — |
| Body mass index, kg/m2 | 28.6 ± 0.17 | 28.5 ± 0.50 | 27.8 ± 0.44 | .002 |
| Maximum AP aortic diameter, mm | 55.2 ± 0.68 | 55.2 ± 0.82 | 54.8 ± 0.70 | <.001 |
| Insurance status | .87 | |||
| Medicare | 67.2 (346) | 68.6 (59) | 70.0 (56) | |
| Commercial | 32.8 (169) | 31.4 (27) | 30.0 (24) | |
| ASA class | .80 | |||
| 1 | 0.69 (5) | 0 (0) | 0.72 (1) | |
| 2 | 11.8 (86) | 12.3 (16) | 7.91 (11) | |
| 3 | 77.2 (561) | 78.5 (102) | 79.9 (111) | |
| 4 | 10.3 (75) | 9.23 (12) | 11.5 (16) | |
| Unfit for open surgery | 0.39 (3) | 0.72 (1) | 0.69 (1) | .81 |
| Comorbidities | ||||
| Hypertension | 91.6 (701) | 88.5 (123) | 91.0 (132) | .49 |
| Diabetes | 4.44 (34) | 5.04 (7) | 4.14 (6) | .93 |
| Smoking | .01 | |||
| Never | 0.78 (6) | 4.32 (6) | 3.45 (5) | |
| Prior | 60.4 (462) | 54.7 (76) | 57.2 (83) | |
| Current | 38.8 (297) | 41.0 (57) | 39.3 (57) | |
| CAD | 17.4 (133) | 15.8 (22) | 24.1 (35) | .12 |
| CHF | 0 (0) | 0 (0) | 0 (0) | — |
| COPD | 12.8 (98) | 17.3 (24) | 17.2 (25) | .18 |
| Dialysis | 0 (0) | 0 (0) | 0 (0) | — |
| Pertinent surgical history | ||||
| Prior CABG or PCI | 25.4 (131) | 20.0 (17) | 32.1 (26) | .20 |
| Prior aortic surgery | 1.44 (11) | 2.88 (4) | 1.38 (2) | .38 |
| Prior aneurysm repair | 0 (0) | 0 (0) | 0 (0) | — |
| Prior PVI | 0 (0) | 0 (0) | 0 (0) | — |
| Living status, home | 100 (765) | 100 (139) | 100 (145) | — |
| Functional status | .004 | |||
| Ambulatory | 99.0 (483) | 98.8 (82) | 93.8 (76) | |
| Ambulatory with assistance | 1.02 (5) | 1.20 (1) | 6.17 (5) | |
| Wheelchair dependent | 0 (0) | 0 (0) | 0 (0) | |
| Bedridden | 0 (0) | 0 (0) | 0 (0) | |
| Perioperative details | ||||
| Anesthesia | .44 | |||
| Local | 2.48 (19) | 2.17 (3) | 4.83 (7) | |
| Regional | 4.58 (35) | 4.35 (6) | 2.76 (4) | |
| General | 92.8 (710) | 93.5 (129) | 91.7 (133) | |
| Procedure time, minutes | 122 ± 2.0 | 120 ± 4.2 | 109 ± 4.0 | .005 |
| Contrast material volume, mL | 94.6 ± 1.9 | 98.8 ± 4.5 | 98.1 ± 5.1 | .09 |
| Total RBC units transfused | 0.003 ± 0.002 | 0.007 ± 0.007 | 0.007 ± 0.007 | .59 |
| Endoleak at completion | .01 | |||
| None | 75.7 (579) | 64.8 (90) | 70.3 (102) | |
| Type I | 1.31 (10) | 1.44 (2) | 4.83 (7) | |
| Type II | 21.8 (167) | 30.9 (43) | 21.4 (31) | |
| Type III | 0.52 (4) | 1.44 (2) | 1.38 (2) | |
| Postoperative vasopressors | 0 (0) | 0 (0) | 0 (0) | — |
| Postoperative complication | ||||
| Myocardial infarction | 0 (0) | 0 (0) | 0 (0) | — |
| Heart failure | 0 (0) | 0 (0) | 0 (0) | — |
| Respiratory | 0 (0) | 0 (0) | 0 (0) | — |
| Kidney injury | 0 (0) | 0 (0) | 0 (0) | — |
| Lower extremity thrombosis | 0.13 (1) | 0 (0) | 0 (0) | .83 |
| Wound complication | 0.26 (2) | 0.72 (1) | 0 (0) | .51 |
| Return to OR | 0 (0) | 0 (0) | 0 (0) | — |
| Stroke | 0 (0) | 0 (0) | 0 (0) | — |
| ICU length of stay, days | 0.05 ± 0.01 | 0.11 ± 0.03 | 0.12 ± 0.03 | .49 |
| Postoperative length of stay, days | 1.19 ± 0.01 | 1.23 ± 0.04 | 1.23 ± 0.04 | .70 |
| Discharge status, home | 100 (765) | 100 (139) | 100 (145) | — |
AP, Anteroposterior; ASA, American Society of Anesthesiologists; CABG, coronary artery bypass graft; CAD, coronary artery disease; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; ICU, intensive care unit; OR, operating room; PCI, percutaneous coronary intervention; PVI, peripheral vascular intervention; RBC, red blood cell.
Categorical data are presented as percentage (number). Continuous data are presented as mean values ± standard error.
Within the matched cohort, estimated 5-year survival after EVAR for LTF vs phone call follow-up vs in-person follow-up was 84.9% vs 84.8% vs 91.9%, respectively (log-rank, P < .001; Fig 2, B). Risk of death remained significantly higher for the LTF group (HR, 3.9; 95% CI, 2.5-6.1) and was similarly poor for the phone call follow-up group (HR, 3.2; 95% CI, 2.1-4.9) compared with patients with in-person follow-up (P < .001).
Secondary outcomes
At a mean follow-up of 36.6 ± 0.3 months, documented postoperative imaging at last follow-up was available in 56.1% (n = 685) of patients with phone call follow-up compared with 85.1% (n = 8848) of patients with in-person follow-up (P < .001). The need for secondary interventions was not significantly different between the in-person and phone call follow-up groups (2.9% vs 2.0%; P = .06; Table III).
To assess whether there was a potential association between endoleak and survival, we created two Kaplan-Meier curves showing post-EVAR survival stratified by both follow-up method and the presence or absence of immediate postoperative (Fig 3, A) or long-term (Fig 3, B) endoleak. There are no significant differences in survival for each group based on endoleak status (all, P = NS).
Fig 3.
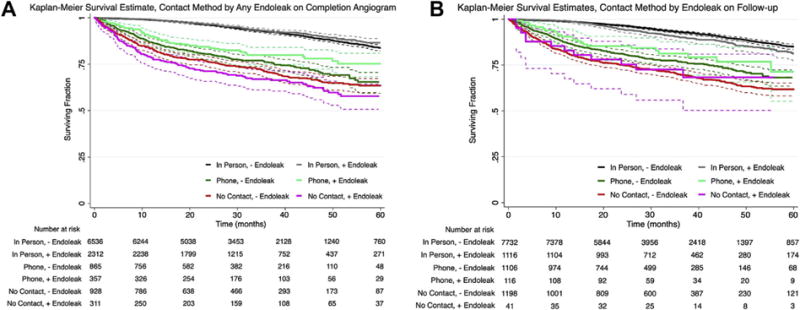
Kaplan-Meier curves showing survival after endovascular aneurysm repair (EVAR) stratified by both follow-up method and the presence or absence of immediate postoperative (A) or long-term (B) endoleak. There are no significant differences in survival for each group based on endoleak status (all, P = NS).
DISCUSSION
Durability has become the most important difference between open aneurysm repair and EVAR. Contemporary research suggests that, unlike open abdominal aortic aneurysm repair, EVAR requires vigilant postimplantation monitoring to rescue late device failure.2,8 As such, current SVS guidelines advise surveillance with computed tomography scans at 1 month and 12 months postoperatively and then annually thereafter to detect endoleaks and aneurysmal enlargement.1 However, recent reports have called the utility of these guidelines into question.5,9-11 In addition, it is unclear whether in-person follow-up is needed as an adjunct to postoperative surveillance imaging for post-EVAR patients.
In the current study, we examined whether there is an association between patient follow-up visits after EVAR and postoperative survival. Our data suggest that patients LTF have inferior long-term survival in comparison with those patients who followed up in person. Patients who are LTF tend to have worse baseline health status, poor functional status, more complicated perioperative courses, and ultimately worse long-term mortality than patients with in-person follow-up. Even after matching patients to account for baseline group differences, the risk of mortality was higher among those patients LTF. Interestingly, remote surveillance by phone call does not appear to be an adequate surrogate for an in-person consultation, as patients with phone call follow-up also have worse mortality outcomes than patients with in-person follow-up. Phone call follow-up is associated with a lower rate of postoperative surveillance imaging as well. Taken together, these data suggest that in-person follow-up after EVAR is associated with better survival outcomes.
Our findings are somewhat contrary to those published by other groups. In a review of 9503 Medicare patients, Garg et al recently demonstrated that nonadherence to SVS post-EVAR imaging guidelines was not associated with poor outcomes.5 Similar findings have been demonstrated using institutional cohorts as well.9-11 However, our study is different from these prior reports on post-EVAR imaging surveillance because it deals specifically with patient follow-up visits after EVAR. Notably, of the 22,000 patients undergoing EVAR during the study period, <60% had recorded follow-up; 11% of patients were LTF, 11% of patients were restricted to phone call follow-up only, and 42% of all EVAR patients had no entry in the follow-up data field in the VQI database. These data suggest that between 20% and 40% of all patients undergoing EVAR by vascular surgeons who engage in the SVS VQI are receiving only one postoperative follow-up visit (and in many cases none).
The question of whether in-person follow-up is necessary after relatively minimally invasive procedures such as EVAR is relatively new and stems largely from an increasing push toward performing elective EVARs at centers of excellence.12 Recent studies have demonstrated better survival outcomes among patients treated with EVAR at high-volume institutions, especially when the patients have multiple comorbidities.13,14 With this centralization of EVAR comes a concern about compliance of the patient with postoperative surveillance. Although some data exist suggesting that distance from a tertiary care center is not a limiting factor in the patient’s adherence to follow-up after EVAR,15 others have shown that shorter driving distances are independent predictors of compliance with postoperative imaging surveillance.3 As such, one potential solution to improve the compliance of patients while minimizing cost and time burdens would be to limit the number of in-person follow-up visits needed after EVAR.
There has been some investigation into the efficacy of telehealth, or remotely provided health care, as a means of follow-up after surgery in other fields. In a pediatric surgery population, a paradigm shift from in-person clinic postoperative visits to postoperative phone call follow-up demonstrated that postoperative phone call follow-up can be an effective tool that improves patient and physician efficiency and satisfaction; 93% of patients were satisfied with phone call follow-up alone, and postoperative hospital costs decreased by 89%.16 More recently, an observational study designed to test the feasibility of a telehealth home monitoring program in 20 patients after liver transplantation showed good compliance of the patients and enhanced monitoring of vital signs postoperatively.17 These home-based health care models are based on previous data suggesting that telehealth programs can improve outcomes in certain patients with chronic diseases, specifically those with higher severity heart failure and diabetes and those with poor access to standard health care.18
No similar in-person vs phone call follow-up study has been reported within a vascular surgery population. However, if it is executed correctly, there could be substantial financial benefits to moving to a phone call follow-up process in certain patients.19 For example, patients could obtain their appropriate follow-up imaging study locally but then do a telehealth visit with vascular providers at the original EVAR center. If they are recovering uneventfully, a time-consuming in-person visit could be avoided. Some of the largest barriers to achieving in-person follow-up after surgery are likely to include inconvenience and the patient’s inability to travel because of either lack of access or deconditioning.19 By implementing a mechanism for remote follow-up using any number of the currently available telecommunication tools, patients would be less burdened to drive long distances for brief visits with their surgeons and thereby may be more inclined to adhere to appropriate follow-up imaging and evaluation guidelines. Follow-up compliance rates could also potentially be improved by having institutions or societal bodies implement automated phone systems that remind patients to schedule their post-EVAR follow-up visits at the appropriate times in whichever form (in-person or remote) is most convenient for them.
Unfortunately, we did not find this to be the case in our study. Survival among patients with phone call follow-up was significantly lower than among patients who followed up in person, to the point that it was equivalent to that of patients who were completely LTF. However, postoperative imaging was available in 85% of patients who followed up in person, compared with only 56% of patients who had phone call follow-up, which may explain the differences in outcomes. Although existing reports suggest that routine postoperative surveillance after EVAR may not be necessary in all patients,2,5,9,11 our data suggest that in-person follow-up is associated with both better survival and better postoperative imaging surveillance compliance compared with phone call follow-up. It is unclear whether phone call follow-up with appropriate post-EVAR imaging would mitigate this difference, especially in cases in which the imaging is performed locally; centers not experienced with postoperative EVAR surveillance may not have the same quality or expertise of ultrasound imaging as centers with substantial EVAR experience.
Whether the benefits that we observe with in-person follow-up after EVAR are directly related to post-EVAR mortality remains to be determined. Although postoperative surveillance imaging was less frequent in the phone call follow-up group compared with the in-person follow-up group, we found a similar rate of secondary interventions among the patients who did receive imaging, suggesting a possible underdiagnosis of EVAR complications in the phone call follow-up group. Consistent with this notion, our 2% to 3% reintervention rate after EVAR is somewhat lower than the 6% to 10% reported by prior studies.5,11 It is also possible that in-person follow-up visits after EVAR allow early identification of potential postoperative complications and multidisciplinary care for existing or evolving comorbidities, thus leading to earlier treatment and ultimately better outcomes for affected patients. In our study, patients who are LTF tended to be older, more frequently female, sicker, and less fit for surgery than patients who have in-person or phone call follow-up. They have more complicated perioperative courses, more postoperative complications, and longer intensive care unit and hospital stays. These findings are consistent with existing literature, which has previously shown independent predictors of lack of follow-up surveillance after EVAR to include older age, multiple comorbidities, urgent or emergent repairs, Medicaid eligibility, and longer driving distances.3,4,20 As such, it is possible that those patients who are LTF have poor compliance or limited health care access that puts them at higher risk for death of any cause (not necessarily related to EVAR) long term. Future studies investigating the association between the patient’s frailty and compliance with recommended post-EVAR imaging and evaluation would be of interest to better define this trend.
To address this possibility, we performed two advanced statistical techniques to test the association between follow-up method after EVAR and death. On multivariable analysis, both phone call follow-up and LTF were associated with a higher risk of death than in-person follow-up after adjusting for baseline differences between groups. In CEM, patients were matched on 30 preoperative, intraoperative, and postoperative variables, thus allowing a comparison of like groups. Similar to our multivariable findings, lack of in-person follow-up was associated with worse post-EVAR survival. Both analyses adjust for potential confounding in different ways,7 but the outcomes were similar. Taken together, it appears that there is a benefit with in-person follow-up after EVAR that is not observed with phone call follow-up alone.
The major potential limitations to this study relate to its dependence on accurate entry of patient data, our limited definition of follow-up, and a lack of detailed anatomic information. The VQI database is a well-recognized, granular database that is designed and collected as part of a quality improvement initiative by vascular surgeons.21 However, there are always concerns with missing, inaccurate, or omitted data with any large database study. For example, the current VQI EVAR database does not report data on the presence or absence of aneurysm sac shrinkage postoperatively, and the available long-term endoleak data are limited. In our study, the presence of an endoleak was not associated with worse outcomes, but it is unclear whether this is due to a true lack of association between endoleak and survival or rather a reflection of the relatively short follow-up or inadequate imaging obtained postoperatively in many of the patients in the database. Furthermore, 42% of patients in the existing VQI EVAR database were missing follow-up classifications. Each of these issues represents an opportunity for database improvement and highlights a need for the SVS to promote improved compliance with data entry by all centers participating in the VQI. In terms of follow-up methods, we defined the in-person, phone call, and LTF groups according to published VQI definitions.6 We were unable to account for how many times a patient followed up, the travel distance from the patient’s home to treating hospital, or whether a patient had follow-up with a physician other than the treating surgeon. Given that we demonstrate worse outcomes for patients who do not follow up in person with the operating surgeon, we would argue that follow-up with unrelated physicians is associated with a survival disadvantage that is similar to no follow-up. Finally, we were unable to match patients on the basis of detailed anatomic aneurysm data or the adherence of surgeons to implanting EVAR according to published instructions for use criteria. Certainly, the LTF group appeared to have more severe disease on univariable analysis. Given that we replicated our findings using two different advanced statistical methods, we do not think that subtle changes in aortic anatomy would substantially change our findings. However, our analysis is missing information on aneurysm sac volume and neck diameter, length, and angulation, all of which have previously been shown to have an impact on the utility of post-EVAR surveillance imaging.22,23
CONCLUSIONS
EVAR patients with more comorbidities and a higher incidence of in-hospital complications tend to be more frequently LTF and ultimately have worse survival outcomes. In-person follow-up is associated with better post-EVAR survival and a higher rate of postoperative imaging. Phone follow-up appears to confer a mortality risk equivalent to lack of follow-up, possibly as a result of inadequate postoperative imaging. Surgeons should stress the importance of office-based postoperative follow-up to all EVAR patients, particularly those with poor baseline health and functional status and more complicated perioperative courses. The utility of phone call follow-up with appropriate post-EVAR imaging remains to be determined.
ARTICLE HIGHLIGHTS.
Type of Research: Retrospective analysis of prospectively collected Vascular Quality Initiative (VQI) data
Take Home Message: This study of 11,309 elective endovascular aneurysm repair patients revealed that patients who are lost to follow-up and those with phone call follow-up have higher 5-year all-cause mortality compared with patients who follow up in-person.
Recommendation: The authors suggest that lack of follow-up after endovascular aneurysm repair is a risk factor for increased 5-year mortality.
Footnotes
Author conflict of interest: J.H.B. is a consultant for Cook Medical and Medtronic.
The editors and reviewers of this article have no relevant financial relationships to disclose per the JVS policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
Presented as a Plenary Presentation at the 2016 Vascular Annual Meeting of the Society for Vascular Surgery, National Harbor, Md, June 8-11, 2016.
AUTHOR CONTRIBUTIONS
Conception and design: CH, DZ, PG, MM
Analysis and interpretation: CH, DZ, IB, DS, JB, JE-J, PG, MM
Data collection: CH, DZ, IB, PG, MM
Writing the article: CH, DZ
Critical revision of the article: CH, DZ, IB, DS, JB, JE-J, PG, MM
Final approval of the article: CH, DZ, IB, DS, JB, JE-J, PG, MM
Statistical analysis: CH, DZ, PG, MM
Obtained funding: Not applicable
Overall responsibility: CH
References
- 1.Chaikof EL, Brewster DC, Dalman RL, Makaroun MS, Illig KA, Sicard GA, et al. SVS practice guidelines for the care of patients with an abdominal aortic aneurysm: executive summary. J Vasc Surg. 2009;50:880–96. doi: 10.1016/j.jvs.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 2.Hoel AW, Schanzer A. Follow-up surveillance after endovas-cular aneurysm repair: less is more? JAMA Surg. 2015;150:964. doi: 10.1001/jamasurg.2015.1334. [DOI] [PubMed] [Google Scholar]
- 3.Wu CY, Chen H, Gallagher KA, Eliason JL, Rectenwald JE, Coleman DM. Predictors of compliance with surveillance after endovascular aneurysm repair and comparative survival outcomes. J Vasc Surg. 2015;62:27–35. doi: 10.1016/j.jvs.2015.02.023. [DOI] [PubMed] [Google Scholar]
- 4.Garg T, Baker LC, Mell MW. Adherence to postoperative surveillance guidelines after endovascular aortic aneurysm repair among Medicare beneficiaries. J Vasc Surg. 2015;61:23–7. doi: 10.1016/j.jvs.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 5.Garg T, Baker LC, Mell MW. Postoperative surveillance and long-term outcomes after endovascular aneurysm repair among Medicare beneficiaries. JAMA Surg. 2015;150:957–63. doi: 10.1001/jamasurg.2015.1320. [DOI] [PubMed] [Google Scholar]
- 6.Society for Vascular Surgery. Vascular Quality Initiative Updated. 2016 Available at: http://www.vascularqualityinitiative.org/. Accessed March 16, 2016.
- 7.Blackwell M, King G, Iacus S, Porro G. CEM: Coarsened exact matching in Stata. Stata J. 2010;9:524–46. [Google Scholar]
- 8.Sternbergh WC, 3rd, Greenberg RK, Chuter TA, Tonnessen BH, Zenith Investigators Redefining postoperative surveillance after endovascular aneurysm repair: recommendations based on 5-year follow-up in the US Zenith multicenter trial. J Vasc Surg. 2008;48:278–84. doi: 10.1016/j.jvs.2008.02.075. discussion: 284-5. [DOI] [PubMed] [Google Scholar]
- 9.Kret MR, Azarbal AF, Mitchell EL, Liem TK, Landry GJ, Moneta GL. Compliance with long-term surveillance recommendations following endovascular aneurysm repair or type B aortic dissection. J Vasc Surg. 2013;58:25–31. doi: 10.1016/j.jvs.2012.12.046. [DOI] [PubMed] [Google Scholar]
- 10.Leurs LJ, Laheij RJ, Buth J, EUROSTAR Collaborators What determines and are the consequences of surveillance intensity after endovascular abdominal aortic aneurysm repair? Ann Vasc Surg. 2005;19:868–75. doi: 10.1007/s10016-005-7751-2. [DOI] [PubMed] [Google Scholar]
- 11.Dias NV, Riva L, Ivancev K, Resch T, Sonesson B, Malina M. Is there a benefit of frequent CT follow-up after EVAR? Eur J Vasc Endovasc Surg. 2009;37:425–30. doi: 10.1016/j.ejvs.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 12.Gifford ED, de Virgilio C. Aortic centers of excellence: shifting the focus. JAMA Surg. 2016;151:845. doi: 10.1001/jamasurg.2016.0838. [DOI] [PubMed] [Google Scholar]
- 13.Mousa AY, Bozzay J, Broce M, Yacoub M, Stone PA, Najundappa A, et al. Novel risk score model for prediction of survival following elective endovascular abdominal aortic aneurysm repair. Vasc Endovascular Surg. 2016;50:261–9. doi: 10.1177/1538574416638760. [DOI] [PubMed] [Google Scholar]
- 14.Hicks CW, Canner JK, Arhuidese I, Obeid T, Black JH, 3rd, Malas MB. Comprehensive assessment of factors associated with in-hospital mortality after elective abdominal aortic aneurysm repair. JAMA Surg. 2016;151:838–45. doi: 10.1001/jamasurg.2016.0782. [DOI] [PubMed] [Google Scholar]
- 15.Sarangarm D, Knepper J, Marek J, Biggs KL, Robertson D, Langsfeld M. Post-endovascular aneurysm repair patient outcomes and follow-up are not adversely impacted by long travel distance to tertiary vascular surgery centers. Ann Vasc Surg. 2010;24:1075–81. doi: 10.1016/j.avsg.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 16.Fischer K, Hogan V, Jager A, von Allmen D. Efficacy and utility of phone call follow-up after pediatric general surgery versus traditional clinic follow-up. Perm J. 2015;19:11–4. doi: 10.7812/TPP/14-017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ertel AE, Kaiser TE, Abbott DE, Shah SA. Use of video-based education and tele-health home monitoring after liver transplantation: results of a novel pilot study. Surgery. 2016;160:869–76. doi: 10.1016/j.surg.2016.06.016. [DOI] [PubMed] [Google Scholar]
- 18.Flodgren G, Rachas A, Farmer AJ, Inzitari M, Shepperd S. Interactive telemedicine: effects on professional practice and health care outcomes. Cochrane Database Syst Rev. 2015;9:CD002098. doi: 10.1002/14651858.CD002098.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dorsey ER, Topol EJ. State of telehealth. N Engl J Med. 2016;375:154–61. doi: 10.1056/NEJMra1601705. [DOI] [PubMed] [Google Scholar]
- 20.Schanzer A, Messina LM, Ghosh K, Simons JP, Robinson WP, 3rd, Aiello FA, et al. Follow-up compliance after endovascular abdominal aortic aneurysm repair in Medicare beneficiaries. J Vasc Surg. 2015;61:16–22.e1. doi: 10.1016/j.jvs.2014.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cronenwett JL, Kraiss LW, Cambria RP. The Society for Vascular Surgery Vascular Quality Initiative. J Vasc Surg. 2012;55:1529–37. doi: 10.1016/j.jvs.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 22.Bastos Goncalves F, van de Luijtgaarden KM, Hoeks SE, Hendriks JM, ten Raa S, Rouwet EV, et al. Adequate seal and no endoleak on the first postoperative computed tomography angiography as criteria for no additional imaging up to 5 years after endovascular aneurysm repair. J Vasc Surg. 2013;57:1503–11. doi: 10.1016/j.jvs.2012.11.085. [DOI] [PubMed] [Google Scholar]
- 23.Schanzer A, Greenberg RK, Hevelone N, Robinson WP, Eslami MH, Goldberg RJ, et al. Predictors of abdominal aortic aneurysm sac enlargement after endovascular repair. Circulation. 2011;123:2848–55. doi: 10.1161/CIRCULATIONAHA.110.014902. [DOI] [PubMed] [Google Scholar]


